Abstract
Introduction
In some cases, immunoglobulin (IgA)-mediated antiglomerular basement membrane (anti-GBM) disease has been reported. Whether circulating IgA anti-GBM antibodies affect the clinico-pathologic characteristics and outcome of typical anti-GBM disease deserves further study.
Methods
Circulating IgA anti-α3(IV)NC1 antibodies were examined by enzyme-linked immunosorbent assay (ELISA) using recombinant human α3(IV)NC1 as solid phase antigens in 107 patients with anti-GBM disease and 115 controls. Clinical, pathological, and follow-up data of patients were retrospectively analyzed.
Results
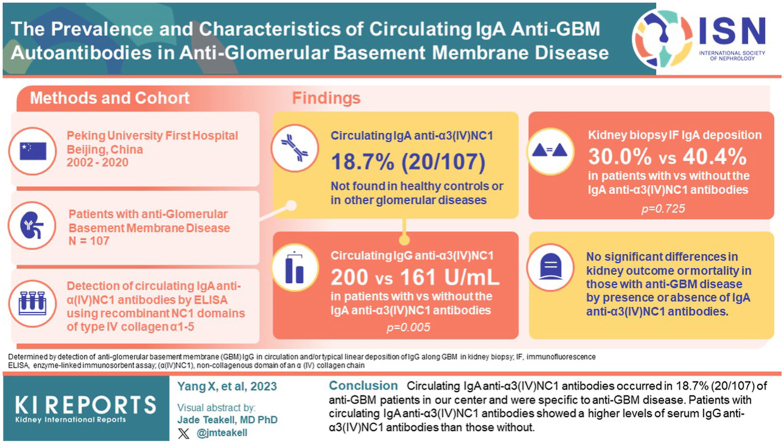
Circulating IgA anti-α3(IV)NC1 antibodies were found in 18.7% (20/107) of patients with anti-GBM disease but were not detected in healthy controls or in patients with other glomerular diseases. The positivity of circulating IgA anti-α3(IV)NC1 antibodies was not associated with whether the patient was with combined IgA nephropathy or other glomerulonephritis. Kidney immunofluorescence showed no statistical difference in IgA deposition between patients with circulating IgA anti-α3(IV)NC1 antibodies and patients without (30.0% vs. 40.4%, P = 0.725). The titers of circulating immunoglobulin G (IgG) anti-α3(IV)NC1 antibodies in patients with circulating IgA anti-α3(IV)NC1 antibodies were significantly higher than those without (200 [183.3, 200] vs. 161 [85.5, 200] U/ml, P = 0.005). There were no significant differences in kidney outcome and mortality between the 2 groups.
Conclusion
Circulating IgA anti-α3(IV)NC1 antibodies occurred in 18.7% (20/107) of patients with anti-GBM in our center and were specific to anti-GBM disease. Patients with circulating IgA anti-α3(IV)NC1 antibodies showed a higher levels of serum IgG anti-α3(IV)NC1 antibodies than those without.
Keywords: Anti-glomerular basement membrane disease, clinical, IgA, IgG, IgA anti-α3(IV)NC1 antibodies, pathological
Graphical abstract
Anti-GBM disease, also known as Goodpasture’s disease, is a rare and severe disease that usually presents as rapidly progressive glomerulonephritis with or without pulmonary hemorrhage. Histopathologic features encompass extensive crescent formation and linear deposition of IgG along the GBM. Typically, the pathogenic antibodies deposited along GBM are IgG directed against the noncollagenous (NC1) domain of the α3(IV) collagen chain (α3(IV)NC1).1 Rapid detection of serum IgG anti-GBM antibodies by commercial ELISA kits has facilitated the early diagnosis of anti-GBM disease. However, anti-GBM disease mediated by other immunoglobulins, especially IgA, have been sporadically reported in individual cases.2, 3, 4, 5, 6, 7, 8 These cases are usually characterized by linear deposition of IgA along the GBM or the presence of IgA anti-GBM antibodies in circulation. The clinical presentations and outcomes of those cases are diverse, which could bring new challenges to the diagnosis and treatment of anti-GBM disease.
Whether circulating IgA antibodies against GBM, particularly α3(IV)NC1, are associated with the disease or kidney injuries are still unknown. Therefore, we investigated the prevalence of circulating IgA antibodies against 5 out of 6 α chains of type IV collagen in a large cohort of anti-GBM disease. We found unexpectedly that nearly 20% of patients with classical anti-GBM disease had detectable circulating IgA antibodies against α3(IV)NC1, especially in those possessing high levels of anti-α3(IV)NC1 IgG. Moreover, the prevalence of circulating IgA anti-GBM antibodies had no correlation with IgA deposition in kidney biopsies, either along GBM or in the mesangial areas. Patients with circulating IgA anti-α3(IV)NC1 antibodies showed a higher levels of serum IgG anti-α3(IV)NC1 antibodies than those without.
Methods
Patients and Samples
A total of 107 patients with anti-GBM disease from August 2002 to June 2020 at Peking University First Hospital were included in the study. The diagnosis was made by the detection of anti-GBM antibodies in circulation and/or typical linear deposition of IgG along the GBM in kidney biopsy, excluding other causes of linear fluorescence including diabetes mellitus and paraproteinemia. Commercial ELISA kits (Euroimmun, Luebeck, Germany) was used to detect circulating IgG anti-GBM antibodies.
Demographic and clinical data were collected at the time of diagnosis. Original kidney biopsy reports were reviewed for pathological analysis. Clinical data, histopathological findings, treatments, and prognosis were collected from medical records at the time of diagnosis and during follow-up.
The primary end point (renal survival) was set as end-stage kidney disease (ESKD) defined as dialysis dependence for >3 months. Patients who had not progressed to ESKD before death were treated as censored data when analyzing renal survival.
Disease control groups included 20 patients with crescentic IgA nephropathy, 20 with antineutrophil cytoplasmic antibodies-associated vasculitis, 15 with thrombotic microangiopathy, 20 with membranous nephropathy, and 20 with diabetic kidney disease. Sera from 20 healthy donors were used as normal controls. Sera or plasmapheresis effluents from all the patients were collected at the time of diagnosis or on the day of kidney biopsy. All samples were stored at −80 °C until detection. This research complied with the ethical principles stated in the Declaration of Helsinki and approved by the ethics committee of Peking University First Hospital.
Preparation of Recombinant Human α(IV)NC1
The recombinant human α(IV)NC1 were produced as described previously.9 In brief, cDNA from the NC1 domain of human type IV collagen α1, α2, α3, α4, and α5 were ligated to a type X collagen triple-helix leader sequence and subcloned into the pcDNA3 vector. We then stably transfected the constructs into a human embryonic kidney cell line (HEK 293 or EBNA). Recombinant proteins were harvested and purified from the medium and designated rHα1(IV)NC1, rHα2(IV)NC1, rHα3(IV)NC1, rHα4(IV)NC1, and rHα5(IV)NC1.10
Detection of Circulating IgA Anti-α(IV)NC1 Antibodies by ELISA
The recombinant human α (IV)NC1 were coated at 2μg/ml with 0.05 mol/l bicarbonate buffer (pH 9.6) into half of the 96-well plates at 37 °C for 60 minutes. The other half was coated with 0.05 mol/l bicarbonate buffer as antigen-free wells to exclude nonspecific binding. Sera or plasmapheresis samples, diluted 1:100 in phosphate-buffered saline and 0.1% Tween 20 (PBST), were added to each well at 37 °C for 60 minutes. Incubation resumed for 60 minutes with horseradish peroxidase-conjugated mouse monoclonal antihuman IgA antibodies (Fc specific, Abcam, Cambridge, United Kingdom) diluted 1:5000. The plates were washed thrice with PBST between each step. Then 3,3’,5,5’-tetramethylbenzidine liquid was applied as substrate. 1mM sulfuric acid was used to terminate the color development approximately 15 minutes later. The absorbance was read at 450 nm. All assays were run in duplicate, and when standard errors >10% were found, samples were re-analyzed. Sera from 20 healthy donors were measured and the cutoff values were set at 2 SDs above the mean.
Detection of Circulating IgA anti-α3(IV)NC1 Antibodies by Western Blot Analysis
Circulating IgA anti-α3(IV)NC1 antibodies were detected by Western blot analysis as described previously.11 Recombinant human α3(IV)NC1 were electrophoresed on a 12.5% sodium dodecyl sulfate polyacrylamide gel under nonreducing conditions and transferred to a nitrocellulose membrane (Schleicher and Schuell, Kent, United Kingdom) using semidry blotting. The membrane was blocked in TBSTM buffer (0.01 mol/l Tris-HCl, pH 7.2, 0.15 mol/l NaCl, 0.1% Tween 20, 20 g/l skimmed milk) for 30 minutes at room temperature, incubated overnight with sera diluted 1:50 in TBSTM at 4 °C, washed and incubated with Goat Anti-Human IgA alpha chain (horseradish peroxidase) preadsorbed secondary antibodies (1:5000, Fc specific, Abcam, Cambridge, United Kingdom) for 1 hour at room temperature. The antibody binding was detected using an enhanced chemiluminescent detection kit (WBKLS0500, Sigma-Aldrich) on an Western blot detection system (Amersham ImageQuant 800, Cytiva).
Statistical Analysis
SPSS (version 22.0, IBM, Chicago, IL) was used for statistical analysis. Quantitative variables were presented as mean and standard deviation (mean ± SD) when they were normally distributed and as median (interquartile range) when they were not normally distributed. Qualitative variables were exhibited as frequency and percentage (n [%]). Differences of continuous variables were assessed using t-test for data that were normally distributed or Mann–Whitney U test for data that were not normally distributed. Categorical variables were compared with chi-square or Fisher exact test. Survival analyses were conducted using Kaplan-Meier curves. Univariate survival analysis was processed by log-rank test. Spearman correlation analysis was used for correlation analysis. All statistical analyses were 2-tailed and a value of P < 0.05 was considered significant. GraphPad Prism 8 (GraphPad Software, San Diego, CA) was used to draw charts.
Results
Demographic and Clinical Characteristics
A total of 107 patients with anti-GBM disease were enrolled in this study, including 51 males and 56 females. The demographic and clinical data are shown in Table 1. The mean age at the time of diagnosis was 54.4 ± 17.0 years. Thirty-five (35/107, 32.7%) patients were smokers, and 49 (49/107, 45.8%) patients had prodromal infections before disease onset. Hemoptysis was observed in 26 (24.3%) patients. Among all the patients, 104 (97.2%) presented with acute kidney injury or acute kidney disease on diagnosis with a median level of serum creatinine at 659.6 (interquartile range, 412.0–932.0) μmol/l. Initial kidney replacement therapy was required in 72 (67.3%) patients. Serum antineutrophil cytoplasmic antibodies were positive in 27 (25.2%) patients, with 26 sera reactive to myeloperoxidase and 1 to proteinase 3. There was no correlation between circulating IgG antibody titer and kidney outcome (P = 0.61) or patient outcome (P = 0.229).
Table 1.
Clinical characteristics of patients with anti-GBM disease according to circulating IgA anti-α3(IV)NC1 antibodies status
| Characteristics | Total patients (n = 107) | IgA Anti-α3(IV)NC1 antibodies Positive (n = 20) | IgA Anti-α3(IV)NC1 antibodies Negative (n = 87) | P-value |
|---|---|---|---|---|
| Demography | ||||
| Gender (male/female) | 51/56 | 11/9 | 40/47 | 0.466 |
| Age (years) | 54.4 ± 17.0 | 55.5 ± 17.7 | 54.2 ± 16.9 | 0.767 |
| Clinical features | ||||
| Smoking | 35 (32.7%) | 7 (35.0%) | 28 (32.2%) | 0.809 |
| Prodromal infection | 49 (45.8%) | 10 (50.0%) | 39 (44.8%) | 0.675 |
| Hemoptysis | 26 (24.3%) | 6 (30.0%) | 20 (23.0%) | 0.566 |
| AKD and AKI | 104 (97.2%) | 20 (100.0%) | 84 (96.6%) | 1.000 |
| SCr on diagnosis (μmol/l) | 659.6 (412.0, 932.0) | 697.3 (439.0, 975.2) | 650 (364, 932) | 0.832 |
| Oliguria/anuria | 53 (49.5%) | 13(65.0%) | 40 (46.0%) | 0.125 |
| Gross hematuria | 46 (43.0%) | 9(45.0%) | 37 (42.5%) | 0.840 |
| Microscopic hematuria | 56 (52.3%) | 11(55.0%) | 45 (51.7%) | 0.791 |
| Proteinuria | 101 (94.4%) | 19(95.0%) | 82 (94.3%) | 1.000 |
| Nephrotic syndrome | 14 (13.1%) | 1(5.0%) | 13 (14.9%) | 0.460 |
| Serum albumin (g/l) | 30.4 ± 5.4 | 29.0 ± 6.5 | 30.7 ± 5.2 | 0.231 |
| Anemia | 98 (91.6%) | 16 (80.0%) | 82 (94.3%) | 0.061 |
| Hemoglobin (g/l) | 91.5 ± 21.7 | 95.2 ± 28.2 | 90.7 ± 20.0 | 0.507 |
| ANCA | 27 (25.2%) | 6 (30.0%) | 21 (24.1%) | 0.586 |
| MPO-ANCA/PR3-ANCA/both | 26/1/0 | 6/0/0 | 20/1/0 | 1.000 |
| Anti GBM antibodies (ELISA) | 107 (100.0%) | 20 (100.0%) | 87 (100.0%) | - |
| Anti-GBM antibodies (U/ml) | 184 (105.5, 200) | 200 (183.3, 200) | 161 (85.5, 200) | 0.005a |
| Combined GN | ||||
| IgAN and HSPGN | 12 (11.2%) | 2 (10.0%) | 10 (11.5%) | 1.000 |
| MN | 12 (11.2%) | 2 (10.0%) | 10 (11.5%) | 1.000 |
| AAV | 27 (25.2%) | 6 (30.0%) | 21 (24.1%) | 0.586 |
| Other GN | 1 (0.9%) | 0 | 1 (1.1%) | 1.000 |
| Time between onset and diagnosis (median, mo) | 1.0 (0.7, 2.0) | 0.7 (0.5, 2.0) | 1.0 (0.8, 2.0) | 0.271 |
| Treatment | ||||
| Dialysis on diagnosis | 72 (67.3%) | 13 (65.0%) | 59 (67.8%) | 0.809 |
| Plasmapheresis | 106 (99.1%) | 20 (100.0%) | 86 (98.9%) | 1.000 |
| Corticosteroids | 105 (98.1%) | 20 (100.0%) | 85 (97.7%) | 1.000 |
| Corticosteroid pulses | 94 (87.9%) | 19 (95.0%) | 75 (86.2%) | 0.460 |
| Cyclophosphamide | 81 (75.7%) | 16 (80.0%) | 65 (74.7%) | 0.776 |
| Rituximab | 7 (6.5%) | 2 (10.0%) | 5 (5.7%) | 0.613 |
| Other immunosuppressive agent | 3 (2.8%) | 0 | 3(3.4%) | 1.000 |
| Outcome | ||||
| Follow-up duration (median, mo) | 30.8 (12.0, 59.0) | 27.8 (16.3, 59.8) | 32.2 (9.3, 58.4) | 0.857 |
| ESKD | 59 (55.1%) | 7 (35.0%) | 52 (59.8%) | 0.045a |
| Death | 18 (16.8%) | 6 (30.0%) | 12 (13.8%) | 0.100 |
AAV, ANCA-associated vasculitis; AKD, acute kidney disease; AKI, acute kidney injury; ANCA, antineutrophil cytoplasmic antibodies; CKD, chronic kidney disease; ESKD, end stage kidney disease; GBM, glomerular basement membrane; GN, glomerulonephritis; HSPGN, Henoch–Schönlein purpura glomerulonephritis; IgAN, IgA nephropathy; MN, membranous nephropathy; MPO, myeloperoxidase; PR3, proteinase 3; SCr, serum creatinine.
Unless otherwise indicated, values are given as mean ± SD, frequency (percentage), or median (interquartile range). P-values is the comparison between IgA Anti-α3(IV)NC1 antibodies positive and negative group.
Significant P-values.
Plasmapheresis were applied in 106 (106/107, 99.1%) patients. Of the patients, 105 (98.1%) received corticosteroids, 81 (81/107, 75.7%) received cyclophosphamide, and 7 (7/107, 6.5%) received rituximab. The median duration of follow-up time was 30.8 months (interquartile range, 12.0–59.0). At the end of follow-up, 59 (59/107, 55.1%) patients progressed to ESKD, and 18 (18/107, 16.8%) deaths had occurred.
Circulating IgA Anti-α(IV)NC1 Antibodies
Circulating IgA anti-α(IV)NC1 antibodies were detected by ELISA, using recombinant human α(IV)NC1 as solid phase antigens. In total, 20 sera (20/107, 18.7%) of the patients with anti-GBM disease showed positive circulating IgA antibodies binding to α3(IV)NC1. None of the sera from disease controls were found positive, including patients with crescentic IgA nephropathy or other kidney diseases (Figure 1a). Among the 20 patients with circulating IgA anti-α3(IV)NC1 antibodies, serum IgA antibodies against other α chains were only detected in 2 sera, with one reactive to α1, α2 and α4(IV)NC1 and 1 to α4(IV)NC1. Circulating IgA anti-α3(IV)NC1 antibodies were further confirmed by Western blot analysis (Figure 1b).
Figure 1.
(a) Investigation of circulating IgA anti-α3 (IV)NC1 antibodies by ELISA. Sera or plasmapheresis effluents from patients with anti-GBM disease/Good pasture’s disease (GP disease, n = 107), crescentic IgA nephropathy (cIgAN, n = 20), ANCA-associated vasculitis (AAV, n = 20), thrombotic microangiopathy (TMA, n = 15), membranous nephropathy (MN, n = 20), diabetic kidney disease (DKD, n = 20) and normal controls (NC, n = 20) were assayed for circulating IgA anti-α3 (IV)NC1 antibodies by indirect ELISA. The scatterplot shows human IgA binding, expressed as OD. The dotted line shows the cutoff values, defined as 2 SD above the mean OD of normal control sera. (b) Western blot analysis of circulating IgA anti-α3(IV)NC1 antibodies. AAV, ANCA-associated vasculitis; cIgAN, crescentic IgA nephropathy; DKD, diabetic kidney disease; GP, Good pasture’s; M, molecular weight marker; N1 and N2, sera from 2 negative controls; NC, normal control; P1 and P2, sera from 2 patients with circulating IgA anti-α3(IV)NC1 antibodies detected by ELISA; TMA, thrombotic microangiopathy.
Comparison of Patients With Anti-GBM Disease With or Without Circulating IgA Anti-α3(IV)NC1 Antibodies
Patients with anti-GBM disease possessing circulating IgA antibodies against α3(IV)NC1 showed significantly higher levels of serum IgG anti-α3(IV)NC1 antibodies, compared to those without circulating IgA anti-α3(IV)NC1 antibodies (median levels of antibody: 200 vs. 161 RU/ml, P = 0.005). There were no significant differences between the 2 groups with or without circulating IgA anti-α3(IV)NC1 antibodies in gender, age, smoking, prodromal infection, and hemoptysis.
Among the 107 patients with anti-GBM disease enrolled in this study, 59 had kidney biopsies. Kidney pathologic characteristics of the biopsied patients with anti-GBM with or without circulating IgA anti-α3(IV)NC1 antibodies were further compared, as shown in Table 2. Immunofluorescence examination was done in 57 patients, because 2 of the 59 biopsied patients had no glomeruli on immunofluorescence tissues. Of the 57 patients who underwent immunofluorescence detection, 4 patients had no linear IgG deposition, including 1 patient with circulating IgA anti-α3(IV)NC1 antibodies and 3 patients without circulating IgA anti-α3(IV)NC1 antibodies. However, immunofluorescence tissues detected by immunofluorescence of these 4 cases contained only sclerotic glomeruli, which probably contributed to the negativity of IgG detection. IgA deposition were observed in 3 (3/10, 30.0%; 1 along the GBM and 2 in the mesangial areas) patients with circulating IgA anti-α3(IV)NC1 antibodies, and 19 (19/47, 40.4%; 2 along the GBM and 17 in the mesangial areas) without the circulating IgA anti-α3(IV)NC1 antibodies (P = 0.725). There were no differences in IgA deposition either along the GBM (P = 0.446) or in the mesangial areas (P = 0.469) in the patients with anti-GBM with or without circulating IgA anti-GBM antibodies. No differences were observed in IgG, IgM, C3, C1q, or FRA deposition in patients with or without circulating IgA anti-α3(IV)NC1 antibodies. Deposition of electric dense deposits observed by electron microscope were comparable in the 2 groups (25% vs. 34.9%, P = 0.703). Combined IgA nephropathy or Henoch–Schönlein purpura glomerulonephritis was present in 2 of 10 patients with circulating IgA anti-α3(IV)NC1 antibodies, and in 10 of 47 patients without the circulating IgA anti-α3(IV)NC1 antibodies. There were no significant differences in the percentage of crescent formation and tubular-interstitial lesions between the 2 groups.
Table 2.
Pathological characteristics of patients with anti-GBM disease according to circulating IgA anti-α3(IV)NC1 antibodies status
| Characteristics | IgA anti-α3(IV)NC1 antibodies Positive (n = 10) | IgA anti-α3(IV)NC1 antibodies Negative (n = 49) | P-value |
|---|---|---|---|
| Immunofluorescence | 10 | 47 | |
| IgG linear deposition, n (%) | 9 (90%) | 44 (93.6%) | 0.548 |
| IgA deposition, n (%) | 3 (30%) | 19 (40.4%) | 0.725 |
| Mesangial region, n (%) | 2 (20%) | 17 (36.2%) | 0.469 |
| Along GBM, n (%) | 1 (10%) | 2 (4.2%) | 0.446 |
| IgM deposition, n (%) | 3 (30%) | 21 (44.7%) | 0.494 |
| C3 deposition, n (%) | 8 (80%) | 37 (78.7%) | 1.000 |
| C1q deposition, n (%) | 4 (40%) | 12 (25.5%) | 0.443 |
| FRA deposition, n (%) | 5 (50%) | 20 (42.6%) | 0.735 |
| Light microscopy | 10 | 49 | |
| Number of glomerulus | 18.5 (12.0, 30.8) | 24.0 (18.5, 33.0) | 0.097 |
| Crescent formation, n (%) | 10 (100%) | 47 (95.9%) | 1.000 |
| Percentage of crescents (%) | 87.6 (75.0, 97.6) | 81.3 (60.1, 95.6) | 0.484 |
| Percentage of Cellular crescents (%) | 54.0 (19.2, 90.9) | 25.0 (12.3, 55.8) | 0.119 |
| Percentage of Cellular-fibrous crescents (%) | 27.3 (0, 46.6) | 38.9 (5.9, 59.0) | 0.459 |
| Percentage of Fibrous crescents (%) | 0 (0, 3.8) | 0 (0, 8.2) | 0.311 |
| TA/IF, n (%) | 9 (90%) | 43 (87.8%) | 1.000 |
| Electron microscopy | 8 | 43 | |
| Electric dense deposit, n (%) | 2 (25%) | 15 (34.9%) | 0.703 |
| Location (subepithelial/basement membrance/subendothelial/mesangial area), n | 2/1/0/2 | 9/2/1/10 |
FRA, fibrinogen-fibrin related antigens; IgG, immunoglobulin G; IgA, immunoglobulin A; IgM, immunoglobulin M; TA/IF, tubular atrophy and interstitial fibrosis.
Values are given as frequency (percentage), or median (interquartile range).
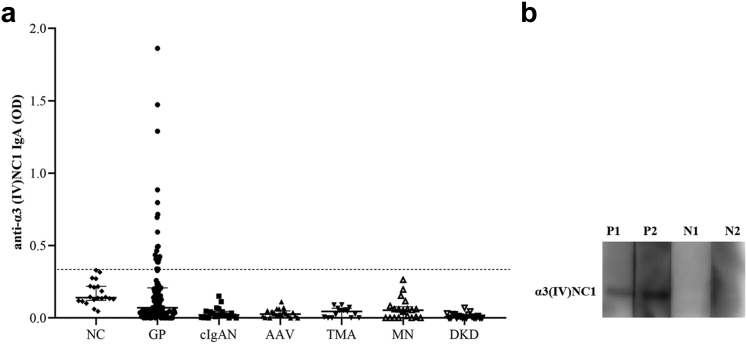
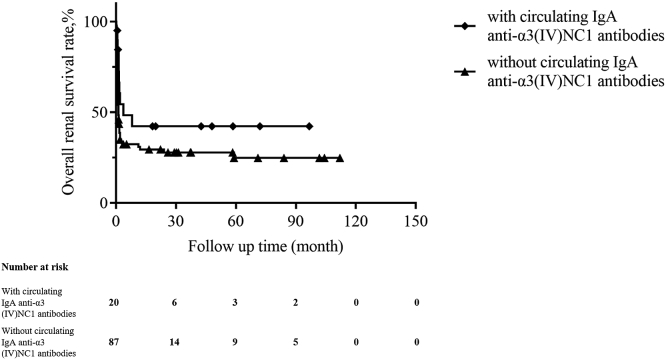
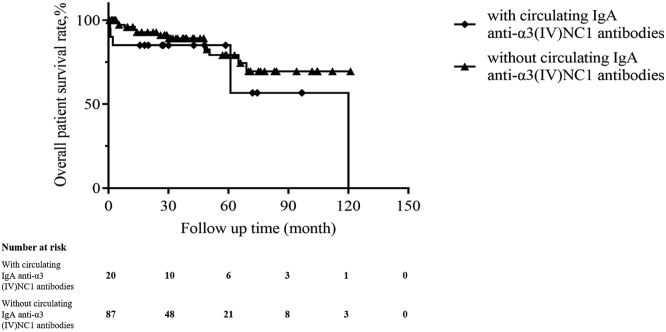
Follow-up duration between the 2 groups were comparable (median, 27.8 vs. 32.2 months, P = 0.857), as were treatments including immunosuppressive therapy and plasmapheresis (Table 1). A lower percentage of patients with the circulating IgA anti-α3(IV)NC1 antibodies progressed to ESKD than those without the antibodies (35.0% vs. 59.8%, P = 0.045; Table 1). Univariate survival analysis showed that patients with circulating IgA anti-α3(IV)NC1 antibodies had a tendency of better kidney survival than those without the antibodies; however, there was no statistical significance (P = 0.094; Figure 2). Patients who had not progressed to ESKD before death were treated as censored data when analyzing renal survival. Mortality was higher in the group of patients with circulating IgA anti-α3(IV)NC1 antibodies (30% vs. 13.8%); however, there was no statistical significance (P = 0.100). Univariate patient survival analysis showed that patients with or without circulating IgA anti-α3(IV)NC1 antibodies had comparable patient survival rates (Figure 3).
Figure 2.
Kaplan-Meier analysis for renal survival compared between patients with and without circulating IgA anti-α3 (IV)NC1 antibodies (P = 0.094).
Figure 3.
Kaplan-Meier analysis for patient survival compared between patients with and without circulating IgA anti-α3 (IV)NC1 antibodies (P = 0.235).
Discussion
To our best knowledge, this is the first large-scale investigation of circulating IgA anti-GBM antibody in patients with anti-GBM disease. It was shown that circulating IgA anti-α3(IV)NC1 antibody could be detected in 18.7% of patients with anti-GBM disease, but not in other kidney diseases including crescentic IgA nephropathy. The presence of circulating IgA antibodies against α3 had no correlation with IgA deposition or the presence of combined IgA nephropathy in kidney biopsies of patients with anti-GBM.
In the present study, most patients with serum IgA anti-GBM antibodies (18/20) had isolated recognition of α3(IV)NC1 alone, 1 serum was reactive to α1, α2, and α4(IV)NC1, and 1 to α4(IV)NC1 simultaneously. Because the standard assays for circulating anti-GBM antibodies are designed to detect IgG, IgA anti-GBM antibodies were not routinely detected in clinical practices. It has been demonstrated that IgG anti-α3(IV)NC1 antibodies recognized 2 major conformational epitopes, designated as EA and EB.12 On Western-blot analysis, sera possessing IgA anti-α3(IV)NC1 antibodies also recognized α3 chain under nonreducing condition. Therefore, we speculated that these IgA antibodies might also recognize the same conformational epitopes of EA and EB. However, further studies might be needed to confirm the specific epitopes recognized by these IgA antibodies. Anti-GBM disease mediated by IgA has been sporadically reported in individual cases.2, 3, 4, 5, 6, 7, 8 These cases are usually characterized by linear deposition of IgA along the GBM, which are different from ours. In the rare cases of IgA-mediated anti-GBM disease reported, only 6 cases had evaluated the specificity of target antigens,2,3,7,13, 14, 15 reviewed in Table 3. Zou et al.3 reported the case of a 37-year-old woman with anti-GBM disease. Sera of the patient had both IgG and IgA autoantibodies targeting α3(IV)NC1, which was similar to our cases. After 2 weeks of treatment, including plasmapheresis, steroids and cyclophosphamide, her serum creatinine level had decreased from 310 μmol/l to 83.4 μmol/l. Four years later, her serum creatinine was still within the normal range. Other cases reported IgA antibodies against different target antigens on GBM other than α3 chain: 1 case with both skin and kidney involvement had IgA autoantibodies to the α5 and α6(IV)NC113; 1 had IgA autoantibodies targeting α1(IV)NC12; and 1 with a history of proliferative lupus nephritis was detected with serum IgA binding to a 38–48 kD collagenase solubilized antigen from human GBM.7 A 54-year-old man with IgA-kappa mediated anti-GBM disease had circulating monoclonal IgA-kappa antibody; however, it did not recognize NC1 monomeric components of the 6-chains of type IV collagen.15 Five years after the diagnosis, he underwent kidney transplantation for ESKD, but relapsed 2 years later.14 A serum monoclonal IgA1-kappa antibody was detected then targeting collagenase-sensitive epitopes within α1/α2(IV) collagen.14
Table 3.
Clinical and pathological data of previously reported IgA-mediated anti-GBM disease with the investigations on target antigens
| Characteristics |
1 |
2 |
3 |
4 |
5 |
6 |
|---|---|---|---|---|---|---|
| Authors | Antonelou et al.2 | Zou et al.3 | Ho et al.7 | Ghohestani et al.13 | Borza et al.14 | Fervenza et al.15 |
| Publication yr | 2019 | 2021 | 2008 | 2003 | 2005 | 1999 |
| Gender /age, yr | M/37 | F/37 | F/74 | M/72 | M/62 | M/54 |
| Hemoptysis | N | N | N | N | Y | Y |
| AKD or AKI | Y | Y | N | Y | Y | N |
| SCr on diagnosis (μmol/l) | 1108 | 310 | 144 | 495 | 177 | 106 |
| Oliguria/anuria | N | NA | NA | NA | N | N |
| Gross hematuria | N | N | N | N | N | N |
| Microscopic hematuria | Y | Y | Y | Y | Y | Y |
| Proteinuria | Y | Y | Y | Y | NA | Y |
| Serum albumin (g/l) | NA | 24 | NA | 29 | NA | 41 |
| Hemoglobin (g/l) | 72 | 124 | NA | 80 | NA | 115 |
| ANCA | negative | negative | negative | negative | negative | negative |
| Anti-GBM (IgG) antibodies (ELISA) | negative | positive (104 U/ml) | negative | negative | negative | negative |
| Anti-GBM (IgA) antibodies | α1(IV)NC1 | a3(IV)NC1 | 38–48 kDa antigens (collagenasesolubilized from human GBM) | α5/α6(IV) NC1 | α1/α2(IV) NC1 | did not recognize α1∼α6(IV) NC1 |
| IgA linear deposition along the GBM | Y | Y | Y | Y | Y | Y |
| Combined GN | N | N | A history of LN | N | N | N |
| Treatment | ||||||
| Dialysis on diagnosis | Y | N | N | N | N | N |
| Plasmapheresis | Y | Y | N | Y | Y | Y |
| Corticosteroids | Y | Y | Y | Y | Y | Y |
| Corticosteroid pulses | N | Y | N | Y | Y | Y |
| Cyclophosphamide | N | Y | Y | Y | N | Y |
| Other immunosuppressive agent | MMF | N | N | N | Sirolimus, MMF | N |
| Follow-up duration | 12 mo | 48 mo | 4 mo | 8 mo | 2 mo | 36 mo |
| Outcome (renal function) | Renal transplant | Normal | Improved | ESKD | ESKD | Stable |
AKD, acute kidney disease; AKI, acute kidney injury; ANCA, antineutrophil cytoplasmic antibodies; ESKD, end- stage kidney disease; F, female; GBM, glomerular basement membrane; GN, glomerulonephritis; LN, lupus nephritis; M, male; MMF, mycophenolate mofetil; N, no; NA, not available; SCr, serum creatinine; Y, yes.
We observed an interesting phenomenon that patients with circulating IgA anti-α3(IV)NC1 antibodies presented with higher level of circulating IgG anti-α3(IV)NC1 antibodies on diagnosis than the patients without. In addition, patients with circulating IgA antibodies had a higher proportion of cellular crescents. These patients might be diagnosed and received treatment at an earlier stage of the disease, which might result in a better kidney prognosis. However, given the small sample of this rare entity, no direct conclusions can be drawn, and further investigations are needed in the future to elucidate the clinical significance of these IgA antibodies. The relationship between the circulating IgA anti-α3(IV)NC1 antibodies and circulating IgG anti-α3(IV)NC1 antibodies is unclear. Two chronological sequences might be possible. First, IgA anti-α3(IV)NC1 antibodies may be a concomitant phenomenon during IgG autoimmune responses. Second, IgA anti-α(IV)NC1 antibodies might be the primary antibodies in part of the patients with anti-GBM. It was shown that prodromal infections and fever were closely associated with the anti-GBM disease.16 IgA is the second most abundant immunoglobulin in serum, and the most abundant ones at mucosal sites,17 which plays an important role in mucosal defense against pathogenic microorganisms. It might be possible that autoimmune reactions of IgA might be elicited by infections. In the cases with “double positive antibodies,” further studies are needed to elucidate the potential relationships between anti-GBM disease and circulating IgA anti-α(IV)NC1 antibodies.
No correlation was observed between circulating IgA anti-α3(IV)NC1 antibodies and pathologically deposited IgA. In patients with circulating IgA anti-α3(IV)NC1 antibodies, only 3 (30%) patients had IgA deposition in glomeruli on immunofluorescence, and it had no difference when compared to the patients without the circulating IgA anti-α3(IV)NC1 antibodies. Moreover, the presence of circulating IgA anti-α3(IV)NC1 antibodies was not associated with whether the patient had combined IgA nephropathy or other glomerulonephritis. Whether the IgA deposited in the glomerulus is homologous with the circulating IgA targeting α3(IV)NC1 merits further exploration.
In conclusion, circulating IgA anti-α3(IV)NC1 antibodies occurred in about 18.7% of patients with classical anti-GBM disease and were specific to α3(IV)NC1. Patients with circulating IgA anti-α3(IV)NC1 antibodies showed a higher levels of serum IgG anti-α3(IV)NC1 antibodies than those without. Further investigations might be needed to elucidate the immune modulation effects of circulating IgA anti-GBM antibodies and their value as potential biomarkers.
Disclosure
All the authors declared no competing interests.
Acknowledgments
Funding
This work was supported by the National Natural Science Foundation of China (82090020 to MHZ, 82070732 to ZC, 82270763 to XYJ, and 82200789 to CRS), the Beijing Municipal Science and Technology Commission Foundation (Z221100007922041 to ZC), the CAMS Innovation Fund for Medical Sciences (2019-I2M-5-046), Peking University First Hospital Fund for commercialization of scientific and technological achievements (2022CX12).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Footnotes
STROBE Statement.
Supplementary Material
STROBE Statement.
References
- 1.Turner N., Mason P.J., Brown R., et al. Molecular cloning of the human Goodpasture antigen demonstrates it to be the alpha 3 chain of type IV collagen. J Clin Invest. 1992;89:592–601. doi: 10.1172/JCI115625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antonelou M., Henderson S.R., Bhangal G., et al. Binding truths: atypical anti-glomerular basement membrane disease mediated by IgA anti-glomerular basement membrane antibodies targeting the alpha1 chain of Type IV collagen. Kidney Int Rep. 2019;4:163–167. doi: 10.1016/j.ekir.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zou G., Lu H., Zhuo L., Zou W., Li W. Anti-glomerular basement membrane disease mediated by IgG and IgA: a case report. Ren Fail. 2021;43:774–778. doi: 10.1080/0886022X.2021.1914658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bacalja J., Zibar L., Ljubanovic D.G. IgA-mediated anti-glomerular basement membrane disease. A case report. Nefrol (Engl Ed) 2018;38:339–341. doi: 10.1016/j.nefro.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Ke C.L., Wen Y.K., Chen M.L. IgA variant of anti-glomerular basement membrane glomerulonephritis associated with pulmonary hemorrhage and microangiopathic hemolytic anemia. Ren Fail. 2012;34:657–660. doi: 10.3109/0886022X.2012.664807. [DOI] [PubMed] [Google Scholar]
- 6.Moulis G., Huart A., Guitard J., Fortenfant F., Chauveau D. IgA-mediated anti-glomerular basement membrane disease: an uncommon mechanism of Goodpasture’s syndrome. Clin Kidney J. 2012;5:545–548. doi: 10.1093/ckj/sfs087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ho J., Gibson I.W., Zacharias J., Fervenza F., Colon S., Borza D. Antigenic heterogeneity of IgA anti-GBM disease: new renal targets of IgA autoantibodies. Am J Kidney Dis. 2008;52:761–765. doi: 10.1053/j.ajkd.2008.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wen Y.K., Wen K.I. An unusual case of IgA-mediated anti-glomerular basement membrane disease. Int Urol Nephrol. 2013;45:1229–1234. doi: 10.1007/s11255-012-0162-8. [DOI] [PubMed] [Google Scholar]
- 9.Chen L., Hellmark T., Wieslander J., Bolton W.K. Immunodominant epitopes of alpha3(IV)NC1 induce autoimmune glomerulonephritis in rats. Kidney Int. 2003;64:2108–2120. doi: 10.1046/j.1523-1755.2003.00332.x. [DOI] [PubMed] [Google Scholar]
- 10.Jia X.Y., Qu Z., Cui Z., Yang R., Zhao J., Zhao M.H. Circulating anti-glomerular basement membrane autoantibodies against alpha3(IV)NC1 undetectable by commercially available enzyme-linked immunosorbent assays. Nephrol (Carlton) 2012;17:160–166. doi: 10.1111/j.1440-1797.2011.01511.x. [DOI] [PubMed] [Google Scholar]
- 11.Cui Z., Zhao M., Jia X., et al. Antibodies to α5 chain of collagen IV are pathogenic in Goodpasture’s disease. J Autoimmun. 2016;70:1–11. doi: 10.1016/j.jaut.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Netzer K.O., Leinonen A., Boutaud A., et al. The goodpasture autoantigen. Mapping the major conformational epitope(s) of alpha3(IV) collagen to residues 17–31 and 127–141 of the NC1 domain. J Biol Chem. 1999;274:11267–11274. doi: 10.1074/jbc.274.16.11267. [DOI] [PubMed] [Google Scholar]
- 13.Ghohestani R.F., Rotunda S.L., Hudson B., et al. Crescentic glomerulonephritis and subepidermal blisters with autoantibodies to alpha5 and alpha6 chains of type IV collagen. Lab Investig. 2003;83:605–611. doi: 10.1097/01.lab.0000067497.86646.4d. [DOI] [PubMed] [Google Scholar]
- 14.Borza D., Chedid M.F., Colon S., Lager D.J., Leung N., Fervenza F.C. Recurrent Goodpasture’s disease secondary to a monoclonal IgA1-κ antibody autoreactive with the α1/α2 chains of type IV collagen. Am J Kidney Dis. 2005;45:397–406. doi: 10.1053/j.ajkd.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 15.Fervenza F.C., Terreros D., Boutaud A., et al. Recurrent Goodpasture’s disease due to a monoclonal IgA-kappa circulating antibody. Am J Kidney Dis. 1999;34:549–555. doi: 10.1016/s0272-6386(99)70084-3. [DOI] [PubMed] [Google Scholar]
- 16.Gu Q.H., Xie L.J., Jia X.Y., et al. Fever and prodromal infections in anti-glomerular basement membrane disease. Nephrol (Carlton) 2018;23:476–482. doi: 10.1111/nep.13040. [DOI] [PubMed] [Google Scholar]
- 17.de Sousa-Pereira P., Woof J.M. IgA: structure, function, and developability. Antibodies (Basel) 2019;8:57. doi: 10.3390/antib8040057. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.