Highlights
-
•
Alternative resistance training (RT) methods should be considered in potential exercise prescription for people with cancer and may be of interest for specific cancer populations, stage of disease, or cancer and treatment-related comorbidities.
-
•
Robust evidence from research investigating eccentric, cluster sets, and blood flow restriction training in older adults and other clinical populations corroborates its possible use in cancer research.
-
•
Alternative RT methods may potentially elicit equal or even greater effects compared to traditional RT on muscle strength, hypertrophy, physical function, and body composition.
Keywords: Blood flow restriction, Cluster set, Eccentric training, Resistance training
Abstract
Exercise has emerged as fundamental therapeutic medicine in the management of cancer. Exercise improves health-related outcomes, including quality of life, neuromuscular strength, physical function, and body composition, and it is associated with a lower risk of disease recurrence and increased survival. Moreover, exercise during or post cancer treatments is safe, can ameliorate treatment-related side effects, and may enhance the effectiveness of chemotherapy and radiation therapy. To date, traditional resistance training (RT) is the most used RT modality in exercise oncology. However, alternative training modes, such as eccentric, cluster set, and blood flow restriction are gaining increased attention. These training modalities have been extensively investigated in both athletic and clinical populations (e.g., age-related frailty, cardiovascular disease, type 2 diabetes), showing considerable benefits in terms of neuromuscular strength, hypertrophy, body composition, and physical function. However, these training modes have only been partially or not at all investigated in cancer populations. Thus, this study outlines the benefits of these alternative RT methods in patients with cancer. Where evidence in cancer populations is sparse, we provide a robust rationale for the possible implementation of certain RT methods that have shown positive results in other clinical populations. Finally, we provide clinical insights for research that may guide future RT investigations in patients with cancer and suggest clear practical applications for targeted cancer populations and related benefits.
Graphical Abstract
1. Introduction
The World Health Organization (WHO) defines physical activity as any bodily movement produced by skeletal muscles that requires energy expenditure.1 Exercise for general well-being, health and, most recently, medical treatment is a growing area of interest over the past few decades. The WHO has provided exercise recommendations in the management of 4 types of noncommunicable diseases: (a) cardiovascular diseases (CVDs), (b) chronic respiratory diseases, (c) type 2 diabetes mellitus, and (d) cancer.1,2 The WHO expert panel recommends at least 150–300 min or 75–150 min of moderate or vigorous aerobic physical activity, respectively.1 In addition, the WHO advocates for performing strengthening exercises involving all major muscle groups at moderate or greater intensity twice per week to counteract the possible onset of noncommunicable diseases.1,3,4 Among these diseases, cancer represents the biggest contributor to morbidity and mortality worldwide. For example, it has been estimated that a total of 19.3 million cases and 10.0 million deaths occurred in 2020 due to various forms of cancer, with breast, lung, colon and rectum, and prostate cancer being the most common cancer types and contributing most to mortality.5 Unfortunately, the prevalence of cancer is rapidly increasing and, as a result, strategies to prevent or treat cancer are needed.5
Exercise has emerged as a new and fundamental therapeutic medicine in the management of cancer.6, 7, 8, 9 Not only is it widely acknowledged that exercise lowers the risk of at least 7 different types of cancer, but it has also been associated with lower risk of cancer recurrence as well as with higher survival rates in patients with cancer.10 Furthermore, there is strong evidence of the safety and effectiveness of exercise as a medicine to address health-related cancer outcomes, including fatigue, quality of life (QoL), cardiorespiratory capacity, neuromuscular strength, physical function (e.g., 6-minute walk test (6MWT)), body composition (e.g., fat and lean mass), anxiety, and depressive symptoms.11, 12, 13, 14, 15 In summary, exercise either during or post cancer treatments (i.e., chemotherapy or radiation therapy) is safe, provides physical and mental health benefits, and ameliorates treatment-related side effects (e.g., fatigue, nausea, weight loss or gain).8,16
When considering the type of exercise undertaken, 2 distinct modes are commonly used: (a) resistance training (RT) and (b) aerobic training. The American College of Sports Medicine defines RT as an intense physical activity of very short duration generating physical exertion against a resistance (e.g., bodyweight, barbell, dumbbell, stretch bands, or machine) with the purpose of improving muscular strength and eliciting muscle hypertrophy.17 RT movements are performed against an external force sufficient to limit the number of repetitions due to the accumulation of neuromuscular fatigue. This stimulates the body to respond by producing structural, physiological, and chemical adaptations so the individual can produce higher muscular force. It should be acknowledged that bodyweight RT could be a sufficient overload to elicit positive adaptation for some populations (e.g., older cancer patients, patients with low muscle mass, or those with other accompanying health conditions). By contrast, aerobic training encompasses continuous or intermittent physical activities involving repetitive movements that can be maintained over longer durations (e.g., walking, swimming, rowing, cycling) aimed at improving cardiorespiratory fitness and reducing body fat.18
However, more nuanced training modalities have recently gained attention, not only in athletic populations, but in clinical as well (e.g., CVD, type 2 diabetes mellitus, age-related frailty). For example, it has been shown that eccentric (ECC) training (i.e., active lengthening of muscle tissue against an external force or load) induces superior improvements in muscle strength and hypertrophy in older adults when compared to traditional RT.19,20 An additional benefit of ECC training is lower metabolic cost and muscle activity for equal levels of exerted force demand; thus, it has also become an attractive method for patients suffering from CVD.21 In addition, manipulation of traditional RT parameters through the alteration of set configuration and rest periods (e.g., cluster sets (CS)) was recently examined with respect to CVD management; the result was less perceived fatigue, which consequently lead to greater intensity being achieved during RT.22
Blood flow restriction (BFR) training has also been shown to induce hypertrophic adaptations with lower intensities (i.e., from bodyweight to ∼50% of 1 repetition maximum (1RM)) in healthy subjects.23 Considerable benefit for preserving muscle mass has been reported for older patients in intensive care when BFR was coupled with passive mobilization.24 Taken together, the aforementioned training methods may be of interest to patients with cancer and their clinicians, considering the commonly associated cancer-related comorbidities often reported (e.g., weight gain, CVDs, bone and muscle mass loss). With this in mind, it should be noted that these training modes either haven't been investigated in cancer populations or have been only partially investigated.25, 26, 27, 28, 29, 30 Findings are more conclusive in other clinical populations (i.e., older adults, CVDs, diabetes), which provides a basis for their application in patients with cancer.21,22,24,31, 32, 33
Therefore, the primary aim of this narrative review was to outline potential alternative RT methods to enhance muscle strength, hypertrophy, physical function, and body composition that could be considered for investigation in patients with cancer. Additionally, where evidence in cancer populations is sparse, we have provided a rationale for possible future studies of certain RT methods that have shown positive results in other clinical populations.
2. Benefits of traditional RT
It is well-established that RT provides multiple benefits for clinical populations. For many years, researchers have reported that RT results in reversal of muscle loss, reduced fat mass, as well as increased bone mineral density, muscle strength, and muscle mass.34 In addition, RT is considered an efficient method for improving metabolic and cardiorespiratory health, leading to a decrease in CVD and type 2 diabetes mellitus, in some cases, equivalent to aerobic training.35 Furthermore, it has been reported that RT is associated with reduced risk of all-cause, CVD, and cancer-specific mortality by ∼14%.36
More specifically, in cancer patients undergoing chemotherapy or radiation therapy, a recent meta-analysis revealed significant small to moderate increases in lean mass (standardized mean difference (MD) = 0.23), lower and upper limb muscle strength (standardized MD: 0.57–0.58), and handgrip strength (standardized MD = 1.32).37 Similarly, in a meta-analysis of patients undergoing neoadjuvant therapy (i.e., before surgery) as well as adjuvant therapy (i.e., after surgery), RT improved muscle strength with overall MDs of 23.4 kg and 28.6 kg, respectively.38 When examining the effects of RT in cancer survivors, RT significantly enhanced lower limb muscle strength as measured by load lifted during the leg press (weighted MD = 18.2 kg) and reduced percentage of body fat (weighted MD = 4.0%)39 with significant increases in lean mass (i.e., –0.01% to 11.8%) also noted.40 In addition, measures of physical function have also been assessed after RT interventions in cancer survivors, including the timed up-and-go test (TUG), 30-second chair stand, 6MWT, 400-m walk, and stair climb test, all of which showed improvement, by 8.2%, 14.0%, 7.6%, 7.9%, and 8.5%, respectively.41
Examining the RT parameters applied in these studies, most interventions involved 8–12 repetitions of 2–4 sets, consisting of concentric and ECC phases of 1 s with intensity adapted according to a specific %RM or set number of repetitions,37,39,40,42 which corresponds with common traditional RT protocols.8 However, alternative RT methods (e.g., BFR or ECC training) are currently used in clinical populations. In this regard, further research is necessary to investigate whether such alternative RT methods provide comparable (or better) benefits in health-related outcomes for cancer patients.
3. Benefits of alternative RT methods
Trials adopting alternative RT methods in cancer patients are listed in Table 1. In order to clearly elucidate the effects on the outcome measures, calculations of effect sizes (ES) have been conducted and are presented below.
Table 1.
Summary of studies using alternative RT methods in cancer populations.
| Training mode | Population | Training intervention | Result |
|---|---|---|---|
| Eccentric training | Prostate cancer patients undergoing ADT (n = 5) and no ADT (n = 5)46 | Eccentric leg press from 5 min and RPE light to 20 min RPE somewhat hard; 3 d/w for 12 weeks | 6MWT (meter): ADT gr ↑ (ES = 0.37 (95%CI: –1.10 to 1.84)), no ADT gr ↑ (ES = 0.22 (95%CI: –1.24 to 0.69)) TUG(s): ADT gr ↓ (ES = 0.39 (95%CI: –1.09 to 1.86)), no ADT gr ↓ (ES = 0.41 (95%CI: –1.07 to 1.89)) Maximal knee extension isometric peak force right (Nm): ADT gr ↑ (ES = 0.50 (95%CI: –1.99 to 0.98)), no ADT gr ↑ (ES = 0.74 (95%CI: –0.78 to 2.26)) Maximal knee extension isometric peak force left (Nm): ADT gr ↑ (ES = 0.34 (95%CI: –1.14 to 1.81)), no ADT gr ↓ (ES = –0.07 (95%CI: –1.53 to 1.39)) Quadriceps volume right (cm3): ADT gr ↑ (ES = 0.10 (95%CI: –1.36 to 1.56)), no ADT gr ↑ (ES = 0.26 (95%CI: –1.21 to 1.72)) Quadriceps volume left (cm3): ADT gr ↑ (ES = 0.04 (95%CI: –1.42 to 1.50)), no ADT gr ↑ (ES = 0.42 (95%CI: –1.06 to 1.90)) |
| Breast, prostate, colorectal, and lymphoma cancer survivors (n = 20)27 | Eccentric leg press from 5 min and RPE light to 20 min RPE somewhat hard; 3 d/w for 12 weeks | Maximal knee extension isometric peak force (N): ↑ (ES = 0.28 (95%CI: –0.92 to 0.37)) TUG (s): ↓ (ES = 0.47 (95%CI: –0.18 to 1.12)) |
|
| Breast, prostate, colorectal, lung, and lymphoma cancer survivors (n = 40)26 | Eccentric leg press from 5 min and RPE light to 20 min RPE somewhat hard vs. CON: Usual care; 3 d/w for 12 weeks | Quadriceps lean CSA (cm2): INT gr ↑ (ES = 0.16), CON gr ↓ (ES = 0.01) Maximal knee extension isometric peak force (N): INT gr ↑ (ES = 0.28), CON gr ↑ (ES = 0.04) Stair climbing (W): INT gr ↑ (ES = 0.71), CON gr ↑ (ES = 0.22) 6MWT (m): NT gr ↑ (ES = 0.39), CON gr ↑ (ES = 0.09) Stair descent (s): INT gr ↓ (ES = 0.40), CON gr ↓ (ES = 0.14) |
|
| Blood flow restriction training | Abdominal cancer patients before surgery (n = 24)28 | Nine RT exercises 20–30 repetitions × 3 sets with BFR plus 15 min walking with BFR; 6 d/w (RT and AT alternated) for 4 weeks | Fat mass (kg): ↓ (ES = 0.06 (95%CI: –0.52 to 0.65)) Lean mass (kg): ↑ (ES = 0.06 (95%CI: –0.52 to 0.65)) Appendicular lean mass (kg): ↑ (ES = 0.06 (95%CI: –0.52 to 0.64)) Trunk fat mass (kg): ↓ (ES = 0.05 (95%CI: –0.53 to 0.63)) Trunk lean mass (kg): ↑ (ES = 0.03 (95%CI: –0.54 to 0.61)) Hand grip strength (kg): ↑ (ES = 0.02 (95%CI: –0.57 to 0.60)) 5 times sit-to-stand (s): ↓ (ES = 0.56 (95%CI: –0.03 to 1.15)) |
Note: ↑ denotes increase; ↓ denotes decrease.
Abbreviations: 6MWT = 6-minute walk test; 95%CI = 95% confidence interval; ADT = androgen deprivation therapy; AT = aerobic training; BFR = blood flow restriction; CON = control; CSA = cross-sectional area; d/w = day per week; ES = effect size; gr = group; INT = intervention; N = Newton; Nm = Newton-meter; RPE = rate of perceived exertion; RT = resistance training; TUG = timed up-and-go test; W = watt.
3.1. ECC training
Among the alternative RT methods, ECC training has been the most used in exercise interventions with cancer populations, albeit that is only 3 trials. By definition, ECC muscle contractions involve the active lengthening of muscle against an external load.20 The unique trait of ECC training is the combination of high muscle force production with a relatively low energy cost.43 From a physiological perspective, compared to traditional RT, ECC training drives greater anabolic signaling, satellite cell activation, and motor unit recruitment which, in turn, contribute to developing greater levels of muscle mass.44 Consequently, this enables greater expression of force production, motor unit discharge rate, and muscle tendon unit stiffness, which collectively help to increase neuromuscular strength.45
Hansen et al.46 investigated the effects of ECC leg press in 10 prostate cancer patients (5 who were undergoing androgen deprivation therapy), 3 times per week for 12 weeks. There was improvement in 6MWT (ES: 0.22–0.37), TUG (ES: 0.39–0.41), maximal knee extension isometric peak force (ES: –0.07 to 0.74), and quadriceps volume (ES: 0.04–0.42). In addition, LaStayo et al.27 examined the same training in 20 breast, prostate, colorectal, and lymphoma cancer survivors with thrice weekly training sessions for 12 weeks. Measures of maximal knee extension isometric peak force (ES = 0.28) and TUG (ES = 0.47) significantly increased between time points. Another similar pilot study led by the same research group26 implemented the same exercise prescription (i.e., ECC leg press) 3 times a week over a 12-week period in 40 breast, prostate, colorectal, lung, and lymphoma cancer survivors compared to a usual care (control) group, which did not include any recommendation about exercising. Maximal knee extension isometric peak force (ES = 0.28), quadriceps cross-sectional area (ES = 0.16), 6MWT (ES = 0.39), stair descent (ES = 0.40), and stair climbing (ES = 0.71) favored the intervention compared to the control group from pre- to post-intervention.
Taken together, such findings are promising. Enhanced physical fitness, muscle strength, cross-sectional area, and physical function all positively impact QoL, the ability to cope with daily functional activities, and tolerance to cancer treatments (i.e., chemotherapy and radiation therapy).47, 48, 49, 50, 51 Thus, ECC training appears to elicit physical benefits in cancer patients and survivors. However, some limitations in the aforementioned studies are worth mentioning. First, the ECC leg press training protocol adopted an intensity ranging from light to somewhat hard (via the rating of perceived exertion scale) and a duration of up to 20 min using only this exercise; so, given the importance of variety in optimizing adaptations, this protocol may limit further possible positive adaptations from RT. Typically, 6–8 exercises involving major muscle groups and 8–12 repetitions at an intensity of 60%–80% 1RM are recommended,8 which are in contrast with current ECC intervention studies in cancer. Moreover, it is unclear whether the training selected was accentuated ECC only or also included the concentric phase. No studies reported the duration of the ECC phase, which is unusual given that one of the specific goals of ECC training is to slow down the ECC phase to provide enhanced strength and hypertrophy adaptations.52 Further, owing to the high heterogeneity in the different cancer populations examined and stages of related treatments, future investigations are required. Still, the researchers reported overall improvements in several outcomes, including muscle strength and a range of other physical functions.
To further support its implementation in cancer patients, ECC training has been previously investigated in other clinical populations. In a recent review, ECC training was reported to be superior for improvements in isometric knee strength, TUG, 2-min sit-to-stand test, and 30-s sit-to-stand test compared to traditional RT in older adults, while also being safe and feasible for frail and unwell people.19 Further, ECC training has also been used in the management of obesity, diabetes, cardiorespiratory, and chronic diseases (e.g., stroke, osteoarthritis, Parkinson's disease),53, 54, 55, 56 because it has the capability to produce lower metabolic cost and muscle activity for equal levels of exerted force with reduced demand on the cardiovascular system.21 Thus, ECC training may be highly indicated for cancer patients and survivors who are older, frail, and who have low physical function and cardiovascular impairments after cancer treatment.57 However, caution should be applied when administering ECC training in case of neuromuscular impairment (e.g., chemotherapy-induced peripheral neuropathy), as this may be a potential contraindication.58 Therefore, RT modalities such as ECC training, which improve muscular strength and hypertrophy without creating excessive cardiovascular stress (e.g., shortness of breath), may represent an important alternative training intervention for consideration.
Preliminary findings show that ECC training can be safely introduced in cancer populations to promote positive morphological and physiological changes.26,27,46 However, further research is necessary to clearly elucidate the effects of ECC training in different cancer types, treatments, and stages of disease. Among the several outcomes worth investigating, it may be assumed that muscle strength and hypertrophy could be favorably enhanced through ECC training, as has been shown in preclinical models.59, 60, 61, 62 Indeed, positive adaptations in skeletal muscles may occur owing to the greater anabolic signals driven by ECC training as compared to traditional RT which, in turn, stimulate satellite cell proliferation.44,63 This is of critical importance for cancer patients presenting with skeletal muscle mass loss (e.g., sarcopenia or cachexia).64,65
3.2. CS training
Another alternative RT method is CS training. Compared to traditional RT, CS uses short intra-set or inter-repetition rest periods.66, 67, 68 These rest periods can be used between a few repetitions or even between single repetitions, with durations ranging from 15–45 s.22 From a practical point of view, traditional RT is based on a given number of repetitions performed in a continuous manner, while CS incorporates a short rest period or periods throughout the set.69 CS has been demonstrated to be an efficacious tool for a wide range of healthy populations, regardless of gender, age, and training experience.70 Additionally, it is well-established that traditional RT causes greater mechanical fatigue and lactate concentrations compared to CS.71 Thus, one of the benefits of CS is to reduce fatigue and increase recovery compared to traditional RT protocols.72 This is likely to translate into a greater intensity which, in turn, may also promote greater strength and muscle size adaptations.66,73 Furthermore, it has also been postulated that training tolerance can be improved, with an increase in adherence.74,75
However, to date only a single study protocol has been published utilizing CS training in cancer patients.25 Implementation of CS training in oncological care requires further research, but the premise of potential clinical application in cancer populations is worth investigating. The underlying rationale is derived from recent studies conducted in older adults that show CS was not inferior but even advantageous for some outcomes. For example, compared to traditional RT in a range of physical function outcomes, including 10-m walking speed test (CS: +15.1%; RT: +6.6%; ES = 0.85), 8-foot up-and-go test (CS: +15.1%; RT: +8.9%; ES = 0.46), and sit-to-stand (CS: +19.9%; RT: +13.7%; ES = 0.21), all in favor of CS.76 Additionally, equal improvements were observed in traditional RT and CS training in muscle strength (RT: 34.3%–41.2%; CS: 30.7%–34.9%).77 CS training has also been used in cardiac rehabilitation because of its tendency to reduce the total load on the cardiovascular system compared to traditional RT, resulting in lower heart rate and systolic blood pressure during resistance exercise with intra-set rest periods.78 In addition, CS training appears to mitigate fatigue and perception of physical and mental effort and so is suggested for treatment of different vulnerable populations, such as in patients with Parkinson's disease, motor neuron disease, stroke, and chronic obstructive pulmonary disease.22
Although it is still a novelty in the clinical setting, the underlying mechanisms suggest that greater physical benefits can be achieved through CS training. Thus, application and evaluation may be of particular interest especially for cancer patients displaying fatigue (i.e., cancer-related fatigue (CRF), for example, during chemotherapy) or deconditioning (e.g., cancer patients or survivors with obesity).79, 80, 81 Our assumption is that CS training may help cancer patients to overcome physical and mental barriers detrimental to exercise adherence with the use of intra-set or inter-repetition rest periods.66,68,82 Moreover, CS may be especially useful in tailoring training intensity and total dosage to the patient's needs, which is in line with the current Exercise and Sports Science Australia (ESSA) guidelines.7 As shown for other clinical populations, a lower perception of fatigue as well as positive physical adaptations in muscle strength makes CS training a potentially attractive modality for future research in cancer settings (i.e., during and after cancer treatments). With this in mind, our suggestion is to implement research into CS training specifically for cancer patients undergoing treatments (e.g., chemotherapy), in particular for those struggling to exercise due to impaired cardiovascular function (e.g., lung cancer) or CRF.
3.3. BFR training
BFR is an additional alternative RT method consisting of partially restricting arterial inflow and fully restricting venous outflow in working musculature during exercise.83 Generally, external pressure is applied (e.g., tourniquet, pressurized cuff, elastic banding), leading to a gradual mechanical compression of the lower or upper limbs.84 The occlusion of venous outflow reduces blood flow overall, resulting in greater hypoxia within the muscles.85 Exercising with BFR produces a hypertrophic response at lower training intensities. For example, RT with BFR performed with load <50% 1RM provided substantial changes in muscle mass equal to traditional high-load RT (i.e., >65% 1RM) in a healthy population.23 Although there are possible contraindications to BFR, including unstable hypertension, peripheral vascular disease, venous thromboembolism, and cardiopulmonary conditions, it has been proven safe in clinical settings.84
Despite the beneficial effects on skeletal muscles, very few BFR studies have been conducted in patients with cancer. Wooten et al.28 investigated the effects of a 4-week BFR program in 24 patients with abdominal cancer waiting for surgery. The training protocol consisted of 3 sets of 20–30 repetitions, including both upper and lower body BFR resistance exercises and walking sessions wearing BFR bands, 6 days per week. Negligible changes were found in body composition (ES: 0.03–0.06) and hand grip strength (ES = 0.02), while 6MWT and TUG significantly (p < 0.05) improved by approximately 50 meters and 1 s, respectively (although no raw scores or ES data were reported). However, it should be noted that the absence of specific training load parameters (e.g., %RM or rating of perceived exertion) and the short intervention duration may limit the positive adaptations of BFR. In addition, a recently published study protocol adopting BFR in early-stage breast cancer patients has outlined a future investigation of potential BFR effects on QoL, physical function, and body composition.86
As is often the case, BFR has recently gained attention in clinical settings despite the paucity of studies in cancer patients.87,88 In a recent review, exercising with BFR as compared to traditional RT resulted in similar improvements in muscle strength and even greater increases in muscle size (ES: 0.11–3.6; measured using cross-sectional area, volume, mass, and thickness) and muscle strength (ES: 0.55–4.34) in older adults.89,90 Furthermore, it is worth mentioning the role of BFR in attenuating muscle atrophy and strength loss following immobilization. Indeed, passive mobilization using BFR led to 19% muscle mass loss compared to 25% in the limb that did not receive BFR.24 In addition, a significant attenuation in knee extensor–flexor strength loss in ECC (BFR: –4.7% to –0.6%; control (CON): –23.5% to –18.9%), concentric (BFR: –6.9% to –2.9%; CON: –22.1% to –18.6%), and isometric (BFR: –4.4% to –3.7%; CON: –22.1% to –20.9%) actions91,92 was observed when BFR was delivered to immobilized patients as compared to controls.
Once again, despite the limited empirical investigations involving BFR training, it should be considered a training method worthy of examination in future research. In fact, the potential to promote muscle growth and strength with body weight or low loads should not be underestimated in cancer settings, in particular for those who have been immobilized following surgery.93 It is well-established that deconditioned cancer patients performing traditional RT respond well to body weight exercises for increasing muscle strength and hypertrophy.94 In this regard, BFR may provide additional positive physical adaptations in the first phase of cancer treatment, gradually stimulating muscle hypertrophy via a synergistic response to metabolic stress and mechanical tension.95 Furthermore, BFR could be used as a strategy for hospitalized cancer patients who experience considerable decline in physical function (e.g., 6MWT, TUG) and muscle atrophy.96, 97, 98 Indeed, BFR training during hospitalization may attenuate decline in physical deconditioning.96 Finally, BFR can also be used in home-based exercise prescription in the absence of any contraindications.28 It is established that home-based exercise has the potential to overcome the barriers that limit access for patients with cancer to participate in programs under direct supervision.99 However, it is difficult to implement the same intensity with traditional RT in the home environment due to limitations of equipment and supervision possibly constraining physical adaptations.99 BFR may allow patients to train at sufficient intensity to induce significant muscle gains while exercising at home.93
Notably, BFR has been found to be safe in different clinical conditions (e.g., in subjects with obesity), meaning that its implementation can be tested in cancer settings.84,100 Indeed, evidence shows that RT with BFR did not elevate the risk of venous thromboembolism after 12 weeks in older adults,101,102 and did not increase blood coagulation factors in patients with ischemic heart disease.103 Additionally, the exercise-induced muscle damage in response to this alternative RT method has been investigated, showing that when performed with low loads (i.e., <50% 1RM) BFR did not induce substantial muscle damage.104, 105, 106, 107 However, considering the possible discomfort induced by BFR, training adherence should be monitored closely in cancer populations. Caution is necessary as each individual may respond differently, and special attention should be paid to fragile patients with cancer to avoid possible negative side effects (e.g., patients undergoing chemotherapy who may show altered endothelial and vascular functions).108 In line with that, we strongly recommend adapting the recommendations of ESSA to patients’ needs.7 Thus, although there are possible contraindications to the use of BFR, it has been proven safe in clinical settings, including cancer settings.28,84,109,110 Our assumptions are only speculative, with the benefits of BFR being more consistent in hospitalized and immobilized patient based on previous studies in older patients in intensive care.24,89,90 However, if such benefits can be observed in other populations (e.g., older adults), it may be assumed that patients with cancer could also benefit from this training modality before and after treatment. For these reasons, we recommend that future research examine BFR in the oncology setting.
4. Directions for future research
We have provided a rationale for alternative RT methods to be considered in potential exercise prescription for future research in people with cancer, although there is still a need to distinguish these potential alternative RT methods based on cancer type, stage, and demographic characteristics. Although very few studies on ECC, CS, and BFR currently exist in cancer populations, the plausible physiological benefits appear to be robust even though further research is necessary. This is supported by the findings of ongoing studies (i.e., protocol papers),25,86 which will be delivered in the future. Research has already been conducted in older adults and other clinical populations (e.g., subjects with obesity), further corroborating the possible use of alternative RT methods in cancer research. Thus, the following key points should be considered for future research.
-
(1)
ECC training may be an alternative RT method for fostering improvements in muscle strength and mass, thus triggering strong anabolic signaling for muscle growth.44,63 Researchers should investigate not only the benefits in terms of physical outcomes (i.e., muscle strength and hypertrophy) but also the physiological pathways involved. Such research will provide insightful information about the underlying mechanisms that promote muscle growth during cancer treatments and when patients are in remission. CS training is another RT modality that may be examined for patients who are suffering from CRF (e.g., during chemotherapy) or who are deconditioned (e.g., cancer patients with obesity or cardiovascular impairment) because it has the benefit of minimizing fatigue, which represents one of the most reported barriers to exercise and has a major impact on QoL.111 Practically, researchers should determine whether CS training is more tolerable in patients with CRF or deconditioning and, consequently, whether it improves exercise adherence and compliance. Patients with advanced disease and extensive treatment history who are highly deconditioned (e.g., those with lung cancer, high grade glioma, pancreatic cancer) require more sophisticated RT investigations to improve exercise therapy outcomes. BFR interventions should be scrutinized in relation to positive muscle adaptations with lower intensities (i.e., from body weight to <50% 1RM). Since BFR may boost physical adaptations compared to traditional RT, it is an attractive modality for future studies in patients with severe muscle loss, where only body weight exercises are possible, or for in-hospital patients with cancer (i.e., following surgery), which to our knowledge is an area that has received little investigation to date.
-
(2)
Where possible, we also suggest that longer-term intervention studies are conducted, possibly up to 6–12 months. Although it is well-established that adaptations occur in 8–12 weeks in healthy subjects,112 it is also widely accepted that training is a process, where consistency helps to drive greater positive adaptations over time.113 In some patients with cancer (e.g., patients with lung cancer), lower dosages of exercise are required because they are unable to tolerate and adapt to the generic recommendations, but this then requires longer programs to achieve meaningful benefits. Related to this, future study designs should carefully consider their fundamental aspects, such as exercise selection, intensity, volume, and frequency. To clearly elucidate the benefits of alternative RT methods, we suggest that traditional RT should be used as a control condition with total volume (i.e., set × repetition × intensity) remaining equal between training methods so that inferences can be made about what adaptations have occurred.
Another important direction for future research in exercise oncology is to determine how these alternative RT methods influence morbidity and mortality. Similarly, future research should aim to understand how responses differ based on disease stage (e.g., localized vs. advanced diseases).
5. Practical applications
New and upcoming studies should explore the effects of ECC, CS, and BFR training modalities in cancer patients. In Table 2 we propose alternative RT modalities with high potential for future investigations, as well as possible outcomes of interest. Importantly, our assumptions are in line with current ESSA recommendations that intensity, frequency, duration, dosage, progression, periodization, and autoregulation should be carefully tailored to fit patient needs, stage of disease, and on-going treatment.7
Table 2.
An overview of methods to practically apply different RT methods in patients with cancer, including proposed benefits.
| Training mode | Repetition | Set | Load/intensity | RPE | Frequency(d/w) | Possible outcome of interest | Target population | Benefits |
|---|---|---|---|---|---|---|---|---|
| Eccentric training | 8–12 | 1–4 | 60%–80% 1RM; 3 s ecc and 1 s conc | 6–8 | 3–5 | Muscle strength, muscle mass, body composition | Cancer patient undergoing treatments | Attenuate muscle loss |
| Accentuated eccentric training | 6–8 | >80% 1RM; 3 s ecc and 1 s conc | 8–10 | Cancer survivors accustomed to RT | ↑ Muscle strength and hypertrophy | |||
| Cluster sets training | 4 + 4 + 4; 30 s intra-set rest | 1–3 | 60%–80% 1RM | 6–8 | 2–3 | Muscle strength, QoL | Cancer patients suffering from CRF | ↓ Fatigue |
| 2 + 2 + 2; 15 s intra-set rest | 60%–80% 1RM | 6–8 | Cancer patients deconditioned | ↓ Fatigue | ||||
| Blood flow restriction training | 10–15 | 1–3 | BW to 50% 1RM | 5–8 | 3–5 | Muscle strength, muscle mass, body composition | Cancer patients with moderate-to-severe muscle loss | ↑ Muscle strength and mass |
| 20–25 | BW to 30% 1RM | 3–6 | Inpatient cancer | Attenuate muscle loss and atrophy |
Notes: Suggested intensity and volume need to be prescribed according to subjects’ physical capacities, health status, type and stage of cancer. ↑ denotes increase; ↓ denotes decrease.
Abbreviations: BW = body weight; conc = concentric phase; CRF = cancer-related fatigue; d/w = day per week; ecc = eccentric phase; QoL = quality of life; RM = repetition maximum; RPE = rate of perceived exertion; RT = resistance training.
Based on the limited evidence thus far, ECC training can be delivered on resistance machines to enhance safety for cancer patients, especially those who have limited RT experience. For example, if the primary aim was to attenuate muscle loss, a protocol of 6–12 repetitions at an intensity of 60%–80% 1RM with approximately 3 s under tension during the ECC phase20 could be explored. Patients who are unaccustomed to this type of training may experience some muscle soreness, so it should be progressed gradually by limiting training volume initially and continually monitoring it from session to session. In contrast, patients accustomed to RT may benefit from accentuated ECC training,114 which safely allows loads >80% 1RM, for which enhanced muscle strength and hypertrophy are the primary desired outcomes.
CS training may be indicated for patients with CRF or those who have difficulties with traditional RT prescription. We have proposed 2 examples with different rest period schemes (i.e., 15 s and 30 s) in order to attenuate fatigue during a traditional RT protocol based on 6–12 repetitions. Intra-set rest periods can be tailored to each patient's capacity. Thus, if the primary goal is to attenuate fatigue while maximizing muscle strength, then CS training may be a suitable strategy for patients with cancer.
In addition, BFR may be an option for those patients who show severe muscle loss, and potentially for those who are hospitalized. As BFR intensity is generally lower than that of traditional RT (intensity could be up to 50% 1RM and >10 repetitions, which may be suitable to attenuate muscle loss and atrophy). This form of training may require 3–5 sessions per week to promote substantial anabolic signaling. In practice, this training mode may require time for patients to learn proper technique, expertise from professionals in its administration. Finally, BFR is limited to upper (upper arm and forearm) and lower limbs (thigh and lower leg).
Lastly, regardless of the alternative RT modalities implemented, exercise selection, intensity, volume, and frequency must be tailored to each patient's physical capacities, health status, type, and stage of cancer as well as to the setting and level of supervision.
6. Conclusion
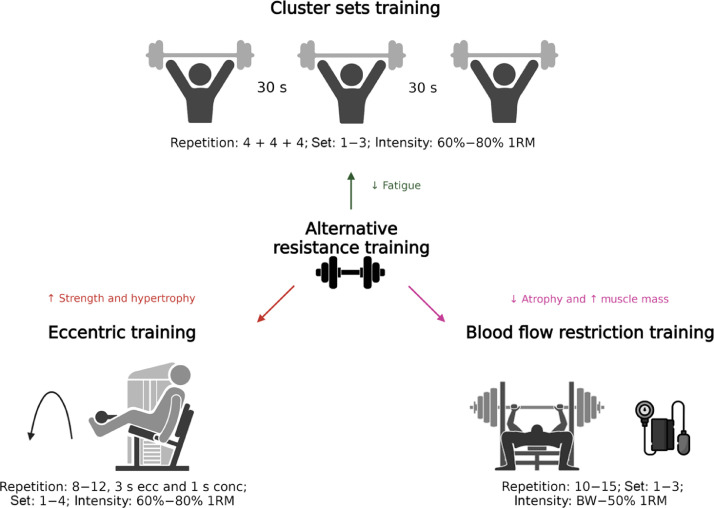
Although exercise oncology has dramatically advanced over the past 2 decades, the full therapeutic potential of exercise in patients with cancer may be masked by the current use of generic prescriptions.115 Given their potentially equal or greater effects on muscle strength, hypertrophy, physical function, and body composition compared to traditional RT, alternative RT methods should be assessed and implemented for future research (Fig. 1). Further, the peculiarities of each modality could be of interest for specific cancer populations, stages of disease, or cancer and treatment-related comorbidities. Here we have summarized the benefits driven by these RT methods, the underlying mechanisms, directions for future research, and practical applications in the cancer setting.
Fig. 1.
Potential proposed benefits of alternative RT methods. ↑ denotes increase; ↓ denotes decrease. BW = body weight; conc = concentric phase; ecc = eccentric phase; RM = repetition maximum; RT = resistance training.
Authors’ contributions
FB drafted the manuscript; CB, DRT, DAG, LM, and RUN edited and revised the manuscript. All authors have read and approved the final version of the manuscript, and agree with the presentation of order of the authors.
Data availability statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
Supplementary materials
References
- 1.World Health Organization . World Health Organization; Geneva: 2014. Global status report on noncommunicable diseases 2014. [Google Scholar]
- 2.Lobelo F, Stoutenberg M, Hutber A. The exercise is medicine global health initiative: A 2014 update. Br J Sports Med. 2014;48:1627–1633. doi: 10.1136/bjsports-2013-093080. [DOI] [PubMed] [Google Scholar]
- 3.6th ed. Lippincott Williams & Wilkins; Philadelphia, PA: 2000. American College of Sports Medicine. ACSM's guidelines for exercise testing and prescription. [Google Scholar]
- 4.Momma H, Kawakami R, Honda T, Sawada SS. Muscle-strengthening activities are associated with lower risk and mortality in major non-communicable diseases: A systematic review and meta-analysis of cohort studies. Br J Sports Med. 2022;56:755–763. doi: 10.1136/bjsports-2021-105061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 6.Ligibel JA, Bohlke K, May AM, et al. Exercise, diet, and weight management during cancer treatment: ASCO guideline. J Clin Oncol. 2022;40:2491–2507. doi: 10.1200/JCO.22.00687. [DOI] [PubMed] [Google Scholar]
- 7.Hayes SC, Newton RU, Spence RR, Galvão DA. The Exercise and Sports Science Australia position statement: Exercise medicine in cancer management. J Sci Med Sport. 2019;22:1175–1199. doi: 10.1016/j.jsams.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Campbell KL, Winters-Stone KM, Wiskemann J, et al. Exercise guidelines for cancer survivors: Consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc. 2019;51:2375–2390. doi: 10.1249/MSS.0000000000002116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmitz KH, Campbell AM, Stuiver MM, et al. Exercise is medicine in oncology: Engaging clinicians to help patients move through cancer. CA Cancer J Clin. 2019;69:468–484. doi: 10.3322/caac.21579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedenreich CM, Neilson HK, Farris MS, Courneya KS. Physical activity and cancer outcomes: A precision medicine approach. Clin Cancer Res. 2016;22:4766–4775. doi: 10.1158/1078-0432.CCR-16-0067. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt K, Vogt L, Thiel C, Jäger E, Banzer W. Validity of the six-minute walk test in cancer patients. Int J Sports Med. 2013;34:631–636. doi: 10.1055/s-0032-1323746. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez-Rodriguez EJ, Sanchez-Gomez C, Mendez-Sanchez R, et al. Multimodal physical exercise and functional rehabilitation program in oncological patients with cancer-related fatigue—A randomized clinical trial. Int J Environ Res Public Health. 2023;20:4938. doi: 10.3390/ijerph20064938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia-Unciti M, Palacios Samper N, Méndez-Sandoval S, Idoate F, Ibáñez-Santos J. Effect of combining impact-aerobic and strength exercise, and dietary habits on body composition in breast cancer survivors treated with aromatase inhibitors. Int J Environ Res Public Health. 2023;20:4872. doi: 10.3390/ijerph20064872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newton RU, Galvão DA, Spry N, et al. Exercise mode specificity for preserving spine and hip bone mineral density in prostate cancer patients. Med Sci Sports Exerc. 2019;51:607–614. doi: 10.1249/MSS.0000000000001831. [DOI] [PubMed] [Google Scholar]
- 15.Kang DW, Fairey AS, Boulé NG, Field CJ, Wharton SA, Courneya KS. A randomized trial of the effects of exercise on anxiety, fear of cancer progression and quality of life in prostate cancer patients on active surveillance. J Urol. 2022;207:814–822. doi: 10.1097/JU.0000000000002334. [DOI] [PubMed] [Google Scholar]
- 16.Bland KA, Zadravec K, Landry T, Weller S, Meyers L, Campbell KL. Impact of exercise on chemotherapy completion rate: A systematic review of the evidence and recommendations for future exercise oncology research. Crit Rev Oncol Hematol. 2019;136:79–85. doi: 10.1016/j.critrevonc.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Kraemer WJ, Adams K, Cafarelli E, et al. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2002;34:364–380. doi: 10.1097/00005768-200202000-00027. [DOI] [PubMed] [Google Scholar]
- 18.Garber CE, Blissmer B, Deschenes MR, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43:1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 19.Čretnik K, Pleša J, Kozinc Ž, Löfler S, Šarabon N. The effect of eccentric vs. traditional resistance exercise on muscle strength, body composition, and functional performance in older adults: A systematic review with meta-analysis. Front Sports Act Living. 2022;4:873718. doi: 10.3389/fspor.2022.873718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suchomel T, Wagle J, Douglas J, et al. Implementing eccentric resistance training—Part 1: A brief review of existing methods. J Funct Morphol Kinesiol. 2019;4:38. doi: 10.3390/jfmk4020038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoppeler H. Moderate load eccentric exercise: A distinct novel training modality. Front Physiol. 2016;7:483. doi: 10.3389/fphys.2016.00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Latella C, Peddle-McIntyre C, Marcotte L, Steele J, Kendall K, Fairman CM. Strengthening the case for cluster set resistance training in aged and clinical settings: Emerging evidence, proposed benefits and suggestions. Sports Med. 2021;51:1335–1351. doi: 10.1007/s40279-021-01455-4. [DOI] [PubMed] [Google Scholar]
- 23.Krzysztofik M, Wilk M, Wojdała G, Gołaś A. Maximizing muscle hypertrophy: A systematic review of advanced resistance training techniques and methods. Int J Environ Res Public Health. 2019;16:4897. doi: 10.3390/ijerph16244897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barbalho M, Rocha AC, Seus TL, Raiol R, Del Vecchio FB, Coswig VS. Addition of blood flow restriction to passive mobilization reduces the rate of muscle wasting in elderly patients in the intensive care unit: A within-patient randomized trial. Clin Rehabil. 2019;33:233–240. doi: 10.1177/0269215518801440. [DOI] [PubMed] [Google Scholar]
- 25.Fairman CM, Owens OL, Kendall KL, et al. Study protocol: Investigating the feasibility of a hybrid delivery of home-based cluster set resistance training for individuals previously treated for lung cancer. Pilot Feasibility Stud. 2022;8:102. doi: 10.1186/s40814-022-01065-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.LaStayo PC, Marcus RL, Dibble LE, Smith SB, Beck SL. Eccentric exercise versus usual-care with older cancer survivors: The impact on muscle and mobility–An exploratory pilot study. BMC Geriatr. 2011;11:5. doi: 10.1186/1471-2318-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LaStayo PC, Larsen S, Smith S, Dibble L, Marcus R. The feasibility and efficacy of eccentric exercise with older cancer survivors: A preliminary study. J Geriatr Phys Ther. 2010;33:135–140. [PMC free article] [PubMed] [Google Scholar]
- 28.Wooten SV, Fleming RYD, Wolf JS, et al. Prehabilitation program composed of blood flow restriction training and sports nutrition improves physical functions in abdominal cancer patients awaiting surgery. Eur J Surg Oncol. 2021;47:2952–2958. doi: 10.1016/j.ejso.2021.05.038. [DOI] [PubMed] [Google Scholar]
- 29.Bigaran A, Howden EJ, Foulkes S, et al. Prescribing exercise in early-stage breast cancer during chemotherapy: A simple periodized approach to align with the cyclic phases of chemotherapy. J Strength Cond Res. 2022;36:2934–2941. doi: 10.1519/JSC.0000000000003990. [DOI] [PubMed] [Google Scholar]
- 30.Winters-Stone KM, Leo MC, Schwartz A. Exercise effects on hip bone mineral density in older, post-menopausal breast cancer survivors are age dependent. Arch Osteoporos. 2012;7:301–306. doi: 10.1007/s11657-012-0071-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klijn P, van Keimpema A, Legemaat M, Gosselink R, van Stel H. Nonlinear exercise training in advanced chronic obstructive pulmonary disease is superior to traditional exercise training. A randomized trial. Am J Respir Crit Care Med. 2013;188:193–200. doi: 10.1164/rccm.201210-1829OC. [DOI] [PubMed] [Google Scholar]
- 32.Nikseresht M, Agha-Alinejad H, Azarbayjani MA, Ebrahim K. Effects of nonlinear resistance and aerobic interval training on cytokines and insulin resistance in sedentary men who are obese. J Strength Cond Res. 2014;28:2560–2568. doi: 10.1519/JSC.0000000000000441. [DOI] [PubMed] [Google Scholar]
- 33.Hinton PS, Nigh P, Thyfault J. Effectiveness of resistance training or jumping-exercise to increase bone mineral density in men with low bone mass: A 12-month randomized, clinical trial. Bone. 2015;79:203–212. doi: 10.1016/j.bone.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Westcott WL. Resistance training is medicine: Effects of strength training on health. Curr Sports Med Rep. 2012;11:209–216. doi: 10.1249/JSR.0b013e31825dabb8. [DOI] [PubMed] [Google Scholar]
- 35.Strasser B, Schobersberger W. Evidence for resistance training as a treatment therapy in obesity. J Obes. 2011;2011:482564. doi: 10.1155/2011/482564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shailendra P, Baldock KL, Li LSK, Bennie JA, Boyle T. Resistance training and mortality risk: A systematic review and meta-analysis. Am J Prev Med. 2022;63:277–285. doi: 10.1016/j.amepre.2022.03.020. [DOI] [PubMed] [Google Scholar]
- 37.McGovern A, Mahony N, Mockler D, Fleming N. Efficacy of resistance training during adjuvant chemotherapy and radiation therapy in cancer care: A systematic review and meta-analysis. Support Care Cancer. 2022;30:3701–3719. doi: 10.1007/s00520-021-06708-6. [DOI] [PubMed] [Google Scholar]
- 38.Padilha CS, Marinello PC, Galvão DA, et al. Evaluation of resistance training to improve muscular strength and body composition in cancer patients undergoing neoadjuvant and adjuvant therapy: A meta-analysis. J Cancer Surviv. 2017;11:339–349. doi: 10.1007/s11764-016-0592-x. [DOI] [PubMed] [Google Scholar]
- 39.Strasser B, Steindorf K, Wiskemann J, Ulrich CM. Impact of resistance training in cancer survivors: A meta-analysis. Med Sci Sports Exerc. 2013;45:2080–2090. doi: 10.1249/MSS.0b013e31829a3b63. [DOI] [PubMed] [Google Scholar]
- 40.Lønbro S. The effect of progressive resistance training on lean body mass in post-treatment cancer patients – A systematic review. Radiother Oncol. 2014;110:71–80. doi: 10.1016/j.radonc.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 41.Hanson ED, Wagoner CW, Anderson T, Battaglini CL. The independent effects of strength training in cancer survivors: A systematic review. Curr Oncol Rep. 2016;18:31. doi: 10.1007/s11912-016-0511-3. [DOI] [PubMed] [Google Scholar]
- 42.Lee J. The effects of resistance training on muscular strength and hypertrophy in elderly cancer patients: A systematic review and meta-analysis. J Sport Health Sci. 2022;11:194–201. doi: 10.1016/j.jshs.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Isner-Horobeti ME, Dufour SP, Vautravers P, Geny B, Coudeyre E, Richard R. Eccentric exercise training: Modalities, applications and perspectives. Sports Med. 2013;43:483–512. doi: 10.1007/s40279-013-0052-y. [DOI] [PubMed] [Google Scholar]
- 44.Hyldahl RD, Olson T, Welling T, Groscost L, Parcell AC. Satellite cell activity is differentially affected by contraction mode in human muscle following a work-matched bout of exercise. Front Physiol. 2014;5:485. doi: 10.3389/fphys.2014.00485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.MO Harris-Love, Gollie JM, Keogh JWL. Eccentric exercise: Adaptations and applications for health and performance. J Funct Morphol Kinesiol. 2021;6:96. doi: 10.3390/jfmk6040096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hansen PA, Dechet CB, Porucznik CA, LaStayo PC. Comparing eccentric resistance exercise in prostate cancer survivors on and off hormone therapy: A pilot study. PM R. 2009;1:1019–1024. doi: 10.1016/j.pmrj.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 47.Yoo JS, Yang HC, Lee JM, Kim MS, Park EC, Chung SH. The association of physical function and quality of life on physical activity for non-small cell lung cancer survivors. Support Care Cancer. 2020;28:4847–4856. doi: 10.1007/s00520-020-05302-6. [DOI] [PubMed] [Google Scholar]
- 48.Shin WK, Song S, Jung SY, et al. The association between physical activity and health-related quality of life among breast cancer survivors. Health Qual Life Outcomes. 2017;15:132. doi: 10.1186/s12955-017-0706-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buffart LM, Kalter J, Sweegers MG, et al. Effects and moderators of exercise on quality of life and physical function in patients with cancer: An individual patient data meta-analysis of 34 RCTs. Cancer Treat Rev. 2017;52:91–104. doi: 10.1016/j.ctrv.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 50.Groen WG, Naaktgeboren WR, van Harten WH, et al. Physical fitness and chemotherapy tolerance in patients with early-stage breast cancer. Med Sci Sports Exerc. 2022;54:537–542. doi: 10.1249/MSS.0000000000002828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Piraux E, Caty G, Aboubakar Nana F, Reychler G. Effects of exercise therapy in cancer patients undergoing radiotherapy treatment: A narrative review. SAGE Open Med. 2020;8 doi: 10.1177/2050312120922657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Azevedo PHSM, Oliveira MGD, Schoenfeld BJ. Effect of different eccentric tempos on hypertrophy and strength of the lower limbs. Biol Sport. 2022;39:443–449. doi: 10.5114/biolsport.2022.105335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Julian V, Thivel D, Miguet M, et al. Eccentric cycling training improves health-related quality of life in adolescents with obesity. Obes Facts. 2020;13:548–559. doi: 10.1159/000509961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kudiarasu C, Rohadhia W, Katsura Y, Koeda T, Singh F, Nosaka K. Eccentric-only versus concentric-only resistance training effects on biochemical and physiological parameters in patients with type 2 diabetes. BMC Sports Sci Med Rehabil. 2021;13:162. doi: 10.1186/s13102-021-00384-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roig M, Shadgan B, Reid WD. Eccentric exercise in patients with chronic health conditions: A systematic review. Physiother Can. 2008;60:146–160. doi: 10.3138/physio.60.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ellis R, Shields N, Lim K, Dodd KJ. Eccentric exercise in adults with cardiorespiratory disease: A systematic review. Clin Rehabil. 2015;29:1178–1197. doi: 10.1177/0269215515574783. [DOI] [PubMed] [Google Scholar]
- 57.Ruddy KJ, Patel SR, Higgins AS, Armenian SH, Herrmann J. Cardiovascular health during and after cancer therapy. Cancers (Basel) 2020;12:3737. doi: 10.3390/cancers12123737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Winters-Stone KM, Horak F, Jacobs PG, et al. Falls, functioning, and disability among women with persistent symptoms of chemotherapy-induced peripheral neuropathy. J Clin Oncol. 2017;35:2604–2612. doi: 10.1200/JCO.2016.71.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kon M, Tanabe K, Lee H, Kimura F, Akimoto T, Kono I. Eccentric muscle contractions induce greater oxidative stress than concentric contractions in skeletal muscle. Appl Physiol Nutr Metab. 2007;32:273–281. doi: 10.1139/H06-115. [DOI] [PubMed] [Google Scholar]
- 60.Tatebayashi D, Himori K, Yamada R, Ashida Y, Miyazaki M, Yamada T. High-intensity eccentric training ameliorates muscle wasting in colon 26 tumor-bearing mice. PLoS One. 2018;13:e0199050. doi: 10.1371/journal.pone.0199050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hardee JP, Mangum JE, Gao S, et al. Eccentric contraction-induced myofiber growth in tumor-bearing mice. J Appl Physiol (1985) 2016;120:29–37. doi: 10.1152/japplphysiol.00416.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hardee JP, Fix DK, Koh HJ, Wang X, Goldsmith EC, Carson JA. Repeated eccentric contractions positively regulate muscle oxidative metabolism and protein synthesis during cancer cachexia in mice. J Appl Physiol (1985) 2020;128:1666–1676. doi: 10.1152/japplphysiol.00908.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gonzalez AM, Hoffman JR, Stout JR, Fukuda DH, Willoughby DS. Intramuscular anabolic signaling and endocrine response following resistance exercise: Implications for muscle hypertrophy. Sports Med. 2016;46:671–685. doi: 10.1007/s40279-015-0450-4. [DOI] [PubMed] [Google Scholar]
- 64.Anjanappa M, Corden M, Green A, et al. Sarcopenia in cancer: Risking more than muscle loss. Tech Innov Patient Support Radiat Oncol. 2020;16:50–57. doi: 10.1016/j.tipsro.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rolland Y, Abellan van Kan G, Gillette-Guyonnet S, Vellas B. Cachexia versus sarcopenia. Curr Opin Clin Nutr Metab Care. 2011;14:15–21. doi: 10.1097/MCO.0b013e328340c2c2. [DOI] [PubMed] [Google Scholar]
- 66.Haff GG, Burgess SJ, Stone MH. Cluster training: Theoretical and practical applications for the strength and conditioning professional. Prof Strength Cond. 2008;12:12–17. [Google Scholar]
- 67.Lawton TW, Cronin JB, Lindsell RP. Effect of interrepetition rest intervals on weight training repetition power output. J Strength Cond Res. 2006;20:172–176. doi: 10.1519/R-13893.1. [DOI] [PubMed] [Google Scholar]
- 68.Gieβsing J, Fisher J, Steele J, Rothe F, Raubold K, Eichmann B. The effects of low-volume resistance training with and without advanced techniques in trained subjects. J Sports Med Phys Fitness. 2016;56:249–258. [PubMed] [Google Scholar]
- 69.Moir GL, Graham BW, Davis SE, Guers JJ, Witmer CA. Effect of cluster set configurations on mechanical variables during the deadlift exercise. J Hum Kinet. 2013;39:15–23. doi: 10.2478/hukin-2013-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Latella C, Teo WP, Drinkwater EJ, Kendall K, Haff GG. The acute neuromuscular responses to cluster set resistance training: A systematic review and meta-analysis. Sports Med. 2019;49:1861–1877. doi: 10.1007/s40279-019-01172-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pareja-Blanco F, Rodríguez-Rosell D, Aagaard P, et al. Time course of recovery from resistance exercise with different set configurations. J Strength Cond Res. 2020;34:2867–2876. doi: 10.1519/JSC.0000000000002756. [DOI] [PubMed] [Google Scholar]
- 72.Morán-Navarro R, Pérez CE, Mora-Rodríguez R, et al. Time course of recovery following resistance training leading or not to failure. Eur J Appl Physiol. 2017;117:2387–2399. doi: 10.1007/s00421-017-3725-7. [DOI] [PubMed] [Google Scholar]
- 73.Tufano JJ, Brown LE, Haff GG. Theoretical and practical aspects of different cluster set structures: A systematic review. J Strength Cond Res. 2017;31:848–867. doi: 10.1519/JSC.0000000000001581. [DOI] [PubMed] [Google Scholar]
- 74.Collado-Mateo D, Lavín-Pérez AM, Peñacoba C, et al. Key factors associated with adherence to physical exercise in patients with chronic diseases and older adults: An umbrella review. Int J Environ Res Public Health. 2021;18:2023. doi: 10.3390/ijerph18042023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Halperin I, Emanuel A. Rating of perceived effort: Methodological concerns and future directions. Sports Med. 2020;50:679–687. doi: 10.1007/s40279-019-01229-z. [DOI] [PubMed] [Google Scholar]
- 76.Ramirez-Campillo R, Alvarez C, Garcìa-Hermoso A, et al. High-speed resistance training in elderly women: Effects of cluster training sets on functional performance and quality of life. Exp Gerontol. 2018;110:216–222. doi: 10.1016/j.exger.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 77.Dias RKN, Penna EM, Noronha ASN, et al. Cluster-sets resistance training induce similar functional and strength improvements than the traditional method in postmenopausal and elderly women. Exp Gerontol. 2020;138:111011. doi: 10.1016/j.exger.2020.111011. [DOI] [PubMed] [Google Scholar]
- 78.Ribeiro-Torres O, de Sousa AFM, Iglesias-Soler E, et al. Lower cardiovascular stress during resistance training performed with inter-repetition rests in elderly coronary patients. Medicina (Kaunas) 2020;56:264. doi: 10.3390/medicina56060264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Savina S, Zaydiner B. Cancer-related fatigue: Some clinical aspects. Asia Pac J Oncol Nurs. 2019;6:7–9. doi: 10.4103/apjon.apjon_45_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wagner LI, Cella D. Fatigue and cancer: Causes, prevalence and treatment approaches. Br J Cancer. 2004;91:822–828. doi: 10.1038/sj.bjc.6602012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mitchell SA. Cancer-related fatigue: State of the science. PM R. 2010;2:364–383. doi: 10.1016/j.pmrj.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 82.Baillot A, Chenail S, Barros Polita N, et al. Physical activity motives, barriers, and preferences in people with obesity: A systematic review. PLoS One. 2021;16 doi: 10.1371/journal.pone.0253114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Scott BR, Loenneke JP, Slattery KM, Dascombe BJ. Exercise with blood flow restriction: An updated evidence-based approach for enhanced muscular development. Sports Med. 2015;45:313–325. doi: 10.1007/s40279-014-0288-1. [DOI] [PubMed] [Google Scholar]
- 84.Patterson SD, Hughes L, Warmington S, et al. Blood flow restriction exercise: Considerations of methodology, application, and safety. Front Physiol. 2019;10:533. doi: 10.3389/fphys.2019.00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Larkin KA, Macneil RG, Dirain M, Sandesara B, Manini TM, Buford TW. Blood flow restriction enhances post-resistance exercise angiogenic gene expression. Med Sci Sports Exerc. 2012;44:2077–2083. doi: 10.1249/MSS.0b013e3182625928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lopez-Garzon M, Cantarero-Villanueva I, Legerén-Alvarez M, et al. Prevention of chemotherapy-induced peripheral neuropathy with PRESIONA, a therapeutic exercise and blood flow restriction program: A randomized controlled study protocol. Phys Ther. 2022;102:pzab282. doi: 10.1093/ptj/pzab282. [DOI] [PubMed] [Google Scholar]
- 87.Ferraz RB, Gualano B, Rodrigues R, et al. Benefits of resistance training with blood flow restriction in knee osteoarthritis. Med Sci Sports Exerc. 2018;50:897–905. doi: 10.1249/MSS.0000000000001530. [DOI] [PubMed] [Google Scholar]
- 88.DePhillipo NN, Kennedy MI, Aman ZS, Bernhardson AS, O'Brien LT, LaPrade RF. The role of blood flow restriction therapy following knee surgery: Expert opinion. Arthroscopy. 2018;34:2506–2510. doi: 10.1016/j.arthro.2018.05.038. [DOI] [PubMed] [Google Scholar]
- 89.Baker BS, Stannard MS, Duren DL, Cook JL, Stannard JP. Does blood flow restriction therapy in patients older than age 50 result in muscle hypertrophy, increased strength, or greater physical function? A systematic review. Clin Orthop Relat Res. 2020;478:593–606. doi: 10.1097/CORR.0000000000001090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vechin FC, Libardi CA, Conceição MS, et al. Low-intensity resistance training with partial blood flow restriction and high-intensity resistance training induce similar changes in skeletal muscle transcriptome in elderly humans. Appl Physiol Nutr Metab. 2019;44:216–220. doi: 10.1139/apnm-2018-0146. [DOI] [PubMed] [Google Scholar]
- 91.Kubota A, Sakuraba K, Koh S, Ogura Y, Tamura Y. Blood flow restriction by low compressive force prevents disuse muscular weakness. J Sci Med Sport. 2011;14:95–99. doi: 10.1016/j.jsams.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 92.Kubota A, Sakuraba K, Sawaki K, Sumide T, Tamura Y. Prevention of disuse muscular weakness by restriction of blood flow. Med Sci Sports Exerc. 2008;40:529–534. doi: 10.1249/MSS.0b013e31815ddac6. [DOI] [PubMed] [Google Scholar]
- 93.Pearson SJ, Hussain SR. A review on the mechanisms of blood-flow restriction resistance training-induced muscle hypertrophy. Sports Med. 2015;45:187–200. doi: 10.1007/s40279-014-0264-9. [DOI] [PubMed] [Google Scholar]
- 94.Lopez P, Newton RU, Taaffe DR, Winster-Stone K, Galvão DA, Buffart LM. Moderators of resistance-based exercise programs' effect on sarcopenia-related measures in men with prostate cancer previously or currently undergoing androgen deprivation therapy: An individual patient data meta-analysis. J Geriatr Oncol. 2023;14:101535. doi: 10.1016/j.jgo.2023.101535. [DOI] [PubMed] [Google Scholar]
- 95.Cognetti DJ, Sheean AJ, Owens JG. Blood flow restriction therapy and its use for rehabilitation and return to sport: Physiology, application, and guidelines for implementation. Arthrosc Sports Med Rehabil. 2022;4:e71–e76. doi: 10.1016/j.asmr.2021.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fu JB, Raj VS, Guo Y. A guide to inpatient cancer rehabilitation: Focusing on patient selection and evidence-based outcomes. PM R. 2017;9(Suppl.2):S324–S334. doi: 10.1016/j.pmrj.2017.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fu JB, Lee J, Tran KB, et al. Symptom burden and functional gains in a cancer rehabilitation unit. Int J Ther Rehabil. 2015;22:517–523. doi: 10.12968/ijtr.2015.22.11.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kroenke K, Johns SA, Theobald D, Wu J, Tu W. Somatic symptoms in cancer patients trajectory over 12 months and impact on functional status and disability. Support Care Cancer. 2013;21:765–773. doi: 10.1007/s00520-012-1578-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Batalik L, Winnige P, Dosbaba F, Vlazna D, Janikova A. Home-based aerobic and resistance exercise interventions in cancer patients and survivors: A systematic review. Cancers (Basel) 2021;13:1915. doi: 10.3390/cancers13081915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sun L. Effects of blood flow restriction training on anthropometric and blood lipids in overweight/obese adults: Meta-analysis. Front Physiol. 2022;13:1039591. doi: 10.3389/fphys.2022.1039591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yasuda T, Fukumura K, Uchida Y, et al. Effects of low-load, elastic band resistance training combined with blood flow restriction on muscle size and arterial stiffness in older adults. J Gerontol A Biol Sci Med Sci. 2015;70:950–958. doi: 10.1093/gerona/glu084. [DOI] [PubMed] [Google Scholar]
- 102.Yasuda T, Fukumura K, Iida H, Nakajima T. Effects of detraining after blood flow-restricted low-load elastic band training on muscle size and arterial stiffness in older women. Springerplus. 2015;4:348. doi: 10.1186/s40064-015-1132-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Madarame H, Kurano M, Fukumura K, Fukuda T, Nakajima T. Haemostatic and inflammatory responses to blood flow-restricted exercise in patients with ischaemic heart disease: A pilot study. Clin Physiol Funct Imaging. 2013;33:11–17. doi: 10.1111/j.1475-097X.2012.01158.x. [DOI] [PubMed] [Google Scholar]
- 104.Wernbom M, Paulsen G, Nilsen TS, Hisdal J, Raastad T. Contractile function and sarcolemmal permeability after acute low-load resistance exercise with blood flow restriction. Eur J Appl Physiol. 2012;112:2051–2063. doi: 10.1007/s00421-011-2172-0. [DOI] [PubMed] [Google Scholar]
- 105.Umbel JD, Hoffman RL, Dearth DJ, Chleboun GS, Manini TM, Clark BC. Delayed-onset muscle soreness induced by low-load blood flow-restricted exercise. Eur J Appl Physiol. 2009;107:687–695. doi: 10.1007/s00421-009-1175-6. [DOI] [PubMed] [Google Scholar]
- 106.Sieljacks P, Matzon A, Wernbom M, Ringgaard S, Vissing K, Overgaard K. Muscle damage and repeated bout effect following blood flow restricted exercise. Eur J Appl Physiol. 2016;116:513–525. doi: 10.1007/s00421-015-3304-8. [DOI] [PubMed] [Google Scholar]
- 107.Nielsen JL, Aagaard P, Prokhorova TA, et al. Blood flow restricted training leads to myocellular macrophage infiltration and upregulation of heat shock proteins, but no apparent muscle damage. J Physiol. 2017;595:4857–4873. doi: 10.1113/JP273907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Soultati A, Mountzios G, Avgerinou C, et al. Endothelial vascular toxicity from chemotherapeutic agents: Preclinical evidence and clinical implications. Cancer Treat Rev. 2012;38:473–483. doi: 10.1016/j.ctrv.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 109.Brandner CR, May AK, Clarkson MJ, Warmington SA. Reported side-effects and safety considerations for the use of blood flow restriction during exercise in practice and research. Tech Orthop. 2018;33:1. doi: 10.1097/BTO.0000000000000259. [DOI] [Google Scholar]
- 110.Rolnick N, Kimbrell K, Cerqueira MS, Weatherford B, Brandner C. Perceived barriers to blood flow restriction training. Front Rehabil Sci. 2021;2:697082. doi: 10.3389/fresc.2021.697082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ng AH, Ngo-Huang A, Vidal M, et al. Exercise barriers and adherence to recommendations in patients with cancer. JCO Oncol Pract. 2021;17:e972–e981. doi: 10.1200/OP.20.00625. [DOI] [PubMed] [Google Scholar]
- 112.Hughes DC, Ellefsen S, Baar K. Adaptations to endurance and strength training. Cold Spring Harb Perspect Med. 2018;8 doi: 10.1101/cshperspect.a029769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cleather D. Independently Published; UK: 2021. Force: The biomechanics of training. [Google Scholar]
- 114.Wagle JP, Taber CB, Cunanan AJ, et al. Accentuated eccentric loading for training and performance: A review. Sports Med. 2017;47:2473–2495. doi: 10.1007/s40279-017-0755-6. [DOI] [PubMed] [Google Scholar]
- 115.Sasso JP, Eves ND, Christensen JF, Koelwyn GJ, Scott J, Jones LW. A framework for prescription in exercise-oncology research. J Cachexia Sarcopenia Muscle. 2015;6:115–124. doi: 10.1002/jcsm.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.