Abstract
Introduction
Evocalcet is an oral calcimimetic agent with proven efficacy and safety in treating secondary hyperparathyroidism (SHPT) in Japanese patients on dialysis.
Methods
This randomized, double-blind, intrapatient dose-adjustment, parallel-group, international multicenter study compared the efficacy and safety of evocalcet versus cinacalcet for 52 weeks in East Asian hemodialysis patients with SHPT.
Results
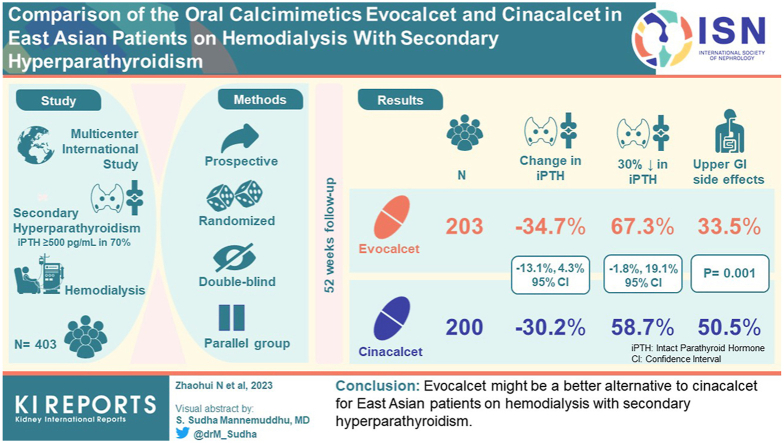
In total, 203 and 200 patients were randomized to receive evocalcet or cinacalcet, respectively (overall, 70.1% had baseline intact parathyroid hormone (PTH) levels ≥500 pg/ml, with no between-group difference). Mean percentage changes in intact PTH levels from baseline were −34.7% and −30.2% in the evocalcet and cinacalcet groups at 52 weeks (between-group difference −4.4%, 95% confidence interval [CI] −13.1%, 4.3%, below the predefined 15% noninferiority margin). Overall, 67.3% and 58.7% of patients in the evocalcet and cinacalcet groups, respectively, achieved ≥30% decrease in intact PTH levels from baseline (between-group difference 8.6%; 95% CI −1.8%, 19.1%). No major safety concerns were observed. Gastrointestinal adverse events (AEs) were significantly less frequent with evocalcet compared with cinacalcet (33.5% vs. 50.5%, P = 0.001), whereas the incidence of hypocalcemia did not differ.
Conclusion
Evocalcet might be a better alternative to cinacalcet for East Asian patients on hemodialysis with SHPT.
Keywords: cinacalcet, evocalcet, gastrointestinal, East Asia, randomized controlled trial, secondary hyperparathyroidism
Graphical abstract
The number of patients under maintenance dialysis therapy is increasing in East Asian countries, such as China, South Korea, and Japan.1,2 Mineral and bone disorder due to chronic kidney disease (CKD-MBD) is prevalent in patients on dialysis.3 SHPT is one of the common complications in CKD-MBD, triggered by hyperphosphatemia, decreased renal production of active vitamin D3, failure in renal calcium (Ca) reabsorption with decreased intestinal Ca absorption, and resulting hypocalcemia. In addition, PTH, which inhibits phosphorus (P) reabsorption from urine and stimulates active vitamin D3 production, is secreted from the parathyroid gland. Therefore, hyperphosphatemia and hypocalcemia further persist, and the chronic stimulation leads to parathyroid hyperplasia and a state of excessive PTH secretion.4,5
SHPT leads to a series of pathological conditions including bone fragility, ectopic calcification, and pain, in addition to vascular calcifications and atherosclerosis.5 It is, therefore, necessary to control PTH, P, and Ca levels in patients with SHPT. Treatment goals (i.e., target ranges) have been established in international practice guidelines such as Kidney Disease Outcome Quality Initiative guidelines and The Kidney Disease Improving Global Outcomes.6,7 Current SHPT treatment options include active vitamin D preparations, phosphate binders, the oral calcimimetic agent cinacalcet hydrochloride (hereafter referred to as cinacalcet), and the intravenous agent etelcalcetide,8 which has recently become available in East Asia.9
Cinacalcet has been shown to reduce intact PTH (iPTH) and concurrently high levels of Ca and P,10, 11, 12, 13 thus improving CKD-MBD management regarding cardiovascular outcomes, fractures, and attenuation of vascular and cardiac valve calcifications.14,15 In the EVOLVE trial, cinacalcet led to nominally significant decreases in the risk of death or first myocardial infarction, hospitalization for unstable angina, heart failure, or a peripheral vascular event.14 Furthermore, in the ADVANCE trial, cinacalcet combined with vitamin D decreased vascular and cardiac valve calcification scores.15
Cinacalcet can result in gastrointestinal AEs16, 17, 18 that patients perceive as burdensome and that can interfere with dose adjustments or hinder treatment adherence.14,19 In addition, cinacalcet strongly inhibits cytochrome P450 (CYP) 2D6 and is metabolized by CYP3A4.20 Etelcalcetide is an intravenous calcimimetic approved for treating SHPT in patients undergoing hemodialysis.21 It has become a suitable option, particularly in patients with poor treatment adherence21; however, in a head-to-head comparison of etelcalcetide and cinacalcet, no significant difference was observed between the 2 in gastrointestinal AEs, such as self-reported nausea and vomiting.8 Therefore, there is a need for new-generation drugs with fewer gastrointestinal AEs and a lower risk of drug interactions.
Evocalcet is a new oral calcimimetic agent with long-term efficacy and safety for SHPT demonstrated in clinical studies in Japan.22, 23, 24, 25, 26, 27, 28 Evocalcet has been shown to be noninferior to cinacalcet, and patients treated with evocalcet had a lower incidence of gastrointestinal drug-related AEs than with cinacalcet.27 Evocalcet does not strongly inhibit major CYP isoforms; therefore, it is considered likely to become an easy-to-use treatment option in terms of drug interactions.29 Considering that the current evidence on evocalcet safety and efficacy for SHPT is from Japan only, it is necessary to confirm these results in other East Asian populations. Therefore, this study aimed to compare the efficacy and safety of evocalcet orally administered once daily for 52 weeks in East Asian patients with SHPT receiving hemodialysis.
Methods
Study Design, Setting, and Procedures
This was a randomized, double-blind, intrapatient, dose-adjustment, parallel-group study conducted at 44 sites in East Asia, including mainland China, South Korea, Taiwan, and Hong Kong Special Administrative Region between April 9, 2019 and September 23, 2021. The ethical review boards of the participating sites approved the study protocol. The study was conducted following the principles described in the Declaration of Helsinki, The International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use, Good Clinical Practice Guidelines, and the regulations in each region. All patients provided written informed consent to participate in this study. The study was registered at Clinicaltrials.gov under the identifier NCT03822507.
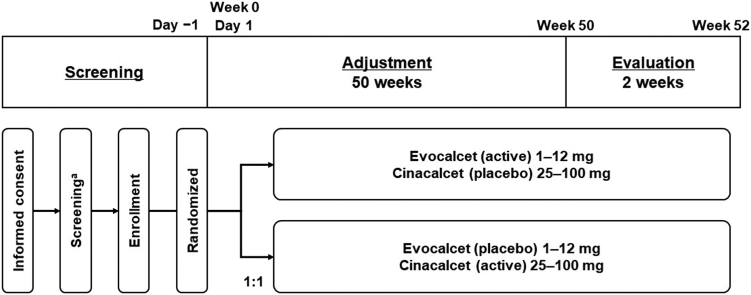
Cinacalcet was the active control, and the treatment duration was 52 weeks, comprising a 50-week dose adjustment period and a 2-week evaluation period. Screening assessments were conducted 30 days before the initial dose of the study drug (Figure 1).
Figure 1.
Study design. aThe screening assessments were conducted within 30 days prior to the first dose of study drug.
For randomization, eligible patients were allocated in a 1:1 ratio to the evocalcet or cinacalcet group using a dynamic allocation procedure. iPTH level at screening (<500 pg/ml, ≥500 and <1000 pg/ml, or ≥1000 pg/ml), status of cinacalcet hydrochloride use, country, and investigative site were used as stratification factors. Randomization was performed using an interactive web response system. The blinding method used was a double-dummy, double-blind design. The master randomization list of the study and created code-break were securely stored until unblinding or in case of emergency.
Study treatment was started before the dialysis session on the day of the longest dialysis interval. Patients were administered the study drug orally. The dose-adjustment period was from week 0 to 49 (until the day before the week 50 visit). The starting dose of evocalcet was 1 mg in patients with an iPTH level of <500 pg/ml and 2 mg in those with an iPTH level of ≥500 pg/ml, as measured at screening. The starting dose of cinacalcet hydrochloride was 25 mg for all patients, regardless of the iPTH level at screening. The evaluation period was from week 50 to 52. The dose prescribed at week 49 was maintained without further adjustments throughout the evaluation. However, dose reduction or interruption was allowed.
The dose was adjusted based on the following criteria and was increased (by 1 mg for evocalcet and 25 mg for cinacalcet) if the current dose was maintained for ≥3 weeks and the iPTH level at the last scheduled visit before the dose change was >300 pg/ml; corrected serum Ca level at the last scheduled visit before the dose change was ≥8.4 mg/dl; or the investigator determined that the dose increase would not affect the patient’s safety. If the dose of cinacalcet reached 100 mg, only the dose of evocalcet was increased. The dose was reduced (by 1 mg for evocalcet and by 25 mg for cinacalcet) if the iPTH level decreased to <150 pg/ml or the investigator determined that the dose reduction was necessary due to AE onset and to ensure patient safety. The dose was interrupted if the corrected serum Ca level decreased to ≤7.5 mg/dl or the investigator determined that the dose interruption was necessary due to AE onset.
Prohibited medications or procedures were cinacalcet, bisphosphonates, denosumab, teriparatide, parathyroidectomy/parathyroid intervention, and peritoneal dialysis. Active vitamin D preparations and derivatives, phosphate binders, and Ca preparations were permitted but restricted. Detailed criteria for these concomitant medications and therapies are provided in the Supplementary Methods.
Patients
This study targeted patients with SHPT receiving hemodialysis. Patients were included if they were aged ≥18 years at the time of consent (the cut-off age depends on local laws) with stable kidney failure, undergoing hemodialysis 3 times per week for at least 12 weeks before screening, and had centrally measured iPTH of >300 pg/ml and serum-corrected Ca of ≥9.0 mg/dl at screening.
The main exclusion criteria were as follows: treatment with cinacalcet hydrochloride within 2 weeks before screening; change in the dose or dosing regimen of an activated vitamin D drug or its derivative, phosphate binder, or Ca preparation within 2 weeks before screening; or the start of treatment with such drugs within 2 weeks before screening; and change in prescribed conditions of dialysis (dialysate Ca concentration, prescribed dialysis time, and prescribed number of dialysis sessions per week) within 2 weeks before screening. Full eligibility criteria are provided in the Supplementary Methods.
Efficacy End Points
The primary end point was the mean percentage change in iPTH level from baseline. Several secondary end points were evaluated, including the number and percentage of patients achieving a mean percentage decrease in iPTH level of ≥30% (percentage change ≤ −30%) from baseline. The number and percentage of patients achieving a mean iPTH level of ≥150 pg/ml and ≤300 pg/ml and iPTH level, corrected serum Ca level, and serum P level during the evaluation period were also assessed.
Exploratory end points were whole PTH level, intact fibroblast growth factor 23 (FGF23) level, and corrected serum Ca–P product and bone metabolic markers (bone-specific alkaline phosphatase [BAP], tartrate-resistant acid phosphatase 5b [TRACP-5b], and total N-terminal propeptide of type 1 procollagen [P1NP]).
Safety
Safety evaluations included the frequency of AEs, AEs associated with upper gastrointestinal disorders, clinically relevant changes in laboratory values, vital signs, or 12-lead electrocardiogram. MedDRA Version 24.0 was used to code AEs.
Laboratory Measurements
All clinical parameters were measured at a central laboratory. For intact PTH measurements, plasma samples were obtained and analyzed by electrochemiluminescence assay (Cobas e601; Roche Diagnostics GmbH, Mannheim, Germany). For iFGF23, plasma samples were analyzed by enzyme-linked immunosorbent assay (human FGF23 [intact]; Immutopics Inc., San Clemente, CA, USA).
Statistical Analysis
Sample size rationale and calculations are described in the Supplementary Methods. The data sets analyzed were the primary efficacy set (referred to as the full analysis set [FAS]), per-protocol set, and safety analysis set. For the primary end point, descriptive statistics, and corresponding 95% CI were calculated for each treatment group. For the secondary end points, the number and percentage of patients achieving the target for each of the different end points were calculated for categorical data; descriptive statistics were calculated for continuous data.
For the primary analysis, the difference in the mean percentage change in iPTH level from baseline between treatment groups (evocalcet group−cinacalcet group) and the 95% CI for the difference was calculated. When iPTH was missing, missing data were imputed using multiple imputation analysis. Noninferiority was demonstrated when the upper bound of the 2-sided 95% CI for the difference between the treatment groups (evocalcet group−cinacalcet group) was under the noninferiority margin of 15%. Furthermore, if noninferiority was confirmed in the primary end point, the noninferiority of the secondary end point, number, and percentage of patients achieving a mean percentage decrease in iPTH level of ≥30% from baseline, was demonstrated when the lower bound of the 2-sided 95% CI for the difference between the treatment groups was over the noninferiority margin of −15%.
For the incidences of gastrointestinal AEs (i.e., vomiting, abdominal discomfort, abdominal distension, nausea, and decreased appetite) by treatment group, the Clopper–Pearson method was used to calculate the 95% CI for evocalcet and cinacalcet. The 95% CI for between-group differences between evocalcet and cinacalcet was calculated using the Wald test method and the P-values using Fisher exact test.
The significance level was 5%, and statistical hypothesis tests were 2-sided. The statistical software used for the analysis was SAS version 9.4 (SAS Institute, Cary, NC).
Results
Patient Disposition
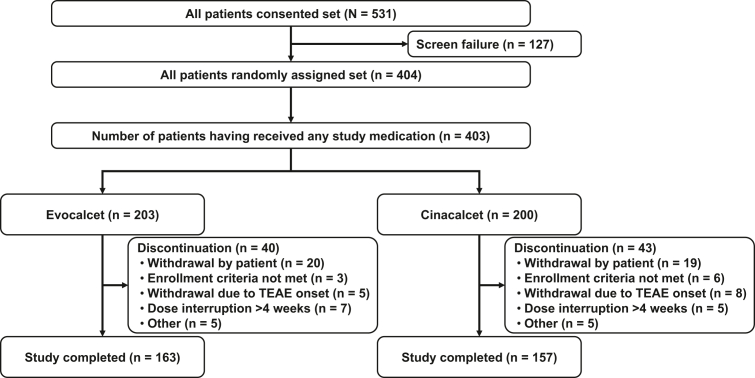
Of the 531 patients who consented to participate, 404 patients met the study criteria and were randomly assigned to treatment: 203 and 200 patients to the evocalcet and cinacalcet groups, respectively. All randomized patients received the study medication, except 1e in the cinacalcet group, who withdrew at their own decision.
Overall, 83 patients (20.6%) discontinued the study: 40 patients (19.7%) and 43 patients (21.5%) in the evocalcet and cinacalcet groups (SAF), respectively. The most common reason for discontinuation was withdrawal by the patient (20 [9.9%], evocalcet group; 19 [9.5%], cinacalcet group), followed by withdrawal by the investigator due to AE onset (5 [2.5%], evocalcet group; 8 [4.0%], cinacalcet group), withdrawal due to continuous dose interruption for >4 weeks (7 [3.4%], evocalcet group; 5 [2.5%], cinacalcet group), and other reasons (5 [2.5%] in each group) (Figure 2).
Figure 2.
Patient disposition. TEAE, treatment-emergent adverse event.
Patient Characteristics
In both groups (FAS), most patients were male (62.8% and 64.8% in the evocalcet and cinacalcet groups, respectively) with a mean age (± SD) of 53.0 (12.1) and 52.0 (13.2) years, respectively; in each group, only 20.1% and 17.3%, respectively of patients were aged ≥65 years. In the evocalcet and cinacalcet groups, respectively, most patients were from China (including Hong Kong Special Administrative Region) (69.3% and 69.4%), followed by Taiwan (19.6% and 20.9%) and South Korea (11.1% and 9.7%).
In both groups, more than 65% of patients had iPTH level ≥500 pg/ml, more than 60% had ≥9.5 mg/dl corrected serum Ca level, and more than 65% had serum P ≥5.5 mg/dl at baseline. Furthermore, over 50% had used cinacalcet and/or active vitamin D preparations and had continued dialysis for <10 years. Regarding underlying conditions at baseline, most patients in both treatment groups did not have diabetic nephropathy. In general, no notable differences between the evocalcet and cinacalcet groups were observed regarding demographic and baseline characteristics (Table 1). The mean dosage during the evaluation period (ad hoc) was 4.3 ± 3.2 mg in the evocalcet group and 49.4 ± 32.5 mg in the cinacalcet group.
Table 1.
Demographics and other characteristics at baseline (FAS)
| Patient characteristics | Evocalcet (n = 199) | Cinacalcet (n = 196) | Total (N = 395) | P-value |
|---|---|---|---|---|
| Agea, yrs, mean (SD) | 53.0 (12.13) | 52.0 (13.16) | 52.5 (12.65) | 0.449d |
| Median (min, max) | 54.0 (26, 79) | 53.0 (26, 90) | 53.0 (26, 90) | 0.425e |
| ≥65 yrs, n (%) | 40 (20.1) | 34 (17.3) | 74 (18.7) | 0.483f |
| Sex, n (%) | ||||
| Male | 125 (62.8) | 127 (64.8) | 252 (63.8) | 0.681f |
| Female | 74 (37.2) | 69 (35.2) | 143 (36.2) | |
| Region, n (%) | ||||
| China (including Hong Kong SAR) | 138 (69.3) | 136 (69.4) | 274 (69.4) | 0.877f |
| Taiwan | 22 (11.1) | 19 (9.7) | 41 (10.4) | |
| South Korea | 39 (19.6) | 41 (20.9) | 80 (20.3) | |
| Body mass indexb (kg/m2), mean ± SD | 24.0 ± 4.0 | 23.9 ± 4.1 | 24.0 ± 4.0 | 0.786d |
| Serum iPTH (pg/ml), mean ± SD | 778.4 ± 421.24 | 807.7 ± 517.67 | - | 0.537d |
| <500 | 53 (26.6) | 65 (33.2) | 118 (29.9) | 0.182f |
| 500–1000 | 98 (49.2) | 79 (40.3) | 177 (44.8) | |
| ≥1000 | 48 (24.1) | 52 (26.5) | 100 (25.3) | |
| Corrected serum Ca level (mg/dl) | 9.77 ± 0.653 | 9.73 ± 0.768 | - | 0.625d |
| Baseline serum P levelc (mg/dl) | 6.35 ± 1.633 | 6.35 ± 1.931 | 0.999 | |
| <5.5 | 61 (30.7) | 67 (34.2) | 128 (32.4) | 0.432f |
| ≥5.5 | 138 (69.3) | 128 (65.3) | 266 (67.3) | |
| Missing | 0 | 1 (0.5) | 1 (0.3) | |
| Use of cinacalcet hydrochloride | ||||
| Yes | 111 (55.8) | 111 (56.6) | 222 (56.2) | 0.864f |
| No | 88 (44.2) | 85 (43.4) | 173 (43.8) | |
| Use of active vitamin D preparations | ||||
| Yes | 119 (59.8) | 122 (62.2) | 241 (61.0) | 0.618f |
| No | 80 (40.2) | 74 (37.8) | 154 (39.0) | |
| Presence or absence of underlying diabetic nephropathy | ||||
| Yes | 17 (8.5) | 21 (10.7) | 38 (9.6) | 0.464f |
| No | 182 (91.5) | 175 (89.3) | 357 (90.4) | |
| Dialysis history (yrs) | ||||
| <10 | 123 (61.8) | 114 (58.2) | 237 (60.0) | 0.497f |
| ≥10 | 76 (38.2) | 81 (41.3) | 157 (39.7) | |
| Missing | 0 | 1 (0.5) | 1 (0.3) | |
| Ca concentration in dialysis solution (mEq/l) | ||||
| 2.5 | 49 (24.6) | 52 (26.5) | 101 (25.6) | 0.682f |
| 2.75 | 0 | 0 | 0 | |
| 3.0 | 143 (71.9) | 138 (70.4) | 281 (71.1) | |
| Other | 7 (3.5) | 6 (3.1) | 13 (3.3) | |
| Dialysis type | ||||
| HD | 120 (60.3) | 110 (56.1) | 230 (58.2) | 0.114f |
| HDF | 13 (6.5) | 6 (3.1) | 19 (4.8) | |
| Other | 66 (33.2) | 80 (40.8) | 146 (37.0) | |
| Initial dose of study drug | ||||
| Evocalcet 1 mg | 62 (31.2) | 65 (33.2) | 127 (32.2) | 0.540f |
| Evocalcet 2 mg | 137 (68.8) | 131 (66.8) | 268 (67.8) |
Ca, calcium; FAS, full analysis set; HD, hemodialysis; HDF, hemodiafiltration; P, phosphorus; PTH, parathyroid hormone; SAR, Special Administrative Region.
The percentage of patients in each category was relative to the total number of patients in the relevant analysis set.
Calculated relative to informed consent date for non-Koreans. Age was collected on case report forms for South Koreans.
Body mass index (kg/m2) = weight (kg) / height at baseline (m)2.
Baseline was defined as the last nonmissing measurement taken prior to the date of first administration of any study medication.
P-value based on t-test.
P-value based on generalized Wilcoxon test.
P-value based on chi-squared test.
Efficacy
Primary End Point
Mean percentage changes in iPTH levels from baseline during the evaluation period were −34.7% in the evocalcet group and −30.2% in the cinacalcet group, with a between-group difference of −4.4% (95% CI −13.1%, 4.3%) (Table 2). The upper limit of the 2-sided 95% CI of the between-group difference was 4.3%, below the noninferiority margin of 15%; thus, evocalcet was demonstrated to be noninferior to cinacalcet.
Table 2.
Summary of results of primary and secondary efficacy measures (FAS)
| Measures | Evocalcet (n = 199) | Cinacalcet (n = 196) | Difference between groups (evocalcet − cinacalcet), P-value |
|---|---|---|---|
| Mean percentage change in iPTH (95% CI) | −34.7 (−40.8, −28.5) | −30.2 (−36.3, −24.2) | −4.4 (−13.1, 4.3), 0.000 |
| Target iPTH level ≥30% (percentage change ≤ −30%) from baseline (95% CI) | 67.3 (60.2, 74.5) | 58.7 (51.2, 66.2) | 8.6 (−1.8, 19.1), 0.000 |
| Target iPTH level (≥150 pg/ml and ≤300 pg/ml) (95% CI) | 33.8 (26.8, 40.8) | 34.1 (26.9, 41.2) | −0.2 (−10.2, 9.8), 0.003 |
CI, confidence interval; FAS, full analysis set, PTH, parathyroid hormone.
95% CI values were derived by t statistic. The noninferiority margin was 15%. P-values were calculated by t-test.
Secondary End Points
The percentage of patients achieving a mean percentage decrease in iPTH level of ≥30% (percentage change ≤ −30%) from baseline was 67.3% with evocalcet and 58.7% with cinacalcet (with a between-group difference in the achievement ratio of 8.6% [95% CI −1.8%, 19.1%]; Table 2), indicating evocalcet was noninferior to cinacalcet.
The percentage of patients achieving mean iPTH levels of ≥150 pg/ml and ≤300 pg/ml during the evaluation period were similar in both groups (33.8% and 34.1%), with a between-group difference in the achievement ratio of −0.2% (95% CI −10.2%, 9.8%).
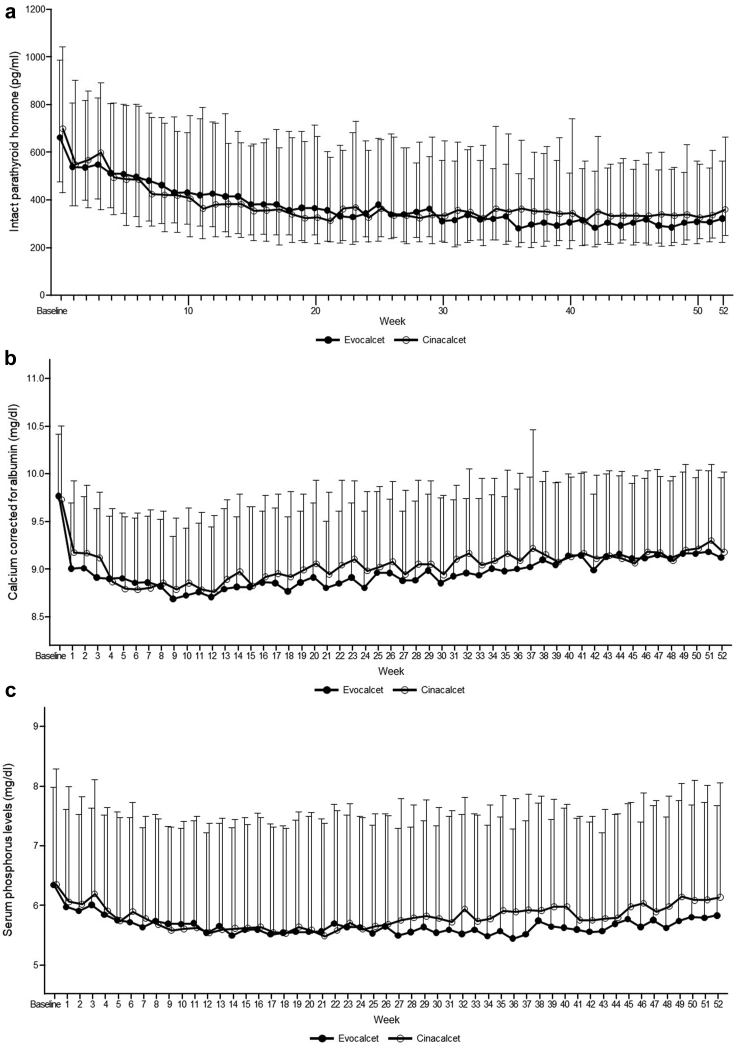
The time courses of iPTH level, corrected serum Ca level, and serum P level are shown in Figure 3a–c. Median baseline iPTH levels were 664.20 and 700.05 pg/ml in the evocalcet and cinacalcet groups and decreased to 325.00 and 361.10 pg/ml at week 52, respectively. Median percentage changes from baseline at week 52 were −48.60% and −43.51% in the evocalcet and cinacalcet groups, respectively. In the evocalcet and cinacalcet groups, median corrected serum Ca levels were comparable at baseline (9.70 and 9.60 mg/dl) and remained similar at week 52 (9.10 and 9.10 mg/dl), with median changes from baseline at week 52 of −0.60 and −0.45 mg/dl, respectively. Median serum P levels were also comparable (6.20 and 5.90 mg/dl) at baseline and decreased slightly at week 52 (5.75 and 5.90 mg/dl) with median changes from baseline of −0.60 and −0.30 mg/dl in the evocalcet and cinacalcet groups, respectively.
Figure 3.
Time course of (a) iPTH (Median [Q1–Q3]), (b) corrected serum Ca (Mean + SD), and (c) serum P levels (Mean + SD) during the study in the FAS. Ca, calcium; FAS, full analysis set; P, phosphorus; PTH, parathyroid hormone; Q, quartile.
Exploratory End Points
Whole PTH (Supplementary Figure S1) over time from baseline and the median (quartile 1 [Q1], Q3) percentage changes in whole PTH level from baseline at week 52 were comparable between the groups (−51.2% [–68.74%, –19.16%] and −46.6% [−67.37%, −15.02%], respectively). Other measures, including corrected serum Ca–P product (Supplementary Figure S2) and the bone metabolic markers BAP levels (Supplementary Figure S3), TRACP-5b (Supplementary Figure S4), total P1NP (Supplementary Figure S5), and intact FGF23 levels (Supplementary Figure S6), generally decreased over time.
In both treatment groups, the higher the iPTH level at baseline, the higher the mean percentage change in iPTH from baseline during the evaluation period (Supplementary Tables S1, S2).
Safety
Summary of AEs
AEs occurred in 197 of 203 patients (97.0%) and 195 of 200 patients (97.5%) in the evocalcet and cinacalcet groups, respectively. Drug-related AEs occurred in 156 of 203 patients (76.8%) in the evocalcet and 168 of 200 patients (84.0%) in the cinacalcet groups. The most common AEs were hypocalcemia (53.2% and 50.5%) (Table 3) in the respective groups.
Table 3.
Drug-related AEs by PT that occurred in ≥3% of patients in any treatment group (SAF)
| [SOC] PT | Evocalcet (n = 203) n (%) E | Cinacalcet (n = 200) n (%) E | Total (N = 403) n (%) E | P-value |
|---|---|---|---|---|
| Number of patients with at least one drug-related AE | 156 (76.8) 991 | 168 (84.0) 1390 | 324 (80.4) 2381 | 0.070 |
| [Metabolism and nutrition disorders] | 115 (56.7) 559 | 119 (59.5) 589 | 234 (58.1) 1148 | 0.562 |
| Hypocalcemia | 108 (53.2) 490 | 101 (50.5) 448 | 209 (51.9) 938 | 0.587 |
| Decreased appetite | 14 (6.9) 23 | 36 (18.0) 97 | 50 (12.4) 120 | 0.000 |
| Hypoproteinemia | 11 (5.4) 20 | 9 (4.5) 21 | 20 (5.0) 4 | 0.671 |
| [Gastrointestinal disorders] | 65 (32.0) 228 | 95 (47.5) 527 | 160 (39.7) 755 | 0.001 |
| Nausea | 25 (12.3) 49 | 49 (24.5) 157 | 74 (18.4) 206 | 0.001 |
| Vomiting | 19 (9.4) 32 | 40 (20.0) 127 | 59 (14.6) 159 | 0.002 |
| Abdominal discomfort | 23 (11.3) 51 | 35 (17.5) 77 | 58 (14.4) 128 | 0.077 |
| Abdominal distension | 16 (7.9) 39 | 31 (15.5) 70 | 47 (11.7) 109 | 0.017 |
| Diarrhea | 11 (5.4) 22 | 21 (10.5) 36 | 32 (7.9) 58 | 0.059 |
| Abdominal pain upper | 5 (2.5) 11 | 8 (4.0) 11 | 13 (3.2) 22 | 0.382 |
| Flatulence | 0 | 8 (4.0) 18 | 8 (2.0) 18 | 0.003 |
| Gastroesophageal reflux disease | 7 (3.4) 7 | 1 (0.5) 1 | 8 (2.0) 8 | 0.033 |
| [Investigations] | 40 (19.7) 133 | 44 (22.0) 173 | 84 (20.8) 306 | 0.570 |
| Blood calcium decreased | 16 (7.9) 31 | 18 (9.0) 50 | 34 (8.4) 81 | 0.686 |
| Electrocardiogram QT prolonged | 15 (7.4) 29 | 18 (9.0) 48 | 33 (8.2) 77 | 0.555 |
| Calcium ionized decreased | 9 (4.4) 65 | 9 (4.5) 59 | 18 (4.5) 124 | 0.974 |
| [Musculoskeletal and connective tissue disorders] | 8 (3.9) 12 | 8 (4.0) 22 | 16 (4.0) 34 | 0.975 |
| Muscle spasms | 5 (2.5) 9 | 6 (3.0) 18 | 11 (2.7) 27 | 0.740 |
| [Nervous system disorders] | 6 (3.0) 7 | 9 (4.5) 14 | 15 (3.7) 21 | 0.412 |
| Dizziness | 0 | 7 (3.5) 10 | 7 (1.7) 10 | 0.007 |
AE, adverse event; PT, preferred term; SAF, safety analysis set; SOC, system organ class.
“n” represents the number of patients, % is the percentage of patients in each category, and E represents the number of drug-related AEs. AEs were defined as AEs that started or worsened in severity on or after the first dose of study medication. MedDRA Version 24.0 was used to code AEs. All P-values were calculated by chi-squared test.
The proportions of patients who presented Ca decrease-related AEs were similar (125 [61.6%] and 122 [61.0%]) in the evocalcet and cinacalcet groups. Electrocardiogram QT prolonged occurred in 16 patients (7.9%) in the evocalcet group and 20 patients (10.0%) in the cinacalcet group. No torsade de pointes AEs occurred during the trial.
Serious AEs occurred in 49 (24.1%) and 40 patients (20.0%) in the evocalcet and cinacalcet groups; these were considered drug-related AEs in 6 (3.0%) and 2 (1.0%) patients in the evocalcet and cinacalcet groups, respectively. Serious AEs that resulted in death occurred in 3 (1.5%) and 2 (1.0%) patients in the evocalcet and cinacalcet groups, respectively. One event of cardiorespiratory arrest occurred in a patient with congenital heart disease in the evocalcet group, which was considered related to the study drug.
AEs Associated with Upper Gastrointestinal Disorders
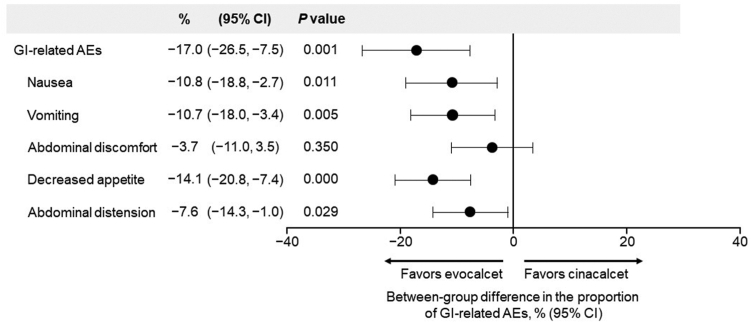
Upper gastrointestinal disorders occurred in 68 (33.5%) and 101 (50.5%) patients in the evocalcet and cinacalcet groups, respectively, with a difference in the incidence of −17.0% (95% CI −26.5%, −7.5%; P = 0.001). The most common upper gastrointestinal disorder in both treatment groups was nausea (34 [16.7%], evocalcet group; 55 [27.5%], cinacalcet group). Comparing the evocalcet and cinacalcet groups, the incidences of upper gastrointestinal disorders, including nausea (−10.8 [95% CI −18.8, −2.7]; P = 0.011), vomiting (−10.7 [−18.0, −3.4]; P = 0.005), decreased appetite (−14.1 [−20.8, −7.4]; P = 0.000), and abdominal distension (−7.6 [−14.3, −1.0]; P = 0.029) were significantly lower in the evocalcet group than in the cinacalcet group (Figure 4). Drug-related AEs involving upper gastrointestinal disorders occurred in 54 patients (26.6%) in the evocalcet group and 87 patients (43.5%) in the cinacalcet group, with a difference in incidence of −16.9% (95% CI −26.1%, −7.7%). The difference in incidences between the 2 groups was consistent for AEs and drug-related AEs.
Figure 4.
Forest plot quantifying the risk difference of GI-related AEs by treatment group. For the evocalcet group, the 95% CIs were calculated using the Clopper–Pearson method. For the cinacalcet group, 95% CIs were calculated using the Wald test method. The p-value was based on Fisher’s exact test. MedDRA Version 24.0 was used to code AEs. A patient was counted only once per AE category and once per unique PT within the AE category. AE, adverse event; CI, confidence interval; GI, gastrointestinal; PT, preferred term; SAF, safety analysis set.
Discussion
This double-blind, double-dummy, randomized, noninferiority trial of evocalcet and cinacalcet in patients with SHPT receiving hemodialysis demonstrated that evocalcet was noninferior to cinacalcet in reducing iPTH levels. For the secondary end points, the proportion of participants with ≥30% decrease in iPTH from baseline was also noninferior to cinacalcet. In addition, no significant concerns were observed with safety overall in the evocalcet group, and the occurrence of AEs was comparable with cinacalcet. The exception was for upper gastrointestinal disorders, whereby the incidence was significantly lower for patients receiving evocalcet compared with cinacalcet. Furthermore, the incidence of almost all components (i.e., vomiting, nausea, abdominal discomfort, decreased appetite, and abdominal distension) was significantly lower with evocalcet than with cinacalcet, which indicates a clear risk reduction in the incidence of upper gastrointestinal disorders. Therefore, based on these results, evocalcet may improve adherence to SHPT treatment.
Notably, this study enrolled 48 (24.1%) and 52 (26.5%) patients with iPTH ≥1000 pg/ml in the evocalcet and cinacalcet groups. Evocalcet was shown to be effective in such patients, with mean (± SD) percentage changes in iPTH of −46.07 (± 35.081) and −37.13 (± 39.112) pg/ml with evocalcet and cinacalcet, respectively. Such patients (with iPTH ≥1000 pg/ml) generally have severe SHPT. Nodular hyperplasia is also associated with resistance to cinacalcet therapy,30 and surgical parathyroidectomy is applied to refractory patients.31 Of note, in the Japanese phase 3 trial, efficacy in patients with such high iPTH remained unknown because of the low number of patients with iPTH ≥1000 pg/ml enrolled in that study.27 Therefore, according to the present results, evocalcet may provide a therapeutic option for severe SHPT.
The phase 2b study suggested a dose of 2-mg evocalcet elicited an iPTH-lowering effect similar to 25 mg cinacalcet.23 It is meaningful that the dose of evocalcet can be increased to 12 mg, which is hypothetically equivalent to cinacalcet 150 mg. A higher treatment effect could be achieved with a higher dose. High doses (9–12 mg) were administered to a higher percentage of patients with iPTH baseline level ≥1000 pg/ml than patients with iPTH baseline <1000 pg/ml (data not shown), which could increase the iPTH-lowering effect in a subpopulation with iPTH baseline ≥1000 pg/ml. Conversely, cinacalcet can cause upper gastrointestinal AEs and hypocalcemia, which are major factors affecting patient adherence and dose increases. Importantly, adherence to calcimimetics impacts the clinical outcome. A previous report showed that combining evocalcet with a vitamin D receptor activator, a commonly used SHPT treatment, can suppress PTH levels while reducing hypocalcemia32; therefore, concomitant use of a vitamin D receptor activator and evocalcet may provide a more effective and safer treatment for severe SHPT patients with high PTH.
This study had some limitations, such as the 52-week treatment period, and longer-term data on East Asian populations are still needed. Changes in and new initiation of vitamin D preparations and changes to prescribed dialysis conditions were restricted in the study, which does not reflect real-world clinical practice. Furthermore, a surrogate end point (lowering iPTH) was used to evaluate CKD-MBD treatment. Finally, the study only enrolled patients in China, South Korea, Taiwan, and Hong Kong Special Administrative Region; therefore, the results are not necessarily generalizable to other populations.
Conclusion
The mean percentage change from baseline in mean iPTH levels during the evaluation period confirmed that evocalcet was noninferior to cinacalcet. No major safety concerns were observed overall, with a significantly lower incidence of upper gastrointestinal drug-related AEs in the evocalcet group compared with the cinacalcet group. Based on these efficacy and safety findings, evocalcet might be a better alternative to cinacalcet for SHPT in East Asian hemodialysis patients with SHPT.
Appendix
List of the Orchestra Study Group
Liang Xinling (Principle Investigator)1, Liu Shuangxin1, Li Sijia1, Xu Lixia1, Ye Zhiming1, Feng Zhonglin1, Huang Renwei1, Li Zhilian1, Chen Wei (Principle Investigator)2, Zheng Xunhua2, Huang Naya2, Ai Zhen2, Wang Xin2, Zheng Xunhua (former PI)3, Zhaohui Ni (present PI)3, Lu Renhua3, Shen Jianxiao3, Zhou Yijun3, Lin Xinghui3, Xie Yuanyuan3, Zhang Jiahui3, Che Miaolin3, Fang Yan3, Pang Huihua3, Su Xinyu3, Gu Leyi3, Jin Wei3, Zhao Peipei3, Shen Yiwei3, Zao Liou3, Lu Wei (Principle Investigator)4, Huang Haidong4, Ji Gang4, Li Hao (former PI)5, Wang Deguang (present PI)5, Wang Deguang5, Yuan Liang5, Ding Lihong5, Wang Xuerong5, Li Huai5, Liu Hong (Principle Investigator)6, Yuan Fang6, Song Panai6, Zhou An6, Chen Xiaojun6, Li Xiejia6, He Liyu6, Tan Xia6, Chen Jing (Principle Investigator)7, Zhang Minmin7, Zhang Qian7, Qian Jing7, Kong Yaozhong (Principle Investigator)88, Chen Youyuan8, Shen Wei8, Xiao Guanqing8, Chen Dezhen8, Li Dao8, Hou Aizhen8, Li Xiaolei8, He Hanchang8, Ye Huizhen8, Sun Zhuxing (Principle Investigator)9, Zhang Xiran9, Shan Weiwei9, Xue Jing9, Chen Yong9, Xing Changying (Principle Investigator)10, Li Li10, Yu Xiangbao10, Liu Kang10, Ge Yifei10, Xu Yili10, Huang Zhimin10, Wu Jingjing10, Liu Bicheng (Principle Investigator)11, Tu Yan11, Pan Mingming11, Lin Hongli (Principle Investigator)12, Wang Dapeng12, Meng Qingyang12, Luo Renna12, Ding Guohua (Principle Investigator)13, Shi Ming13, Qiu Changjian13, Lv Xifeng13, Zhang Guojuan (Principle Investigator)14, Jiang Liping14, Ding Ning14, Zhao Huiying14, Bao Shumin14, Chen Wei14, Chen Shen14, Liang Qiaojing14, Zhang Mei14, Peng Kanfu (Principle Investigator)15, Xie Pan15, Yuan Qian15, Zhuo Yan15, Li Shaohua15, Mao Yonghui (Principle Investigator)16, Zhao Ban16, Wang Songlan16, Chen Xianguang16, Chen Xiaonong (Principle Investigator)17, Gao Chenni17, Yu Haijin17, Weng Qinjie17, Jin Yuanmeng17, Ma Xiaobo17, Luo Ping (Principle Investigator)18, Gao Dan18, Wu Man18, Qi Yonghui18, Zhang Ping (Principle Investigator)19, Du Xiaoying19, Qu Lihui19, Xu Chunping19, Sheng Kaixiang19, Yang Yi19, Wang Song (Principle Investigator)20, Tian Xinkui20, Guo Hongxia20, Bao Wenhan20, Lin Weifeng20, Zhou Sijia20, Cui Zhuan20, Yang Wenling20, Su Kaijie20, He Lian20, Zhou Zhihong (Principle Investigator)21, Zheng Yu21, Zheng Shubei21, Jin Lingwei21, Chen Yan21, Pan Min21, Zhang Guojuan (Principle Investigator)22, Jiang Liping22, Ding Ning22, Zhao Huiying22, Bao Shumin22, Chen Wei22, Chen Shen22, Liang Qiaojing22, Zhang Mei22, Chia-Chao Wu (Principle Investigator)23, Chih-Chien Sung23, Shuei-Liong Lin (Principle Investigator)24, Ming-Shiou Wu24, Jenq-Wen Huang24, Wen Chih Chiang24, Chih-Kang Chiang24, Shao-Yu Yang24, Vin-Cent Wu24, Tao-Min Huang24, Yi-Ting Chen24, Tai-Shuan Lai24, Chun-Fu Lai24, Der-Cherng Tarng (Principle Investigator)25, Shuo-Ming Ou25, Chih-Yu Yang25, Wei-Cheng Tseng25, Yao-Ping Lin25, Junne-Ming Sung (Principle Investigator)26, Te-Hui Kuo26, Yu-Tzu Chang26, An-Bang Wu26, Wei-Hung Lin26, Hua-Chang Fang (Principle Investigator)27, Hsin-Yu Chen27, Chih-Yang Hsu27, Po-Tsang Lee27, Chien-Liang Chen27, Kang-Ju Chou27, Tzung-Yu Ho27, Chien-Te Lee (Principle Investigator)28, Hwee-Yeong Ng28, Yueh-Ting Lee28, Yi-Wen Chiu (Principle Investigator)29, Hung-Tien Kuo29, Chi-Chih Hung29, Mei-Chuan Kuo29, Jia-Jung Lee29, Jer-Chia Tsai29, Jer-Ming Chang29, Lee-Moay, Lim29, Shang-Jyh Hwang29, Jyh-Chang Hwang (Principle Investigator)30, Hsien-Yi Wang30, Wei-Chih Kan30, Chia-Chun Wu30, Ming-Yan Jiang30, Chih-Chiang Chien30, Ming-Ju Wu (Principle Investigator)31, Shang-Feng Tsai31, Cheng-Hsu Chen31, Hsi-Hsien Chen (Principle Investigator)32, Chih-Chin Kao32, Yen-Chung Lin32, Yueh-Lin Wu32, Shu-Ching Yeh32, Daniel Tak Mao Chan (Principle Investigator)33, Maggie Ming Yee Mok33, Lorraine Pui Yuen Kwan33, Gary Chi Wang Chan33, Yong-Lim Kim (Principle Investigator)34, Jang-Hee Cho34, Jeong-Hoon Lim34, Hee-Yeon Jung34, Sun-Hee Park34, Chan-Duck Kim34, Kyu Yeun Kim34, Jung Tak Park (Principle Investigator)35, Tae-Hyun Yoo35, Seung Hyeok Han35, Wookyung Chung (Principle Investigator)36, Ji Yong Jung36, Hyun Hee Lee36, Jae Hyun Chang36, Han Ro36, Ae Jin Kim36, Jong Soo Lee (Principle Investigator)37, Jongha Park37, Kyung Sun Park37, Kyoung Don Yoo37, Tae Ik Chang (Principle Investigator)38, Ea Wha Kang38, Kyoung Sook Park38, Kyubok Jin (Principle Investigator)39, Yaerim Kim39, Jinhyuk Paek39, Wooyeong Park39, Seungyeup Han39, Ohyun Kwon39, Sung Bae Park39, Myung-gyu Kim (Principle Investigator)40, SeWon Oh40, Jung Pyo Lee (Principle Investigator)41, Jeonghwan Lee41, Jihoon Jung41, Cheol Whee Park (Principle Investigator)42, Hyung Duk Kim42, Sunggyun Kim (Principle Investigator)43, Youngrim Song43, Narae Joo43, Hyungsuk Lee43, Bum-Soon Choi (Principle Investigator)44, Hoon Suk Park44, Tae Hyun Ban44
1Department of Nephrology, Guangdong Provincial People‘s Hospital, Mainland China
2Department of Nephrology, The First Affiliated Hospital of Sun Yat-sen University, Mainland China
3Department of Nephrology, Renji Hospital Shanghai Jiaotong University School of Medicine, Mainland China
4Department of Nephrology, Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Mainland China
5Department of Nephrology, The Second Hospital of Anhui Medical University, Mainland China
6Department of Nephrology, The Second Xiangya Hospital of Central South University, Mainland China
7Department of Nephrology, Huashan Hospital, Fudan University, Mainland China
8Department of Nephrology, The First People's Hospital of Foshan, Mainland China
9Department of Nephrology, Wuxi People's Hospital, Mainland China
10Department of Nephrology, Jiangsu Province Hospital, Mainland China
11Department of Nephrology, Zhong Da Hospital, Southeast University, Mainland China
12Department of Nephrology, The First Affiliated Hospital of Dalian Medical University, Mainland China
13Nephrology Department, Renmin Hospital, Wuhan University, Mainland China
14Department of Nephrology, Beijing Tongren Hospital, Capital Medical University, Mainland China
15Dept.of Nephrology, The First Hospital Affiliated to AMU (Southwest Hospital, Mainland China
16Department of Nephrology, Beijing Hospital, Mainland China
17Department of Nephrology, Ruijin Hospital of Shanghai Jiaotong University School of Medicine, Mainland China
18Nephrology Department, The Second Hospital of Jilin University, Mainland China
19Nephrology Department, The First Affiliated Hospital of Zhejiang University school of medicine, Mainland China
20Nephrology Department, Peking University Third Hospital, Mainland China
21Nephrology Department, The 2nd Affiliated Hospital and Yuying Children's Hospital of Wenzhou Medical University, Mainland China
22Department of Nephrology, Beijing Tongren Hospital, Capital Medical University, Mainland China
23Department of Nephrology, Tri-Service General Hospital, Taiwan
24Department of Nephrology, National Taiwan University Hospital, Taiwan
25Department of Nephrology, Taipei Veterans General Hospital, Taiwan
26Department of Internal Medicine, National Cheng Kung University Hospital, Taiwan
27Department of Nephrology, Kaohsiung Veterans General Hospital, Taiwan
28Department of Nephrology, Kaohsiung Chang Gung Memorial Hospital, Taiwan
29Department of Nephrology, Kaohsiung Medical University Chung-Ho Memorial Hospital, Taiwan
30Department of Nephrology, Chi Mei Medical Center, Taiwan
31Department of Nephrology, Taichung Veterans General Hospital, Taiwan
32Department of Nephrology, Taipei Medical University Hospital, Taiwan
33Department of Medicine, Queen Mary Hospital, Taiwan
34Department of Nephrology, Kyungpook National University Hospital, Taiwan
35Department of Nephrology, Severance Hospital, Yonsei University Health System, Taiwan
36Department of Nephrology, Gachon University Gil Medical Center, Taiwan
37Department of Nephrology, Ulsan University Hospital, Taiwan
38Department of Nephrology, National Health Insurance Service Ilsan Hospital, Taiwan
39Department of Nephrology, Keimyung University Dongsan Hospital, Taiwan
40Department of Nephrology, Korea University Anam Hospital, Taiwan
41Department of Nephrology, Boramae Medical Center, Taiwan
42Department of Nephrology, The Catholic University of Korea, Seoul St. Mary’s Hospital, Taiwan
43Department of Nephrology, Hallym University Sacred Hospital, Korea
44Department of Nephrology, The Catholic University of Korea Eunpyeong St.Mary's Hospital, Korea
Disclosure
Y-LK, K-CL, TMC, MF, and XY were advisory board members of this study, and KCL, TMC, MF, and XY have received personal fees from Kyowa Kirin Co., Ltd., during the conduct of the study. MF has received personal fees from Ono pharmaceutical Co., Ltd., Sanwa Kagaku Kenkyusyo Co., Ltd., Bayer Yakuhin, Ltd., and Kissei Pharmaceutical Co., Ltd.; and grants and personal fees from Kyowa Kirin Co., Ltd., outside the submitted work. JK, CN, and MK are employees of Kyowa Kirin. ZN, XL, C-CWu, and KJ have no conflicts of interest to disclose in relation to the present work.
Acknowledgments
The authors wish to thank Keyra Martinez Dunn, MD of Edanz (www.edanz.com), for providing medical writing support, which was funded by Kyowa Kirin Co., Ltd. This work was supported by Kyowa Kirin Co., Ltd. The sponsor had a role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Data Availability
Research data are not shared.
Author Contributions
ZN, XL, KJ, and C-CW contributed significantly to patient recruitment, drafted the main manuscript, and provided final approval for submission. Y-LK, K-CL, TMC, MF, and XY contributed to the study design, data interpretation, drafted the main manuscript, and provided final approval for submission. JK, CN, and MK contributed to the study design, data analysis, reviewed the manuscript critically for important intellectual content and provided final approval for submission.
Footnotes
Supplementary Methods.
Figure S1. Whole parathyroid hormone levels over time (Median [Q1–Q3]) (FAS).
Figure S2. Corrected serum Ca–P product over time (Mean + SD) (FAS).
Figure S3. BAP levels over time (Mean + SD) (FAS).
Figure S4. TRACP-5b levels over time (Mean + SD) (FAS).
Figure S5. P1NP levels over time (Mean + SD) (FAS).
Figure S6. Intact FGF23 over time (Median [Q1–Q3]).
Table S1. Mean percentage change from baseline in intact PTH during evaluation period by myocardial infarction by subgroup (FAS).
Table S2. Mean dosage at last observation period by baseline iPTH subgroup (FAS).
CONSORT Checklist.
Contributor Information
Xueqing Yu, Email: yuxueqing@gdph.org.cn.
Orchestra Study Group:
Liang Xinling, Liu Shuangxin, Li Sijia, Xu Lixia, Ye Zhiming, Feng Zhonglin, Huang Renwei, Li Zhilian, Chen Wei, Zheng Xunhua, Huang Naya, Ai Zhen, Wang Xin, Zheng Xunhua, former PI, Zhaohui Ni, present PI, Lu Renhua, Shen Jianxiao, Zhou Yijun, Lin Xinghui, Xie Yuanyuan, Zhang Jiahui, Che Miaolin, Fang Yan, Pang Huihua, Su Xinyu, Gu Leyi, Jin Wei, Zhao Peipei, Shen Yiwei, Zao Liou, Lu Wei, Huang Haidong, Ji Gang, Li Hao, former PI, Wang Deguang, present PI, Wang Deguang, Yuan Liang, Ding Lihong, Wang Xuerong, Li Huai, Liu Hong, Yuan Fang, Song Panai, Zhou An, Chen Xiaojun, Li Xiejia, He Liyu, Tan Xia, Chen Jing, Zhang Minmin, Zhang Qian, Qian Jing, Kong Yaozhong, Chen Youyuan, Shen Wei, Xiao Guanqing, Chen Dezhen, Li Dao, Hou Aizhen, Li Xiaolei, He Hanchang, Ye Huizhen, Sun Zhuxing, Zhang Xiran, Shan Weiwei, Xue Jing, Chen Yong, Xing Changying, Li Li, Yu Xiangbao, Liu Kang, Ge Yifei, Xu Yili, Huang Zhimin, Wu Jingjing, Liu Bicheng, Tu Yan, Pan Mingming, Lin Hongli, Wang Dapeng, Meng Qingyang, Luo Renna, Ding Guohua, Shi Ming, Qiu Changjian, Lv Xifeng, Zhang Guojuan, Jiang Liping, Ding Ning, Zhao Huiying, Bao Shumin, Chen Wei, Chen Shen, Liang Qiaojing, Zhang Mei, Peng Kanfu, Xie Pan, Yuan Qian, Zhuo Yan, Li Shaohua, Mao Yonghui, Zhao Ban, Wang Songlan, Chen Xianguang, Chen Xiaonong, Gao Chenni, Yu Haijin, Weng Qinjie, Jin Yuanmeng, Ma Xiaobo, Luo Ping, Gao Dan, Wu Man, Qi Yonghui, Zhang Ping, Du Xiaoying, Qu Lihui, Xu Chunping, Sheng Kaixiang, Yang Yi, Wang Song, Tian Xinkui, Guo Hongxia, Bao Wenhan, Lin Weifeng, Zhou Sijia, Cui Zhuan, Yang Wenling, Su Kaijie, He Lian, Zhou Zhihong, Zheng Yu, Zheng Shubei, Jin Lingwei, Chen Yan, Pan Min, Zhang Guojuan, Jiang Liping, Ding Ning, Zhao Huiying, Bao Shumin, Chen Wei, Chen Shen, Liang Qiaojing, Zhang Mei, Chia-Chao Wu, Chih-Chien SungShuei-Liong Lin, Ming-Shiou Wu, Jenq-Wen Huang, Wen Chih Chiang, Chih-Kang Chiang, Shao-Yu Yang, Vin-Cent Wu, Tao-Min Huang, Yi-Ting Chen, Tai-Shuan Lai, Chun-Fu Lai, Der-Cherng Tarng, Shuo-Ming Ou, Chih-Yu Yang, Wei-Cheng Tseng, Yao-Ping Lin, Junne-Ming Sung, Te-Hui Kuo, Yu-Tzu Chang, An-Bang Wu, Wei-Hung Lin, Hua-Chang Fang, Hsin-Yu Chen, Chih-Yang Hsu, Po-Tsang Lee, Chien-Liang Chen, Kang-Ju Chou, Tzung-Yu Ho, Chien-Te Lee, Hwee-Yeong Ng, Yueh-Ting Lee, Yi-Wen Chiu, Hung-Tien Kuo, Chi-Chih Hung, Mei-Chuan Kuo, Jia-Jung Lee, Jer-Chia Tsai, Jer-Ming Chang, Lee-Moay, Lim, Shang-Jyh Hwang, Jyh-Chang Hwang, Hsien-Yi Wang, Wei-Chih Kan, Chia-Chun Wu, Ming-Yan Jiang, Chih-Chiang Chien, Ming-Ju Wu, Shang-Feng Tsai, Cheng-Hsu Chen, Hsi-Hsien Chen, Chih-Chin Kao, Yen-Chung Lin, Yueh-Lin Wu, Shu-Ching Yeh, Daniel Tak Mao Chan, Maggie Ming Yee Mok, Lorraine Pui Yuen Kwan, Gary Chi Wang Chan, Yong-Lim Kim, Jang-Hee Cho, Jeong-Hoon Lim, Hee-Yeon Jung, Sun-Hee Park, Chan-Duck Kim, Kyu Yeun Kim, Jung Tak Park, Tae-Hyun Yoo, Seung Hyeok Han, Wookyung Chung, Ji Yong Jung, Hyun Hee Lee, Jae Hyun Chang, Han Ro, Ae Jin Kim, Jong Soo Lee, Jongha Park, Kyung Sun Park, Kyoung Don Yoo, Tae Ik Chang, Ea Wha Kang, Kyoung Sook Park, Kyubok Jin, Yaerim Kim, Jinhyuk Paek, Wooyeong Park, Seungyeup Han, Ohyun Kwon, Sung Bae Park, Myung-gyu Kim, SeWon Oh, Jung Pyo Lee, Jeonghwan Lee, Jihoon Jung, Cheol Whee Park, Hyung Duk Kim, Sunggyun Kim, Youngrim Song, Narae Joo, Hyungsuk Lee, Bum-Soon Choi, Hoon Suk Park, and Tae Hyun Ban
Supplementary Material
Supplementary Methods.
Figure S1. Whole parathyroid hormone levels over time (Median [Q1–Q3]) (FAS).
Figure S2. Corrected serum Ca–P product over time (Mean + SD) (FAS).
Figure S3. BAP levels over time (Mean + SD) (FAS).
Figure S4. TRACP-5b levels over time (Mean + SD) (FAS).
Figure S5. P1NP levels over time (Mean + SD) (FAS).
Figure S6. Intact FGF23 over time (Median [Q1–Q3]).
Table S1. Mean percentage change from baseline in intact PTH during evaluation period by myocardial infarction by subgroup (FAS).
Table S2. Mean dosage at last observation period by baseline iPTH subgroup (FAS).
CONSORT Checklist.
References
- 1.Hong Y.A., Ban T.H., Kang C.Y., et al. Trends in epidemiologic characteristics of end-stage renal disease from 2019 Korean Renal Data System (KORDS) Kidney Res Clin Pract. 2021;40:52–61. doi: 10.23876/j.krcp.20.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nitta K., Masakane I., Hanafusa N., et al. Annual dialysis data report 2017, JSDT Renal Data Registry. Ren Replace Ther. 2019;5:53. doi: 10.1186/s41100-019-0248-1. [DOI] [Google Scholar]
- 3.Williams M.E. Chronic kidney disease/bone and mineral metabolism: the imperfect storm. Semin Nephrol. 2009;29:97–104. doi: 10.1016/j.semnephrol.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Cannata-Andía J.B., Martín-Carro B., Martín-Vírgala J., et al. Chronic kidney disease-mineral and bone disorders: pathogenesis and management. Calcif Tissue Int. 2021;108:410–422. doi: 10.1007/s00223-020-00777-1. [DOI] [PubMed] [Google Scholar]
- 5.Cunningham J., Locatelli F., Rodriguez M. Secondary hyperparathyroidism: pathogenesis, disease progression, and therapeutic options. Clin J Am Soc Nephrol. 2011;6:913–921. doi: 10.2215/CJN.06040710. [DOI] [PubMed] [Google Scholar]
- 6.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group Kidney disease: improving global outcomes (KDIGO) CKD-MBD update work group. KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD) Kidney Int Suppl (2011) 2017;7:1–59. doi: 10.1016/j.kisu.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Kidney Foundation K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42(4 suppl 3):S1–S201. [PubMed] [Google Scholar]
- 8.Block G.A., Bushinsky D.A., Cheng S., et al. Effect of etelcalcetide vs Cinacalcet on serum parathyroid hormone in patients receiving hemodialysis with secondary hyperparathyroidism: a randomized clinical trial. JAMA. 2017;317:156–164. doi: 10.1001/jama.2016.19468. [DOI] [PubMed] [Google Scholar]
- 9.FOSUN Fosun pharma and Amgen announce collaboration to accelerate two innovative medicines benefitting Chinese patients. https://en.fosun.com/content/details74_6903.html
- 10.Akizawa T., Kido R., Fukagawa M., et al. Decreases in PTH in Japanese hemodialysis patients with secondary hyperparathyroidism: associations with changing practice patterns. Clin J Am Soc Nephrol. 2011;6:2280–2288. doi: 10.2215/CJN.11501210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beladi-Mousavi S.S., Faramarzi M. Calcimimetic agents in the management of secondary hyperparathyroidism among patients with end-stage renal disease; a review article. J Parathyroid Dis. 2015;3:12–19. [Google Scholar]
- 12.Fukagawa M., Yumita S., Akizawa T., et al. Cinacalcet (KRN1493) effectively decreases the serum intact PTH level with favorable control of the serum phosphorus and calcium levels in Japanese dialysis patients. Nephrol Dial Transplant. 2008;23:328–335. doi: 10.1093/ndt/gfm534. [DOI] [PubMed] [Google Scholar]
- 13.Mei C., Chen N., Ding X., et al. Efficacy and safety of Cinacalcet on secondary hyperparathyroidism in Chinese chronic kidney disease patients receiving hemodialysis. Hemodial Int. 2016;20:589–600. doi: 10.1111/hdi.12410. [DOI] [PubMed] [Google Scholar]
- 14.EVOLVE Trial Investigators. Chertow G.M., Block G.A., et al. Effect of Cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med. 2012;367:2482–2494. doi: 10.1056/NEJMoa1205624. [DOI] [PubMed] [Google Scholar]
- 15.Raggi P., Chertow G.M., Torres P.U., et al. The ADVANCE study: a randomized study to evaluate the effects of Cinacalcet plus low-dose vitamin D on vascular calcification in patients on hemodialysis. Nephrol Dial Transplant. 2011;26:1327–1339. doi: 10.1093/ndt/gfq725. [DOI] [PubMed] [Google Scholar]
- 16.Komaba H., Nakanishi S., Fujimori A., et al. Cinacalcet effectively reduces parathyroid hormone secretion and gland volume regardless of pretreatment gland size in patients with secondary hyperparathyroidism. Clin J Am Soc Nephrol. 2010;5:2305–2314. doi: 10.2215/CJN.02110310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sterrett J.R., Strom J., Stummvoll H.K., et al. Cinacalcet HCI (Sensipar/Mimpara) is an effective chronic therapy for hemodialysis patients with secondary hyperparathyroidism. Clin Nephrol. 2007;68:10–17. doi: 10.5414/cnp68010. [DOI] [PubMed] [Google Scholar]
- 18.Chokesuwattanaskul R., Thongprayoon C., Tanawuttiwat T., Kaewput W., Pachariyanon P., Cheungpasitporn W. Safety and efficacy of apixaban versus warfarin in patients with end-stage renal disease: meta-analysis. Pacing Clin Electrophysiol. 2018;41:627–634. doi: 10.1111/pace.13331. [DOI] [PubMed] [Google Scholar]
- 19.Akizawa T., Koshikawa S. Clinical study of Cinacalcet in Japan. Ther Apher Dial. 2008;12(Suppl 1):S13–S15. doi: 10.1111/j.1744-9987.2008.00625.x. [DOI] [PubMed] [Google Scholar]
- 20.Nakashima D., Takama H., Ogasawara Y., et al. Effect of cinacalcet hydrochloride, a new calcimimetic agent, on the pharmacokinetics of dextromethorphan: in vitro and clinical studies. J Clin Pharmacol. 2007;47:1311–1319. doi: 10.1177/0091270007304103. [DOI] [PubMed] [Google Scholar]
- 21.Friedl C., Zitt E. Role of etelcalcetide in the management of secondary hyperparathyroidism in hemodialysis patients: a review on current data and place in therapy. Drug Des Dev Ther. 2018;12:1589–1598. doi: 10.2147/DDDT.S134103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akizawa T., Shimazaki R., Shiramoto M., Fukagawa M., Evocalcet Study Group Pharmacokinetics, pharmacodynamics, and safety of the novel calcimimetic agent evocalcet in healthy Japanese subjects: first-in-human phase I study. Clin Drug Investig. 2018;38:945–954. doi: 10.1007/s40261-018-0687-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akizawa T., Shimazaki R., Fukagawa M., Evocalcet Study Group Phase 2b study of evocalcet (KHK7580), A novel calcimimetic, in Japanese patients with secondary hyperparathyroidism undergoing hemodialysis: a randomized, double-blind, placebo-controlled, dose-finding study. PLoS One. 2018;13 doi: 10.1371/journal.pone.0204896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shigematsu T., Shimazaki R., Fukagawa M., Akizawa T. Pharmacokinetics of evocalcet in secondary hyperparathyroidism patients receiving hemodialysis: first-in-patient clinical trial in Japan. Clin Pharmacol. 2018;10:101–111. doi: 10.2147/CPAA.S171044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shigematsu T., Shimazaki R., Fukagawa M., Akizawa T., Evocalcet Study Group Pharmacodynamics of evocalcet for secondary hyperparathyroidism in Japanese hemodialysis patients. Clin Exp Nephrol. 2019;23:258–267. doi: 10.1007/s10157-018-1635-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsuruya K., Shimazaki R., Fukagawa M., Akizawa T., Evocalcet Study Group Efficacy and safety of evocalcet in Japanese peritoneal dialysis patients. Clin Exp Nephrol. 2019;23:739–748. doi: 10.1007/s10157-019-01692-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fukagawa M., Shimazaki R., Akizawa T. Evocalcet study group. Head-to-head comparison of the new calcimimetic agent evocalcet with Cinacalcet in Japanese hemodialysis patients with secondary hyperparathyroidism. Kidney Int. 2018;94:818–825. doi: 10.1016/j.kint.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 28.Koiwa F., Tokunaga S., Asada S., Endo Y., Fukagawa M., Akizawa T. Efficacy of evocalcet in previously Cinacalcet-treated secondary hyperparathyroidism patients. Kidney Int Rep. 2021;6:2830–2839. doi: 10.1016/j.ekir.2021.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Narushima K., Maeda H., Shiramoto M., et al. Assessment of CYP-mediated drug interactions for evocalcet, a new calcimimetic agent, based on in vitro investigations and a cocktail study in humans. Clin Transl Sci. 2019;12:20–27. doi: 10.1111/cts.12588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirai T., Nakashima A., Takasugi N., Yorioka N. Association of nodular hyperplasia with resistance to Cinacalcet therapy for secondary hyperparathyroidism in hemodialysis patients. Ther Apher Dial. 2010;14:577–582. doi: 10.1111/j.1744-9987.2010.00843.x. [DOI] [PubMed] [Google Scholar]
- 31.Fukagawa M., Yokoyama K., Koiwa F., et al. Clinical practice guideline for the management of chronic kidney disease-mineral and bone disorder. Ther Apher Dial. 2013;17:247–288. doi: 10.1111/1744-9987.12058. [DOI] [PubMed] [Google Scholar]
- 32.Shigematsu T., Asada S., Endo Y., Kawata T., Fukagawa M., Akizawa T. Evocalcet with vitamin D receptor activator treatment for secondary hyperparathyroidism. PLoS One. 2022;17 doi: 10.1371/journal.pone.0262829. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Research data are not shared.