Abstract
Drug-induced nephrotoxicity accounts for up to 60% of cases of acute kidney injury (AKI) in hospitalized patients and is associated with increased morbidity and mortality in both adults and children. Antibiotics are one of the most common causes of drug-induced nephrotoxicity. Mechanisms of antibiotic-induced nephrotoxicity include glomerular injury, tubular injury or dysfunction, distal tubular obstruction from casts, and acute interstitial nephritis (AIN) mediated by a type IV (delayed-type) hypersensitivity response. Clinical manifestations of antibiotic-induced nephrotoxicity include acute tubular necrosis (ATN), AIN, and Fanconi syndrome. Given the potential nephrotoxic effects of antibiotics on critically ill patients, the use of novel biomarkers can provide information to optimize dosing and duration of treatment and can help prevent nephrotoxicity when traditional markers, such as creatinine, are unreliable. Use of novel kidney specific biomarkers, such as cystatin C and urinary kidney injury molecule-1 (KIM-1), may result in earlier detection of AKI, dose adjustment, or discontinuation of antibiotic and development of nonnephrotoxic antibiotics.
Keywords: AKI, antibiotics, cystatin C, drug-induced nephrotoxicity, KIM-1, novel biomarkers
Drug-induced nephrotoxicity is a common cause of AKI and antibiotics represent one of the largest group of medications associated with AKI. In this article, we review first the clinical presentations and management of nephrotoxicity from different antibiotic classes. Then, we discuss the mechanisms by which antibiotics cause nephrotoxicity to different areas of the kidney. In our last section, we discuss novel biomarkers that have been shown to be promising in early detection of antibiotic-induced nephrotoxicity. A summary of this is shown in Table 1.
Table 1.
Different classes of antibiotics and their various mechanisms of nephrotoxicity, along with the clinical presentation and proposed biomarkers
| Antibiotic class | Examples | Clinical presentation of toxicity | Mechanisms of toxicity | Biomarkers: pre-clinical | Biomarkers: clinical |
|---|---|---|---|---|---|
| Aminoglycosides | Neomycin Gentamicin Tobramycin |
-ATN (10%–20%) -Fanconi Syndrome |
-Accumulates in proximal tubule cells, causing mitochondrial dysfunction and cell apoptosis. -Causes mesangial cell contraction, decreasing glomerular filtration and surface area |
Serum: cystatin C Urine: --KIM-1 --clusterin --NAG --NGAL --OPN --IL-18 --miRNAs |
Serum: cystatin C Urine: --KIM-1 --NAG --NGAL --OPN --IL-18 --miRNAs |
| Glycopeptides | Vancomycin | -ATN -Non-crystalline cast nephropathy |
-Accumulates in proximal tubule cells, leading to cell injury and apoptosis via ROS induced damage -Causes tubular obstruction through formation of non-crystal vancomycin and uromodulin casts |
Urine: --KIM-1 --NGAL |
Serum: cystatin C Urine: --TIMP2/IGFBP7 --NGAL |
| Beta lactams | Penicillin Cephalosporins Piperacillin/Tazobactam |
-AIN | -Causes interstitial inflammation from delayed T cell mediated (type IV) hypersensitivity reaction. | Urine (in AIN) --MCP1 --NGAL --NAG --MMPs --KIM-1 --C5b-9 --INFα --IL-5, 6, 8, 9 ,12, 17 |
|
| Folate synthesis iInhibitors | TMP/SMX | -Pseudo-AKI -AIN -Crystalline nephropathy -Hyperkalemia |
-Trimethoprim competes with creatinine at the OAT-2 transporter -Interstitial inflammation from delayed T cell mediated (type IV) hypersensitivity reaction -Intratubular obstruction from precipitation of sulfamethoxazole -Inhibits sodium influx via ENaC, reducing the gradient for potassium excretion |
Urine (in AIN) --MCP1 --NGAL --NAG --MMPs --KIM-1 --C5b-9 --INFα --IL-5, 6, 8, 9 ,12, 17 |
|
| Sulfonamides | Sulfadiazine | -Crystalline nephropathy -Stone formation -AIN |
-Crystalline precipitation, causing tubular obstruction and interstitial inflammation | Urine (in AIN) --MCP1 --NGAL --NAG --MMPs --KIM-1 --C5b-9 --INFα --IL- 5, 6, 8, 9 ,12, 17 |
|
| Fluoroquinolones | Ciprofloxacin Levofloxacin Moxifloxacin |
-Crystalline nephropathy -AIN -ATN -Granulomatous interstitial nephritis (rare) |
-Crystalline precipitation, causing tubular obstruction and interstitial inflammation | Urine (in AIN) --MCP1 --NGAL --NAG --MMPs --KIM-1 --C5b-9 --INFα --IL-5, 6, 8, 9 ,12, 17 |
|
| Tetracyclines | Doxycycline Minocycline |
-Fanconi syndrome -AIN -Granulomatous interstitial nephritis (rare) |
-Accumulates in proximal tubular cells, inhibiting ribosomal protein production and causing cell damage -Interstitial inflammation from delayed T cell mediated (type IV) hypersensitivity reaction |
Urine (in AIN) --MCP1 --NGAL --NAG --MMPs --KIM-1 --C5b-9 --INFα --IL-5, 6, 8, 9 ,12, 17 |
|
| Polymixin | Colistin | -ATN (20%–60%) | - Accumulates in proximal tubular cells, causing cell membrane and mitochondrial damage, leading to cell apoptosis | Serum: cystatin C Urine: --KIM 1 --NGAL |
Serum: cystatin C Urine: clusterin |
AIN, acute interstitial nephritis; ATN, acute tubular necrosis; C5b-9, (terminal complement complex); ENaC, epithelial sodium channel; IFNα, interferon-alpha; IL, interleukin; KIM-1, kidney injury molecule-1; MCP-1, monocyte chemoattractant protein-1; MMP, matrix metalloproteinase; NAG, N-acetyl-beta-D-glucosaminidase; NGAL, neutrophil gelatinase associated lipocalin; OAT-2, organic anion transporter-2; OPN, osteopontin; TMP/SMX, trimethoprim-sulfamethoxazole.
Clinical Manifestations of Antibiotic-Induced Nephrotoxicity
Aminoglycosides
Although aminoglycoside use is limited due to their known nephrotoxicity, they are typically used for serious gram-negative infections and infectious endocarditis.1 Aminoglycosides have a long postantibiotic effect and work in a concentration-dependent bactericidal manner. Nephrotoxicity occurs with frequent repeat dosing, which leads to increased accumulation of the antibiotic in the renal parenchyma.2 Of the aminoglycosides, the most nephrotoxic is neomycin, followed by gentamicin, tobramycin, and amikacin.3 Streptomycin appears to be the least nephrotoxic. Aminoglycoside-induced AKI includes ATN, which typically presents 5 to 7 days after initial exposure.4 It can also cause proximal tubular dysfunction, known as Fanconi syndrome, leading to metabolic acidosis, hypophosphatemia, glucosuria, aminoaciduria.4
Strategies to prevent aminoglycoside-induced AKI include using less toxic aminoglycosides, dose adjusting for estimated glomerular filtration rate, frequent monitoring of drug trough levels and extended interval dosing.4,5 Extended interval dosing is the process of administering higher doses of the drug less frequently. It lowers the risk of nephrotoxicity, while maintaining the same efficacy, by supersaturating the megalin complex in the proximal convoluted tubule, thereby reducing the amount of aminoglycoside reabsorbed renally.2,5 Use of other megalin substrates, such as statins, have been evaluated in vitro and in rat studies to competitively inhibit megalin-induced tubular uptake of the aminoglycoside.5 Certain medications such as nifedipine or fosfomycin have been evaluated in small pediatric populations to prevent AKI from aminoglycosides, though the benefit appears unclear.5 Treatment of aminoglycoside-induced nephrotoxicity typically involves supportive cares and cessation of drug.4
Vancomycin
As one of the most commonly used antibiotics, the primary indications for vancomycin include staphylococcal and streptococcal infections. However, AKI from vancomycin is well-documented, and most commonly includes ATN and AIN, and typically occurs 4 to 17 days after exposure.6 Vancomycin-induced ATN is associated with oliguria and an acute rise in serum creatinine. Alternatively, vancomycin-induced AIN will present as a nonoliguric AKI associated with sterile pyuria, nonnephrotic proteinuria 7 to 14 days after exposure.6 Symptomatically, there may be rash, fever, and eosinophilia; however, this triad is seen in less than 10% of patients with AIN.6 Classically, the renal biopsy has interstitial edema and cellular infiltrate of eosinophils and lymphocytes, but can occasionally show granulomatous interstitial nephritis.6,7 Vancomycin can also cause noncrystalline cast nephropathy as discussed above; however, it remains controversial whether these casts can actually lead to AKI.8,9 Risk factors for developing AKI include higher trough levels (>15 mg/l), longer duration of use (>7 days), obesity, critical illness, and underlying chronic kidney disease.9
Prevention of vancomycin-induced AKI is done by monitoring drug troughs to avoid supratherapeutic levels, avoiding use of nephrotoxins, and maintaining good hydration.10 Treatment of vancomycin-induced AKI includes withdrawal of the antibiotic and supportive cares.9 Steroids may be used in cases of AIN.10 In some case reports, high-flux hemodialysis to remove vancomycin has also been suggested as a form of treatment, though this is not commonly used.10
Vancomycin/Piperacillin-Tazobactam
In combination, vancomycin and piperacillin-tazobactam (VPT) is used for empiric coverage of gram-positive, gram-negative and anaerobic organisms, specifically covering methicillin-resistant staphylococcus aureus and Pseudomonas aeruginosa.11 However, the combination has previously been associated with AKI in approximately 15% to 35% of patients. VPT is reported to be a higher risk for AKI than vancomycin alone, and cause AKI earlier (3–5 days after exposure vs. 5–8 days in vancomycin alone.)11,12 More recently, there is evidence that the elevated creatinine following exposure to VPT may be a “pseudo-AKI.”12,13 This occurs because piperacillin-tazobactam is a substrate of the organic anion transporters 1 and 3, and competitively inhibits tubular secretion of creatinine.12,13 A study by Miano et al.13 revealed that although VPT does increase creatinine, it does not increase cystatin C, because cystatin C does not undergo tubular secretion through organic anion transporters 1 and 3. Biopsies done in patients with AKI following exposure to VPT indicate that the etiology is either ATN or AIN.12 Tubular damage may be from vancomycin, whereas the AIN may be from either vancomycin or piperacillin-tazobactam.12 Risk factors described for AKI from VPT include higher vancomycin trough levels, diabetes, and hypertension.11
Prevention of this AKI is predominantly through monitoring trough levels, avoiding concomitant nephrotoxins and/or preemptively using alternative combinations, such as cefepime and vancomycin. Preclinical studies have evaluated the use of antioxidants, such as erythropoietin or vitamins C and E to prevent AKI.14 In one small randomized controlled trial, magnesium infusions targeting a level of 3 mg/dl was evaluated to prevent AKI in patients receiving VPT, which showed a nonsignificant reduction in AKI.14
Beta-lactams
Beta-lactams are a common class of antibiotics that includes penicillin, cephalosporins, carbapenems, and monobactams.15 They are used frequently, both in the outpatient and inpatient setting, to treat a variety of infections. Beta-lactams are often associated with the development of AIN. Of the penicillin derivatives, nafcillin and methicillin are associated with the highest risk of developing AIN.4,15,16 Cephalosporins are also associated with AIN, more commonly seen with first generation cephalosporins. AIN will present as a nonoliguric AKI typically approximately 8 to 10 days following exposure to the drug, with associated pyuria, hematuria, and low grade proteinuria.15 Occasionally, eosinophilia and other allergic type symptoms are seen, but are often absent.4,15 On biopsy, granulomatous AIN has been reported with both penicillins (methicillin, ampicillin, and oxacillin) and cephalosporins.17
Prevention of AKI includes avoiding antibiotics (such as amoxicillin or nafcillin) with a stronger association to AIN. Treatment of AIN typically includes withdrawal of the medication, and occasionally, the use of steroids. The overall benefit of steroids in treating AIN is unclear given that the studies surrounding its use are small and retrospective.18 However, these studies suggest an improved renal outcome with the use of steroids, especially if used earlier in the course of the disease.18,19
Trimethoprim-sulfamethoxazole
Trimethoprim-sulfamethoxazole (TMP/SMX) is useful for treatment and prophylaxis against gram-positive and gram-negative infections in the respiratory, gastrointestinal, and genitourinary tracts.20 However, TMP/SMX is known to be nephrotoxic through various mechanisms. Trimethoprim is also “pseudonephrotoxic” given that it is a substrate of organic anion transporter-2, which leads to a reduction in tubular creatinine secretion, causing a 10% to 28% increase in serum creatinine.21 However, a true AKI from AIN can develop, more commonly from the sulfamethoxazole component, and is associated with relatively rapid onset and allergic symptoms.4 A less common form of AKI is from intratubular obstruction from precipitation of sulfamethoxazole.22 Because sulfamethoxazole is a weak acid, it can precipitate if the urinary pH is 5.5 or less, and can be identified by crystalluria.22,23 Tubular obstruction following exposure to sulfamethoxazole will present as an oliguric AKI approximately 7 days after exposure, correlating to deposition of crystals in the renal parenchyma or medullary rays.22 Rarely, sulfamethoxazole has been reported to cause urolithiasis.24 TMP/SMX is also known to cause hyperkalemia through its interaction with epithelial sodium channel.25 Risk factors for developing AKI from TMP/SMX are hypertension, diabetes mellitus, higher doses of sulfamethoxazole, and hypovolemia (increasing the risk for tubular precipitation.)22 Prevention can be from ensuring good hydration and alkalinizing urine.22
Treatment of AKI from TMP/SMX includes withholding the medication, and consideration of steroids for those with AIN. In the setting of sulfamethoxazole crystal nephropathy, treatment includes increasing intravenous fluid to induce diuresis and alkalinizing urine.22
Sulfadiazine
Sulfadiazine is a sulfonamide antibiotic that is used for prevention and treatment of infections such as chancroid and toxoplasmosis gondii. Similar to sulfamethoxazole, sulfadiazine is known to cause crystalline nephropathy.22 In acidic pH urine or low volume urine, sulfadiazine can precipitate, causing distal tubular obstruction.4,26 Additional forms of AKI include stone formation, along with AIN.26 The AKI from crystalline nephropathy often presents as an asymptomatic rise in creatinine. Evaluation of urine microscopy will show sulfadiazine crystals in a classic “shock of wheat” formation.4 Risk factors for developing crystalline nephropathy include low urine volume, acidic urine, and high dose of sulfadiazine.26 Prevention of this AKI is done by ensuring volume repletion, dose reducing sulfadiazine, or switching to an alternative antibiotic. Additional preventative efforts include forced diuresis with large volume intravenous fluids and attempting to alkalinize the urine.26
Fluoroquinolones
Fluoroquinolones (FQs) are broad spectrum antibiotics, used to cover enteric gram-negative bacteria, respiratory pathogens, and certain gram-positive bacteria. However, AKI is associated with certain FQs such as ciprofloxacin, and less commonly levofloxacin and moxifloxacin.4,15,27 The risk of AKI does not appear to be a class effect, because FQs such as gatifloxacin, norfloxacin, and gemifloxacin have a much rarer risk of AKI.28 The AKI from FQs is typically secondary to AIN, but has also been reported to cause ATN in overdose, and rarely granulomatous interstitial nephritis and crystalluria.15,17,28, 29, 30 Risk factors for developing crystalluria include alkaline urine (pH > 6.8), concomitant use of renin-angiotensin system blockers as well factors that increase risk for precipitation, including low urine volume and high drug concentrations.4,29,30 When the crystals precipitate, it often occurs in the distal tubules, obstructing urine output and causing interstitial inflammation. Crystals isolated in urine samples are stellate-appearing; by biopsy, they are needle shaped within the tubules, and are birefringent by polarized light.4,30
Similar to other antibiotics, AIN from FQs may or may not present with classic inflammatory effects (such as pyuria or eosinophilia) and is typically nonoliguric. In contrast, AKI from ATN may lead to anuria. Crystalline nephropathy from FQs often develops asymptomatically, with an acute increase in creatinine and oliguria. Preventative measures include ensuring good hydration, avoiding alkaline urine, as well as using FQs that are less commonly associated with AKI, such as moxifloxacin or norfloxacin.4,28 Treatment includes stopping the antibiotic or switching to an alternative option.
Tetracyclines
Tetracyclines are a broad-spectrum class that covers gram-positive, gram-negative, and atypical organisms, such as Borrelia and Treponema species. Nephrotoxicity from these antibiotics is most often reported as proximal tubule damage leading to Fanconi syndrome, often after use of degraded or outdated tetracyclines.31 The tubular dysfunction is usually seen approximately 1 week after exposure to tetracyclines, and the most common sign is hypokalemia, as well as phosphaturia and glucosuria.32 This tubular dysfunction usually requires approximately 9 weeks for resolution.33 Less commonly, AIN from minocycline and doxycycline has been reported. As with other forms of AIN, patients may be asymptomatic or have fevers, rash, and eosinophilia.34,35 Patients typically develop nonoliguric AKI with sterile pyuria, and enlarged kidneys on imaging, and symptoms typically develop 2 to 3 weeks after exposure.35 Renal biopsy typically shows classic AIN findings of interstitial inflammation and cellular infiltrate; however, rarely, granulomatous AIN has also been reported.17,35
Risk factors for tetracycline-induced nephrotoxicity (specifically tubular dysfunction) include use of outdated or degraded versions of the medications. Prevention of nephrotoxicity is managed by avoiding these types of drugs. Treatment includes withdrawal of the medication, supportive care, and steroids for AIN.35
Colistin
Colistin is a polymyxin antibiotic that has seen a resurgence in use for serious multidrug-resistant gram-negative organisms, including Pseudomonas or Klebsiella.36,37 However, its use is limited by nephrotoxicity. Typical presentation of AKI from colistin occurs approximately 5 to 7 days after exposure, and the underlying cause of AKI from colistin appears to be ATN.36,37 Risk factors for AKI include high daily doses of colistin (>5 mg/kg), older age, severity of underlying illness, comorbidities, such as chronic kidney or liver disease, and concomitant use of nephrotoxins, such as vancomycin.36, 37, 38
Prevention of AKI from colistin includes judicious dosing practices because high doses of colistin are required to achieve therapeutic serum concentrations; however, doses >5 mg/kg increase risk of AKI37; in addition, avoiding concomitant use of vancomycin or other nephrotoxins and careful patient evaluation prior to use. Treatment involves supportive care and withdrawal of the drug.38
Mechanisms of Antibiotic-Induced Nephrotoxicity
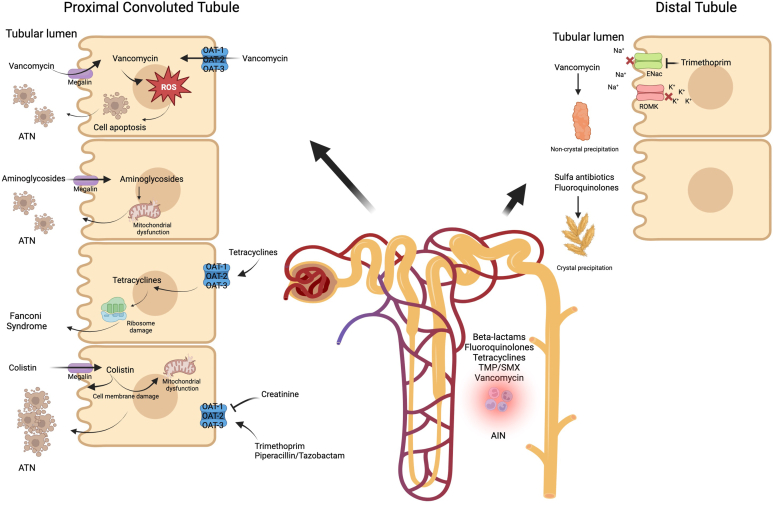
Each antibiotic interacts with different parts of the nephron, leading to nephrotoxicity. These effects are described below, and are organized anatomically. Common mechanisms of nephrotoxicity and their clinical presentation is depicted in Figure 1.
Figure 1.
Mechanisms of antibiotic-induced nephrotoxicity. ATN, acute tubular necrosis.
Glomerular Toxicity
Mesangial cells are located at the core of the glomerular tuft between capillary loops. Mesangial tone exerts a mechanical traction on the glomerular basement membrane and on the endothelial capillary lining, which determines the filtration surface and modulates glomerular vascular resistance. Gentamicin, an aminoglycoside, stimulates mesangial cell contraction and proliferation, leading to reduction in glomerular filtration surface area and rate.39 In addition, with proximal tubular damage, there is increased sodium delivery to the distal part of the nephron. This activates the tubuloglomerular feedback mechanism, leading to afferent arteriolar vasoconstriction and a subsequent decrease in renal blood flow and glomerular filtrate rate.1
Tubular Toxicity
Antibiotic-associated tubular injury can manifest as tubular dysfunction, such as Fanconi’s syndrome, electrolyte and acid-base disorders, and as ATN through increased oxidative stress, alteration of cell metabolism and enzymatic activity, and direct tubular injury.12
Vancomycin enters proximal tubular cells by both receptor-mediated endocytosis from the urine and transporter-mediated secretion from peritubular circulation.40 High intracellular concentration of vancomycin subsequently induces oxidative stress, mitochondrial dysfunction, and cellular apoptosis in the proximal renal tubules.6 Oxygen consumption generates reactive oxygen species (ROS) inside the cells. Oxidative stress refers to an imbalance between ROS and antioxidants within cells. When vancomycin accumulates inside the tubular epithelial cells, it stimulates oxidative phosphorylation and oxygen consumption, leading to increased ROS production. ROS induce lipid peroxidation resulting in mitochondrial membrane damage, which subsequently induces cytochrome C release, caspase activation, and cell apoptosis.41,42 In addition, ROS production causes injury to mitochondrial DNA and promotes the activation of poly-adenosine diphosphate ribose polymerase 1, an enzyme involved in DNA repair. When excessive mitochondrial DNA damage coupled with poly-adenosine diphosphate ribose polymerase 1 overactivation, there is a depletion of intracellular nicotinamide adenine dinucleotide and adenosine triphosphate, leading to cell necrosis. Furthermore, vancomycin accumulates in lysosomes and activates the mitogen-activated protein kinase pathway, resulting in programmed cell death of the proximal tubular cells.41,43
Obstructing tubular casts composed of noncrystalline vancomycin aggregates entangled with uromodulin were noted on renal biopsies in patients with vancomycin-induced AKI.8,44 These casts were mainly found in the distal tubules. Macrophage infiltration was observed surrounding these casts within the interstitium, suggesting that these casts may induce an inflammatory process leading to further damage. Similar vancomycin casts were reproduced experimentally in mice that were given high dose vancomycin. Vancomycin obstructive casts appear very early after administration of vancomycin and are detected as early as 40 minutes postinjection on the kidney sections in the mice models.8
Colistin is reabsorbed by the proximal tubular cells upon binding to megalin at the apical membrane.45 Intracellular accumulation of colistin leads to mitochondrial damage, activation of death receptors, and cell apoptosis.46,47 In addition, colistin increases tubular epithelial cell membrane permeability, resulting in influx of cations, anions, and water, leading to swelling and lysis of the cells.48
Aminoglycosides are freely filtered and subsequently reabsorbed by megalin, an endocytic receptor in proximal tubular cells. Once inside the cell, aminoglycoside concentrates in the lysosomes, endoplasmic reticulum, and Golgi body. When a concentration threshold is reached, aminoglycosides empty into the cytosol, leading to mitochondrial dysfunction and impaired generation of adenosine triphosphate, which induces apoptosis and necrosis of the tubular epithelial cells.1,49 Uptake of aminoglycosides into the proximal tubular cells directly correlates with megalin activity. It has been demonstrated that aminoglycoside-induced nephrotoxicity is completely eliminated in mice with genetically-induced megalin deficiency.50
Tetracyclines enter the proximal tubular epithelial cells through organic anion transporters. Ribosomes are the main intracellular target of tetracycline, causing inhibition of ribosomal protein synthesis.32 Histological examination of kidney tissue after tetracycline exposure showed vacuolization of the tubular epithelium, hypercellularity of glomerular tufts, prominent capillary loops, and basement membrane thickening.33 This damage from tetracycline is associated with proximal tubular dysfunction and Fanconi syndrome.
Trimethoprim use is associated with hyperkalemia. On the basolateral membrane of the cortical collecting duct, trimethoprim inhibits Na-K-ATPase. On the apical membrane of the cortical collecting duct, trimethoprim inhibits influx via epithelial sodium channel, which decreases the net driving force for potassium to exit across the apical cell membrane. As a result, trimethoprim decreases urinary potassium excretion, even in healthy volunteers with normal renal function.25,51
Interstitial Toxicity
AIN is a delayed T cell-mediated (type IV) hypersensitivity reaction that occurs after exposure to a culprit drug. Reexposure to the same drug will result in a faster and more severe reaction. The kidneys are susceptible to developing AIN because they are a major site of drug and drug metabolite excretion, which become antigens that trigger the reaction.52
Antibiotics are thought to be the most common cause of AIN. In a case series of 133 patients with biopsy-proven AIN between 1993 and 2011, 70% of the cases were drug-induced, and antibiotics accounted for almost half these drug-induced AIN cases.53
Of the drugs frequently cited to cause AIN, beta-lactams are the most common. Beta-lactams, such as penicillin and cephalosporin, are eliminated by glomerular filtration and varying degrees of active transport across the tubular epithelial cells. The antigenic determinant of these drugs is formed through binding of their reactive degradation product with albumin. These penicillin-albumin or cephalosporin-albumin complexes then bind to drug-specific T cells, leading to recruitment of inflammatory cells to the renal parenchyma. Infiltrating neutrophils lead to tubulitis seen in severe cases of AIN. Activated fibroblasts proliferate and promote matrix synthesis, eventually causing fibrosis. Infiltrating macrophages release collagenase, elastase, and ROS, magnifying the injury initiated by the lymphocytes.52
Biomarkers of Antibiotic-Induced Nephrotoxicity
Most commonly used serum markers of antibiotic-induced nephrotoxicity such as creatinine or blood urea nitrogen are imperfect markers of kidney function because they are influenced by many renal and nonrenal factors, independent of kidney function.54 For example, antibiotics such as trimethoprim, which is most often given in combination with sulfamethoxazole, alter the tubular secretion of creatinine leading to changes in serum creatinine independent of estimated glomerular filtration rate.55 For example, the cephalosporin antibiotic cefoxitin can interfere with some creatinine assays resulting in falsely high creatinine levels. Serum creatinine may change due to nonrenal factors independent of kidney function such as age, gender, race, muscle mass, nutritional status, total parenteral nutrition, infection, protein intake, catabolic states, and volume status. Alterations in serum creatinine lag several days behind actual changes in glomerular filtration rate. Blood urea nitrogen is also altered by nonrenal factors such as protein intake, catabolic state, upper gastrointestinal bleeding, volume status, and high-dose steroids.56
Earlier detection of antibiotic-induced nephrotoxicity with a kidney specific biomarker may result in earlier detection of AKI, avoidance of nephrotoxic antibiotics, more rational antibiotic dosing, or the development of new nonnephrotoxic antibiotics. For example, cystatin C has been incorporated into some drug-dosing algorithms for vancomycin because it increases earlier in response to vancomycin toxicity than creatinine.57
In 2018, the FDA approved a composite measure composed of 6 urinary biomarkers: cystatin C, KIM-1, clusterin, N-acetyl-beta-D-glucosaminidase (NAG), neutrophil gelatinase-associated lipocalin (NGAL), and osteopontin as a safety composite biomarker panel to be used in conjunction with traditional measures to aid in the detection of tubular injury in phase 1 trials in healthy volunteers when there is an a prior concern that a drug may cause tubular injury.58,59 These FDA-accepted biomarkers will be discussed first. A summary of the novel biomarkers is found in Table 2.
Table 2.
Novel biomarkers and their use in antibiotic induced nephrotoxicity
| Biomarker | Metabolism | Antibiotic with which the biomarker has correlated to nephrotoxicity (preclinical animal studies) | Antibiotic with which the biomarker has correlated to nephrotoxicity (clinical studies) |
|---|---|---|---|
| Cystatin C | -Produced by all nucleated cells -Freely filtered by glomerulus -Serum and urinary cystatin C increased in AKI |
-AGs/Gentamicin -Colistin |
-AGs/gentamicin (pediatrics) -Colistin (adults) -Vancomycin (pediatrics, adults) |
| KIM-1 | -Transmembrane protein expressed on the apical membrane of PCT -Urinary KIM-1 increased in AKI |
-Vancomycin -Colistin |
-AGs/Gentamicin (pediatrics) |
| Clusterin | -Produced in renal tubular cells -Urinary clusterin increased in renal tubular cell injury |
-AGs/Gentamicin -Vancomycin |
NA |
| NAG | -Is a glycosidase located in the lysosomes of PCT epithelial cells -Urinary NAG increased in AKI |
-AGs/Gentamicin | -AGs/Gentamicin (adults) |
| NGAL | -Protein involved in innate immunity, present in leukocytes -Urinary NGAL increased in AKI |
-AGs/Gentamicin -Vancomycin -Colistin |
-AGs (pediatrics) -Vancomycin (adults) |
| OPN | -Glycoprotein involved in immunity and has upregulated expression in tubules and glomeruli with renal injury -Urinary OPN increased in AKI |
-AGs/Gentamicin -Colistin |
-NA |
| TIMP2 x IGFBP7 | -Markers of cell cycle arrest in renal tubular epithelial cells -Urinary TIMP2 x IGFBP7 is increased in ischemic or sepsis induced AKI |
-NA | -VPT (adults) -Vancomycin (adults) -AGs (pediatrics) |
| IL-18 | -Proinflammatory cytokine, present in renal tubules -Urinary IL-18 is increased in tubular injury |
-AGs/Gentamicin | -AGs (pediatrics) |
| miRNAs | -Non-coding RNAs that are important in gene regulation | -AGs/Gentamicin | -AGs/Gentamicin -Vancomycin |
AGs, aminoglycosides; AKI, acute kidney injury; IGFBP7, insulin-like growth factor-binding-protein 7; IL, interleukin; KIM-1, kidney injury molecule-1; NAG, N-acetyl-beta-D-glucosaminidase; NGAL, neutrophil gelatinase associated lipocalin; OPN, osteopontin; PCT, proximal convoluted tubule; TIMP2, tissue inhibitor of metalloproteinases-2; VPT, vancomycin and piperacillin-tazobactam.
Cystatin C
Cystatin C is produced by all nucleated cells, freely filtered by the glomerulus and is completely reabsorbed by the proximal tubules.60 Serum cystatin C, in some situations, may be more accurate than creatinine, because it is not affected by muscle mass, diet, gender, or tubular secretion.61 Serum cystatin C and cystatin C based formulae perform well in estimating glomerular filtration rate.62,63 However, abnormalities of thyroid function, high dose glucocorticoid therapy, and systemic inflammation may increase cystatin C independent of kidney function.54 Based on the number of published studies, serum cystatin C is the most promising biomarker of antibiotic-induced nephrotoxicity.64, 65, 66, 67
Preclinical Studies
Urinary cystatin C, KIM-1, and NGAL, are biomarkers of gentamicin-induced nephrotoxicity that increase before blood urea nitrogen or serum creatinine in rats.68,69 Serum cystatin C increases before serum creatinine in rats treated with colistin.70
Clinical Studies
Serum cystatin C increases before serum creatinine and can predict worsening AKI stage in preterm infants on amikacin and in noncritically ill children receiving aminoglycosides.71,72 In children with cystic fibrosis, serum cystatin C levels and clearance showed greater ability to predict amikacin clearance than creatinine clearance.73,74 In adults on colistin, serum cystatin C is a better marker of renal function than serum creatinine and predictive of persistent AKI.75
In hospitalized patients on vancomycin, the best performing model for dosing utilizes the Chronic Kidney Disease Epidemiology Collaboration creatinine-cystatin C estimated glomerular filtration rate equation. This equation was converted into a bedside applicable algorithm and vancomycin dosing was selected from a bedside algorithm drug nomogram.57,76 The incorporation of cystatin C in the algorithm led to more frequent dosing intervals and a reduction in the number of patients with subtherapeutic trough levels. Use of cystatin C allows better prediction of serum vancomycin concentrations; better prediction of vancomycin clearance; and facilitates initial dosing and dose adjustments in spinal cord injury, in the ICU, in elderly and hospitalized patients and in children with cystic fibrosis.77, 78, 79, 80, 81
In a prospective study in 739 ICU patients, VPT was associated with a higher percentage of creatinine-defined AKI.13 In contrast, VPT was not associated with change in cystatin C or clinical outcomes of dialysis or mortality, suggesting that VPT’s effects on creatinine may represent a pseudotoxicity characterized by isolated effects on tubular creatinine secretion.
In summary, in preclinical and clinical studies, both urine and serum cystatin C can effectively predict nephrotoxicity due to aminoglycosides, vancomycin, and colistin. Use of cystatin C allows better prediction of serum vancomycin concentrations, better prediction of vancomycin clearance, and facilitates initial dosing and dose adjustments.
KIM-1
After cystatin C, the next most studied biomarker of antibiotic nephrotoxicity is KIM-1. KIM-1 is a transmembrane protein expressed on the proximal tubule apical membrane that is increased in ischemic AKI kidneys.82, 83, 84, 85
Preclinical Studies
KIM-1 is increased in proximal tubules and in urine before the increase in serum creatinine in antibiotic-induced nephrotoxicity.86, 87, 88, 89, 90, 91 Urinary KIM-1, clusterin, and osteopontin are more sensitive and specific than cystatin C and NGAL to detect vancomycin-induced AKI as determined by histopathology.92 In rats treated with colistin, urinary KIM-1, albumin, NGAL, clusterin, GST-μ, and osteopontin are elevated in a time-dependent manner.93 Kidney function decline and increased KIM-1 are observed among rats that received vancomycin only, but not those that received piperacillin-tazobactam or VPT.94,95
Clinical Studies
In children with cystic fibrosis receiving tobramycin, there is increased urinary KIM-1 and NGAL in the absence of serum creatinine changes.96 In premature neonates on gentamicin, significant increases are observed in urinary KIM-1.97
Despite many preclinical and clinical studies that show that KIM-1 is an early marker of antibiotic-induced nephrotoxicity, the use of urinary KIM-1 in patients to better predict serum antibiotic concentrations and clearance (in order to facilitate initial dosing and dose adjustments and to prevent nephrotoxicity) has not yet been detailed.
Clusterin
Clusterin is a protein found in renal tubules where it mediates clearance of cellular debris and apoptosis.98,99 After tubular injury, clusterin gene expression is upregulated. Clusterin is not filtered by the glomeruli, so its detection in urine is a specific marker of damage to tubular cells.100 In animal studies, urinary clusterin outperforms blood urea nitrogen, serum creatinine, and urinary NAG in detecting proximal tubular injury caused by gentamicin and vancomycin.59,69,101 In summary, there are no human studies of clusterin as a biomarker of antibiotic-mediated nephrotoxicity.
N-acetyl-beta-D-glucosaminidase
NAG is a glycosidase found in proximal tubular epithelial cell lysosomes and urinary NAG increases with tubular injury. NAG has a high molecular weight and is not filtered through the glomerulus; therefore, its increase in urine is caused exclusively by secretion from proximal tubular cell lysosomes.102
Preclinical Studies
In gentamicin toxicity in rats, urinary NAG increases earlier than creatinine.101,103,104 However, urinary KIM-1 and urinary clusterin are more sensitive and specific for early gentamicin-induced nephrotoxicity than urinary NAG.50,91
Clinical Studies
In patients on gentamicin, increased urinary NAG predicts the development of renal failure, increases before serum creatinine, and correlates with increased serum cystatin C.74,105,106
In summary, newly discovered biomarkers have improved sensitivity and specificity for antibiotic-induced nephrotoxicity than urinary NAG.74,89,91,97
Neutrophil Gelatinase-Associated Lipocalin
NGAL is a protein that is participates in innate immunity and is massively increased in urine after ischemic AKI in rats and mice.107
Preclinical Studies
In rats treated with gentamicin, vancomycin, or colistin, urinary NGAL is an early biomarker of AKI that increases before serum creatinine.59,68,69,87,92,93,108,109
Clinical Studies
In children with cystic fibrosis receiving tobramycin and in adults that develop AKI, urinary NGAL and KIM-1 increase before serum creatinine.96,110 NGAL is present in leukocytes and serum NGAL may not be accurate in septic patients with leukocytosis.111 In a prospective cohort study of 94 hospitalized patients receiving vancomycin, urinary NGAL predicts AKI, the time of vancomycin use, and higher plasma vancomycin concentrations.112
In summary, there are many preclinical and clinical studies that demonstrate that urinary NGAL increases before creatinine in antibiotic-mediated nephrotoxicity. However, urinary NGAL has not yet been used clinically for antibiotic dosing or for early discontinuation of nephrotoxic antibiotics to prevent development of clinical AKI.
Osteopontin
Osteopontin is a glycoprotein that regulates immunity and inflammation. After renal injury, osteopontin expression is significantly upregulated in all tubule segments and glomeruli.113 Urinary ostepontin is increased more than 10-fold in gentamicin-treated and colistin-treated rats.59,93 In preterm infants, amikacin administration is associated with higher urinary cystatin C but not urinary osteopontin or NGAL.72 In summary, in preclinical studies of gentamicin and colistin toxicity, urinary osteopontin is an early biomarker that increases before creatinine. However, the same is yet to be demonstrated in clinical studies.
Other Biomarkers of Antibiotic-Induced Nephrotoxicity
Besides the FDA-accepted biomarker panel discussed above, there are other important novel urinary biomarkers of antibiotic-induced nephrotoxicity: TIMP2 and IGFBP7 (also known as Nephrocheck), interleukin-18 (IL-18), and miRNAs.
TIMP2 and IGFBP7
TIMP2 and IGFBP7 are markers of cell cycle arrest in renal tubular epithelial cells usually after ischemia or sepsis-induced AKI.114 AKI-induced urinary TIMP2/IGFBP7 elevations are due to increased filtration, decreased tubule reabsorption, and proximal tubule cell TIMP2/IGFBP7 urinary leakage, rather than stress-induced gene transcription.115 A combination of urinary TIMP2 multiplied by IGFBP7, known as NephroCheck, is the first FDA-approved platform as a biomarker of ischemia and sepsis-induced AKI in critically ill patients.116, 117, 118
AKI, as determined by TIMP2 x IGFBP7, occurs more frequently in critically ill patients receiving VPT than in those receiving piperacillin–tazobactam alone.119 The risk of death or dialysis is greatest for VPT versus monotherapy. This study is in direct contrast to studies with urinary KIM-1 or cystatin C, that do not show increased nephrotoxicity biomarkers with VPT compared to monotherapy.13,94 In a prospective cohort study of 94 hospitalized patients receiving vancomycin, TIMP2 x IGFBP-7 is a prognostic factor for nonrecovery of kidney function at discharge.112
Studies are emerging on the role of TIMP-2 x IGFBP7 in the diagnosis of antibiotic-induced nephrotoxicity and development and deployment of pharmacist-directed cystatin C and TIMP-2/ IGFBP7-based antibiotic dosing and monitoring protocols presents an opportunity to decrease antibiotic nephrotoxicity.73
Interleukin-18
IL-18 is a proinflammatory cytokine that is important in both innate and acquired immunity.120 IL-18 is present in the renal tubules and released into the urine by tubular injury.121,122 In rats, IL-18 increases in kidney after gentamicin.123 In a prospective study of children that received aminoglycosides and developed AKI, urinary IL-18, KIM-1, or TIMP2 x IGFBP7 were modest biomarkers for predicting AKI.124
Urinary miRNAs
MiRNAs are small noncoding RNAs that are important in gene regulation and a novel class of therapeutic targets. In gentamicin-treated rats, miR-26-3p, 192-5p, and 378a-3p have sensitivities comparable to clinical biomarkers and urinary KIM-1 to detect tubular injury; and urinary miR-138-5p, miR-1971, miR-218-1-3p, and miR-489 increase before creatinine.125,126 In gentamicin -treated patients with sepsis, miR-15a-5p is increased; and in patients on vancomycin, miR-155-5p and miR-192-5p positively correlate with creatinine and NGAL values.127 Therefore, miRNAs may serve as diagnostic or therapeutic tool in patients with sepsis treated with vancomycin or gentamicin.
Biomarkers of AIN
The gold standard for diagnosis of AIN is the presence of tubulointerstitial eosinophil infiltrate on kidney biopsy. Urinary cytokines and chemokines that may reflect kidney local inflammation are the most promising urinary biomarkers of AIN.128 In patients with drug-induced AIN, numerous studies have found an increase in many different urinary cytokines and chemokines: MCP-1, NGAL, NAG, metalloproteinase 2, metalloproteinase 9, KIM-1, C5b9, IFNα, IL-6, IL-8, IL-12, IL-17, TNFα, IL-9, and IL-5.128 Therefore, although many cytokines and chemokines increase in the urine in drug-induced AIN, further research is needed on the precise sensitivity and specificity of these biomarkers and whether these biomarkers predict clinical outcomes.
Conclusion
For life-saving antibiotics with potential nephrotoxic effects in critically ill patients, the simultaneous use of both functional and damage biomarkers has the potential to provide important information to optimize dosing and duration of treatment and prevent nephrotoxicity, especially in situations when traditional markers such as creatinine may be unreliable.129,130 The discovery of novel biomarkers of antibiotic-mediated nephrotoxicity and research on their use may hasten the development of new nonnephrotoxic antibiotics and may improve medication effectiveness and safety by improving medication dosing and monitoring.
Disclosure
All the authors have declared no competing interests.
Acknowledgments
The authors acknowledge BioRender.com in the production of Figure 1.
References
- 1.Lopez-Novoa J.M., Quiros Y., Vicente L., Morales A.I., Lopez-Hernandez F.J. New insights into the mechanism of aminoglycoside nephrotoxicity: an integrative point of view. Kidney Int. 2011;79:33–45. doi: 10.1038/ki.2010.337. [DOI] [PubMed] [Google Scholar]
- 2.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179–c184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 3.Bentley M.L., Corwin H.L., Dasta J. Drug-induced acute kidney injury in the critically ill adult: recognition and prevention strategies. Crit Care Med. 2010;38(6):S169–S174. doi: 10.1097/CCM.0b013e3181de0c60. (suppl) [DOI] [PubMed] [Google Scholar]
- 4.Perazella M.A., Rosner M.H. Drug-induced acute kidney injury. Clin J Am Soc Nephrol. 2022;17:1220–1233. doi: 10.2215/CJN.11290821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McWilliam S.J., Antoine D.J., Smyth R.L., Pirmohamed M. Aminoglycoside-induced nephrotoxicity in children. Pediatr Nephrol. 2017;32:2015–2025. doi: 10.1007/s00467-016-3533-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kan W.C., Chen Y.C., Wu V.C., Shiao C.C. Vancomycin-associated acute kidney injury: a narrative review from pathophysiology to clinical application. Int J Mol Sci. 2022;23:2052. doi: 10.3390/ijms23042052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hong S., Valderrama E., Mattana J., et al. Vancomycin-induced acute granulomatous interstitial nephritis: therapeutic options. Am J Med Sci. 2007;334:296–300. doi: 10.1097/MAJ.0b013e3180a6ec1e. [DOI] [PubMed] [Google Scholar]
- 8.Luque Y., Louis K., Jouanneau C., et al. Vancomycin-associated cast nephropathy. J Am Soc Nephrol. 2017;28:1723–1728. doi: 10.1681/ASN.2016080867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stokes M.B., Stevens J.S. Vancomycin-associated cast nephropathy: reality or fantasy? Kidney360. 2022;3:372–375. doi: 10.34067/KID.0007282021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sawada A., Kawanishi K., Morikawa S., et al. Biopsy-proven vancomycin-induced acute kidney injury: a case report and literature review. BMC Nephrol. 2018;19:72. doi: 10.1186/s12882-018-0845-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ciarambino T., Giannico O.V., Campanile A., et al. Acute kidney injury and vancomycin/piperacillin/tazobactam in adult patients: a systematic review. Intern Emerg Med. 2020;15:327–331. doi: 10.1007/s11739-020-02287-2. [DOI] [PubMed] [Google Scholar]
- 12.Blair M., Cote J.M., Cotter A., Lynch B., Redahan L., Murray P.T. Nephrotoxicity from vancomycin combined with piperacillin-tazobactam: a comprehensive review. Am J Nephrol. 2021;52:85–97. doi: 10.1159/000513742. [DOI] [PubMed] [Google Scholar]
- 13.Miano T.A., Hennessy S., Yang W., et al. Association of vancomycin plus piperacillin-tazobactam with early changes in creatinine versus cystatin C in critically ill adults: a prospective cohort study. Intensive Care Med. 2022;48:1144–1155. doi: 10.1007/s00134-022-06811-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khalili H., Rahmani H., Mohammadi M., Salehi M., Mostafavi Z. Intravenous magnesium sulfate for prevention of vancomycin plus piperacillin-tazobactam induced acute kidney injury in critically ill patients: an open-label, placebo-controlled, randomized clinical trial. Daru. 2021;29:341–351. doi: 10.1007/s40199-021-00411-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morales-Alvarez M.C. Nephrotoxicity of antimicrobials and antibiotics. Adv Chronic Kidney Dis. 2020;27:31–37. doi: 10.1053/j.ackd.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Lazarus B., Davies M.R.P., Trubiano J.A., Pellicano R. Time to acute kidney injury in beta-lactam-induced acute interstitial nephritis. Kidney Int Rep. 2020;5:1068–1070. doi: 10.1016/j.ekir.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah S., Carter-Monroe N., Atta M.G. Granulomatous interstitial nephritis. Clin Kidney J. 2015;8:516–523. doi: 10.1093/ckj/sfv053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernandez-Juarez G., Perez J.V., Caravaca-Fontan F., et al. Duration of treatment with corticosteroids and recovery of kidney function in acute interstitial nephritis. Clin J Am Soc Nephrol. 2018;13:1851–1858. doi: 10.2215/CJN.01390118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moledina D.G., Perazella M.A. Treatment of drug-induced acute tubulointerstitial nephritis: the search for better evidence. Clin J Am Soc Nephrol. 2018;13:1785–1787. doi: 10.2215/CJN.12001018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cockerill F.R., Edson R.S. Trimethoprim-sulfamethoxazole. Mayo Clin Proc. 1991;66:1260–1269. doi: 10.1016/s0025-6196(12)62478-1. [DOI] [PubMed] [Google Scholar]
- 21.Delanaye P., Mariat C., Cavalier E., Maillard N., Krzesinski J.M., White C.A. Trimethoprim, creatinine and creatinine-based equations. Nephron Clin Pract. 2011;119:c187–c193. doi: 10.1159/000328911. [DOI] [PubMed] [Google Scholar]
- 22.Perazella M.A. Crystal-induced acute renal failure. Am J Med. 1999;106:459–465. doi: 10.1016/s0002-9343(99)00041-8. [DOI] [PubMed] [Google Scholar]
- 23.Shrishrimal K., Wesson J. Sulfamethoxazole crystalluria. Am J Kidney Dis. 2011;58:492–493. doi: 10.1053/j.ajkd.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roedel M.M., Nakada S.Y., Penniston K.L. Sulfamethoxazole-induced sulfamethoxazole urolithiasis: a case report. BMC Urol. 2021;21:133. doi: 10.1186/s12894-021-00894-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Don B.R. The effect of trimethoprim on potassium and uric acid metabolism in normal human subjects. Clin Nephrol. 2001;55:45–52. [PubMed] [Google Scholar]
- 26.Perazella M.A., Herlitz L.C. The crystalline nephropathies. Kidney Int Rep. 2021;6:2942–2957. doi: 10.1016/j.ekir.2021.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ansari F.A., Manuel S., Dwivedi R., et al. A rare case of acute kidney injury due to levofloxacin-induced crystal nephropathy. Indian J Nephrol. 2019;29:424–426. doi: 10.4103/ijn.IJN_295_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bird S.T., Etminan M., Brophy J.M., Hartzema A.G., Delaney J.A. Risk of acute kidney injury associated with the use of fluoroquinolones. CMAJ. 2013;185:E475–E482. doi: 10.1503/cmaj.121730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hajji M., Jebali H., Mrad A., et al. Nephrotoxicity of ciprofloxacin: five cases and a review of the literature. Drug Saf Case Rep. 2018;5:17. doi: 10.1007/s40800-018-0073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khan M., Ortega L.M., Bagwan N., Nayer A. Crystal-induced acute kidney injury due to ciprofloxacin. J Nephropathol. 2015;4:29–31. doi: 10.12860/jnp.2015.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montoliu J., Carrera M., Darnell A., Revert L. Lactic acidosis and Fanconi’s syndrome due to degraded tetracycline. Br Med J (Clin Res Ed) 1981;283:1576–1577. doi: 10.1136/bmj.283.6306.1576-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zietse R., Zoutendijk R., Hoorn E.J. Fluid, electrolyte and acid-base disorders associated with antibiotic therapy. Nat Rev Nephrol. 2009;5:193–202. doi: 10.1038/nrneph.2009.17. [DOI] [PubMed] [Google Scholar]
- 33.Izzedine H., Launay-Vacher V., Isnard-Bagnis C., Deray G. Drug-induced Fanconi’s syndrome. Am J Kidney Dis. 2003;41:292–309. doi: 10.1053/ajkd.2003.50037. [DOI] [PubMed] [Google Scholar]
- 34.Bihorac A., Ozener C., Akoglu E., Kullu S. Tetracycline-induced acute interstitial nephritis as a cause of acute renal failure. Nephron. 1999;81:72–75. doi: 10.1159/000045249. [DOI] [PubMed] [Google Scholar]
- 35.Sharma K., Geagan N., Tengsupakul S. Severe acute interstitial nephritis secondary to minocycline use in an adolescent girl. SAGE Open Med Case Rep. 2020;8 doi: 10.1177/2050313X20943069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arrayasillapatorn N., Promsen P., Kritmetapak K., Anunnatsiri S., Chotmongkol W., Anutrakulchai S. Colistin-induced acute kidney injury and the effect on survival in patients with multidrug-resistant gram-negative infections: significance of drug doses adjusted to ideal body weight. Int J Nephrol. 2021;2021 doi: 10.1155/2021/7795096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shields R.K., Anand R., Clarke L.G., et al. Defining the incidence and risk factors of colistin-induced acute kidney injury by KDIGO criteria. PLoS One. 2017;12 doi: 10.1371/journal.pone.0173286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prasannan B.K., Mukthar F.C., Unni V.N., Mohan S., Vinodkumar K. Colistin nephrotoxicity-age and baseline kidney functions hold the key. Indian J Nephrol. 2021;31:449–453. doi: 10.4103/ijn.IJN_130_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martinez-Salgado C., Lopez-Hernandez F.J., Lopez-Novoa J.M. Glomerular nephrotoxicity of Aminoglycosides. Toxicol Appl Pharmacol. 2007;223:86–98. doi: 10.1016/j.taap.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 40.Perazella M.A. Drug-induced acute kidney injury: diverse mechanisms of tubular injury. Curr Opin Crit Care. 2019;25:550–557. doi: 10.1097/MCC.0000000000000653. [DOI] [PubMed] [Google Scholar]
- 41.Nishino Y., Takemura S., Minamiyama Y., et al. Targeting superoxide dismutase to renal proximal tubule cells attenuates vancomycin-induced nephrotoxicity in rats. Free Radic Res. 2003;37:373–379. doi: 10.1080/1071576031000061002. [DOI] [PubMed] [Google Scholar]
- 42.Oktem F., Arslan M.K., Ozguner F., et al. In vivo evidences suggesting the role of oxidative stress in pathogenesis of vancomycin-induced nephrotoxicity: protection by erdosteine. Toxicology. 2005;215:227–233. doi: 10.1016/j.tox.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 43.Pais G.M., Liu J., Zepcan S., et al. Vancomycin-induced kidney injury: animal models of toxicodynamics, mechanisms of injury, human translation, and potential strategies for prevention. Pharmacotherapy. 2020;40:438–454. doi: 10.1002/phar.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tantranont N., Luque Y., Hsiao M., et al. Vancomycin-associated tubular casts and vancomycin nephrotoxicity. Kidney Int Rep. 2021;6:1912–1922. doi: 10.1016/j.ekir.2021.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suzuki T., Yamaguchi H., Ogura J., Kobayashi M., Yamada T., Iseki K. Megalin contributes to kidney accumulation and nephrotoxicity of colistin. Antimicrob Agents Chemother. 2013;57:6319–6324. doi: 10.1128/AAC.00254-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dai C., Li J., Tang S., Li J., Xiao X. Colistin-induced nephrotoxicity in mice involves the mitochondrial, death receptor, and endoplasmic reticulum pathways. Antimicrob Agents Chemother. 2014;58:4075–4085. doi: 10.1128/AAC.00070-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nation R.L., Rigatto M.H.P., Falci D.R., Zavascki A.P. Polymyxin acute kidney injury: dosing and other strategies to reduce toxicity. Antibiotics (Basel) 2019;8:24. doi: 10.3390/antibiotics8010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ordooei Javan A., Shokouhi S., Sahraei Z. A review on colistin nephrotoxicity. Eur J Clin Pharmacol. 2015;71:801–810. doi: 10.1007/s00228-015-1865-4. [DOI] [PubMed] [Google Scholar]
- 49.Wargo K.A., Edwards J.D. Aminoglycoside-induced nephrotoxicity. J Pharm Pract. 2014;27:573–577. doi: 10.1177/0897190014546836. [DOI] [PubMed] [Google Scholar]
- 50.Schmitz C., Hilpert J., Jacobsen C., et al. Megalin deficiency offers protection from renal aminoglycoside accumulation. J Biol Chem. 2002;277:618–622. doi: 10.1074/jbc.M109959200. [DOI] [PubMed] [Google Scholar]
- 51.Muto S., Tsuruoka S., Miyata Y., Fujimura A., Kusano E. Effect of trimethoprim-sulfamethoxazole on Na and K+ transport properties in the rabbit cortical collecting duct perfused in vitro. Nephron Physiol. 2006;102:51–60. doi: 10.1159/000089682. [DOI] [PubMed] [Google Scholar]
- 52.Raghavan R., Shawar S. Mechanisms of drug-induced interstitial nephritis. Adv Chronic Kidney Dis. 2017;24:64–71. doi: 10.1053/j.ackd.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 53.Muriithi A.K., Leung N., Valeri A.M., et al. Biopsy-proven acute interstitial nephritis, 1993–2011: a case series. Am J Kidney Dis. 2014;64:558–566. doi: 10.1053/j.ajkd.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 54.Edelstein C.L. In: Biomarkers of Kidney Disease. Edelstein C.L., editor. Elsevier; 2017. Biomarkers in acute kidney injury; pp. 241–303. [Google Scholar]
- 55.Stevens L.L., RA P R.D., Levey A.S. In: Diseases of the Kidney and Urinary Tract. 8th ed. Schrier R.W., editor. Lippincott, Williams and Watkins; 2007. Laboratory evaluation of kidney function; pp. 299–336. [Google Scholar]
- 56.Walser M. Determinants of ureagenesis, with particular reference to renal failure. Kidney Int. 1980;17:709–721. doi: 10.1038/ki.1980.84. [DOI] [PubMed] [Google Scholar]
- 57.Frazee E., Rule A.D., Lieske J.C., et al. Cystatin C-guided vancomycin dosing in critically ill patients: a quality improvement project. Am J Kidney Dis. 2017;69:658–666. doi: 10.1053/j.ajkd.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 58.Sauer J.M., Porter A.C. Qualification of translational safety biomarkers. Exp Biol Med (Maywood) 2021;246:2391–2398. doi: 10.1177/15353702211002153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tengstrand E., Zhang H., Liu N., Dunn K., Hsieh F. A multiplexed UPLC-MS/MS assay for the simultaneous measurement of urinary safety biomarkers of drug-induced kidney injury and phospholipidosis. Toxicol Appl Pharmacol. 2019;366:54–63. doi: 10.1016/j.taap.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 60.Butler E.A., Flynn F.V. The occurrence of post-gamma protein in urine: a new protein abnormality. J Clin Pathol. 1961;14:172–178. doi: 10.1136/jcp.14.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Westhuyzen J., Cystatin C. a promising marker and predictor of impaired renal function. Ann Clin Lab Sci. 2006;36:387–394. [PubMed] [Google Scholar]
- 62.Grubb A., Nyman U., Bjork J., et al. Simple cystatin C-based prediction equations for glomerular filtration rate compared with the modification of diet in renal disease prediction equation for adults and the Schwartz and the Counahan-Barratt prediction equations for children. Clin Chem. 2005;51:1420–1431. doi: 10.1373/clinchem.2005.051557. [DOI] [PubMed] [Google Scholar]
- 63.Simonsen O., Grubb A., Thysell H. The blood serum concentration of cystatin C (gamma-trace) as a measure of the glomerular filtration rate. Scand J Clin Lab Investig. 1985;45:97–101. doi: 10.3109/00365518509160980. [DOI] [PubMed] [Google Scholar]
- 64.Delanaye P., Cavalier E., Morel J., et al. Detection of decreased glomerular filtration rate in intensive care units: serum cystatin C versus serum creatinine. BMC Nephrol. 2014;15:9. doi: 10.1186/1471-2369-15-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Flannery A.H., Bosler K., Ortiz-Soriano V.M., et al. Kidney biomarkers and major adverse kidney events in critically ill patients. Kidney360. 2021;2:26–32. doi: 10.34067/KID.0003552020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ravn B., Prowle J.R., Martensson J., Martling C.R., Bell M. Superiority of serum cystatin C over creatinine in prediction of long-term prognosis at discharge from ICU. Crit Care Med. 2017;45:e932–e940. doi: 10.1097/CCM.0000000000002537. [DOI] [PubMed] [Google Scholar]
- 67.Zhang Z., Lu B., Sheng X., Jin N. Cystatin C in prediction of acute kidney injury: a systemic review and meta-analysis. Am J Kidney Dis. 2011;58:356–365. doi: 10.1053/j.ajkd.2011.02.389. [DOI] [PubMed] [Google Scholar]
- 68.Hoffmann D., Fuchs T.C., Henzler T., et al. Evaluation of a urinary kidney biomarker panel in rat models of acute and subchronic nephrotoxicity. Toxicology. 2010;277:49–58. doi: 10.1016/j.tox.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 69.Udupa V., Prakash V. Gentamicin induced acute renal damage and its evaluation using urinary biomarkers in rats. Toxicol Rep. 2019;6:91–99. doi: 10.1016/j.toxrep.2018.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ghlissi Z., Hakim A., Mnif H., et al. Evaluation of colistin nephrotoxicity administered at different doses in the rat model. Ren Fail. 2013;35:1130–1135. doi: 10.3109/0886022X.2013.815091. [DOI] [PubMed] [Google Scholar]
- 71.Lau L., Al-Ismaili Z., Harel-Sterling M., et al. Serum cystatin C for acute kidney injury evaluation in children treated with Aminoglycosides. Pediatr Nephrol. 2017;32:163–171. doi: 10.1007/s00467-016-3450-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Martini S., Vitali F., Capelli I., et al. Impact of nephrotoxic drugs on urinary biomarkers of renal function in very preterm infants. Pediatr Res. 2022;91:1715–1722. doi: 10.1038/s41390-021-01905-9. [DOI] [PubMed] [Google Scholar]
- 73.Barreto E.F., Rule A.D., Voils S.A., Kane-Gill S.L. Innovative use of novel biomarkers to improve the safety of renally eliminated and nephrotoxic medications. Pharmacotherapy. 2018;38:794–803. doi: 10.1002/phar.2149. [DOI] [PubMed] [Google Scholar]
- 74.Halacova M., Kotaska K., Kukacka J., et al. Serum cystatin C level for better assessment of glomerular filtration rate in cystic fibrosis patients treated by amikacin. J Clin Pharm Ther. 2008;33:409–417. doi: 10.1111/j.1365-2710.2008.00932.x. [DOI] [PubMed] [Google Scholar]
- 75.Larki R.A., Jamali B., Meidani M., Mousavi S. Serum cystatin C for evaluation of acute kidney injury in adults treated with colistin. J Res Pharm Pract. 2018;7:178–181. doi: 10.4103/jrpp.JRPP_18_53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Frazee E.N., Rule A.D., Herrmann S.M., et al. Serum cystatin C predicts vancomycin trough levels better than serum creatinine in hospitalized patients: a cohort study. Crit Care. 2014;18:R110. doi: 10.1186/cc13899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.DeCarolis D.D., Thorson J.G., Marraffa R.A., Clairmont M.A., Kuskowski M.A. Comparison of equations with estimate renal function to predict serum vancomycin concentration in patients with spinal cord injury--does the use of cystatin C improve accuracy? Ther Drug Monit. 2014;36:632–639. doi: 10.1097/FTD.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 78.Kees M.G., Hilpert J.W., Gnewuch C., Kees F., Voegeler S. Clearance of vancomycin during continuous infusion in Intensive Care Unit patients: correlation with measured and estimated creatinine clearance and serum cystatin C. Int J Antimicrob Agents. 2010;36:545–548. doi: 10.1016/j.ijantimicag.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 79.Okamoto G., Sakamoto T., Kimura M., et al. Serum cystatin C as a better marker of vancomycin clearance than serum creatinine in elderly patients. Clin Biochem. 2007;40:485–490. doi: 10.1016/j.clinbiochem.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 80.Olsen K.M., Rudis M.I., Rebuck J.A., et al. Effect of once-daily dosing vs. multiple daily dosing of tobramycin on enzyme markers of nephrotoxicity. Crit Care Med. 2004;32:1678–1682. doi: 10.1097/01.ccm.0000134832.11144.cb. [DOI] [PubMed] [Google Scholar]
- 81.Tanaka A., Suemaru K., Otsuka T., et al. Estimation of the initial dose setting of vancomycin therapy with use of cystatin C as a new marker of renal function. Ther Drug Monit. 2007;29:261–264. doi: 10.1097/FTD.0b013e31803bcfd2. [DOI] [PubMed] [Google Scholar]
- 82.Ichimura T., Asseldonk E.J., Humphreys B.D., Gunaratnam L., Duffield J.S., Bonventre J.V. Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J Clin Invest. 2008;118:1657–1668. doi: 10.1172/JCI34487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ichimura T., Bonventre J.V., Bailly V., et al. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem. 1998;273:4135–4142. doi: 10.1074/jbc.273.7.4135. [DOI] [PubMed] [Google Scholar]
- 84.Ichimura T., Hung C.C., Yang S.A., Stevens J.L., Bonventre J.V. Kidney injury molecule-1: a tissue and urinary biomarker for nephrotoxicant-induced renal injury. Am J Physiol Ren Physiol. 2004;286:F552–F563. doi: 10.1152/ajprenal.00285.2002. [DOI] [PubMed] [Google Scholar]
- 85.Vaidya V.S., Ramirez V., Ichimura T., Bobadilla N.A., Bonventre J.V. Urinary kidney injury molecule-1: a sensitive quantitative biomarker for early detection of kidney tubular injury. Am J Physiol Ren Physiol. 2006;290:F517–F529. doi: 10.1152/ajprenal.00291.2005. [DOI] [PubMed] [Google Scholar]
- 86.Bonventre J.V. Kidney injury molecule-1 (KIM-1): a urinary biomarker and much more. Nephrol Dial Transplant. 2009;24:3265–3268. doi: 10.1093/ndt/gfp010. [DOI] [PubMed] [Google Scholar]
- 87.Luo Q.H., Chen M.L., Sun F.J., et al. KIM-1 and NGAL as biomarkers of nephrotoxicity induced by gentamicin in rats. Mol Cell Biochem. 2014;397:53–60. doi: 10.1007/s11010-014-2171-7. [DOI] [PubMed] [Google Scholar]
- 88.Sieber M., Hoffmann D., Adler M., et al. Comparative analysis of novel noninvasive renal biomarkers and metabonomic changes in a rat model of gentamicin nephrotoxicity. Toxicol Sci. 2009;109:336–349. doi: 10.1093/toxsci/kfp070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vaidya V.S., Ozer J.S., Dieterle F., et al. Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat Biotechnol. 2010;28:478–485. doi: 10.1038/nbt.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang E.J., Snyder R.D., Fielden M.R., Smith R.J., Gu Y.Z. Validation of putative genomic biomarkers of nephrotoxicity in rats. Toxicology. 2008;246:91–100. doi: 10.1016/j.tox.2007.12.031. [DOI] [PubMed] [Google Scholar]
- 91.Zhou Y., Vaidya V.S., Brown R.P., et al. Comparison of kidney injury molecule-1 and other nephrotoxicity biomarkers in urine and kidney following acute exposure to gentamicin, mercury, and chromium. Toxicol Sci. 2008;101:159–170. doi: 10.1093/toxsci/kfm260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pais G.M., Avedissian S.N., O’Donnell J.N., et al. Comparative performance of urinary biomarkers for vancomycin-induced kidney injury according to timeline of injury. Antimicrob Agents Chemother. 2019;63 doi: 10.1128/AAC.00079-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Keirstead N.D., Wagoner M.P., Bentley P., et al. Early prediction of polymyxin-induced nephrotoxicity with next-generation urinary kidney injury biomarkers. Toxicol Sci. 2014;137:278–291. doi: 10.1093/toxsci/kft247. [DOI] [PubMed] [Google Scholar]
- 94.Chang J., Pais G.M., Valdez K., Marianski S., Barreto E.F., Scheetz M.H. Glomerular function and urinary biomarker changes between vancomycin and vancomycin plus piperacillin-tazobactam in a translational rat model. Antimicrob Agents Chemother. 2022;66 doi: 10.1128/aac.02132-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pais G.M., Liu J., Avedissian S.N., et al. Lack of synergistic nephrotoxicity between vancomycin and piperacillin/tazobactam in a rat model and a confirmatory cellular model. J Antimicrob Chemother. 2020;75:1228–1236. doi: 10.1093/jac/dkz563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McWilliam S.J., Antoine D.J., Jorgensen A.L., Smyth R.L., Pirmohamed M. Urinary biomarkers of aminoglycoside-induced nephrotoxicity in cystic fibrosis: kidney injury Molecule-1 and neutrophil gelatinase-associated lipocalin. Sci Rep. 2018;8:5094. doi: 10.1038/s41598-018-23466-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McWilliam S.J., Antoine D.J., Sabbisetti V., et al. Mechanism-based urinary biomarkers to identify the potential for aminoglycoside-induced nephrotoxicity in premature neonates: a proof-of-concept study. PLoS One. 2012;7 doi: 10.1371/journal.pone.0043809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rosenberg M.E., Silkensen J. Silkensen J. Clusterin: physiologic and pathophysiologic considerations. Int J Biochem Cell Biol. 1995;27:633–645. doi: 10.1016/1357-2725(95)00027-m. [DOI] [PubMed] [Google Scholar]
- 99.Shannan B., Seifert M., Boothman D.A., Tilgen W., Reichrath J. Clusterin and DNA repair: a new function in cancer for a key player in apoptosis and cell cycle control. J Mol Histol. 2006;37:183–188. doi: 10.1007/s10735-006-9052-7. [DOI] [PubMed] [Google Scholar]
- 100.Vlasakova K., Erdos Z., Troth S.P., et al. Evaluation of the relative performance of 12 urinary biomarkers for renal safety across 22 rat sensitivity and specificity studies. Toxicol Sci. 2014;138:3–20. doi: 10.1093/toxsci/kft330. [DOI] [PubMed] [Google Scholar]
- 101.Gautier J.C., Zhou X., Yang Y., et al. Evaluation of novel biomarkers of nephrotoxicity in Cynomolgus monkeys treated with gentamicin. Toxicol Appl Pharmacol. 2016;303:1–10. doi: 10.1016/j.taap.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 102.Kim S.R., Lee Y.H., Lee S.G., et al. Urinary N-acetyl-beta-D-glucosaminidase, an early marker of diabetic kidney disease, might reflect glucose excursion in patients with type 2 diabetes. Med (Baltim) 2016;95:e4114. doi: 10.1097/MD.0000000000004114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fauconneau B., Favreliere S., Pariat C., et al. Nephrotoxicity of gentamicin and vancomycin given alone and in combination as determined by enzymuria and cortical antibiotic levels in rats. Ren Fail. 1997;19:15–22. doi: 10.3109/08860229709026256. [DOI] [PubMed] [Google Scholar]
- 104.Whiting P.H., Brown P.A. The relationship between enzymuria and kidney enzyme activities in experimental gentamicin nephrotoxicity. Ren Fail. 1996;18:899–909. doi: 10.3109/08860229609047716. [DOI] [PubMed] [Google Scholar]
- 105.Etherington C., Bosomworth M., Clifton I., Peckham D.G., Conway S.P. Measurement of urinary N-acetyl-b-D-glucosaminidase in adult patients with cystic fibrosis: before, during and after treatment with intravenous antibiotics. J Cyst Fibros. 2007;6:67–73. doi: 10.1016/j.jcf.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 106.Wiland P., Szechcinski J. Proximal tubule damage in patients treated with gentamicin or amikacin. Pol J Pharmacol. 2003;55:631–637. [PubMed] [Google Scholar]
- 107.Mishra J., Ma Q., Prada A., et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14:2534–2543. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- 108.He L., Peng X., Zhu J., et al. Protective effects of curcumin on acute gentamicin-induced nephrotoxicity in rats. Can J Physiol Pharmacol. 2015;93:275–282. doi: 10.1139/cjpp-2014-0459. [DOI] [PubMed] [Google Scholar]
- 109.Kai K., Yamaguchi T., Yoshimatsu Y., Kinoshita J., Teranishi M., Takasaki W. Neutrophil gelatinase-associated lipocalin, a sensitive urinary biomarker of acute kidney injury in dogs receiving gentamicin. J Toxicol Sci. 2013;38:269–277. doi: 10.2131/jts.38.269. [DOI] [PubMed] [Google Scholar]
- 110.Pang H.M., Qin X.L., Liu T.T., et al. Urinary kidney injury molecule-1 and neutrophil gelatinase-associated lipocalin as early biomarkers for predicting vancomycin-associated acute kidney injury: a prospective study. Eur Rev Med Pharmacol Sci. 2017;21:4203–4213. [PubMed] [Google Scholar]
- 111.Park H.D., Seo J.Y., Lee S.Y. The relationship between serum neutrophil gelatinase-associated lipocalin and renal function in patients with vancomycin treatment. Ann Clin Lab Sci. 2012;42:7–13. [PubMed] [Google Scholar]
- 112.Sampaio de Souza Garms D., Cardoso Eid K.Z., Burdmann E.A., et al. The role of urinary biomarkers as diagnostic and prognostic predictors of acute kidney injury associated with vancomycin. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.705636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Verstrepen W.A., Persy V.P., Verhulst A., Dauwe S., De Broe M.E. Renal osteopontin protein and mRNA upregulation during acute nephrotoxicity in the rat. Nephrol Dial Transplant. 2001;16:712–724. doi: 10.1093/ndt/16.4.712. [DOI] [PubMed] [Google Scholar]
- 114.Kellum J.A., Chawla L.S. Cell-cycle arrest and acute kidney injury: the light and the dark sides. Nephrol Dial Transplant. 2016;31:16–22. doi: 10.1093/ndt/gfv130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Johnson A.C.M., Zager R.A. Mechanisms underlying increased TIMP2 and IGFBP7 urinary excretion in experimental AKI. J Am Soc Nephrol. 2018;29:2157–2167. doi: 10.1681/ASN.2018030265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bihorac A., Chawla L.S., Shaw A.D., et al. Validation of cell-cycle arrest biomarkers for acute kidney injury using clinical adjudication. Am J Respir Crit Care Med. 2014;189:932–939. doi: 10.1164/rccm.201401-0077OC. [DOI] [PubMed] [Google Scholar]
- 117.Kashani K., Al-Khafaji A., Ardiles T., et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013;17:R25. doi: 10.1186/cc12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Koyner J.L., Shaw A.D., Chawla L.S., et al. Tissue inhibitor Metalloproteinase-2 (TIMP-2)⋅IGF-binding Protein-7 (IGFBP7) levels are associated with adverse long-term outcomes in patients with AKI. J Am Soc Nephrol. 2015;26:1747–1754. doi: 10.1681/ASN.2014060556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kane-Gill S.L., Ostermann M., Shi J., Joyce E.L., Kellum J.A. Evaluating Renal Stress Using pharmacokinetic Urinary biomarker Data in Critically Ill Patients Receiving vancomycin and/or piperacillin-tazobactam: a Secondary Analysis of the Multicenter sapphire Study. Drug Saf. 2019;42:1149–1155. doi: 10.1007/s40264-019-00846-x. [DOI] [PubMed] [Google Scholar]
- 120.Dinarello C.A., Fantuzzi G. Interleukin-18 and host defense against infection. J Infect Dis. 2003;187(suppl 2):S370–S384. doi: 10.1086/374751. [DOI] [PubMed] [Google Scholar]
- 121.Melnikov V.Y., Ecder T., Fantuzzi G., et al. Impaired IL-18 processing protects caspase-1-deficient mice from ischemic acute renal failure. J Clin Invest. 2001;107:1145–1152. doi: 10.1172/JCI12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Melnikov V.Y., Faubel S., Siegmund B., Lucia M.S., Ljubanovic D., Edelstein C.L. Neutrophil-independent mechanisms of caspase-1- and IL-18-mediated ischemic acute tubular necrosis in mice. J Clin Invest. 2002;110:1083–1091. doi: 10.1172/JCI15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Samarghandian S., Azimi-Nezhad M., Mehrad-Majd H., Mirhafez S.R. Thymoquinone ameliorates acute renal failure in gentamicin-treated adult male rats. Pharmacology. 2015;96:112–117. doi: 10.1159/000436975. [DOI] [PubMed] [Google Scholar]
- 124.Chui H., Caldwell J., Yordanova M., et al. Tubular injury and cell-cycle arrest biomarkers to predict acute kidney injury in noncritically ill children receiving Aminoglycosides. Biomark Med. 2020;14:879–894. doi: 10.2217/bmm-2019-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jeon B.S., Lee S.H., Hwang S.R., et al. Identification of urinary microRNA biomarkers for in vivo gentamicin-induced nephrotoxicity models. J Vet Sci. 2020;21:e81. doi: 10.4142/jvs.2020.21.e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhou X., Qu Z., Zhu C., et al. Identification of urinary microRNA biomarkers for detection of gentamicin-induced acute kidney injury in rats. Regul Toxicol Pharmacol. 2016;78:78–84. doi: 10.1016/j.yrtph.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 127.Petejova N., Martinek A., Zadrazil J., et al. Expression and 7-day time course of circulating microRNAs in septic patients treated with nephrotoxic antibiotic agents. BMC Nephrol. 2022;23:111. doi: 10.1186/s12882-022-02726-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Martinez Valenzuela L., Draibe J., Fulladosa X., Torras J. New biomarkers in acute tubulointerstitial nephritis: a novel approach to a classic condition. Int J Mol Sci. 2020;21:4690. doi: 10.3390/ijms21134690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Levin A., Stevens P.E. Summary of KDIGO 2012 CKD Guideline: behind the scenes, need for guidance, and a framework for moving forward. Kidney Int. 2014;85:49–61. doi: 10.1038/ki.2013.444. [DOI] [PubMed] [Google Scholar]
- 130.Ostermann M., Zarbock A., Goldstein S., et al. Recommendations on acute kidney injury biomarkers from the acute disease quality initiative consensus conference: a consensus statement. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.19209. [DOI] [PubMed] [Google Scholar]