Abstract
Introduction
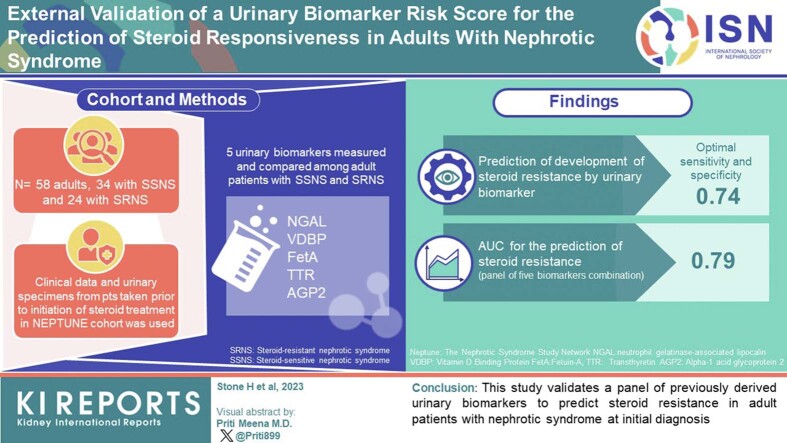
In idiopathic nephrotic syndrome, response to corticosteroids remains the best indicator of prognosis. Noninvasive markers to predict a patient’s response to steroids would allow improved prognostication and a more personalized approach to management. We have previously derived a urinary biomarker risk score which can differentiate steroid sensitive nephrotic syndrome (SSNS) from steroid resistant nephrotic syndrome (SRNS) in children. The goal of this study was to validate this previously derived biomarker risk score in a cohort of steroid-naïve adult patients, to determine whether the panel could be used to predict steroid responsiveness at the time of initial diagnosis.
Methods
In this external validation study, clinical data, and urinary specimens (obtained before initiation of steroid treatment) from adult patients were used in the Nephrotic Syndrome Study Network (NEPTUNE) cohort. A panel of 5 previously identified and validated urinary biomarkers, including neutrophil gelatinase-associated lipocalin (NGAL), vitamin D binding protein (VDBP), Fetuin-A (FetA), Transthyretin (TTR), and alpha-1 acid glycoprotein 2 (AGP2) was measured. A summary risk score for steroid resistance was calculated based on biomarker concentrations. Receiver operating characteristic curves were created for each log-transformed biomarker concentration and for the individual and combined biomarker risk score.
Results
The urine biomarker risk score predicted development of steroid resistance, with optimal sensitivity and specificity of 0.74, and area under the receiver operating characteristic curve (AUC) of 0.79 using both absolute and creatinine-corrected concentrations.
Conclusion
This study validates the previously derived urinary biomarker risk score to predict steroid resistance in adult patients with nephrotic syndrome at initial diagnosis.
Keywords: biomarkers, FSGS, minimal change disease, nephrotic syndrome, risk score, steroid resistance
Graphical abstract
Nephrotic syndrome is a common primary glomerular disease in both children and adults. It is characterized by proteinuria, hypoalbuminemia, hyperlipidemia, and edema; and can lead to serious complications such as infections, thromboses, and acute kidney injury. Minimal change disease (MCD) accounts for 15% to 20% of primary nephrotic syndrome in adults. Other common pathological lesions in adults include focal segmental glomerulosclerosis (FSGS) and membranous nephropathy. The prognosis in primary nephrotic syndrome largely depends on the response to steroids. Those with SSNS typically have MCD and exhibit excellent outcomes, with many achieving long-term remission and maintaining normal kidney function.1 However, some patients with childhood-onset MCD may continue having relapses through adult life, requiring additional courses of steroids for remission. Conversely, patients with SRNS are at high risk for progression to chronic kidney disease (CKD) and end-stage kidney disease during adulthood, and recurrence of disease after kidney transplantation.2 The threshold for performing kidney biopsy in adults with idiopathic nephrotic syndrome is lower than in children. However, we still need noninvasive markers to predict steroid responsiveness in adults, which would allow us to identify those patients who would benefit from earlier biopsy and conversion to second line immunosuppression instead of unnecessarily incurring the well-known toxicities of steroids. This is particularly pertinent to adults with a clinical presentation of MCD, in whom controversies still exist regarding initiating treatment with steroids versus other immunosuppressive regimens, including calcineurin inhibitors and mycophenolate mofetil.3
Our previous work4 used unbiased proteomic profiling methodologies and downstream confirmation to discover and derive novel urinary biomarkers to differentiate steroid responsiveness in childhood nephrotic syndrome. We identified 5 biomarkers with excellent collective predictive capability, with AUC of 0.85. The markers included NGAL, VDBP, FetA, TTR, and AGP2. Using these urinary biomarker concentrations, we derived an algorithm to calculate a risk score for SRNS.4 Two limitations of the previous study included the following: (i) most patients had received steroid treatment prior to enrollment, hindering our ability to reliably use the panel to predict steroid responsiveness, and (ii) all study participants were children. Therefore, the objective of the present study was to externally validate this previously derived biomarker risk score using urine specimens from treatment naïve adult patients. We used samples from adult patients in the multicenter NEPTUNE cohort to determine the predictive value of the biomarker risk score for response to steroids in adults with nephrotic syndrome.
Methods
Source of Data
NEPTUNE is a prospective cohort study comprised of adult and pediatric patients with nephrotic syndrome.5 All enrolled patients have undergone renal biopsy and are categorized into a histological study cohort: FSGS, MCD, membranous nephropathy, or other. Demographic and clinical data, renal biopsy tissue, urine and blood samples are available for study participants. The NEPTUNE Cohort Study Design and flow diagram has been previously published.5
Participants and Patient Samples
For the present validation study, we employed the Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis recommendations for the reporting of studies developing, validating, or updating a prediction model.6 Pertinent clinical data and baseline urine specimens were requested and obtained for adult patients in the NEPTUNE cohort who presented with their first episode of primary nephrotic syndrome, had a baseline urine sample collected before initiation of treatment with corticosteroids, and who subsequently received steroid treatment. Patients with nephrotic syndrome secondary to systemic disease, patients with active infection or gross hematuria at the time of baseline sample collection, and those already receiving corticosteroids at the time of enrollment were excluded. At the time of the request, 58 samples were available from NEPTUNE and were used as convenience samples.
Outcomes
The dose of steroids (and of any other medications) used in the NEPTUNE cohort was at the discretion of the prescribing physician. A patient was designated steroid sensitive if complete remission (defined as a urine protein-to-creatinine ratio [UPCR] <0.3) was achieved after screening and during the initial study period of the NEPTUNE cohort. Patients who failed to achieve complete remission (UPCR ≥ 0.3) after 8 weeks of steroid treatment were labeled steroid resistant.
Sample Size and Power Calculation
Our derivation study4 was performed using a total sample size of 50 patients, including 30 with SSNS and 20 with SRNS. For this validation study, we used a similar sample size. Fifty-eight patients from the NEPTUNE cohort met our inclusion criteria. Of these, 34 achieved complete remission (SSNS group) whereas 24 failed to achieve complete remission during the study period (SRNS group). A power analysis evaluated the 95% confidence interval of AUC under the receiver operating characteristic curve using a 2-sided test, assuming an expected AUC of 0.85 which would suggest that the study is powered to produce a confidence interval width of 0.098. Our obtained AUC estimate of 0.79 falls right within the range of 0.85 ± 0.098.
Specimen Processing
Baseline urine samples were received from the NEPTUNE biorepository and stored at −80 °C until batch analysis. No more than 2 freeze-thaw cycles were used per sample.
Predictive Variables: Urine Biomarker Measurements
Commercially available enzyme-linked immunosorbent assay (ELISA) kits were used to measure each urinary biomarker in all samples as previously described.4 Briefly, VDBP was measured using a commercially available ELISA kit (R&D Systems, Minneapolis, MN), with test variability coefficients of variation (CVs) of 5.9% and 6.2% for intra-assay and interassay, respectively. NGAL was measured using an ELISA kit from Bioporto (NGAL ELISA Kit 036; BioPorto, Grusbakken, Denmark). This test has an intra-assay CV of 2.1% and interassay CV of 9.1%. AGP2 was measured with a commercially available ELISA kit (Abnova, Taipei City, Taiwan), with intra-assay CV of 4.4% and interassay CV of 7.2%. FetA was measured using a commercially available ELISA with intra-assay CV of 5.5% and interassay CV of 7.6%. TTR was measured using a commercially available ELISA (Assaypro, St. Charles, MO) with intra-assay of CV 4.6% and interassay CV of 9.0%. Urine creatinine was measured using the Siemens enzymatic creatinine test (Siemens Dimension RXL Max clinical chemistry system) and was used to standardize the concentrations of the measured biomarkers. Results are reported for both unadjusted and urine creatinine-adjusted biomarker concentrations. Individuals performing the laboratory assessments were blinded to the identity of the patients, including their steroid responsiveness status.
Statistical Analysis
Demographic and clinical characteristics were compared for patients in the SSNS and SRNS groups, using 2-sample t test or Mann-Whitney test (for continuous variables) or χ2 test (for categorical variables). We evaluated both absolute (nonstandardized) biomarker concentrations and values standardized for urine creatinine concentrations. A log2 transformation of all biomarker values was performed to correct for right skewness. Log2 transformed values for each biomarker were compared for the SRNS and SSNS groups using a 2-sample t-test (or Mann-Whitney if nonparametric). These biomarker values were also compared to patients’ demographic and clinical data, to determine whether the biomarkers provide valuable information independent of these other relevant disease factors. Our group has previously published a summary risk score, which is a linear function of the 5-biomarker panel.4 Briefly, we log-transformed biomarker concentrations, multiplied each biomarker by a multiplier factor, and added this to the equation derived from logistic regression analysis to obtain the risk score. We used a similar approach to calculate a summarized risk score in this study, then performed AUC analysis using this risk score. The Delong test was used to compare the AUCs for each model versus a model which combined all biomarkers. An optimal cutoff for the risk score to identify SRNS was derived by optimizing the Youden Index in the AUC analysis.
Results
The Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis Reporting checklist for the reporting of this validation study6 is shown in Supplementary Table S1.
Patient Characteristics
Our derivation study4 was performed using a total sample size of 50 children, including 30 with SSNS and 20 with SRNS. For this validation study, we used a similar sample size. Fifty-eight adult patients from the NEPTUNE cohort5 met our inclusion criteria for the present validation study. Of these, 34 achieved complete remission (SSNS group) while 24 failed to achieve complete remission during the study period (SRNS group). Patient demographics and baseline clinical characteristics are described in Table 1. Patients with steroid-resistant disease were significantly younger (41 vs. 53 years, P = 0.002) and more likely to be Hispanic (38% vs. 6%, P = 0.0025) than those with SSNS. The groups were similar for sex, race, body mass index, and histologic diagnosis. Although all patients displayed nephrotic range proteinuria, the SRNS group exhibited significantly higher absolute values for urine albumin-to-creatinine ratio (UACR) and UPCR at initial diagnosis.
Table 1.
Patient demographics for SSNS and SRNS groups at the time of patient recruitment
| Variable | SSNS (N = 34) | SRNS (N = 24) | P-value |
|---|---|---|---|
| Male | 24 (71%) | 16 (67%) | 0.7505 |
| Age | 53 (43, 62) | 41 (36, 47) | 0.0019 |
| Race | 0.5509 | ||
| White/Caucasian | 22 (65%) | 13 (54%) | |
| Black/African American | 5 (15%) | 3 (13%) | |
| Other | 7 (21%) | 8 (33%) | |
| Ethnicity (Hispanic) | 2 (6%) | 9 (38%) | 0.0025 |
| BMI | 30.8 (26.45, 33.14) | 31.7 (26.76, 35.15) | 0.6764 |
| BMI >35 | 7 (21%) | 6 (25%) | 0.6915 |
| BMI >40 | 4 (12%) | 4 (17%) | 0.5939 |
| Follow up (mo) | 45 (36, 55) | 41 (30, 53.5) | 0.2979 |
| Hypertension at baseline | 25 (74%) | 19 (79%) | 0.6212 |
| eGFR (ml/min/1.73 m2) | 71 (50, 88) | 69 (38, 108) | 0.8059 |
| Histology | 0.7454 | ||
| MN | 13 (38 %) | 7 (29%) | |
| MCD | 3 (9%) | 1 (4%) | |
| Other | 8 (24%) | 7 (29%) | |
| FSGS | 10 (29%) | 9 (38%) | |
| UACR | 2686 (751, 3959) | 4150 (2256, 5188) | 0.0084 |
| UPCR | 3.84 (0.81, 5.12) | 5.46 (2.59, 7.36) | 0.0197 |
BMI, body mass index; eGFR, estimated glomerular filtration rate using CKD-EPI equation; FSGS, focal segmental glomerulosclerosis; MCD, minimal change disease; MN, membranous nephropathy; SRNS, steroid resistant nephrotic syndrome; SSNS, steroid sensitive nephrotic syndrome; UACR, urine albumin to creatinine ratio; UPCR, urine protein to creatinine ratio.
Reported as median and interquartile ranges (Q1, Q3) for continuous variables and n (%) for categorical variables.
Predictor Selection: Urinary Biomarker Concentrations
Urinary biomarker levels were measured in each sample and compared between the SSNS and SRNS cohorts. In Table 2, we show median and interquartile range for each log-transformed biomarker. NGAL, VDBP, and FetA levels were significantly higher in the SRNS group than the SSNS group after log2 transformation. For VDBP and FetA, this difference persisted after correcting for urine creatinine.
Table 2.
Comparison of biomarker levels for SSNS versus SRNS, reported as median (Q1, Q3)
| Log2 biomarker | SSNS (N = 34) | SRNS (N = 24) | Fold (SRNS/SSNS) | P-value |
|---|---|---|---|---|
| UACR | 10.59 (9.89, 11.95) | 11.72 (11.14, 12.34) | 1.11 (1.03, 1.19) | 0.0072 |
| UPCR | 0.74 (−0.31, 2.36) | 2.11 (1.37, 2.88) | 2.84 (1.20, 88.74) | 0.0079 |
| NGAL | 4.95 (3.61, 6.11) | 6.17 (4.29, 7.82) | 1.25 (1.03, 1.51) | 0.0240 |
| VDBP | 10.79 (9.08, 13.68) | 13.01 (11.81, 14.98) | 1.21 (1.06, 1.38) | 0.0068 |
| TTR | 10.19 (6.91, 12.96) | 11.74 (11.18, 14.15) | 1.15 (0.97, 1.37) | 0.1025 |
| Fetuin-A | 14.71 (13.01, 16.57) | 16.72 (15.38, 18.57) | 1.14 (1.05, 1.24) | 0.0039 |
| AGP2 | 0.89 (0.49, 1.05) | 0.65 (0.49, 0.76) | 0.73 (0.49, 1.04) | 0.0961 |
| NGAL/creatinine | −14.45 (−16.06, −13.39) | −13.4 (−15.32, −11.4) | 0.93 (0.85, 1.01) | 0.0944 |
| VDBP/creatinine | −8.6 (−10.73, −5.92) | −6.56 (−7.74, −5.15) | 0.76 (0.61, 0.94) | 0.0187 |
| TTR/creatinine | −9.21 (−12.05, −6.2) | −7.84 (−8.82, −5.28) | 0.85 (0.67,1.07) | 0.1689 |
| Fetuin-A/creatinine | −4.68 (−6.82, −3.08) | −2.85 (−3.79, −1.73) | 0.61 (0.39, 0.89) | 0.0135 |
| AGP2/creatinine | −18.51 (−19.33, −17.96) | −18.93 (−19.72, −18.34) | 1.02 (0.99, 1.05) | 0.1151 |
SSNS, steroid sensitive nephrotic syndrome; SRNS, steroid resistant nephrotic syndrome; UACR, urine albumin to creatinine ratio; UPCR, urine protein to creatinine ratio; NGAL, neutrophil gelatinase associated lipocalin; VDP, vitamin D binding protein; TTR, transthyretin; AGP2, alpha-1 acid glycoprotein 2; creat, creatinine.
Receiver Operating Characteristic Curve Analysis
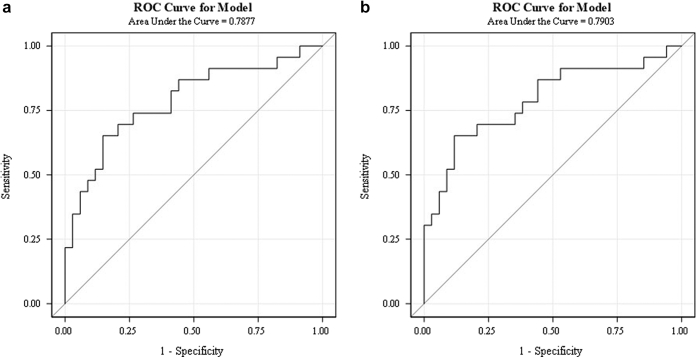
In Table 3, we summarize the AUC for the prediction of steroid resistance for each biomarker alone and for the summary risk score for the combined 5-biomarker panel, using either values unadjusted for or standardized for urine creatinine. The corresponding figures for the AUC derivations are shown in Figures 1 and 2. The risk score of the 5 biomarkers combined yielded an AUC of 0.79, which is significantly higher than that of UACR and UPCR (AUC 0.69) or of any single biomarker alone. Adjusting the concentration of the 5 biomarkers to urine creatinine did not significantly change the diagnostic accuracy (AUC of 0.7877 using unadjusted biomarker values and AUC of 0.7903 using concentrations adjusted for urine creatinine). The addition of UACR and UPCR to the model containing the 5 biomarkers did not improve the score, with the AUC remaining at 0.79. Furthermore, the combined risk score of all 5 biomarkers yielded the best AUC for SRNS prediction, when compared to a panel of 2, 3, or 4 markers, as summarized in Supplementary Table S2.
Table 3.
Summary of AUCs for detecting SRNS
| Biomarker ROC model |
Non standardized |
Standardizeda |
||
|---|---|---|---|---|
| AUC (95% CI) | P-value | AUC (95% CI) | P-value | |
| NGAL | 0.66 (0.55–0.83) | 0.078 | 0.62 (0.46–0.77) | 0.066 |
| VDBP | 0.70 (0.56–0.84) | 0.187 | 0.66 (0.51–0.81) | 0.125 |
| TTR | 0.65 (0.49–0.80) | 0.073 | 0.61 (0.45–0.76) | 0.064 |
| Fetuin-A | 0.74 (0.60–0.87) | 0.344 | 0.69 (0.54–0.83) | 0.203 |
| AGP2 | 0.65 (0.50–0.80) | 0.057 | 0.62 (0.47–0.77) | 0.027 |
| All 5 biomarkers | 0.79 (0.65–0.91) | Reference | 0.79 (0.65–0.91) | Reference |
| UACR & UPCR | 0.69 (0.55–0.83) | 0.220 | 0.69 (0.55–0.83) | 0.219 |
| All 5 biomarkers + UACR and UPCR | 0.79 (0.65–0.91) | 0.963 | 0.79 (0.65–0.91 | 0.963 |
AGP2, alpha-1 acid glycoprotein 2; AUC, area under the receiver operating characteristic curve; NGAL, neutrophil gelatinase associated lipocalin; SRNS, steroid resistant nephrotic syndrome; TTR, transthyretin; UACR, urine albumin to creatinine ratio; UPCR, urine protein to creatinine ratio; VDP, vitamin D binding protein.
Results used biomarker standardized to urine creatinine.
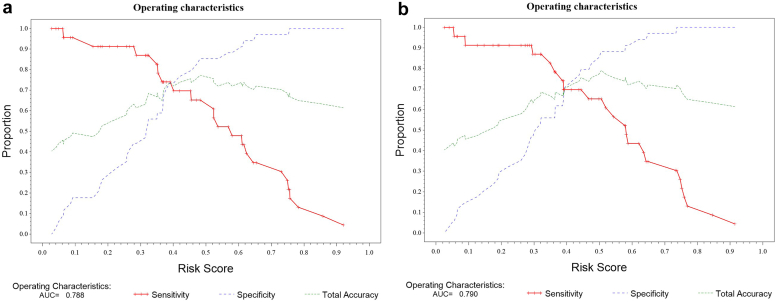
Figure 1.
Sensitivity and specificity characteristics for the combined 5 urinary biomarker panel. (a) is for absolute nonstandardized concentrations of the biomarkers. (b) is for concentrations of the biomarkers adjusted for urine creatinine. AUC, area under the receiver operating characteristic curve.
Figure 2.
Receiver operating characteristic curve analysis for the combined 5 urinary biomarker panel. (a) is for absolute nonstandardized concentrations of the biomarkers. (b) is for concentrations of the biomarkers adjusted for urine creatinine. The AUC (0.79) is almost identical for both approaches when all 5 biomarkers are used in combination.
A cutoff risk score of 0.36 was identified by optimizing the Youden index (Figures 1 and 2), which corresponds to a sensitivity and specificity of 0.74 and 0.74, respectively for the absolute nonstandardized score.
Practical Use of the Summary Risk Score to Identify Steroid Resistance
Using the same formula previously derived from the logistic regression analysis, we calculated the risk score for the prediction of steroid resistance in the current study sample. Next, applying the optimal cutoff score from the receiver operating characteristic analysis, patients at high risk for SRNS were identified. To demonstrate the practical usage of this approach, a list of steps to be followed as well as a patient example are provided as follows.
Step 1: Log2 transform each biomarker concentration
Step 2: Multiply log-transformed values by factor from the following equation:
Logit SRNS = −8.03 + 0.48 × creatinine + 0.15 × NGAL + 0.46 × VDBP − 0.29 × TTR + 0.14 × FetA − 0.69 × AGP2
Step 3: Add to equation to get patient’s risk score
Step 4: Compare the risk score to the optimal cutoff score of 0.36. If a patient’s score is higher than this cutoff value, he or she is at increased risk for steroid resistance.
For example, assume a patient’s urinary biomarker levels are as follows: creatinine 82.7 mg/dl, NGAL 180 ng/ml, VDBP 14,200 ng/ml, TTR 9200 ng/ml, FetA 194,000 ng/ml, AGP2 2.04 ng/ml.
Step 1: Log transformation gives the following: creatinine 6.4, NGAL 7.5, VDBP 13.8, TTR 13.2, FetA 17.6, AGP2 1.0.
Step 2: Multiplying each log-transformed value by the factor from the equation above gives: 0.48 × 6.4 + 0.15 × 180 + 0.46 × 13.8 − 0.29 × 13.2 + 0.14 × 17.6 − 0.69 × 1.0
Step 3: Adding this to the equation: −8.03 + 3.1 + 1.1 + 6.3 − 3.8 + 2.5 − 0.7 = 0.43
Because the patient’s calculated risk score of 0.43 is greater than the cutoff of 0.36, this patient is considered at increased risk for steroid resistance.
Discussion
Noninvasive biomarkers to predict the response to steroids in childhood and adult nephrotic syndrome are unmet needs. The goal of this study was to validate in adult populations a previously derived panel of 5 biomarkers which has been shown to differentiate SSNS from SRNS in children.4 We found that 3 of the 5 urinary biomarkers previously identified to be overexpressed in pediatric SRNS were also significantly higher in concentrations in the baseline pretreatment urine specimens of adult patients who went on to develop steroid resistant disease. When combined, the panel of 5 biomarkers had very good discriminatory power to predict steroid resistance, with AUC of 0.79, which is significantly better than that of traditional markers such as UACR and UPCR, and of any single biomarker alone. The addition of the UACR and UPCR did not yield an improvement in the AUC when compared to the 5 biomarkers alone. The diagnostic accuracy of the 5 biomarkers was not different when comparing concentrations that were adjusted for urine creatinine versus unadjusted. We created a SRNS risk score which could be used early on in a patient’s disease course to predict responsiveness to steroid therapy, thus facilitating personalized approaches to management options.
These results build on our previous findings examining urinary biomarkers to predict steroid resistance in children.4 We previously employed unbiased isobaric tags for relative and absolute quantitation to identify urinary proteins differentially expressed in SSNS versus SRNS. We combined the 4 urinary proteins most upregulated in SRNS by univariate analysis (VDBP, FetA, TTR, and AGP2) with urine NGAL because of the widely reported effectiveness of NGAL as a marker of CKD progression and tubulointerstitial damage in several patient cohorts,7, 8, 9, 10, 11 including subjects with SRNS.12, 13, 14, 15, 16, 17, 18 Using these urinary biomarker concentrations, we previously derived an algorithm to calculate a risk score for SRNS.4 This set of 5 urinary biomarkers showed significant associations with SRNS in children and yielded an AUC of 0.85 for prediction of steroid resistance.4 However, most patients had received steroid treatment prior to enrollment, hindering our ability to use the panel to reliably predict steroid responsiveness. In the present study, we examined the same panel of biomarkers using urine specimens from treatment naïve adult patients, and externally validated the biomarker risk score calculation from our previous pediatric derivation study.
The urinary proteins validated in this study (NGAL, VDBP, FetA, TTR, and AGP2) have strong clinical and biologic plausibility as biomarkers of SRNS. NGAL is one of the most upregulated genes in the kidney following a variety of experimental19, 20, 21 and human22,23 kidney diseases. Furthermore, NGAL protein is upregulated in human kidney tissues (specifically in tubular epithelial cells) in several kidney conditions, in which the degree of NGAL expression is correlated with adverse clinical outcomes. These include impaired graft function after transplantation,24,25 chronic lupus nephritis,26 proteinuric (>1 gm per day) patients with diabetic nephropathy, membranous nephropathy or IgA nephropathy,27 and patients with rapidly progressive CKD due to autosomal dominant polycystic kidney disease, oligomeganephronia or IgA nephropathy.28,29 In animal models of protein overload, albumin induces a tubular protein response prominently including NGAL modulation, which leads to tubular cell apoptosis. Consistent with an essential role of NGAL in CKD progression, experimental NGAL gene ablation decreases tubule cell apoptosis, tubulointerstitial lesions and mortality in proteinuric mice.28 The ability of urine NGAL to predict steroid responsiveness in human nephrotic syndrome is now well-established.12, 13, 14, 15, 16, 17, 18 In the present study, we found that urine NGAL alone displayed a good diagnostic accuracy for prediction of SRNS (AUC of 0.66 and 0.62 for unadjusted and urine creatinine-adjusted values, respectively); however, the combination of 5 biomarkers significantly improved the score (AUC 0.79 for both unadjusted and creatinine-adjusted concentrations).
Subjects with nephrotic range proteinuria and CKD display deficiency of both vitamin D and its carrier, VDBP in serum, resulting largely from urinary losses of VDBP that occur in direct proportion to the degree of albuminuria.30, 31, 32, 33 Mechanistically, because CKD due to SRNS results in pronounced proximal tubular injury, this would be expected to diminish megalin-dependent reabsorption of VDBP, resulting in excessive losses of VDBP in the urine. In support of this hypothesis, rodent models of chronic adriamycin-induced nephrosis display tubular fibrosis, inflammation, and interstitial damage, the degree of which was closely correlated with the urinary excretion of VDBP.33 In subjects with CKD and nephrotic range proteinuria, the urinary VDBP excretion was attenuated by intensification of nephroprotective therapy.33 Recent unbiased urine proteomic analysis using liquid chromatography/mass spectrometry followed by validation using multiple reaction monitoring has also revealed significantly increased concentrations of VDBP in subjects with progressive FSGS in comparison to MCD.34 However, subjects in this study were included at the time of first presentation with nephrotic syndrome and are unlikely to have incurred chronic kidney damage resulting in excessive urinary losses of VDBP. We postulate that mechanisms leading to excessive urinary VDBP loss in primary nephrotic syndrome include excessive filtration of systemic VDBP across a damaged glomerulus (molecular weight of VDBP is 52–59 kDa which is well below the known filtration barrier of 70 kDa exhibited by the normal glomerulus) and possibly, impaired megalin-dependent reabsorption.
FetA is a reverse acute phase serum glycoprotein and systemic inhibitor of calcification that normally is freely filtered by the glomerulus, absorbed by the proximal tubule by megalin-mediated endocytosis, and targeted to lysosomes for degradation.35 The megalin dysfunction characteristic of tubular damage in proteinuric states would be expected to result in excessive urinary FetA losses. Urinary FetA has been shown to be a marker for diabetic nephropathy, with excretion rates directly correlated to progression of albuminuria.36 Unbiased urinary proteomic studies using capillary electrophoresis coupled to mass spectrometry have also identified peptide fragments of FetA in patients with CKD from a variety of causes37 and in patients with diabetic kidney disease38; the latter study also showed correlations between urinary FetA peptides and degree of kidney dysfunction. These findings are further supported by another proteomic analysis by liquid chromatography/mass spectrometry followed by multiple reaction monitoring validation, revealing significantly increased concentrations of FetA in subjects with progressive FSGS in comparison to MCD.34 However, because subjects in this study did not display progressive chronic kidney damage, an additional pertinent mechanism to explain the high concentration of FetA in the urine might include direct glomerular damage (molecular weight of FetA is 51–67 kDa, which is below the normal glomerular filtration barrier.
AGP2, also known as orosomucoid, is a major acute-phase immunomodulatory protein and its serum concentration increases many-fold during inflammation. Early studies using high-performance liquid chromatography showed that urinary excretion of AGP2 was increased in patients with proteinuria.39 Proteomic profiling using isobaric tags for relative and absolute quantitation followed by ELISA have confirmed the strong association between urinary AGP2 concentration and SRNS.40 Increased urinary AGP2 has also been documented in other kidney conditions with chronic tubular damage and proteinuria, including lupus nephritis,41, 42, 43 chronic allograft nephropathy,44 and sickle cell nephropathy.45 Exogenously administered AGP2 reduces proteinuria and inflammation by inducing antiinflammatory macrophages in the kidney in animal models of Adriamycin-induced nephropathy.46
TTR, also known as prealbumin, is one of the most abundant circulating proteins, largely synthesized by the liver. Mutations in the TTR gene are documented to cause kidney amyloidosis, proteinuria, and CKD.47 Serum TTR levels are elevated in CKD,48 and proximal tubular megalin is essential for TTR uptake from the glomerular filtrate.49 It is presumed that tubular damage in proteinuric states would result in megalin dysfunction and consequent loss of TTR in the urine.
This study had several limitations which should be noted. First, this was a small study, with only 58 patients meeting inclusion criteria, although our sample size calculations indicated that the study was adequately powered for validation. Second, we relied on retrospective data from the NEPTUNE study to provide clinical characteristics. Third, the patients in this study are all over the age of 18, and therefore these results may not be generalizable to the pediatric population. Notably, most patients in this study had histology consistent with membranous nephropathy or FSGS, which are seen less frequently in the pediatric population in whom the original derivation study was completed. The present study was not adequately powered to separately analyze biomarker performance in various disease groups, for example, FSGS versus membranous versus MCD. It is possible that urinary biomarker levels could vary based on the underlying histologic diagnosis, and this should be explored in larger future studies. Fourth, we acknowledge that some subjects in the original derivation study were already on steroids when the urine samples were obtained. It is possible that future discovery-based studies completed only in steroid-naïve subjects may yield additional biomarkers for future validation. Fifth, the urinary proteins included in this study are not specific for idiopathic nephrotic syndrome and can be detected in a variety of proteinuric CKD states. Finally, the biomarker measurements in this panel currently require individual ELISA assays for completion. Prior to any direct clinical application, it would be desirable to construct a multiplex assay using platforms that are easily deployable at clinical testing sites, as has been recently reported for other urinary panels for kidney diseases.50
Despite these limitations, we have now demonstrated the ability of a risk score derived from a panel of 5 urinary biomarkers to identify patients with SRNS. This panel was initially used to differentiate steroid-resistant from steroid-sensitive disease in children with nephrotic syndrome at our institution, and we have now validated its use for the prediction of steroid resistance in adults in the multicenter NEPTUNE cohort. This risk score should now be tested in a large, prospective analysis to confirm its predictive capabilities. Future studies should be sufficiently powered to address any differences in biomarker concentration due to disease type, histologic diagnosis, and prognosis. If further validated, the panel could be used at the time of diagnosis to predict the risk of steroid resistance and its outcome. This information could then be used by clinicians to help guide management and personalize therapy. Specifically, those at higher risk for steroid resistance could benefit from closer monitoring, earlier kidney biopsy to establish the histologic diagnosis, and optimization of antiproteinuric therapy. Such an approach would minimize the well-known side effects of steroid therapy in both children and adults, including hyperglycemia, dyslipidemia, adrenal suppression, hypertension, osteoporosis, and psychiatric disturbances. In addition, an early prediction of steroid resistance would enable rapid transition from steroids to trials of other potentially effective immunosuppressive therapies, including rituximab, calcineurin inhibitors, or mycophenolate mofetil.
Disclosure
PD and MRB are coinventors on an approved patent: “Composition and methods for treating steroid-resistant nephrotic syndrome and/or steroid sensitive nephrotic syndrome.” PD is a co-inventor on patents submitted for the use of NGAL as a biomarker of kidney injury. All other authors declared no competing interests.
Acknowledgments
PD and MRB were supported by the NIH Pediatric Center of Excellence in Nephrology (P50DK096418). HKS was supported by the NIH Training Grant in Pediatric Nephrology (T32 DK007695).
The Nephrotic Syndrome Study Network (NEPTUNE) is part of the Rare Diseases Clinical Research Network (RDCRN), which is funded by the National Institutes of Health (NIH) and led by the National Center for Advancing Translational Sciences (NCATS) through its Division of Rare Diseases Research Innovation (DRDRI). NEPTUNE is funded under grant number U54DK083912 as a collaboration between NCATS and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Additional funding and/or programmatic support is provided by the University of Michigan, NephCure Kidney International, Alport Syndrome Foundation, and the Halpin Foundation. RDCRN consortia are supported by the RDCRN Data Management and Coordinating Center (DMCC), funded by NCATS and the National Institute of Neurological Disorders and Stroke (NINDS) under U2CTR002818.
Footnotes
Table S1. The Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD) checklist for prediction model validation.
Table S2. Summary of AUCs for detecting SRNS using various combinations of the 5 biomarkers.
Appendix
Members of the Nephrotic Syndrome Study Network (NEPTUNE)
NEPTUNE Enrolling Centers
Atrium Health Levine Children’s Hospital, Charlotte, SC: S Massengill∗ L Lo#
Children’s Hospital, Los Angeles. C Fajardo∗, S Sharma#: CA: K Lemley.
Children’s Mercy Hospital. Kansas City, MO: T Srivastava∗, K Markus#.
Cleveland Clinic, Cleveland OH: K Dell∗∗, J Sedor∗∗, B Martin#.
Cohen Children’s Hospital. New Hyde Park, NY: C Sethna∗, S Vento #.
Columbia University. New York, NY: P Canetta∗, A Pradhan#.
Duke University Medical Center, Durham NC: R Gbadegesin∗, O Olabisi∗∗ M Smith#.
Emory University, Atlanta. CS Wang∗∗, E Yun#: GA: L Greenbaum.
Mayo Clinic, Rochester MN: F Fervenza∗, M Hogan∗∗, J Lieske∗#.
The Lundquist Institute, Torrence CA. S Adler∗, J LaPage#
John H Stroger Cook County Hospital, Chicago, IL: A Amarah∗, M Itteera#: Baltimore, MD: Johns Hopkins Medicine: M Atkinson∗, M Williams#.
Medical University of South Carolina, D Selewski∗ , C Alston#
Montefiore Medical Center, Bronx NY: F Kaskel∗, M Ross∗, P Flynn#.
New York University Medical Center. New York, NY: L Malaga-Dieguez∗ , O Zhdanova∗∗, LJ Pehrson#: Columbus, OH: The Ohio State University College of Medicine: S Almaani∗ , L Roberts#.
NIDDK. Intramural, Bethesda MD. J Kopp∗.
Stanford University. Stanford, CA: R Lafayette∗, S Dave#.
Temple University. Philadelphia, PA: I Lee∗, Z Pfeffer#
Texas Children’s Hospital at Baylor College of Medicine, Houston, TX: S Shah∗ A Deslandes#.
University Health Network Toronto: H Reich∗, M Hladunewich∗∗. P Ling#, M Romano#.
University of California at San Francisco: San Francisco, CA: P Brakeman∗.
University of Colorado Anschutz Medical Campus, Aurora CO. A Podoll∗ N Rogers#.
University of Kansas Medical Center. Kansas City, KS: E McCarthy∗ E Landry#.
University of Miami, Miami FL. A Fornoni∗, C Bidot#.
University of Michigan. A Williams#, M Stelzer#: Ann Arbor, MI: M Kretzler, D Gipson∗.
University of Minnesota. Minneapolis, MN: P Nachman∗, M Rheault∗∗, V Rao#.
University of North Carolina, Chapel Hill NC:V Derebail, K Gibson∗. A Froment#, F Ochoa-Toro#.
University of Pennsylvania. Philadelphia, PA: L Holzman∗, K Meyers∗∗, K Kallem#, A Swenson#: San Antonio, San Antonio, TX: University of Texas: K Sharma∗.
University of Texas Southwestern. Dallas, TX: K Sambandam∗, E Robles#, M Turk#.
University of Washington, Seattle WA: A Jefferson∗, S Hingorani∗∗. K Tuttle∗∗§, L Manahan #, E Pao#, K Kuykendall K§
Wake Forest University Baptist Health: Winston-Salem, NC: JJ Lin∗ no coordinators identified.
Washington University in St Louis. St. Louis, MO: E Cody∗ no Coordinators Identified.
Data Analysis and Coordinating Center
M Kretzler∗, L Barisoni∗∗, C Gadegbeku∗∗, B Gillespie∗∗, D Gipson∗∗, L Holzman∗∗, L Mariani∗∗, MG Sampson∗∗, J Sedor∗∗, A Smith∗∗, J Zee∗∗, G Alter, H Desmond, S Eddy, D Fermin, W Ju, M Larkina, S Li, CC Lienczewski, T Mainieri, R Scherr, J Troost, A Williams, Y Wang.
Digital Pathology Committee
Carmen Avila-Casado (University Health Network, Toronto), Serena Bagnasco (Johns Hopkins University), Clarissa Cassol (Arakana), Lihong Bu (Mayo Clinic), Shelley Caltharp (Emory University), Dawit Demeke (University of Michigan), Brenda Gillespie (University of Michigan), Jared Hassler (Temple University), Leal Herlitz (Cleveland Clinic), Stephen Hewitt (National Cancer Institute), Jeff Hodgin (University of Michigan), Danni Holanda (Arkana), Neeraja Kambham (Stanford University), Kevin Lemley (Children’s Hospital of Los Angeles), Laura Mariani (University of Michigan), Nidia Messias (Washington University), Alexei Mikhailov (Wake Forest), Behzad Najafian (University of Washington), Matthew Palmer (University of Pennsylvania), Avi Rosenberg (Johns Hopkins University), Virginie Royal (University of Montreal), Barry Stokes (Columbia University), David Thomas (Duke University), Michifumi Yamashita (Cedar Sinai), Hong Yin (Emory University) Jarcy Zee (University of Pennsylvania), Yiqin Zuo (University of Miami), Co-Chairs: Laura Barisoni (Duke University) and Cynthia Nast (Cedar Sinai).
Supplementary Material
Table S1. The Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD) checklist for prediction model validation.
Table S2. Summary of AUCs for detecting SRNS using various combinations of the 5 biomarkers.
References
- 1.Niaudet P. Long-term outcome of children with steroid-sensitive nephrotic syndrome. Clin J Am Soc Nephrol. 2009;4:1547–1548. doi: 10.2215/CJN.05950809. [DOI] [PubMed] [Google Scholar]
- 2.Trautmann A., Schnaidt S., Lipska-Ziętkiewicz B.S., et al. Long-term outcome of steroid-resistant nephrotic syndrome in children. J Am Soc Nephrol. 2017;28:3055–3065. doi: 10.1681/ASN.2016101121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azukaitis K., Palmer S.C., Strippoli G.F., Hodson E.M. Interventions for minimal change disease in adults with nephrotic syndrome. Cochrane Database Syst Rev. 2022;3:CD001537. doi: 10.1002/14651858.CD001537.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett M.R., Pleasant L., Haffner C., et al. A novel biomarker panel to identify steroid resistance in childhood idiopathic nephrotic syndrome. Biomark Insights. 2017;12 doi: 10.1177/1177271917695832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gadegbeku C.A., Gipson D.S., Holzman L.B., et al. Design of the Nephrotic Syndrome Study Network (NEPTUNE) to evaluate primary glomerular nephropathy by a multidisciplinary approach. Kidney Int. 2013;83:749–756. doi: 10.1038/ki.2012.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins G.S., Reitsma J.B., Altman D.G., Moons K.G., TRIPOD Group Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. The TRIPOD group. Circulation. 2015;131:211–219. doi: 10.1161/CIRCULATIONAHA.114.014508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolignano D., Lacquaniti A., Coppolino G., et al. Neutrophil gelatinase-associated lipocalin (NGAL) and progression of chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:337–344. doi: 10.2215/CJN.03530708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nickolas T.L., Forster C.S., Sise M.E., et al. NGAL (Lcn2) monomer is associated with tubulointerstitial damage in chronic kidney disease. Kidney Int. 2012;82:718–722. doi: 10.1038/ki.2012.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou L.T., Lv L.L., Pan M.M., et al. Are urinary tubular injury markers useful in chronic kidney disease? A systematic review and meta analysis. PLoS One. 2016;11 doi: 10.1371/journal.pone.0167334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bullen A.L., Katz R., Jotwani V., et al. Biomarkers of kidney tubule health, CKD progression, and acute kidney injury in Sprint (systolic blood pressure intervention trial) participants. Am J Kidney Dis. 2021;78:361–368.e1. doi: 10.1053/j.ajkd.2021.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin H.Y., Hwang D.Y., Lee S.C., et al. Urinary neutrophil gelatinase-associated lipocalin and clinical outcomes in chronic kidney disease patients. Clin Chem Lab Med. 2015;53:73–83. doi: 10.1515/cclm-2014-0647. [DOI] [PubMed] [Google Scholar]
- 12.Youssef D.M., El-Shal A.A. Urine neutrophil gelatinase-associated lipocalin and kidney injury in children with focal segmental glomerulosclerosis. Iran J Kidney Dis. 2012;6:355–360. [PubMed] [Google Scholar]
- 13.Zhang Q., Jiang C., Tang T., et al. Clinical significance of urinary biomarkers in patients with primary focal segmental glomerulosclerosis. Am J Med Sci. 2018;355:314–321. doi: 10.1016/j.amjms.2017.12.019. [DOI] [PubMed] [Google Scholar]
- 14.Bennett M.R., Piyaphanee N., Czech K., Mitsnefes M., Devarajan P. NGAL distinguishes steroid sensitivity in idiopathic nephrotic syndrome. Pediatr Nephrol. 2012;27:807–812. doi: 10.1007/s00467-011-2075-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korzeniecka-Kozerska A., Wasilewska A., Tenderenda E., Sulik A., Cybulski K. Urinary MMP-9/NGAL ratio as a potential marker of FSGS in nephrotic children. Dis Markers. 2013;34:357–362. doi: 10.3233/DMA-130980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nickavar A., Safaeian B., Sadeghi-Bojd S., dashti L.H. Urine neutrophil gelatinase associated lipocalin to creatinine ratio: a novel index for steroid response in idiopathic nephrotic syndrome. Indian J Pediatr. 2016;83:18–21. doi: 10.1007/s12098-015-1809-0. [DOI] [PubMed] [Google Scholar]
- 17.Choudhary A., Mohanraj P.S., Krishnamurthy S., Rajappa M. Association of urinary vitamin D binding protein and neutrophil gelatinase-associated lipocalin with steroid responsiveness in idiopathic nephrotic syndrome of childhood. Saudi J Kidney Dis Transpl. 2020;31:946–956. doi: 10.4103/1319-2442.301201. [DOI] [PubMed] [Google Scholar]
- 18.Kumar R., Shekhar R., Gupta A.K., et al. Urinary neutrophil gelatinase associated lipocalin: a novel biomarker determining steroid responsiveness in nephrotic syndrome. Cureus. 2023;15 doi: 10.7759/cureus.34503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Supavekin S., Zhang W., Kucherlapati R., Kaskel F.J., Moore L.C., Devarajan P. Differential gene expression following early renal ischemia/reperfusion. Kidney Int. 2003;63:1714–1724. doi: 10.1046/j.1523-1755.2003.00928.x. [DOI] [PubMed] [Google Scholar]
- 20.Mishra J., Ma Q., Prada A., et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14:2534–2543. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- 21.Rudman-Melnick V., Adam M., Potter A., et al. Single-cell profiling of AKI in a murine model reveals novel transcriptional signatures, profibrotic phenotype, and epithelial-to-stromal crosstalk. J Am Soc Nephrol. 2020;31:2793–2814. doi: 10.1681/ASN.2020010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Desanti De Oliveira B., Xu K., Shen T.H., et al. Molecular nephrology: types of acute tubular injury. Nat Rev Nephrol. 2019;15:599–612. doi: 10.1038/s41581-019-0184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menon R., Bomback A.S., Lake B.B., et al. Integrated single-cell sequencing and histopathological analyses reveal diverse injury and repair responses in a participant with acute kidney injury: a clinical-molecular-pathologic correlation. Kidney Int. 2022;101:1116–1125. doi: 10.1016/j.kint.2022.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mishra J., Ma Q., Kelly C., et al. Kidney NGAL is a novel early marker of acute injury following transplantation. Pediatr Nephrol. 2006;21:856–863. doi: 10.1007/s00467-006-0055-0. [DOI] [PubMed] [Google Scholar]
- 25.Bank J.R., van der Pol P., Vreeken D., et al. Kidney injury molecule-1 staining in renal allograft biopsies 10 days after transplantation is inversely correlated with functioning proximal tubular epithelial cells. Nephrol Dial Transplant. 2017;32:2132–2141. doi: 10.1093/ndt/gfx286. [DOI] [PubMed] [Google Scholar]
- 26.Gulati G., Bennett M.R., Abulaban K., et al. Prospective validation of a novel renal activity index of lupus nephritis. Lupus. 2017;26:927–936. doi: 10.1177/0961203316684212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El Karoui K., Viau A., Dellis O., et al. Endoplasmic reticulum stress drives proteinuria-induced kidney lesions via lipocalin 2. Nat Commun. 2016;7 doi: 10.1038/ncomms10330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Viau A., El Karoui K., Laouari D., et al. Lipocalin 2 is essential for chronic kidney disease progression in mice and humans. J Clin Invest. 2010;120:4065–4076. doi: 10.1172/JCI42004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yavas H., Sahin O.Z., Ersoy R., et al. Prognostic value of NGAL staining in patients with IgA nephropathy. Ren Fail. 2013;35:472–476. doi: 10.3109/0886022X.2013.767114. [DOI] [PubMed] [Google Scholar]
- 30.Yousefzadeh P., Shapses S.A., Wang X. Vitamin D binding protein impact on 25-hydroxyvitamin D Levels under different physiologic and pathologic conditions. Int J Endocrinol. 2014;2014 doi: 10.1155/2014/981581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Selewski D.T., Chen A., Shatat I.F., et al. Vitamin D in incident nephrotic syndrome: a Midwest Pediatric Nephrology Consortium study. Pediatr Nephrol. 2016;31:465–472. doi: 10.1007/s00467-015-3236-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bennett M.R., Pordal A., Haffner C., Pleasant L., Ma Q., Devarajan P. Urinary vitamin D-binding protein as a biomarker of steroid-resistant nephrotic syndrome. Biomark Insights. 2016;11:1–6. doi: 10.4137/BMI.S31633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mirković K., Doorenbos C.R., Dam W.A., et al. Urinary vitamin D binding protein: a potential novel marker of renal interstitial inflammation and fibrosis. PLoS One. 2013;8 doi: 10.1371/journal.pone.0055887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chebotareva N.V., Vinogradov A., Brzhozovskiy A.G., et al. Potential urine proteomic biomarkers for focal segmental glomerulosclerosis and minimal change disease. Int J Mol Sci. 2022;23 doi: 10.3390/ijms232012607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsui I., Hamano T., Mikami S., et al. Retention of fetuin-A in renal tubular lumen protects the kidney from nephrocalcinosis in rats. Am J Physiol Ren Physiol. 2013;304:F751–F757F60. doi: 10.1152/ajprenal.00329.2012. [DOI] [PubMed] [Google Scholar]
- 36.Inoue K., Wada J., Eguchi J., et al. Urinary fetuin-A is a novel marker for diabetic nephropathy in type 2 diabetes identified by lectin microarray. PLoS One. 2013;8 doi: 10.1371/journal.pone.0077118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Catanese L., Siwy J., Mavrogeorgis E., et al. A novel urinary proteomics classifier for non-invasive evaluation of interstitial fibrosis and tubular atrophy in chronic kidney disease. Proteomes. 2021;9:32. doi: 10.3390/proteomes9030032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Magalhães P., Zürbig P., Mischak H., Schleicher E. Urinary fetuin-A peptides as a new marker for impaired kidney function in patients with type 2 diabetes. Clin Kidney J. 2020;14:269–276. doi: 10.1093/ckj/sfaa176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arai H., Tomizawa S., Maruyama K., Seki Y., Kuroume T. Reversed-phase high-performance liquid chromatography for analysis of urinary proteins: diagnostic significance of alpha 1-acid glycoprotein. Nephron. 1994;66:278–284. doi: 10.1159/000187823. [DOI] [PubMed] [Google Scholar]
- 40.Suresh C.P., Saha A., Kaur M., et al. Differentially expressed urinary biomarkers in children with idiopathic nephrotic syndrome. Clin Exp Nephrol. 2016;20:273–283. doi: 10.1007/s10157-015-1162-7. [DOI] [PubMed] [Google Scholar]
- 41.Suzuki M., Wiers K., Brooks E.B., et al. Initial validation of a novel protein biomarker panel for active pediatric lupus nephritis. Pediatr Res. 2009;65:530–536. doi: 10.1203/PDR.0b013e31819e4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brunner H.I., Bennett M.R., Mina R., et al. Association of noninvasively measured renal protein biomarkers with histologic features of lupus nephritis. Arthritis Rheum. 2012;64:2687–2697. doi: 10.1002/art.34426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brunner H.I., Bennett M.R., Gulati G., et al. Urine biomarkers to predict response to lupus nephritis therapy in children and young adults. J Rheumatol. 2017;44:1239–1248. doi: 10.3899/jrheum.161128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Higuchi H., Kamimura D., Jiang J.J., et al. Orosomucoid 1 is involved in the development of chronic allograft rejection after kidney transplantation. Int Immunol. 2020;32:335–346. doi: 10.1093/intimm/dxaa003. [DOI] [PubMed] [Google Scholar]
- 45.Jerebtsova M., Saraf S.L., Soni S., et al. Urinary orosomucoid is associated with progressive chronic kidney disease stage in patients with sickle cell anemia. Am J Hematol. 2018;93:E107–E109. doi: 10.1002/ajh.25036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fujimura R., Watanabe H., Nishida K., et al. α1-acid glycoprotein attenuates adriamycin-induced nephropathy via CD163 expressing macrophage induction. Kidney360. 2020;1:343–353. doi: 10.34067/KID.0000782019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Solignac J., Delmont E., Fortanier E., et al. Kidney involvement in hereditary transthyretin amyloidosis: a cohort study of 103 patients. Clin Kidney J. 2022;15:1747–1754. doi: 10.1093/ckj/sfac118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gaipov A., Jackson C.D., Talwar M., et al. Association between serum prealbumin level and outcomes in prevalent kidney transplant recipients. J Ren Nutr. 2019;29:188–195. doi: 10.1053/j.jrn.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 49.Sousa M.M., Norden A.G., Jacobsen C., et al. Evidence for the role of megalin in renal uptake of transthyretin. J Biol Chem. 2000;275:38176–38181. doi: 10.1074/jbc.M002886200. [DOI] [PubMed] [Google Scholar]
- 50.Cody E.M., Bennett M.R., Gulati G., et al. Successful urine multiplex bead assay to measure lupus nephritis activity. Kidney Int Rep. 2021;6:1949–1960. doi: 10.1016/j.ekir.2021.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.