Introduction
Nonsmall cell lung cancer (NSCLC) is the most common type of lung cancer, accounting for approximately 85% of all lung cancer cases.1 NSCLC is a heterogeneous disease with different molecular and histological subtypes that respond differently to treatments.2 The current standard of care for NSCLC includes surgery, radiation therapy, chemotherapy, and targeted therapy.1 However, the prognosis of patients with advanced or metastatic NSCLC remains poor, and new treatment options are urgently needed.
Mesenchymal-epithelial transition (MET) is a receptor tyrosine kinase that plays a crucial role in cell proliferation, survival, invasion, and angiogenesis.3 Dysregulation of the MET pathway has been implicated in the development and progression of various cancers, including NSCLC.3 Patients with NSCLC with MET exon 14 skipping mutation is a molecular subgroup that accounts for approximately 3% to 4% of all NSCLC cases.3 One promising targeted therapy for NSCLC is capmatinib, a potent oral type 1B MET tyrosine kinase inhibitor.3
MET inhibitors, such as crizotinib, tepotinib, and capmatinib, have been linked to a transient increase in serum creatinine (SCr) without evidence of acute kidney injury (AKI).4, 5, 6 This phenomenon, known as pseudo-AKI, is commonly associated with medications like cimetidine, trimethoprim, and pyrimethamine.7 There is mounting evidence that pseudo-AKI is also observed in targeted cancer therapies as well.
Diagnosing pseudo-AKI can be challenging due to various confounding factors and the need for precise noninvasive methods to measure kidney function. However, a comprehensive review of the medications being used, the time since the drug was introduced, and the onset of SCr elevation can aid in the diagnosis.6 The proposed mechanism for pseudo-AKI is that blockage of tubular creatinine secretion leads to an increase in serum levels without a concomitant decrease in glomerular filtration.8,9
This retrospective multicenter cohort study aims to investigate the adverse kidney effects of capmatinib therapy in patients with NSCLC. Specifically, we evaluated the prevalence of pseudo-AKI cases and their potential impact on patient outcomes, including all-cause mortality as a competing factor.
Results
There was a total of 33 patients receiving capmatinib therapy for NSCLC treatment at Mayo Clinic between May 2020 and October 2021. Patients were excluded if they had a concurrent intensive care unit admission at the time of their capmatinib therapy initiation (n = 1), if they had a prior liver transplant (n = 1), or if they did not have any baseline or follow-up SCr measures available after capmatinib therapy administration (n = 2); this resulted in a total of 29 patients remaining in the cohort for analysis (Supplementary Figure S1). Baseline characteristics are reported in Supplementary Table S1. The mean (SD) age at treatment initiation was 73.4 (±9.7) years; 16 (55.2%) of the patients were female, and all patients were Caucasian. Hypertension was present in 13 (44.8%) patients. The baseline median SCr was 0.84 mg/dl (interquartile range 0.71, 1.11] (Supplementary Table S1). None of the initial patient characteristics were significantly associated with an increased risk of AKI in unadjusted analyses (Supplementary Table S2).
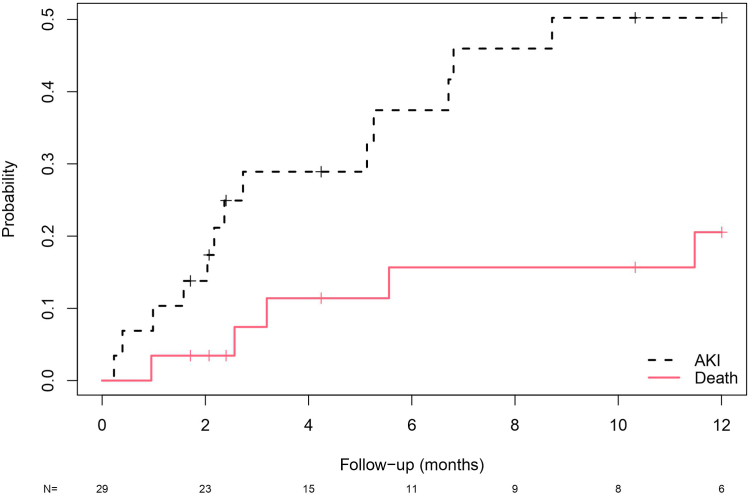
There were 13 (44.8%) AKI events and 5 deaths unrelated to AKI events (17.2%) occurring within 12 months after capmatinib administration and before discontinuation. Cumulative incidence curves for AKI and death as competing factors are plotted in Figure 1. The cumulative incidence of AKI was 50.2%, and death without AKI was 20.5% in the first year (Supplementary Table S3). Among the 13 patients who developed AKI, capmatinib was held for 2 patients, and 1 patient had a dose reduction. All patients had SCr improvement within 95 days of the event without needing kidney replacement therapy. Two patients with AKI had glomerular filtration rate (GFR) measured by cystatin C and iothalamate measurements, and there were no clinically significant differences in GFR compared to the baseline (Supplementary Figure S2). Mortality rates in the AKI group is shown in Supplementary Figure S3.
Figure 1.
Cumulative incidence plot of time from capmatinib administration to AKI, with death as a competing risk. On the y-axis is the probability of the 2 competing events: AKI or death. The x-axis represents time in months. N represents the number of patients each time. AKI, acute kidney injury.
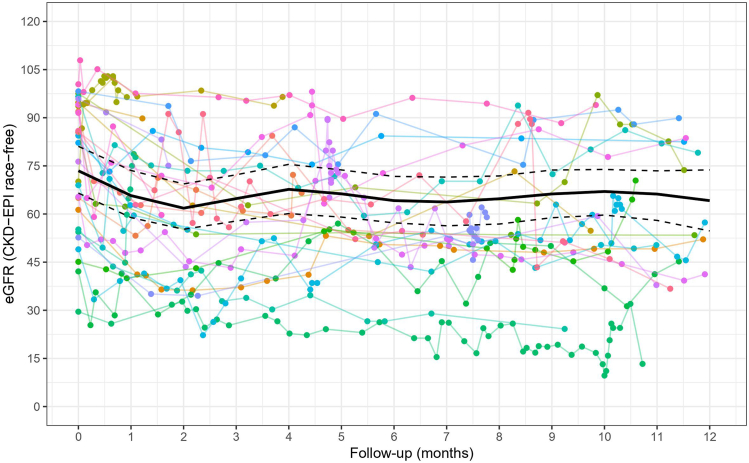
All patients had at least 1 SCr measured within 1 year of capmatinib administration and before stopping treatment, with 406 SCr measurements among all patients. Plots of individual patient trajectories of estimated GFR (eGFR) over time since therapy initiation overlaid with fitted average estimates are illustrated in Figure 2. Predicted eGFR values across time since therapy initiation are presented in Supplementary Table S4. Whereas eGFR values initially showed a pronounced decline after initiation of therapy, this effect was transient, with eGFR levels increasing after 2 months posttreatment and remaining stable over a 1-year follow-up. Among the 11 patients in the cohort who stopped capmatinib due to disease progression (n = 8), hepatotoxicity (n = 2), and SCr rise (n = 1), eGFR increased after drug cessation in all the patients that had SCr measurement after drug interruption (n = 10) (Supplementary Figure S4).
Figure 2.
The plot of eGFR versus time since capmatinib therapy. Each color represents an individual patient trajectory of eGFR up to 12 months after initiation of therapy and is overlaid by a bold line indicating fitted average eGFR versus time using a linear mixed model. The dotted lines indicate the 95% confidence intervals of the fixed effects. eGFR, estimated glomerular filtration rate.
Conclusion
This study characterized adverse kidney outcomes in a retrospective cohort of patients with NSCLC receiving capmatinib therapy. We found that in 29 patients with metastatic NSCLC who received capmatinib for 12 months, there was a cumulative incidence of AKI in approximately 50%, and death without AKI occurred in 20.5% at the end of the follow-up. Although patients initially tended to experience a rise in SCr levels after starting capmatinib treatment, this decrease in eGFR was not sustained and improved for most patients within a year. This reversible SCr rise seems to be more prominent during the first couple of months of therapy, requiring close surveillance to distinguish from a clinically relevant increase in SCr. In addition, 2 patients’ cystatin C measurements during AKI events showed no clinical difference in measured or estimated GFR compared to their baseline values. One of the 2 patients also had stable iothalamate measurements before and after the AKI event; the other did not have iothalamate measurements in the follow-up. Normalization of SCr after discontinuation and the absence of a change in iothalamate clearance during AKI raise the possibility of pseudo-AKI rather than intrinsic renal injury.
One possible mechanistic explanation for the rise in creatinine values without GFR impairment is the competitive inhibition of tubular creatinine transporters. Renal transporters multidrug and toxic extrusion protein 1 and 2-K and organic anion transporter 2 actively secrete creatinine in the proximal renal tubules.8,9 This active secretion can account for up to 40% of total creatinine clearance, depending on the patient’s kidney function. MET inhibitors, such as capmatinib, can inhibit those creatine tubular transporters without interfering with glomerular function.4, 5, 6 This tubular inhibitory mechanism can play a role in the transient SCr increase during the first few months of therapy, yet more studies are needed to reinforce this hypothesis. Despite observing a trend toward an association between lower baseline eGFR and the development of AKI while on capmatinib therapy, we did not identify any significant predictor, possibly due to the small sample size.
Here, we report the first and largest study to assess eGFR trends for up to a year after starting capmatinib therapy. Our findings indicate that relying solely on SCr measurement may be misleading, because distinguishing between pseudo-AKI and true AKI can be difficult with this targeted anticancer agent. This can lead to unnecessary interruption of therapy, in that manner emphasizing the need for more accurate methods of assessing renal function.
Some of the limitations of our study include its retrospective nature, which prevents establishing causality and response to therapy. In addition, none of the patients underwent kidney biopsy because kidney function improved, and cystatin C and iothalamate were measured only in 2 patients.
Pseudo-AKI may negatively impact clinical management, leading to unnecessary interventions and therapy sensation. Our data reinforces the possibility of capmatinib-associated pseudo-AKI development.7 In that manner, we recommend that providers consider assessing kidney function using other methods than GFR estimated by SCr values, such as iothalamate clearance or noncreatinine-based estimates (e.g., cystatin C), before discontinuing capmatinib in patients with elevated SCr, to better characterize patient kidney function.
Disclosure
ASM acknowledges being part of a grant supported by Novartis. SMH is supported by the National Institute of Health K08 DK118120 from the NIDDK and by the Fulk Career Development Award. All the other authors declared no competing interests.
Footnotes
Supplementary Methods.
Figure S1. Flowchart of inclusion and exclusion criteria.
Figure S2. Iothalamate and cystatin C levels before and after AKI.
Figure S3. Cumulative incidence of death among patients with an AKI event.
Figure S4. The plot of eGFR patient trajectories among patients that stopped capmatinib treatment.
Table S1. Patient characteristics at baseline.
Table S2. Univariate associations between baseline patient characteristics and progression to AKI.
Table S3. Cumulative incidence estimates of AKI with death as a competing risk.
Table S4. Predicted eGFR levels after capmatinib therapy initiation using a linear mixed model framework.
STROBE Statement.
Supplementary Material
Supplementary Methods.
Figure S1. Flowchart of inclusion and exclusion criteria.
Figure S2. Iothalamate and cystatin C levels before and after AKI.
Figure S3. Cumulative incidence of death among patients with an AKI event.
Figure S4. The plot of eGFR patient trajectories among patients that stopped capmatinib treatment.
Table S1. Patient characteristics at baseline.
Table S2. Univariate associations between baseline patient characteristics and progression to AKI.
Table S3. Cumulative incidence estimates of AKI with death as a competing risk.
Table S4. Predicted eGFR levels after capmatinib therapy initiation using a linear mixed model framework.
STROBE Statement.
References
- 1.Herbst R.S., Heymach J.V., Lippman S.M. Lung cancer. N Engl J Med. 2008;359:1367–1380. doi: 10.1056/NEJMra0802714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Travis W.D., Brambilla E., Nicholson A.G., et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10:1243–1260. doi: 10.1097/JTO.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 3.Wolf J., Seto T., Han J.Y., et al. Capmatinib in MET exon 14-mutated or MET-amplified non-small-cell lung cancer. N Engl J Med. 2020;383:944–957. doi: 10.1056/NEJMoa2002787. [DOI] [PubMed] [Google Scholar]
- 4.Izzedine H., Brocheriou I., Amoura Z., Mathian A. Acute tubular injury and renal arterial myocyte vacuolization following crizotinib administration. Kidney Int Rep. 2021;6:526–528. doi: 10.1016/j.ekir.2020.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wijtvliet V., Roosens L., De Bondt C., Janssens A., Abramowicz D. Pseudo-acute kidney injury secondary to tepotinib. Clin Kidney J. 2023;16:760–761. doi: 10.1093/ckj/sfac180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohan A., Herrmann S.M. Capmatinib-induced pseudo-acute kidney injury: a case report. Am J Kidney Dis. 2022;79:120–124. doi: 10.1053/j.ajkd.2021.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Nakada T., Kudo T., Ito K. Quantitative consideration of clinical increases in serum creatinine caused by renal transporter inhibition. Drug Metab Dispos. 2023;51:1114–1126. doi: 10.1124/dmd.122.000969. [DOI] [PubMed] [Google Scholar]
- 8.Vanhoutte T., Sprangers B. Pseudo-AKI associated with targeted anti-cancer agents-the truth is in the eye of the filtration marker. Clin Kidney J. 2023;16:603–610. doi: 10.1093/ckj/sfad011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Topletz-Erickson A.R., Lee A.J., Mayor J.G., et al. Tucatinib inhibits renal transporters OCT2 and MATE without impacting renal function in healthy subjects. J Clin Pharmacol. 2021;61:461–471. doi: 10.1002/jcph.1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.