Abstract
Introduction
Kidney outcomes are improved in primary focal segmental glomerulosclerosis (FSGS) by maintaining a remission in proteinuria. However, characteristics associated with relapses are uncertain. We sought to identify these by analyzing each remission.
Methods
We performed a retrospective study in patients with biopsy-proven lesions of FSGS, absent identifiable secondary cause, who had at least 1 remission from nephrotic-range proteinuria. In each patient, we identified every remission, every relapse, and their durations. Using a multilevel logistic regression to account for the clustering of multiple remissions within a patient, we tested which clinical characteristics were independently associated with relapses.
Results
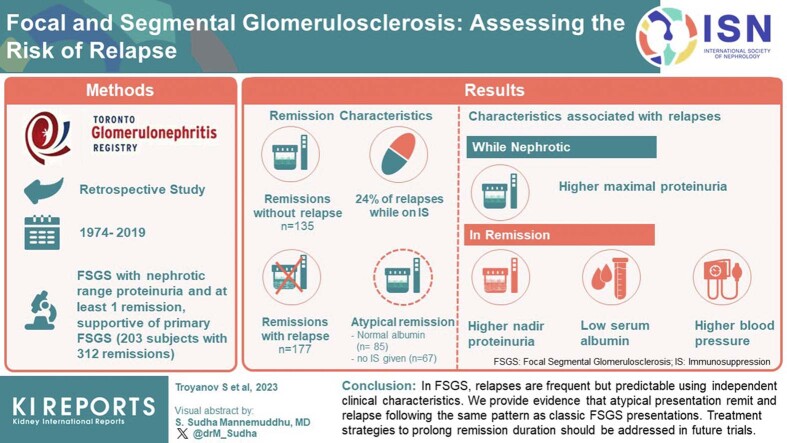
In 203 individuals, 312 remissions occurred, 177 with and 135 without relapse. A minority of remissions were atypical, defined by either absent hypoalbuminemia and/or no immunosuppression (IS), in contrast to the classic nephrotic syndrome that remits with IS. Atypical remission variants were just as likely to relapse as the classical presentation. Only 24% of remission events were on maintenance therapy at relapse. Independent characteristics associated with relapses were higher maximal proteinuria while nephrotic; and in remission, higher nadir proteinuria, lower serum albumin, and higher blood pressure. Using these variables, we created a tool estimating the 1-year risk of relapse ranging from 9% to 80%, well-calibrated to the observed data.
Conclusion
In FSGS, relapses are frequent but predictable using independent clinical characteristics. We also provide evidence that atypical presentations remit and relapse following the same pattern as classic FSGS presentations. Treatment strategies to prolong remission duration should be addressed in future trials.
Keywords: albuminemia, event-analysis, FSGS, immunosuppression, proteinuria, relapse
Graphical abstract
FSGS is one of the most common histologic types of glomerulonephritis leading to end-stage kidney disease in the United States, in both children and adults.1, 2, 3 The proposed classification system of FSGS lesions includes primary (autoimmune), secondary (maladaptive, toxic or viral), genetic, and those of uncertain etiologies labeled of “undetermined cause.”4 In the absence of a gold standard biomarker, a nephrotic syndrome with histology showing FSGS lesions and diffuse foot process effacement (FPE), which remits following IS, best defines primary disease.
It is uncertain how to identify and approach treatment in patients with the FSGS lesion that may lie within the undetermined etiology group. When no evident secondary cause exists, nephrotic range proteinuria even without hypoalbuminemia that subsequently remits supports the diagnosis of a primary FSGS. Certainly, this type of patient has been included in randomized trials of IS.5,6 Although diffuse FPE appears to be highly specific for the autoimmune form, this finding may be insufficiently sensitive in less severe forms of primary FSGS and studies have found exceptions to this rule.7,8 In addition, some patients with FSGS have experienced remissions in proteinuria without IS but subsequently relapse,9, 10, 11 making it even more difficult to delineate the autoimmune forms within the “undetermined cause” group.
Remissions in patients with FSGS with initial high-grade proteinuria and no relapse are the most defining surrogates of long-term outcomes.12, 13, 14 However, none of the immunosuppressive regimens used in induction of remission trials in primary FSGS has been tested to address the risk-benefit of maintenance therapy.6,15, 16, 17, 18, 19, 20 Despite this lack of formal evidence, IS agents are commonly prolonged after achieving remission.14,21,22 However, currently, the literature contains little information about the features associated with the risk of relapse, particularly in milder presentations that lie within the “undetermined cause” group.
We studied patients with biopsy-proven FSGS lesions and established nephrotic range proteinuria who had obtained either a partial or complete remission to examine these uncertainties. We included events displaying a full nephrotic syndrome but also atypical events that experienced remission without having presented with hypoalbuminemia or received IS. We aimed to identify factors associated with a relapse following a remission. We hypothesize that patients with atypical clinical features at presentation will also experience relapses similar to primary FSGS. We also hypothesize that the severity of the nephrotic proteinuria, its subsequent quantitative improvement during the remission phase, and blood pressure control are associated with the risk of subsequent relapse.
Methods
Setting Design and Entry Criteria
We performed an observational cohort study using data extracted from the Toronto Glomerulonephritis Registry from its creation in 1974 to 2019.23 We sought to study autoimmune forms of FSGS and, given the lack of a hallmark definition, we included patients with both full nephrotic syndrome and nephrotic range proteinuria who experienced a remission. We did not limit the definition of FSGS solely to those who had a full nephrotic syndrome or acquired a remission only in the setting of IS, because milder cases of primary FSGS may lie within the “undetermined cause” group.5,6 We did however carefully review all medical history recorded, blood tests (including genetic when available), and radiologic evaluations to rule out classic or well-defined secondary cases.
When the Toronto Glomerulonephritis Registry receives a pathology assessment, a secondary review is performed before classification to ensure that the description is consistent with FSGS. Given the prolonged time frame of this study, a tertiary review of all pathology reports was performed to confirm that the description agreed with current histologic standards.24 Only patients with definite pathologic findings of FSGS (focal and segmental consolidation of the tuft by increased extracellular matrix obliterating the capillary lumen, with or without glomerular adhesions by light microscopy with either negative or only segmental IgM or C3 by immunofluorescence) were considered for this study. In addition, electronic microscopy findings had to be consistent with FSGS, with electron microscopy showing greater than 75% diffuse FPE when performed during a nephrotic period and before IS.7,25 However, this criterion was not a requirement when the biopsy was done outside of their nephrotic period, or if done during immunosuppressive therapy.
The inception time was the first clinical assessment available to the glomerulonephritis registry. Finally, only patients older than 16 years at the entry to the registry and with at least 12 months of observation were included. The ethics committee of the University Health Network approved this retrospective study. This work was carried out in accordance with the declaration of Helsinki.
Variables
In addition to demographics and body mass index, we collected initial and follow-up information on systolic and diastolic blood pressure, serum creatinine and albumin, and 24-hour proteinuria. Race was self-reported. Serum albumin measurements throughout the observation period in the glomerulonephritis registry region of Ontario, Canada, used the bromocresol green method where levels ≤35 g/l defined hypoalbuminemia.26 We recorded the exposure to immunosuppressive agents, including glucocorticoid, antimetabolites (mycophenolate and azathioprine), B-cell therapies (cyclophosphamide and rituximab), and calcineurin inhibitors (cyclosporine and tacrolimus). We noted the number of antihypertensive medications, including renin-angiotensin system blockade.
Definitions
We assessed remissions, either partial or complete, and relapses. A partial remission was defined by a proteinuria value <3.5 g/d plus a 50% reduction from its peak value. A complete remission was defined by a proteinuria value ≤0.3 g/d.14 A relapse was a sustained proteinuria increase to ≥3.5 g/d following a remission.14
“Maintenance” IS during remission was defined by a minimal daily dose of 0.2 mg/kg prednisone, 1 mg/kg azathioprine, 1000 mg mycophenolate mofetil, 2 mg/kg cyclosporine or 0.05 mg/kg tacrolimus. If on a lower dose of IS than above, they were labeled “as tapered.” Patients receiving cyclophosphamide, rituximab, or a combination of calcineurin inhibitors and antimetabolite during remission were considered on maintenance regardless of the dose. Mean arterial pressure was defined as the diastolic pressure plus a third of the pulse pressure. The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration formula.
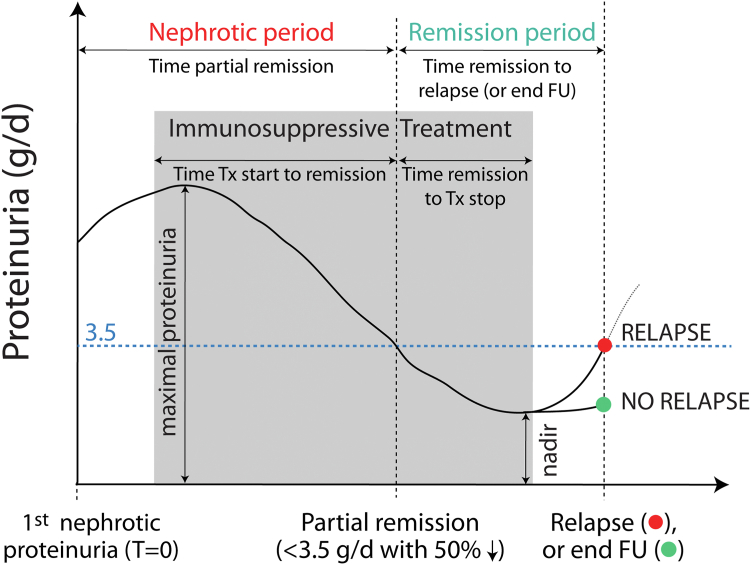
A “remission event” was defined by nephrotic-range proteinuria leading to a remission period ending either with a relapse (and a new nephrotic period) or the end of follow-up (Figure 1). We identified every remission event over the entire follow-up period for each patient and assessed the durations of nephrotic and remission periods. The other variables of interest within each event included those starting at the time of nephrotic proteinuria: eGFR, blood pressure, and antihypertensive medications; and during the nephrotic period: the maximal proteinuria and lowest serum albumin. We also recorded at the time of onset of partial remission, the eGFR, blood pressure, and antihypertensive medications; and during the remission period, the nadir proteinuria and maximum serum albumin.
Figure 1.
Remission event variables of interest. Individuals who relapse can experience subsequent remission events. FU, follow-up; Tx, treatment.
An atypical remission event was defined as occurring without hypoalbuminemia and/or without receiving IS. We also identified specific patients whose course included both classical and atypical remission phenotypes.
Outcomes
There were 3 outcomes of interest. First, the time to relapse from the partial remission threshold. Second, the occurrence of a relapse by 1 year, because we felt identifying this event would assist clinicians when counseling on the need for more prolonged maintenance therapy. Third, the patient survival from a 50% reduction in eGFR, dialysis, kidney transplant, or a persistent eGFR ≤15 ml/min per 1.73 m2 (combined outcome).
Statistical Analyses
Normally distributed variables are presented as means ± SDs and were compared using Student t-test or 1-way analysis of variance. Nonparametric variables are expressed as median with interquartile range, and differences were tested using Mann-Whitney U and Kruskal-Wallis tests. Percentages were compared using the chi-square.
We analyzed each remission event, including multiple ones within a patient, to determine factors associated with the occurrence of a relapse. We did not limit our analysis solely to the first remission event. In turn, this simplified the assessment of time-varying covariates such as sequential treatments that would have been complex to assess over multiple remissions and relapses. To account for clustering by multiple remission events within a patient, we determined independent factors associated with the occurrence of a relapse by the 1-year time-point using a multilevel logistic regression that included variables identified by univariate analyses (level 1 equation), and a random intercept that accounts for multiple remission events per patient (level 2 equation). We verified that the random effect covariance was not statistically different from 0, to confirm a similar risk of relapse following the first and subsequent remissions. Remission periods with less than 1 year of follow-up were discarded for this analysis unless a relapse had occurred within this time. Missing data was not replaced. The goodness of fit of the logistic model was assessed using the C-statistic. To assess calibration, points for each independent predictor were assigned by dividing the β coefficient by the lowest significant β in the model and rounding to the nearest number. We report the observed and predicted risk of relapse according to the sum of these points.27 We also report the observed and predicted risk of relapse in classic versus atypical presentation.
We illustrate the time from remission to relapse using Kaplan-Meier curves and log-Rank test. Finally, we also use Kaplan-Meier curves to illustrate kidney survival from a combined outcome in individuals with and without atypical remission events.
Two-tailed P-values less than 0.05 were considered statistically significant, except when doing subgroup analyses where we use the Bonferroni correction for significance (0.05/number of comparisons). Analyses were performed using SPSS software (version 26, IBM, NY).
Results
Cohort Characteristics
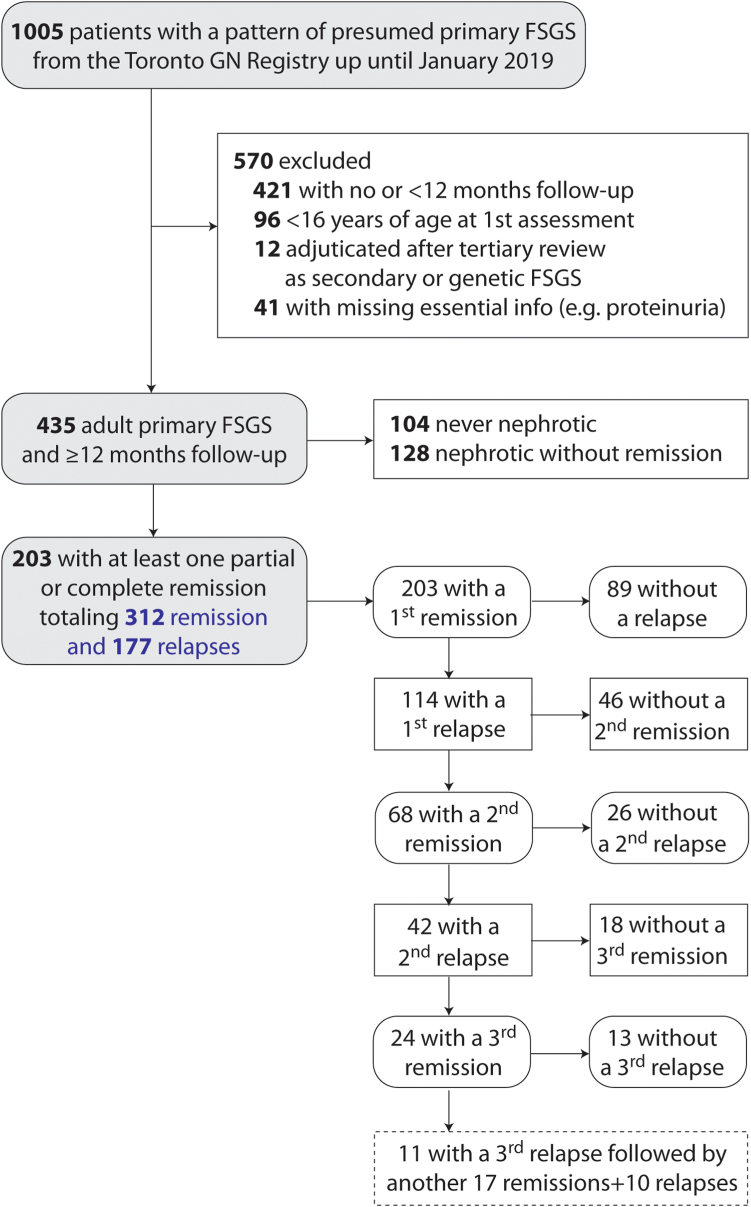
There were 1005 patients with a histologic pattern of FSGS as documented by nephropathologists in the Toronto Glomerulonephritis Registry from its creation until January 2019. After applying exclusions, we identified 203 patients who had had at least 1 remission (Figure 2). These patients were followed-up with for a median of 71 (39–110) months. The number of available measurements per individual was 15 (9–22) for eGFR, 11 (7–29) for proteinuria, 10 (5–15) for serum albumin, and 13 (7–18) for blood pressure. The presenting eGFR was 69 ± 29 ml/min per 1.73 m2, with a body mass index of 27 (23–32), 37% were female; and 11%, 10%, 62%, and 17% were African, Asian, Caucasian, or of other descent, respectively. There were 153 subjects who returned to their physician after 72 (40–109) months and 2 were lost to follow-up because of death of nonrenal cause. There was little missing data: during nephrotic periods, the blood pressure, number of antihypertensives and nadir albumin were unavailable in 13%, 9%, and 10%, respectively. During remission periods, the initial blood pressure and albumin were absent in 4% and 15% of cases. Other variables were missing in ≤1%.
Figure 2.
Patient recruitment and flow-chart. FSGS, focal segmental glomerulosclerosis.
During the follow-up of the 203 patients, there was a total of 312 remissions of which 177 relapsed (Figure 2). Remission events lasted a median 30 (15–61) months. The maximal proteinuria during the nephrotic period leading to the first remission, was 7.0 (5.0–11.2) g/d, and a similar severity was seen in all subsequent nephrotic events, 6.8 (5.0–9.7) g/d.
One hundred subjects had their biopsy done during full nephrotic syndrome (nephrotic proteinuria with hypoalbuminemia) and prior to treatment, and these showed diffuse FPE on electron microscopy. In contrast, 85 patients had their biopsy performed at a time when they did not have nephrotic range proteinuria or hypoalbuminemia and their electron microscopy photographs showed a variable degree of FPE. However, all these subjects subsequently developed nephrotic range proteinuria and 43 of 85 developed hypoalbuminemia. The remaining 42 of 85 were never documented to have hypoalbuminemia. Finally, the remaining 18 of the 203 had their biopsy done >3 months prior to the first clinical assessment accessible to the glomerulonephritis registry.
Factors Associated With a Relapse
Remission events that were followed by a relapse had a higher maximal proteinuria during the nephrotic period, and a higher nadir of proteinuria while in remission (Table 1). Specifically, relapses occurred in 89% of remission events when their proteinuria nadir was 2 to 3.5 g/d, 65% when their nadir was 1 to 2 g/d and only 40% of events that reached a proteinuria nadir ≤1 g/d (P < 0.001). Events that relapsed had, during their remission period, a lower serum albumin (39 ± 6 vs. 42 ± 4 g/l, P < 0.001), a higher mean arterial pressure (97 ± 12 mm Hg vs. 94 ± 11, P = 0.05) despite a higher number of antihypertensive drugs and a smaller reduction in mean arterial pressure from the nephrotic period onset (5 ± 13 mm Hg vs. 8 ± 15, P = 0.04). Neither sex nor age were associated with the risk of a relapse. Type and duration of IS were similar between sustained and unsustained remissions. Overall, after the partial remission threshold was attained, IS was continued for 4 (0–11) months and relapses occurred at 8 (3–21) months (Table 1). Twenty percent of relapses had received no prior IS before the remission event, 37% had stopped, 23% were receiving tapered doses, and only 19% occurred while on maintenance IS.
Table 1.
Comparison of 312 remission events associated with and without a relapse
| Variables | No relapse (n = 135) | Relapse (n = 177) | P-value |
|---|---|---|---|
| Nephrotic period leading to the remission | |||
| Sex, % female | 39 | 33 | 0.28 |
| Initial age, yrs | 44 ± 16 | 43 ± 15 | 0.86 |
| Initial eGFR, ml/min per 1.73 m2 | 68 ± 32 | 70 ± 27 | 0.60 |
| Maximal proteinuria, g/d | 6.8 (4.7–9.6) | 7.1 (5.3–11.1) | 0.05 |
| Nadir albumin, g/l | 30 ± 9 | 28 ± 8 | 0.32 |
| Initial MAP, mm Hg | 103 ± 15 | 102 ± 13 | 0.43 |
| Initial number of blood pressure medication | 1 (0–2) | 1 (0–2) | 0.70 |
| Initial use of RASB, % | 39 | 38 | 0.81 |
| Immunosuppressive treatment | |||
| (%) None, Glucocorticoids, antimetabolites, B-cell therapya, CNI, | 23, 47, 4, 10, 16 | 20, 44, 4, 10, 22 | 0.75 |
| Time from onset of nephrotic proteinuria to immunosuppression start | 0 (0–3) | 0 (0–3) | 0.27 |
| Time from immunosuppression start to partial remission | 4 (2–13) | 4 (1–9) | 0.57 |
| Remission period | |||
| Time to partial remissions | 8 (4–19) | 7 (3–18) | 0.61 |
| eGFR at remission, ml/min per 1.73 m2 | 65 ± 35 | 65 ± 29 | 0.85 |
| Reduction in eGFR from nephrotic period onset | 5 ± 23 | 4 ± 20 | 0.98 |
| Nadir proteinuria, g/d | 0.5 (0.2–1.2) | 1.5 (0.4–2.3) | <0.001 |
| Reduction from maximal value | 5.9 (4.1–9.0) | 5.6 (3.8–9.7) | 0.93 |
| Complete / partial remission, % | 41 / 59 | 21 / 79 | <0.001 |
| Maximal albumin, g/l | 42 ± 4 | 39 ± 6 | <0.001 |
| Increase in albumin from minimal value | 13 ± 9 | 10 ± 9 | 0.04 |
| MAP at remission, mm Hg | 94 ± 11 | 97 ± 12 | 0.05 |
| Reduction from MAP at nephrotic period onset | 8 ± 15 | 5 ± 13 | 0.04 |
| Number of blood pressure medication at remission | 1 (1–2) | 2 (1–2) | 0.49 |
| Use of RASB at remission (%) | 60 | 58 | 0.67 |
| Time from remission to immunosuppression end | 5 (0–16) | 3 (1–9) | 0.52 |
| Time from remission to relapse | - | 8 (3–21) | |
| Immunosuppression at relapse (% not given, stopped, taper, maintenance) | - | 20, 37, 23, 19 | |
| Time from remission to end follow-up (if no relapse) | 26 (11–66) | - | |
| Immunosuppression at end of follow-up (% not given, stopped, taper, maintenance) | 23, 51, 16, 10 | - |
CNI, calcineurin inhibitor; eGFR, estimated glomerular filtration rate; MAP, mean arterial pressure; RASB, renin angiotensin system blockade.
All times are in months.
B-cell therapy consisted of cyclophosphamide (n = 29) and rituximab (n = 1).
Remission Events That Occurred Without IS
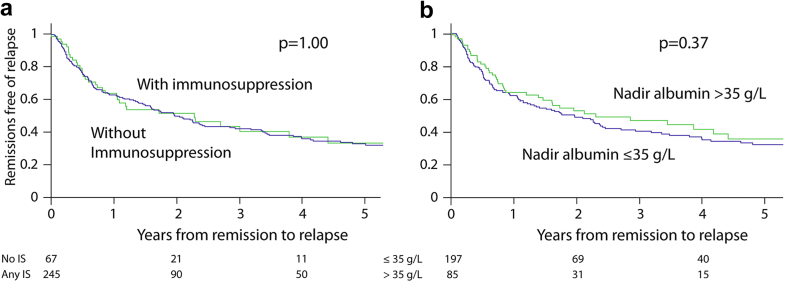
Sixty-seven of the 312 remission events (21%) were atypical given that they occurred without the use of IS (Table 2). Events not given IS had a less severe nephrotic period compared to treated episodes. They experienced less reduction in proteinuria and took longer to reach remission. However untreated remissions lost significantly greater eGFR during their nephrotic period and experienced less reduction in blood pressure. Finally, the proportion and time to relapse were similar between IS and non-IS events (Figure 3a).
Table 2.
Comparison of remission events that occurred with and without immunosuppression
| Variables | Remission events without immunosuppression (n = 67) | Remission events with immunosuppression (n = 245) | P-value |
|---|---|---|---|
| Nephrotic period leading to the remission | |||
| Sex, % female | 36 | 36 | 0.99 |
| Initial age, yrs | 44 ± 15 | 43 ± 15 | 0.58 |
| Initial eGFR, ml/min per 1.73 m2 | 64 ± 27 | 70 ± 29 | 0.16 |
| Maximal proteinuria, g/d | 5.0 (4.1–7.4) | 7.5 (5.4–11.7) | <0.001 |
| Nadir albumin, g/l | 36 ± 6 | 27 ± 8 | <0.001 |
| Initial MAP, mm Hg | 101 ± 13 | 102 ± 14 | 0.63 |
| Initial number of blood pressure medication | 1 (0–2) | 1 (0–2) | 0.45 |
| Initial use of RASB, % | 41 | 38 | 0.67 |
| Remission period | |||
| Time to partial remission | 13 (6–26) | 6 (3-16) | <0.001 |
| eGFR at remission, ml/min per 1.73 m2 | 55 ± 30 | 68 ± 32 | 0.004 |
| Reduction in eGFR from nephrotic period onset | 9 ± 16 | 2 ± 24 | 0.007 |
| Nadir proteinuria, g/d | 1.6 (0.8–2.2) | 0.7 (0.2–1.8) | <0.001 |
| Reduction from maximal value | 3.6 (2.8–5.2) | 6.5 (4.4–10.7) | <0.001 |
| Complete/partial remission, % | 7 / 93 | 36 / 64 | <0.001 |
| Maximal albumin, g/l | 41 ± 5 | 40 ± 5 | 0.31 |
| Increase in albumin from minimal value | 5 ± 5 | 13 ± 9 | <0.001 |
| MAP at remission, mm Hg | 97 ± 10 | 96 ± 12 | 0.28 |
| Drop in MAP from nephrotic period onset | 3 ± 10 | 7 ± 15 | 0.02 |
| Number of blood pressure medication at remission | 2 (1–2) | 1 (1–2) | 0.60 |
| Use of RASB at remission, % | 60 | 58 | 0.85 |
| Relapse, % | 54 | 58 | 0.58 |
| Time from remission to relapse | 8 (4–18) | 8 (3–21) | 0.81 |
| Time from remission to end FU when no relapse | 20 (7–51) | 32 (11–69) | 0.12 |
eGFR, estimated glomerular filtration rate; FU, follow-up; MAP, mean arterial pressure; RASB, renin angiotensin system blockade.
All times are in months.
Figure 3.
Time to relapse (a) in events with and without immunosuppression and (b) in events with and without hypoalbuminemia. IS, immunosuppression.
There were 14 of 203 subjects who experienced both treated and untreated remissions. They had a total of 38 remission events, 21 treated with IS and 17 without IS (Supplementary Figure S1A). Patient survival from a combined event was similar between individuals who only experienced remission(s) with IS, those who only experienced remission(s) without IS, and the 14 subjects who experienced both treated and untreated events (Supplementary Figure S2A).
Remission Events That Occurred Without Hypoalbuminemia
Twenty-seven percent (85/312) remission events were atypical given that they presented with nephrotic range proteinuria but without hypoalbuminemia, whereas 63% had a full nephrotic syndrome (remaining 10% of events had no albumin measurement during their nephrotic period). These events without hypoalbuminemia had marked clinical differences compared to events with it (Table 3). They presented with milder proteinuria, a lower eGFR, and fewer received IS. However, they took longer to reach partial remission, had a greater decline in eGFR while nephrotic, and obtained a nadir proteinuria of only 1.4 (0.7–1.9) g/d compared to a nadir of 0.7 (0.2–1.8) g/d in those who presented with hypoalbuminemia (P = 0.001). The proportion and time to relapse, however, were similar between both groups (Figure 3b).
Table 3.
Comparison of remission events that occurred with and without hypoalbuminemiaa
| Variables | Remission events with hypoalbuminemia (n = 197) | Remission events without hypoalbuminemia (n = 85) | P-value |
|---|---|---|---|
| Nephrotic period leading to the remission | |||
| Sex, % female | 43 | 24 | 0.002 |
| Initial age, yrs | 42 ± 15 | 47 ± 13 | 0.02 |
| Initial eGFR, ml/min per 1.73 m2 | 73 ± 30 | 58 ± 24 | <0.001 |
| Maximal proteinuria, g/d | 8.2 (5.7–12.3) | 5.4 (4.3–7.1) | <0.001 |
| Nadir albumin, g/l | 25 ± 7 | 39 ± 3 | By design |
| Initial MAP, mm Hg | 102 ± 14 | 104 ± 14 | 0.25 |
| Initial number of blood pressure medication | 1 (0–2) | 1 (0–2) | 0.15 |
| Initial use of RASB, % | 35 | 47 | 0.08 |
| Immunosuppressive treatment | |||
| (%) None, Glucocorticoids, antimetabolites, B-cell therapya, CNI | 13, 49, 2, 12, 24 | 46, 28, 7, 6, 13 | <0.001 |
| Remission period | |||
| Time to partial remission | 7 (3–15) | 10 (5–26) | 0.02 |
| eGFR at remission, ml/min per 1.73 m2 | 71 ± 31 | 48 ± 26 | <0.001 |
| Reduction in eGFR from nephrotic period onset | 2 ± 24 | 11 ± 13 | <0.001 |
| Nadir proteinuria, g/d | 0.7 (0.2–1.8) | 1.4 (0.7–1.9) | 0.001 |
| Reduction from maximal value | 7.0 (4.6–11.7) | 4.0 (3.1–5.9) | <0.001 |
| Complete/partial remission, % | 36 / 64 | 16 / 84 | <0.001 |
| Maximal albumin, g/l | 39 ± 5 | 43 ± 4 | <0.001 |
| Increase in albumin from minimal value | 15 ± 9 | 4 ± 3 | <0.001 |
| MAP at remission, mm Hg | 95 ± 13 | 99 ± 8 | 0.001 |
| Drop in MAP from nephrotic period onset | 7 ± 14 | 5 ± 13 | 0.29 |
| Number of blood pressure medication at remission | 1 (1–2) | 2 (1–3) | 0.002 |
| Use of RASB at remission, % | 54 | 72 | 0.005 |
| Relapse, % | 57 | 49 | 0.22 |
| Time from remission to relapse | 7 (3–20) | 8 (4–19) | 0.37 |
| Time from remission to end FU when no relapse | 28 (11–69) | 26 (11–59) | 0.52 |
CNI, calcineurin inhibitor; eGFR, estimated glomerular filtration rate; FU, follow-up; MAP, mean arterial pressure; RASB, Renin angiotensin system blockade.
All times are in months.
10% of remission did not have a measured albumin level and could not be categorized.
There were 12 of 203 subjects who experienced remissions both with and without hypoalbuminemia, for a total of 43 remission, 26 with and 17 without low albumin (Supplementary Figure S1B). Patient survival from a combined event was similar between individuals who experienced only nephrotic events with hypoalbuminemia, those that only had events without hypoalbuminemia, and the 12 patients who experienced multiple events, some with and some without hypoalbuminemia (Supplementary Figure S2B).
Comparisons of Relapses That Occur <1 Year, 1 to 2 Years, and >2 Years After Remission
The characteristics differentiating events that did and did not relapse also distinguished early versus late relapses (Table 4). Early (<1 year, n = 108) relapses followed remission events where the peak proteinuria was 9.3 (5.9–12.0) g/d as opposed to a much lower peak of 6.5 (5.0–11.0) and 5.8 g/d (4.8–7.6) in the relapses that occurred 1–2 years (n = 31) and >2 years (n = 38) after the remission (P < 0.001). In addition, early relapses were distinguished from later ones by a higher nadir proteinuria, lower albumin, and greater need for antihypertensive drugs during their remission period. The specific type of IS treatments during the nephrotic periods and the proportions that remained on maintenance therapy at the time of relapse were similar between early versus late relapses.
Table 4.
Comparison of relapses that occur at ≤1, 1–2, >2 years after a remission event
| Variables | Relapse ≤1 yr (Group 1, n = 108) | Relapse 1–2 yrs (Group 2, n = 31) | Relapse >2 yrs (group 3, n = 38) | P-value |
|---|---|---|---|---|
| Nephrotic period leading to the remission | ||||
| Sex, % female | 34 | 23 | 39 | 0.32 |
| Initial age, yrs | 45 ± 15 | 40 ± 14 | 42 ± 14 | 0.25 |
| Initial eGFR, ml/min per 1.73 m2 | 68 ± 26 | 69 ± 28 | 75 ± 29 | 0.39 |
| Maximal proteinuria, g/d | 9.3 (5.9–12.0) | 6.5 (5.0–11.0) | 5.8 (4.8–7.6) | 0.003 (1>3) |
| Nadir albumin, g/l | 29 ± 8 | 27 ± 9 | 28 ± 8 | 0.68 |
| Initial MAP, mm Hg | 103 ± 12 | 101 ± 13 | 97 ± 15 | 0.02 |
| Initial number of blood pressure medication | 1 (0–2) | 1 (0–2) | 1 (0–2) | 0.51 |
| Initial use of RASB, % | 38 | 36 | 37 | 0.97 |
| Treatments, % | ||||
| % none, glucocorticoids, antimetabolites, B-cell therapy, CNI | 21, 47, 4, 8, 20 | 19, 29, 10, 13, 29 | 21, 45, 3, 10, 21 | 0.70 |
| Time from onset of nephrotic proteinuria to immunosuppression start | 0 (0–4) | 1 (0–4) | 0 (0–2) | 0.76 |
| Time from immunosuppression start to remission | 4 (2–10) | 3 (1–7) | 4 (2–6) | |
| Remission period | ||||
| Time to remission (partial remission criteria) | 9 (3–21) | 7 (4–18) | 5 (3–11) | 0.23 |
| eGFR at remission, ml/min per 1.73 m2 | 63 ± 29 | 69 ± 31 | 70 ± 31 | 0.36 |
| Reduction in eGFR from nephrotic period onset | 5 ± 21 | 1 ± 25 | 5 ± 16 | 0.66 |
| Nadir proteinuria, g/d | 1.6 (0.7–2.6) | 1.9 (0.7–2.4) | 0.6 (0.2–1.5) | <0.001 (1,2<3) |
| Reduction in proteinuria from maximal value | 6.5 (4.1–10.2) | 4.9 (3.1–10.8) | 5 (3.6–6.0) | 0.06 |
| Complete / partial remission, % | 15 / 85 | 23 / 77 | 39 / 61 | 0.006 (1<3) |
| Maximal albumin, g/l | 38 ± 6 | 39 ± 6 | 42 ± 4 | 0.004 (1<3) |
| Increase in albumin from minimal value | 9 ± 7 | 12 ± 10 | 13 ± 9 | 0.04 (1<3) |
| MAP at remission, mm Hg | 98 ± 13 | 97 ± 11 | 95 ±10 | 0.32 |
| Reduction in MAP from nephrotic period onset | 5 ± 13 | 4 ± 15 | 4 ± 14 | 0.78 |
| Number of blood pressure medication at remission | 2 (1–2) | 1 (0–2) | 1 (0–2) | 0.04 (1>3) |
| Use of RASB at remission, % | 63 | 48 | 50 | 0.20 |
| Time from remission to immunosuppression end | 3 (1–6) | 13 (0–18) | 21 (2–32) | <0.001 (1<3) |
| Time from remission to relapse | 4 (3–7) | 17 (14–21) | 40 (28–50) | By definition |
| IS at time of relapse, % not given, stopped, taper, maintenance | 20, 30, 27, 23 | 19, 52, 13, 16 | 21, 47, 21, 11 | 0.17 |
CNI, calcineurin inhibitor; eGFR, estimated glomerular filtration rate; IS, immunosuppression; MAP, mean arterial pressure; RASB, renin angiotensin system blockade.
All times are in months.
Assessment of the Risk of a Relapse at 1 Year
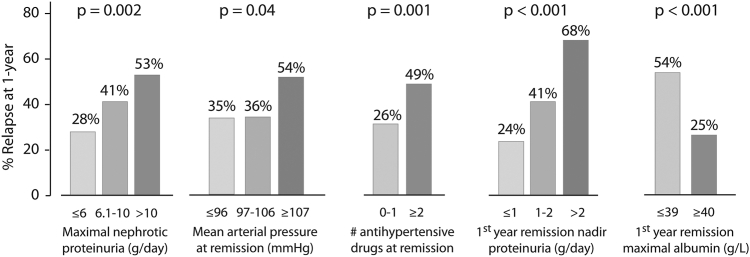
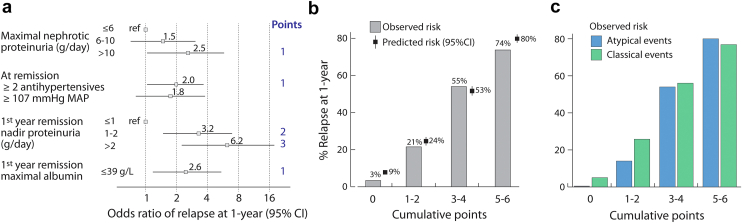
We sought to identify independent factors associated with a relapse within 1-year following a remission, because we felt this is an important clinical milestone. We performed this analysis excluding events that had less than 1 year follow-up following the remission unless they had already relapsed (n = 36). This left 276 remissions and 108 early relapses to examine. There were 5 characteristics associated with the risk of relapse by 1 year by univariate analysis: the maximal proteinuria during the nephrotic period (categorized into 3 levels: ≤6, 6–10 and >10 g/d approximating the tertile cut-offs of 6.2 and 10.4 g/d), the blood pressure at remission, the number of antihypertensive drugs at remission, the nadir proteinuria, and maximal serum albumin within the first year after a remission (Figure 4). We also found that having experienced a previous relapse was not associated with the risk of future relapse (38% relapse rate by 1 year after first remission vs. 42% after subsequent remissions, P = 0.43). Using a multilevel logistic model accounting for multiple remission events within individuals, we found 4 variables independently associated with relapses (Figure 5a), with a C-statistic of 0.81 (95% confidence interval: 0.75–0.86). The predicted risk of relapse according to these variables ranged from 9% to 80% and was well-calibrated to the observed rates (Figure 5b). The model applied similarly to remissions with classical or atypical presentations (Figure 5c) and regardless of whether a first or subsequent remission was obtained (data not shown). We repeated this model excluding those who received maintenance IS during the entire first year in remission (n = 57) to assess the “natural” risk of relapse and found identical risk factors, C-statistic, and calibration (Supplementary Figure S3).
Figure 4.
Univariate factors associated with a relapse by 1-year following a remission when maintenance immunosuppression is tapered, stopped, or not given. The maximal proteinuria during the nephrotic period was categorized into 3 categories: <6, 6–10, and >10 g/d, approximating the tertile cut-offs of 6.2 and 10.4 g per day. The mean arterial pressures of 97 and 107 mmHg approximate blood pressures of 130/80 and 140/90 mm Hg.
Figure 5.
Independent factors (a) associated with a relapse at 1 year following the remission, model calibration (b) and applicability to events with and without atypical features (c). Atypical events are defined by remissions that followed nephrotic range proteinuria without hypoalbuminemia or remissions that occurred without the use of immunosuppression. The predicted risk was the predicted probabilities obtained from the multilevel logistic regression. MAP, mean arterial pressure.
Influence of Maintenance IS on the Risk of a Relapse
Immunosuppressive regimens used in this observational study were variable and frequently multimodal (Supplementary Table S1). We described their usage and report 4 groups: glucocorticoid monotherapy, antimetabolites ± glucocorticoid, B-cell therapy ± any other IS drugs, and calcineurin inhibitor (CNI) ± any other IS drugs excluding B-cell therapies. Prior to the first remission, glucocorticoid monotherapy was used most often (69%), compared to antimetabolites (2%), cytotoxic (9%), and CNI (20%). In comparison to patients prior to their third or later remission, the proportion receiving glucocorticoid monotherapy decreased to 24% compared to antimetabolites (18%), cytotoxic (9%), and CNI (49%). Events in the setting of CNI were associated with a greater eGFR loss and less reduction in mean arterial pressure during the nephrotic period whereas the risk of relapse was similar to other groups. However, as shown earlier, CNI treatments were used more often in remission events that followed multiple relapses.
Discussion
In this analysis of 312 remissions in 203 patients with FSGS, we found that following remissions in proteinuria, relapses occurred frequently, even following remissions where IS was never started or where hypoalbuminemia was not observed. Relapses were more common following events characterized by a higher maximal proteinuria while nephrotic, and when in remission, by a higher nadir proteinuria, lower serum albumin, and higher blood pressure despite more antihypertensive treatment. Using these factors, we could accurately predict the risk of relapse in our cohort at 1-year. This supports our rationale that clinical factors during nephrotic and remissions periods can justify maintenance treatment in patients with high-risk of relapse. It also supports the need to address the duration of therapy in future studies because of the impact of relapses on kidney survival.12 In addition, it provides some evidence that the definition of primary or autoimmune FSGS should not be limited to abrupt onset of nephrotic syndrome that remits following IS therapy because we observed marked variations in presentation severity between events even within the same individual. Events with atypical presentations were just as likely to relapse and had a similar long-term kidney outcome. Although this last finding may appear counterintuitive, we found that atypical presentations were associated, during the nephrotic period, with a statistically greater loss of renal function, a smaller reduction in blood pressure, and a longer time to reach the partial remission threshold, all factors that could adversely impact kidney survival.
The number of potential secondary etiological agents associated with the FSGS lesion has progressively increased over the years. It includes genetic, drug-induced, and maladaptive factors that may all produce significant proteinuria associated with an FSGS lesion on biopsy.28, 29, 30, 31, 32 A distinction between immunologic and nonimmunologic FSGS in adults is evident when comparing extremes in presentations: massive proteinuria with sudden and severe edema and low serum albumin versus asymptomatic proteinuria values of unknown duration and normal albumin, often with a recognizable causative secondary factor.4,11,33 However, our data shows that intermediate phenotypes do occur, and clinicians are tasked to consider potent IS, knowing that this group may still have a responsive immunological component. Currently, although the most specific finding supporting a diagnosis of autoimmune FSGS remains obtaining a remission in high-grade proteinuria after IS, primary FSGS has occasionally been shown to remit spontaneously, making this definition less sensitive to milder forms.9, 10, 11 In our study, 21% of events achieved remission without IS. This could potentially result from dramatic weight loss or better blood pressure control; however, both seem unlikely in our cases because body mass index showed little obesity in the cohort, and blood pressure improved less with spontaneous remission than when induced by IS. The possibility that these events were true spontaneous remissions is also supported by the risk of relapse being similar to those receiving IS therapy, and by the 14 cases where multiple remissions and relapses were observed in the same individual with and without IS (Supplementary Figure S2A). This pattern is perhaps not so unexpected in this group, given they were specifically selected patients because they had at least 1 remission.10,11,33 The same caveat applies to nephrotic range events without hypoalbuminemia (Supplementary Figure S2B). Although considered atypical using the highly-specific definition of primary FSGS, we found they were just as likely to relapse. In addition, though diffuse FPE is highly specific for primary FSGS in adults, not all subjects were biopsied at their worst clinical state and others were on IS, lowering the sensitivity of this finding. It is always possible that a few nonautoimmune forms of FSGS may have been included in this study despite careful review of cases; however, we are reassured that our capacity to predict relapse is applicable to events even with milder presentations.
Little information exists regarding either the prediction or the treatment of relapses in patients with FSGS. We have reported on factors associated with any relapse when studying the relevance of a partial remission.12,14 Banfi et al.21 have previously shown that prolonged IS treatment can lead to a more sustained remission. A recent study reported that CNI and mycophenolate were the most used regimens employed in this circumstance, although maintenance regimens and drugs varied widely.22 The ability to predict relapses that occur early appeared to us more clinically important than predicting late ones. This information might encourage both the clinician and the patient to accept longer periods of therapy to maintain remission to prevent a foreseeable event such as relapse or progressive loss of kidney function. This proposed approach is likely to become even more acceptable with new conservative options and the advent of newer IS drugs with fewer side effects.34, 35, 36, 37
There are limitations to our study. The varying states of remissions and relapses in our cases of FSGS would have made it challenging to demonstrate that factors such as the severity of proteinuria and nadir proteinuria in remission of a single event influence renal survival. We therefore delineated the follow-ups into remission events, a surrogate for long-term outcomes. We had previously found a close relationship between maintenance of remission and long-term survival in this specialized FSGS remission cohort.12 Given the long time span of the study, the use of renin-angiotensin system blockade was not universal, because part of the follow-up time predated the introduction of these drugs, and their universal use could potentially influence the results. In addition, we did not incorporate pathology findings, which have been described as associated with outcomes.24,38 However, the lengthy time between biopsy, remissions, and relapses, reduces the value of pathology compared to short-term studies.24,38, 39, 40, 41 Our model was also perhaps oversimplified by assessing blood pressure and the number of antihypertensive medications simultaneously at remission because of their interdependence and by using the worst and best proteinurias and albuminemias during remission events, regardless of the time of occurrence and instead of a time-average. Finally, our findings require validation in independent cohorts or by using different remission definitions.13 Despite these limitations, we report the largest relapsing and remitting FSGS event-driven cohort to date.
In summary, we found that classic primary FSGS commonly relapses and remits and that the atypical clinical presentations can follow the same pattern when evident secondary causes have been excluded. We propose the use of a composite of basic clinical factors to assess the risk of relapse in both phenotypes that will help the physician to counsel the patient when proposing either prolonging or discontinuing maintenance treatment.
Disclosure
AJ and ST have nothing to declare. DC has served on advisory boards, received consulting fees or grant funding and/or a data monitoring committee member for Alnylam, Alexion, Calliditas, Chemocentryx, Chinook, Dimerix, Genentech, Novartis, Principia, and Vera; and End point adjudicator member for Aurinea. HR has received consulting fees or honoraria for lectures from Calliditas, Novartis, Chinook, Travere, and Omeros. MH has received grants or consulting fees from Calliditas, Pfizer, Ionis, Genentech, GlaxoSmithKline, and Alnylam. Part of this material was published in abstract form only for the World Congress of Nephrology in Montréal, Canada in April 2021.
Acknowledgments
The Toronto Glomerulonephritis Registry is supported in part by the McCann Fund of the Toronto General Hospital Foundation.
Data Availability
The data underlying this article will be shared upon reasonable request to the corresponding authors.
Footnotes
Figure S1. Remission events with and without (a) immunosuppression or (b) hypoalbuminemia.
Figure S2. Survival from a combined event in patients that experienced events only with IS, (a) only without IS, and both with and without IS; (b) and in patients that experienced events only with hypoalbuminemia, only without hypoalbuminemia, and both with and without hypoalbuminemia. IS, immunosuppression.
Figure S3. Independent factors (a) associated with a relapse at 1 year following the remission without maintenance immunosuppression, (b) model calibration and applicability to events with and (c) without atypical features.
Table S1. Remissions events using different immunosuppression drugs.
STROBE Statement.
Contributor Information
Stéphan Troyanov, Email: stephan.troyanov@umontreal.ca.
Daniel C. Cattran, Email: daniel.cattran@uhn.ca.
Toronto Glomerulonephritis Registry Group:
N. Ryan, P. Ling, P. Lam, M. Romano, S. Albert, R. Aslahi, P. Aujla, N. Barrese, M. Barua, M. Berall, A. Berbece, S. Bhandhal, D.R. Birbrager, P. Boll, G. Buldo, C. Cardella, C. Chan, P. Chan, A. Charest, D. Cherney, M. Chidambaram, S. Chow, E. Cole, M. Cummings, S. Donnelly, A. Dunn, A. Elfirjani, S. Fenton, E. Fong, J. Fung, J. Goldstein, Z. Harel, G. Hercz, S.V. Jassal, S. Kajbaf, K. Kamel, A. Kang, S. Karanicolas, V. Ki, S.J. Kim, D.H. Kim, A. Konvalinka, K. Kundhal, V. Langlois, P. Lekas, I. Lenga, C. Licht, J. Lipscombe, C. Lok, J. Ly, M. Manogaran, R. McQuillan, P. McFarlane, H. Mehta, D. Mendelssohn, J.A. Miller, G. Nagai, B. Nathoo, G. Nesrallah, M. Pandes, S. Pandeya, R. Parekh, R. Pearl, Y. Pei, D. Perkins, J. Perl, A. Pierratos, R. Prasad, S. Radhakrishnan, M. Rao, R. Richardson, J. Roscoe, A. Roushdi, J. Sachdeva, D. Sapir, J. Sasal, J. Schiff, J. Scholey, M. Schreiber, X. Shan, N. Siddiqui, T. Sikaneta, C.V. Silva Gomez, S. Singh, R. Singhal, A. Sohal, A. Steele, S. Suneja, E. Szaky, D. Tam, P. Tam, L. Teskey, K. Tinckam, R. Ting, S. Tsui, P.A. Turner, D. Wadehra, J.A. Wadgymar, R. Wald, A. Walele, L. Warner, C. Wei, J. Weinstein, C. Whiteside, S. Wijeyasekaran, G. Wong, G. Wu, T. Yassa, D. Yuen, and J. Zaltzman
Appendix
List of the Toronto Glomerulonephritis Registry Group
N. Ryan, P. Ling, P. Lam, M. Romano, S. Albert, R. Aslahi, P. Aujla, N. Barrese, M. Barua, M. Berall, A. Berbece, S. Bhandhal, D.R. Birbrager, P. Boll, G. Buldo, C. Cardella, C. Chan, P. Chan, A. Charest, D. Cherney, M. Chidambaram, S. Chow, E. Cole, M. Cummings, S. Donnelly, A. Dunn, A. Elfirjani, S. Fenton, E. Fong, J. Fung, J.Goldstein, Z. Harel, G. Hercz, S.V. Jassal, S. Kajbaf, K. Kamel, A. Kang, S. Karanicolas, V. Ki, S.J. Kim, D.H. Kim, A. Konvalinka, K. Kundhal, V. Langlois, P. Lekas, I. Lenga, C. Licht, J. Lipscombe, C. Lok, J. Ly, M. Manogaran, R. McQuillan, P. McFarlane, H. Mehta, D. Mendelssohn, J.A. Miller, G. Nagai, B. Nathoo, G. Nesrallah, M. Pandes, S. Pandeya, R. Parekh, R. Pearl, Y. Pei, D. Perkins, J. Perl, A. Pierratos, R. Prasad, S. Radhakrishnan, M. Rao, R. Richardson, J. Roscoe, A. Roushdi, J. Sachdeva, D. Sapir, J. Sasal, J. Schiff, J. Scholey, M. Schreiber, X. Shan, N. Siddiqui, T. Sikaneta, C.V. Silva Gomez, S. Singh, R. Singhal, A. Sohal, A. Steele, S. Suneja, E. Szaky, D. Tam, P. Tam, L. Teskey, K. Tinckam, R. Ting, S. Tsui, P.A. Turner, D. Wadehra, J.A. Wadgymar, R. Wald, A. Walele, L. Warner, C. Wei, J. Weinstein, C. Whiteside, S. Wijeyasekaran, G. Wong, G. Wu, T. Yassa, D. Yuen, and J. Zaltzman.
Supplementary Material
Figure S1. Remission events with and without (a) immunosuppression or (b) hypoalbuminemia.
Figure S2. Survival from a combined event in patients that experienced events only with IS, (a) only without IS, and both with and without IS; (b) and in patients that experienced events only with hypoalbuminemia, only without hypoalbuminemia, and both with and without hypoalbuminemia. IS, immunosuppression.
Figure S3. Independent factors (a) associated with a relapse at 1 year following the remission without maintenance immunosuppression, (b) model calibration and applicability to events with and (c) without atypical features.
Table S1. Remissions events using different immunosuppression drugs.
STROBE Statement.
References
- 1.Kitiyakara C., Eggers P., Kopp J.B. Twenty-one-year trend in ESRD due to focal segmental glomerulosclerosis in the United States. Am J Kidney Dis. 2004;44:815–825. doi: 10.1016/S0272-6386(04)01081-9. [DOI] [PubMed] [Google Scholar]
- 2.Heaf J.G., Sorensen S.S., Hansen A. Increased incidence and improved prognosis of glomerulonephritis: a national 30-year study. Clin Kidney J. 2021;14:1594–1602. doi: 10.1093/ckj/sfaa169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee H., Kim D.K., Oh K.H., et al. Mortality and renal outcome of primary glomerulonephritis in Korea: observation in 1,943 biopsied cases. Am J Nephrol. 2013;37:74–83. doi: 10.1159/000345960. [DOI] [PubMed] [Google Scholar]
- 4.Lepori N., Zand L., Sethi S., Fernandez-Juarez G., Fervenza F.C. Clinical and pathological phenotype of genetic causes of focal segmental glomerulosclerosis in adults. Clin Kidney J. 2018;11:179–190. doi: 10.1093/ckj/sfx143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gipson D.S., Trachtman H., Kaskel F.J., et al. Clinical trial of focal segmental glomerulosclerosis in children and young adults. Kidney Int. 2011;80:868–878. doi: 10.1038/ki.2011.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cattran D.C., Appel G.B., Hebert L.A., et al. A randomized trial of cyclosporine in patients with steroid-resistant focal segmental glomerulosclerosis. North America nephrotic syndrome study group. Kidney Int. 1999;56:2220–2226. doi: 10.1046/j.1523-1755.1999.00778.x. [DOI] [PubMed] [Google Scholar]
- 7.Deegens J.K., Dijkman H.B., Borm G.F., et al. Podocyte foot process effacement as a diagnostic tool in focal segmental glomerulosclerosis. Kidney Int. 2008;74:1568–1576. doi: 10.1038/ki.2008.413. [DOI] [PubMed] [Google Scholar]
- 8.Sethi S., Glassock R.J., Fervenza F.C. Focal segmental glomerulosclerosis: towards a better understanding for the practicing nephrologist. Nephrol Dial Transplant. 2015;30:375–384. doi: 10.1093/ndt/gfu035. [DOI] [PubMed] [Google Scholar]
- 9.Cattran D.C., Appel G.B., Hebert L.A., et al. Cyclosporine in patients with steroid-resistant membranous nephropathy: a randomized trial. Kidney Int. 2001;59:1484–1490. doi: 10.1046/j.1523-1755.2001.0590041484.x. [DOI] [PubMed] [Google Scholar]
- 10.Deegens J.K., Assmann K.J., Steenbergen E.J., et al. Idiopathic focal segmental glomerulosclerosis: a favourable prognosis in untreated patients? Neth J Med. 2005;63:393–398. [PubMed] [Google Scholar]
- 11.De Vriese A.S., Sethi S., Nath K.A., Glassock R.J., Fervenza F.C. Differentiating primary, genetic, and secondary FSGS in adults: a clinicopathologic approach. J Am Soc Nephrol. 2018;29:759–774. doi: 10.1681/ASN.2017090958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jauhal A., Reich H.N., Hladunewich M., et al. Quantifying the benefits of remission duration in focal and segmental glomerulosclerosis. Nephrol Dial Transplant. 2023;38:950–960. doi: 10.1093/ndt/gfac238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Troost J.P., Trachtman H., Nachman P.H., et al. An outcomes-based definition of proteinuria remission in focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2018;13:414–421. doi: 10.2215/CJN.04780517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Troyanov S., Wall C.A., Miller J.A., Scholey J.W., Cattran D.C. Toronto Glomerulonephritis Registry Group. Focal and segmental glomerulosclerosis: definition and relevance of a partial remission. J Am Soc Nephrol. 2005;16:1061–1068. doi: 10.1681/ASN.2004070593. [DOI] [PubMed] [Google Scholar]
- 15.Ponticelli C., Rizzoni G., Edefonti A., et al. A randomized trial of cyclosporine in steroid-resistant idiopathic nephrotic syndrome. Kidney Int. 1993;43:1377–1384. doi: 10.1038/ki.1993.194. [DOI] [PubMed] [Google Scholar]
- 16.Lieberman K.V., Tejani A. A randomized double-blind placebo-controlled trial of cyclosporine in steroid-resistant idiopathic focal segmental glomerulosclerosis in children. J Am Soc Nephrol. 1996;7:56–63. doi: 10.1681/ASN.V7156. [DOI] [PubMed] [Google Scholar]
- 17.Heering P., Braun N., Mullejans R., et al. Cyclosporine A and chlorambucil in the treatment of idiopathic focal segmental glomerulosclerosis. Am J Kidney Dis. 2004;43:10–18. doi: 10.1053/j.ajkd.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 18.Ren H., Shen P., Li X., Pan X., Zhang W., Chen N. Tacrolimus versus cyclophosphamide in steroid-dependent or steroid-resistant focal segmental glomerulosclerosis: a randomized controlled trial. Am J Nephrol. 2013;37:84–90. doi: 10.1159/000346256. [DOI] [PubMed] [Google Scholar]
- 19.Braun N., Schmutzler F., Lange C., et al. Immunosuppressive treatment for focal segmental glomerulosclerosis in adults. Cochrane Database Syst Rev. 2008;2008:CD003233. doi: 10.1002/14651858.CD003233.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laurin L.P., Nachman P.H., Foster B.J. Calcineurin inhibitors in the treatment of primary focal segmental glomerulosclerosis: a systematic review and meta-analysis of the literature. Can J Kidney Health Dis. 2017;4 doi: 10.1177/2054358117692559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banfi G., Moriggi M., Sabadini E., Fellin G., D’Amico G., Ponticelli C. The impact of prolonged immunosuppression on the outcome of idiopathic focal-segmental glomerulosclerosis with nephrotic syndrome in adults. A collaborative retrospective study. Clin Nephrol. 1991;36:53–59. [PubMed] [Google Scholar]
- 22.Fernandez-Juarez G., Villacorta J., Ruiz-Roso G., et al. Therapeutic variability in adult minimal change disease and focal segmental glomerulosclerosis. Clin Kidney J. 2016;9:381–386. doi: 10.1093/ckj/sfw028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Regional program for the study of glomerulonephritis. Central committee of the Toronto glomerulonephritis registry. Can Med Assoc J. 1981;124:158–161. [PMC free article] [PubMed] [Google Scholar]
- 24.D’Agati V.D., Fogo A.B., Bruijn J.A., Jennette J.C. Pathologic classification of focal segmental glomerulosclerosis: a working proposal. Am J Kidney Dis. 2004;43:368–382. doi: 10.1053/j.ajkd.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 25.Kambham N., Markowitz G.S., Valeri A.M., Lin J., D’Agati V.D. Obesity-related glomerulopathy: an emerging epidemic. Kidney Int. 2001;59:1498–1509. doi: 10.1046/j.1523-1755.2001.0590041498.x. [DOI] [PubMed] [Google Scholar]
- 26.Seimiya M., Ohno S., Asano H., et al. Change in albumin measurement method affects diagnosis of nephrotic syndrome. Clin Lab. 2014;60:1663–1667. doi: 10.7754/clin.lab.2014.131105. [DOI] [PubMed] [Google Scholar]
- 27.Royston P., Moons K.G., Altman D.G., Vergouwe Y. Prognosis and prognostic research: developing a prognostic model. BMJ. 2009;338:b604. doi: 10.1136/bmj.b604. [DOI] [PubMed] [Google Scholar]
- 28.Rosenberg A.Z., Kopp J.B. Focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2017;12:502–517. doi: 10.2215/CJN.05960616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pei Y. INF2 is another piece of the jigsaw puzzle for FSGS. J Am Soc Nephrol. 2011;22:197–199. doi: 10.1681/ASN.2010121293. [DOI] [PubMed] [Google Scholar]
- 30.Yao T., Udwan K., John R., et al. Integration of genetic testing and pathology for the diagnosis of adults with FSGS. Clin J Am Soc Nephrol. 2019;14:213–223. doi: 10.2215/CJN.08750718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bose B., Cattran D. Toronto glomerulonephritis R. Glomerular diseases: FSGS. Clin J Am Soc Nephrol. 2014;9:626–632. doi: 10.2215/CJN.05810513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagai R., Cattran D.C., Pei Y. Steroid therapy and prognosis of focal segmental glomerulosclerosis in the elderly. Clin Nephrol. 1994;42:18–21. [PubMed] [Google Scholar]
- 33.Praga M., Morales E., Herrero J.C., et al. Absence of hypoalbuminemia despite massive proteinuria in focal segmental glomerulosclerosis secondary to hyperfiltration. Am J Kidney. 1999;33:52–58. doi: 10.1016/s0272-6386(99)70257-x. [DOI] [PubMed] [Google Scholar]
- 34.Trachtman H., Nelson P., Adler S., et al. DUET: a Phase 2 study evaluating the efficacy and safety of sparsentan in patients with FSGS. J Am Soc Nephrol. 2018;29:2745–2754. doi: 10.1681/ASN.2018010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tedesco M., Mescia F., Pisani I., et al. The role of rituximab in primary focal segmental glomerular sclerosis of the adult. Kidney Int Rep. 2022;7:1878–1886. doi: 10.1016/j.ekir.2022.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gauckler P., Kronbichler A. The role of rituximab in focal segmental glomerulosclerosis-update from Italy. Kidney Int Rep. 2022;7:1731–1733. doi: 10.1016/j.ekir.2022.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heerspink H.J.L., Stefansson B.V., Correa-Rotter R., et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436–1446. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 38.Thomas D.B., Franceschini N., Hogan S.L., et al. Clinical and pathologic characteristics of focal segmental glomerulosclerosis pathologic variants. Kidney Int. 2006;69:920–926. doi: 10.1038/sj.ki.5000160. [DOI] [PubMed] [Google Scholar]
- 39.Hogg R.J., Friedman A., Greene T., et al. Renal function and proteinuria after successful immunosuppressive therapies in patients with FSGS. Clin J Am Soc Nephrol. 2013;8:211–218. doi: 10.2215/CJN.08330812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laurin L.P., Gasim A.M., Derebail V.K., et al. Renal survival in patients with collapsing compared with not otherwise specified FSGS. Clin J Am Soc Nephrol. 2016;11:1752–1759. doi: 10.2215/CJN.13091215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.D’Agati V.D., Alster J.M., Jennette J.C., et al. Association of histologic variants in FSGS clinical trial with presenting features and outcomes. Clin J Am Soc Nephrol. 2013;8:399–406. doi: 10.2215/CJN.06100612f. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared upon reasonable request to the corresponding authors.