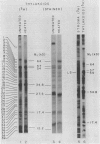
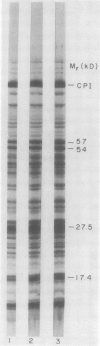
Abstract
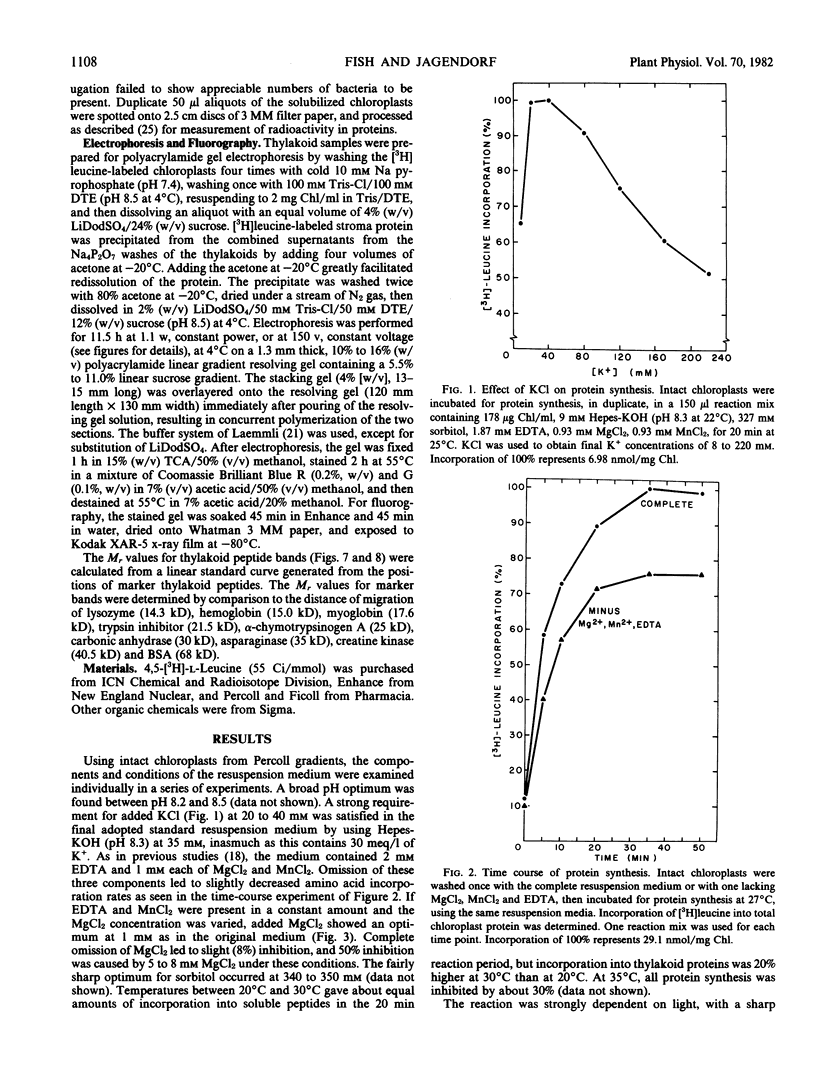
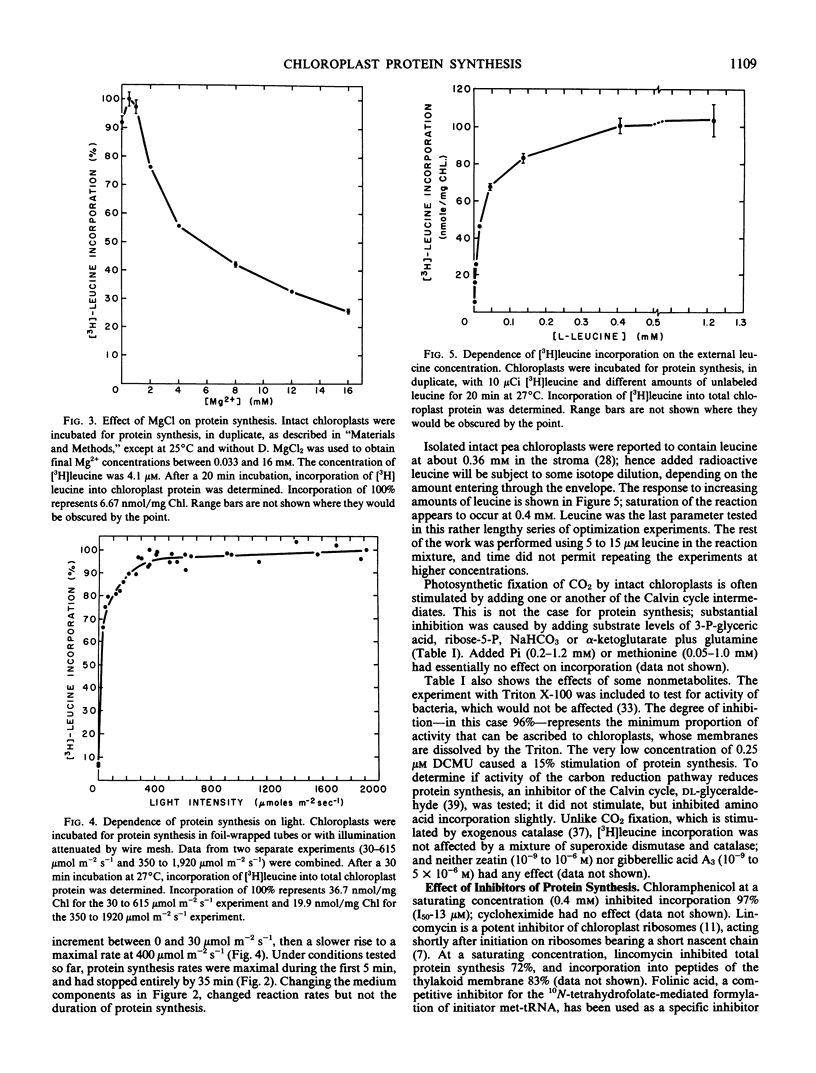
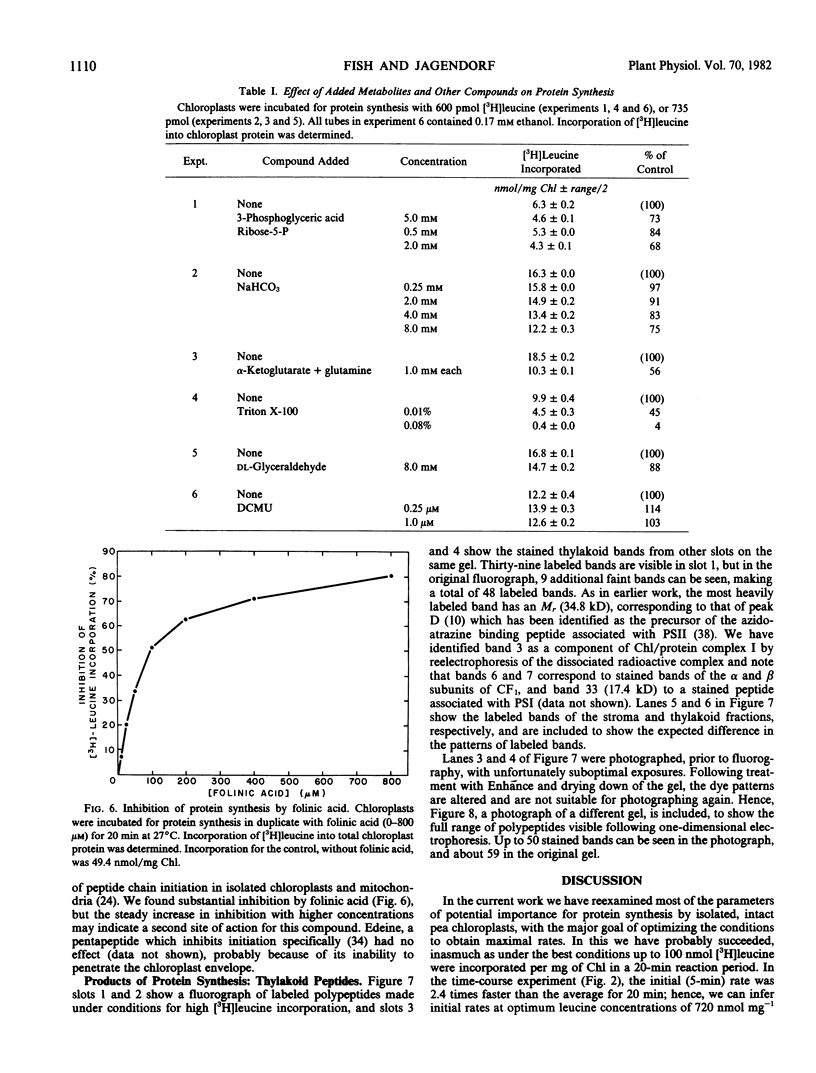
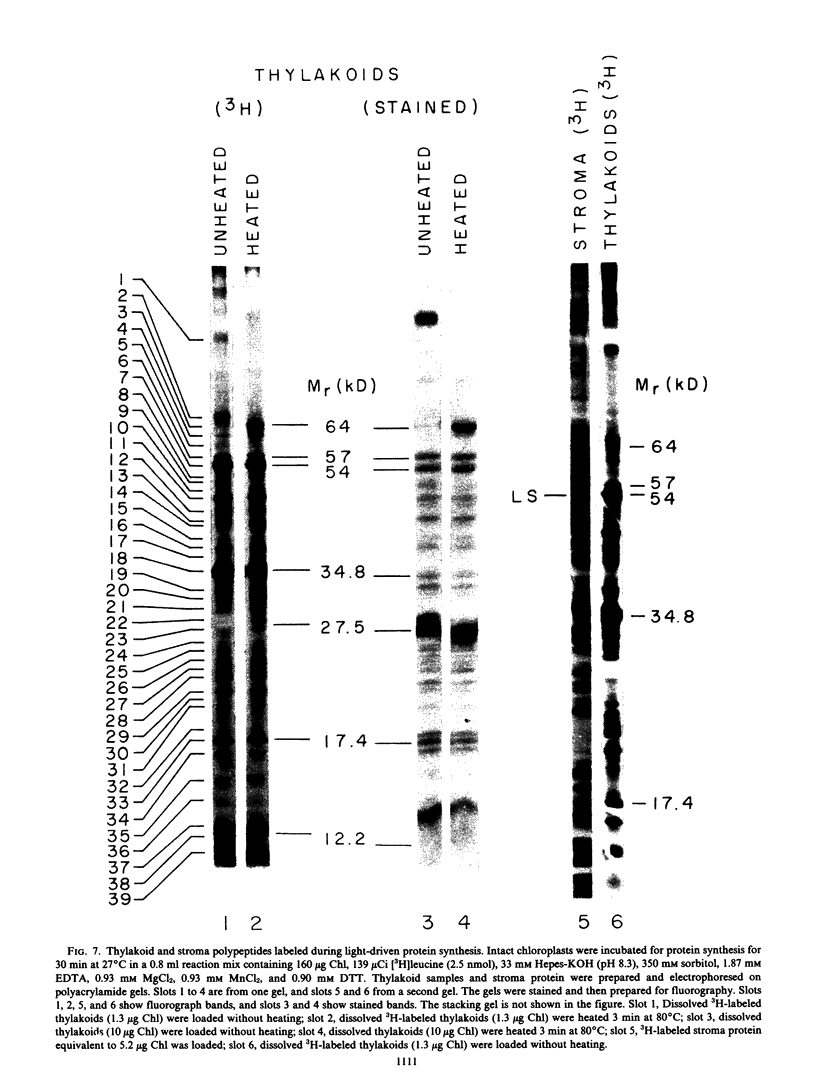
Improvements are described in the preparation and in vitro conditions of an intact pea (Pisum sativum Progress No. 9) chloroplast system which provides high efficiency for translation of endogenous messenger RNA, using light as an energy source. High rates result in the incorporation into protein of up to 100 nanomoles tritiated leucine per milligram chlorophyll. These rates suggest extensive reinitiation, and repeated utilization of the messenger RNA that code for thylakoid proteins. Up to 39 radioactive thylakoid peptide bands were detected by fluorography after labeling with tritiated leucine.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apel K. The light-harvesting chlorophylla a/b.protein complex of the green alga Acetabularia mediterranea. Isolation and characterization of two subunits. Biochim Biophys Acta. 1977 Nov 17;462(2):390–402. doi: 10.1016/0005-2728(77)90137-2. [DOI] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I., Tsujimoto H. Y., McSwain B. D. Ferredoxin and photosynthetic phosphorylation. Nature. 1967 May 6;214(5088):562–566. doi: 10.1038/214562a0. [DOI] [PubMed] [Google Scholar]
- Binder A., Jagendorf A., Ngo E. Isolation and composition of the subunits of spinach chloroplast coupling factor protein. J Biol Chem. 1978 May 10;253(9):3094–3100. [PubMed] [Google Scholar]
- Bottomley W., Spencer D., Whitfeld P. R. Protein synthesis in isolated spinach chloroplasts: comparison of light-driven and ATP-driven synthesis. Arch Biochem Biophys. 1974 Sep;164(1):106–117. doi: 10.1016/0003-9861(74)90012-5. [DOI] [PubMed] [Google Scholar]
- Burns D. J., Cundliffe E. Bacterial-protein synthesis. A novel system for studying antibiotic action in vivo. Eur J Biochem. 1973 Sep 3;37(3):570–574. doi: 10.1111/j.1432-1033.1973.tb03020.x. [DOI] [PubMed] [Google Scholar]
- Chua N. H., Gillham N. W. The sites of synthesis of the principal thylakoid membrane polypeptides in Chlamydomonas reinhardtii. J Cell Biol. 1977 Aug;74(2):441–452. doi: 10.1083/jcb.74.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R. J., Hartley M. R. Sites of synthesis of chloroplast proteins. Nature. 1971 Oct 13;233(5320):193–196. [PubMed] [Google Scholar]
- Fish L. E., Jagendorf A. T. Light-induced increase in the number and activity of ribosomes bound to pea chloroplast thylakoids in vivo. Plant Physiol. 1982 Apr;69(4):814–824. doi: 10.1104/pp.69.4.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geetha V., Gnanam A. Synthesis of soluble, thylakoid and envelope polypeptides by isolated chloroplasts of Sorghum vulgare. Biochim Biophys Acta. 1980 Jul 29;608(2):427–434. doi: 10.1016/0005-2787(80)90188-4. [DOI] [PubMed] [Google Scholar]
- Grebanier A. E., Steinback K. E., Bogorad L. Comparison of the Molecular Weights of Proteins Synthesized by Isolated Chloroplasts with Those Which Appear during Greening in Zea mays. Plant Physiol. 1979 Mar;63(3):436–439. doi: 10.1104/pp.63.3.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green B. R. Protein synthesis by isolated Acetabularia chloroplasts. In vitro synthesis of the apoprotein of the P-700-chlorophyll alpha-protein complex (CP i). Biochim Biophys Acta. 1980 Aug 26;609(1):107–120. doi: 10.1016/0005-2787(80)90205-1. [DOI] [PubMed] [Google Scholar]
- Huber S. C., Maury W. Effects of Magnesium on Intact Chloroplasts: I. EVIDENCE FOR ACTIVATION OF (SODIUM) POTASSIUM/PROTON EXCHANGE ACROSS THE CHLOROPLAST ENVELOPE. Plant Physiol. 1980 Feb;65(2):350–354. doi: 10.1104/pp.65.2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. G., Bassham J. A. Photosynthesis by isolated chloroplasts. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1095–1101. doi: 10.1073/pnas.56.4.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabat D., Chappell M. R. Competition between globin messenger ribonucleic acids for a discriminating initiation factor. J Biol Chem. 1977 Apr 25;252(8):2684–2690. [PubMed] [Google Scholar]
- Kirk P. R., Leech R. M. Amino Acid Biosynthesis by Isolated Chloroplasts during Photosynthesis. Plant Physiol. 1972 Aug;50(2):228–234. doi: 10.1104/pp.50.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lea P. J., Miflin B. J. Alternative route for nitrogen assimilation in higher plants. Nature. 1974 Oct 18;251(5476):614–616. doi: 10.1038/251614a0. [DOI] [PubMed] [Google Scholar]
- Lodish H. F. Translational control of protein synthesis. Annu Rev Biochem. 1976;45:39–72. doi: 10.1146/annurev.bi.45.070176.000351. [DOI] [PubMed] [Google Scholar]
- Lucchini G., Bianchetti R. Initiation of protein synthesis in isolated mitochondria and chloroplasts. Biochim Biophys Acta. 1980 Jun 27;608(1):54–61. doi: 10.1016/0005-2787(80)90133-1. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Maury W. J., Huber S. C., Moreland D. E. Effects of Magnesium on Intact Chloroplasts : II. CATION SPECIFICITY AND INVOLVEMENT OF THE ENVELOPE ATPase IN (SODIUM) POTASSIUM/PROTON EXCHANGE ACROSS THE ENVELOPE. Plant Physiol. 1981 Dec;68(6):1257–1263. doi: 10.1104/pp.68.6.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenthaler J. J., Mendiola-Morgenthaler L. Synthesis of soluble, thylakoid, and envelope membrane proteins by spinach chloroplasts purified from gradients. Arch Biochem Biophys. 1976 Jan;172(1):51–58. doi: 10.1016/0003-9861(76)90046-1. [DOI] [PubMed] [Google Scholar]
- Morgenthaler J. J., Price C. A. Photosynthetic activity of spinach chloroplasts after isopycnic centrifugation in gradients of silica. Plant Physiol. 1974 Oct;54(4):532–534. doi: 10.1104/pp.54.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz W., Reardon E. M., Price C. A. Preparation of chloroplasts from euglena highly active in protein synthesis. Plant Physiol. 1980 Aug;66(2):291–294. doi: 10.1104/pp.66.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parenti F., Margulies M. M. In Vitro Protein Synthesis by Plastids of Phaseolus vulgaris. I. Localization of Activity in the Chloroplasts of a Chloroplast Containing Fraction from Developing Leaves. Plant Physiol. 1967 Sep;42(9):1179–1186. doi: 10.1104/pp.42.9.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez J. M., Campo F. F., Arnon D. I. Photosynthetic phosphorylation as energy source for protein synthesis and carbon dioxide assimilation by chloroplasts. Proc Natl Acad Sci U S A. 1968 Feb;59(2):606–612. doi: 10.1073/pnas.59.2.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slovacek R. E., Hind G. Energetic factors affecting carbon dioxide fixation in isolated chloroplasts. Plant Physiol. 1980 Mar;65(3):526–532. doi: 10.1104/pp.65.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinback K. E., McIntosh L., Bogorad L., Arntzen C. J. Identification of the triazine receptor protein as a chloroplast gene product. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7463–7467. doi: 10.1073/pnas.78.12.7463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes D. M., Walker D. A. Photosynthesis by isolated chloroplasts. Inhibition by DL-glyceraldehyde of carbon dioxide assimilation. Biochem J. 1972 Aug;128(5):1147–1157. doi: 10.1042/bj1281147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasconcelos A. C. Synthesis of Proteins by Isolated Euglena gracilis Chloroplasts. Plant Physiol. 1976 Dec;58(6):719–721. doi: 10.1104/pp.58.6.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Burke J., Autz G., Jagendorf A. T. Bound Ribosomes of Pea Chloroplast Thylakoid Membranes: Location and Release in Vitro by High Salt, Puromycin, and RNase. Plant Physiol. 1981 May;67(5):940–949. doi: 10.1104/pp.67.5.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski R. E., Price C. A. Synthesis of thylakoid membrane proteins by chloroplasts isolated from spinach. Cytochrome b559 and P700-chlorophyll a-protein. J Cell Biol. 1980 May;85(2):435–445. doi: 10.1083/jcb.85.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]