Abstract
Guillain-Barré syndrome (GBS) usually develops after preceding infection, but cardiac surgery can also occasionally cause GBS. Currently, cardiac catheterizations have already become common therapeutic options for heart diseases, but there have been no reports of GBS occurrence after that. Herein, we present a rare case in which GBS occurred following catheterization. An 85-year-old-man with sudden onset chest pain was rushed to our hospital and diagnosed with ST-elevated myocardial infarction. He underwent emergent percutaneous coronary intervention (PCI) to left anterior descending artery, but he still had exertional chest pain. Echocardiography revealed severe aortic stenosis (AS) and our heart team considered AS was the cause of symptom and decided to perform and transcatheter aortic valve implantation (TAVI), 11 days after the PCI. However, 5 days after the TAVI procedure, he presented with symmetrical muscular weakness of extremities. Cranial magnetic resonance imaging showed no significant lesion. Based on several signs including albuminocytologic dissociation in cerebrospinal fluid examination, demyelinating polyneuropathy in nerve conduction study, positive anti-ganglioside antibody, and the lack of preceding infection, he was diagnosed with GBS triggered by cardiac catheterizations. We administered high-dose intravenous immunoglobulin therapy and his motor strength gradually improved, finally discharged with full motor strength after 7 months rehabilitation.
Learning objective
-
•
Cardiac surgery has been already reported as a non-infectious risk factor of Guillain-Barré syndrome (GBS) in previous literatures, and cardiac catheterization such as percutaneous coronary intervention and transcatheter aortic valve implantation, which were relatively less invasive procedure, may be a potential risk factor for GBS occurrence as well.
-
•
If a patient complains of progressive, symmetrical neurological symptoms after cardiac catheterization, GBS should be considered as the possible cause, and nerve conduction study and cerebrospinal fluid examination may be helpful for the diagnosis.
Keywords: Aortic stenosis, Guillain–Barré syndrome, Percutaneous coronary intervention, ST-elevated myocardial infarction, Transcatheter aortic valve implantation, Transcatheter aortic valve replacement, Case report
Introduction
Surgery-related Guillain-Barré syndrome (GBS) is still uncommon, therefore, difficult to diagnose and often overlooked [1]. Because it can rapidly deteriorate and can be life-threatening by respiratory failure, cardiac complications, and thromboembolisms, early diagnosis and therapeutic intervention are essential. After cardiac surgeries, GBS occurrence has been also reported and can be associated with worse outcomes. Currently, the number of transcatheter cardiac interventions has been increasing worldwide, and neurological complications such as cerebral embolism or hypoperfusion are one of unresolved issues, but GBS after cardiac catheterization has not been reported yet. Herein, we report a rare case of GBS occurrence in a patient after percutaneous coronary intervention (PCI) and transcatheter aortic valve implantation (TAVI).
Case presentation
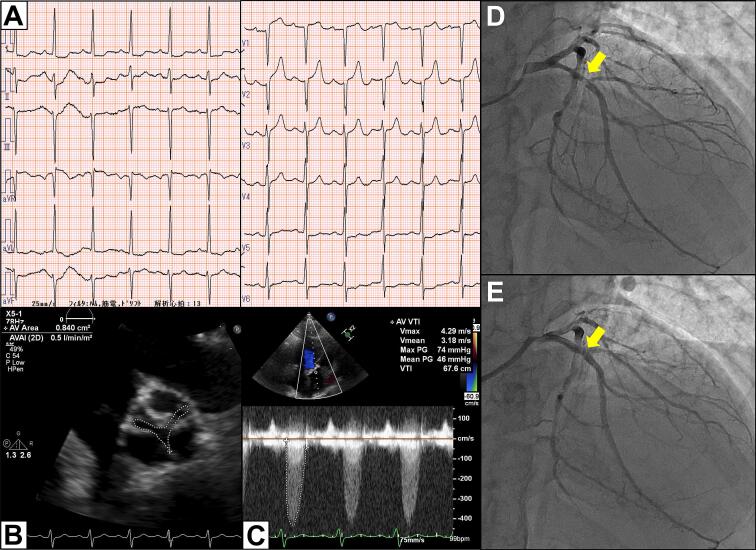
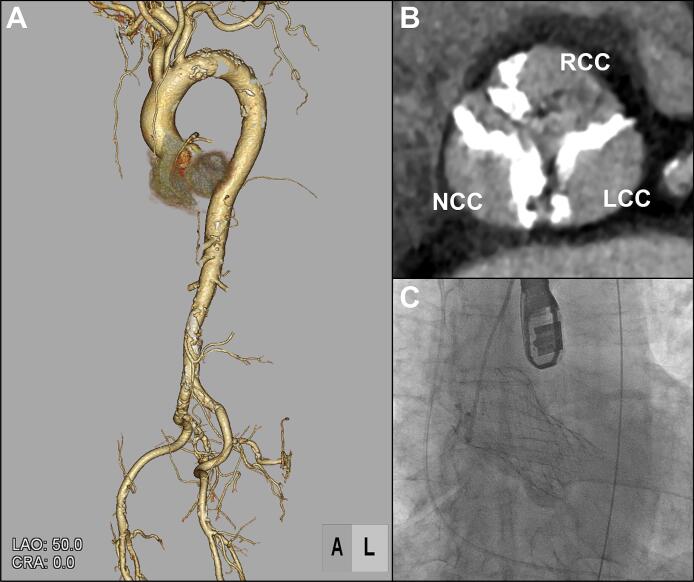
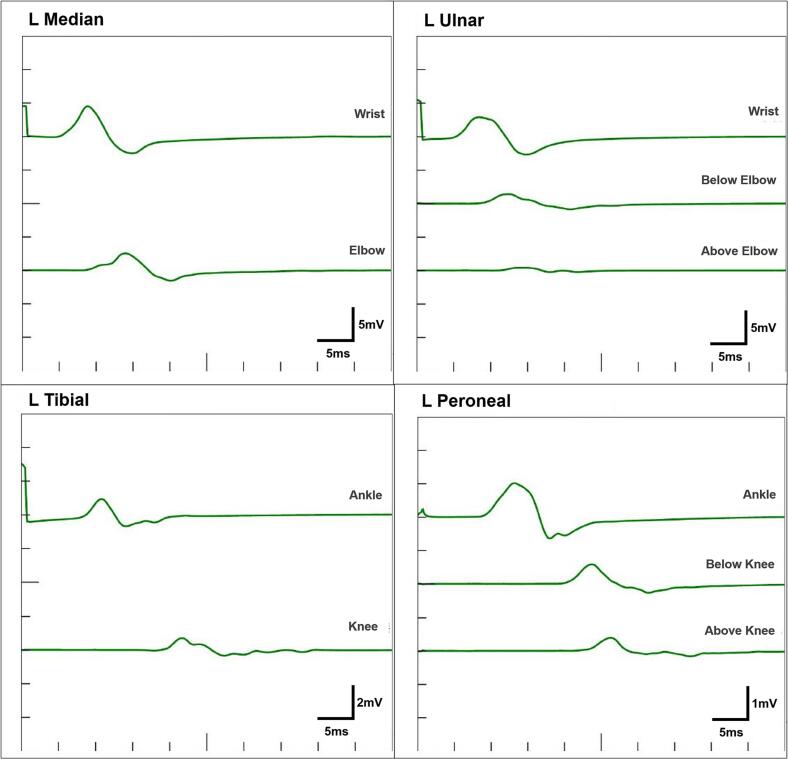
An 85-year-old man with hypertension, dyslipidaemia and chronic kidney disease, was transported to our emergency room with sudden onset of chest pain. Electrocardiogram indicated ST elevation in leads V1-2 with reciprocal changes in lateral leads (Fig. 1A). His vital signs were stable and cardiac auscultation revealed a grade 4/6 systolic ejection murmur propagating to the neck heard at the second right upper sternal border. Echocardiography revealed preserved left ventricular ejection fraction (58 %) with severe hypokinesis at antero-septal wall without thinning, and severe aortic stenosis (AS) (peak velocity, 4.3 m/s; mean pressure gradient, 46 mmHg; valve area, 0.8 cm2; Fig. 1B, C). He was diagnosed with ST-elevated myocardial infarction and underwent emergency coronary angiography (CAG). CAG revealed the significant stenosis of proximal left anterior descending artery (Fig. 1D), and which was treated by a drug-eluting stent with TIMI 3 result (Fig. 1E). However, even after the percutaneous coronary intervention (PCI), he still had exertional chest pain. Primary cause for the symptom was considered severe AS and our local multidisciplinary heart-team decided to subsequently treat AS and chose TAVI owing to his advanced age and higher clinical frailty scale. Pre-procedural computed tomography (CT) showed no major anatomical issues to perform TAVI (Fig. 2A, B). Therefore, 11 days after PCI, he underwent TAVI under general anesthesia via right femoral artery and 29-mm self-expandable valve was successfully implanted without any complications (Fig. 2C; Supplementary Video 1). His peri-operative period remained uneventful, but 5 days after TAVI, he suddenly complained of muscular weakness of extremities. Neurological examination revealed symmetrical muscular weakness of extremities (Medical Research Council scale for muscle strength grades: proximal arms 4, distal arms 3, proximal legs 4, and distal legs 4), absent deep tendon reflexes, dysarthria, and dysphagia. Sensory examination was normal. Cranial CT and magnetic resonance imaging (MRI) showed no significant lesion. Additionally, MRI of cervical spine demonstrated no foraminal narrowing or abnormal intensity. Cerebrospinal fluid examination revealed albuminocytologic dissociation (1 leukocyte cells/μl, protein 60 ml/dl, and glucose 57 mg/dl). Nerve conduction study including F-wave showed motor dominant peripheral polyneuropathy with demyelinating changes such as conduction delay, temporal dispersion, and conduction block (Fig. 3; Supplementary Table 1). Three days after the onset of neurological symptoms, clinical diagnosis of GBS was made. At that time, he was already bedridden because of the muscular weakness and he needed a nasogastric tube for the nutrition because of the difficulty of swallowing, although his respiratory function was preserved and he did not require the mechanical ventilation. In this case, there was no evidence of preceding infections; paired-serum antibody test for cytomegalovirus, Epstein-Barr virus, and mycoplasma were all negative and he did not have diarrhoea suggestive of intestinal infection such as Campylobacter jejuni. Following the day after the diagnosis, we immediately administered high-dose intravenous immunoglobulin (IVIG) therapy 20 g/day for 5 days. After IVIG therapy, his motor strength gradually improved and he was transferred to the other hospital for rehabilitation 37 days after the onset. Here, anti-GD1a antibody was found to be positive.
Fig. 1.
Electrocardiogram, echocardiogram at admission, and coronary angiography.
(A) Electrocardiogram at the emergency room shows ST-segment elevation in leads V1, V2 and down-sloping ST-segment depression in leads I, II, aVL.
(B, C) Echocardiography at the index hospitalization shows severe aortic stenosis (peak velocity, 4.3 m/s; mean pressure gradient, 46 mmHg; valve area, 0.8 cm2)
(D) Left coronary artery showing 99 % stenosis of proximal left anterior descending artery (yellow arrow).
(E) Coronary angiography in the proximal left anterior descending artery after drug-eluting stent implantation (yellow arrow).
Fig. 2.
Pre-procedural CT and intraoperative fluoroscopy.
(A) Iliofemoral vascular access is suitable for TAVI in 3-dimensional CT reconstruction.
(B) CT scan shows aortic valve complex with severe calcification, predominantly on NCC and LCC.
(C) Fluoroscopy showing a 29-mm Evolut PRO+ valve implantation. The 26-mm prosthesis was successfully deployed at proper position without any complication.
CT, computed tomography; NCC, non-coronary cusp; LCC, left-coronary cusp; RCC, right-coronary cusp; TAVI, transcatheter aortic valve implantation.
Fig. 3.
Nerve conduction study.
Temporal dispersion is observed in median and tibial nerves, conduction block is in ulnar and peroneal nerves, and nerve conduction is slowed in all nerves, indicating the demyelinating type of GBS.
GBS, Guillain-Barré syndrome.
Seven months after the development of GBS, he was discharged and regained full motor strength, indicating modified Rankin scale 0. Between day 3 and day 10 after the onset, his Hughes functional grade score was worst; 4 (bedridden or chair-bound), although it completely recovered to 0 (normal clinical performance without any symptoms or signs) after the discharge.
Discussion
GBS is a rare, but potentially fatal, immune-mediated disease of the peripheral nerves and nerve roots, that is classically triggered by infection [2]. However, non-infectious factors like trauma, vaccination and surgery have also been reported as potential triggers of GBS and 4.5–9.5 % of GBS are caused by surgery [1,3]. Several mechanisms of post-surgical GBS have been proposed, such as a release of antigen due to surgery and subsequent antigen autoimmunity [4], infection due to post-operative immunosuppression [5], and hypersecretion of adrenocorticotropic hormone causing immunosuppression after surgery [6], but the underlying pathogenesis has not been fully understood even now. Orthopaedic surgery is known as the most common operation developing GBS [1,6] and GBS after cardiac surgery has been also reported [7]. However, GBS following cardiac catheterization has not been reported yet. In the current case, the patient complained of weakness 16 days after the PCI and 5 days after the TAVI procedure, and there was no evidence of preceding infection; thus, those cardiac catheterizations could potentially be causes of GBS occurrence. Neurological events due to cardiac embolization or hypoperfusion after PCI and TAVI are frequently reported [8], but we should consider the possibility of post cardiac catheterization GBS as well as this case.
We consider one possible reason why GBS occurred in the current case. Basically, the degree of immunosuppression is mostly determined by the magnitude of surgical insult [9], and single catheterization procedure is minimally invasive and its effect on immune system is too small. However, in the current case, two catheterization procedures were performed in short term, and which could exert a larger impact on immune system than isolated catheterization procedure, and might consequently have caused post cardiac catheterization GBS.
According to previous reports, post-surgical GBS has several features and tendencies. Patients with post-surgical GBS often present with muscular atrophy and have high Hughes functional grade score in comparison with GBS caused by other factors. Moreover, among post-surgical GBS cases, the anti-GD1a antibody can be positive with a higher frequency than non post-surgical GBS, and the sub-type is usually acute motor axonal neuropathy [1,10]. In the present case, “post cardiac catheterization GBS”, several clinical presentations were same as typical post-surgical GBS; Hughes functional grade score was high and the anti-GD1a antibody was positive, but the sub-type of this case was acute inflammatory demyelinating polyneuropathy, not acute motor axonal neuropathy. In addition, it was also reported that 47.1 % of post-surgical GBS needed mechanical ventilator assist and sequelae often remained [1], but our patient recover without any sequelae, which might be owing to the immediate diagnosis and treatment.
In post-surgical GBS, the symptoms mostly appear within 2 weeks after surgery and the initial presentation is usually muscular weakness, and which were almost same as the present case [1,6]. Following cardiac catheterization, similar clinical manifestations can be seen in typical neurological complications such as cerebral embolism and hypoperfusion. Therefore, it is necessary to identify the correct cause and consider GBS as probable cause of neurological events after cardiac catheterizations. One of definite differences between GBS and cerebral infarction is that the weakness observed in GBS is usually bilateral, whereas post catheterization stroke is often unilateral. Thus, in patients who exhibit unexplainable symmetrical limb weakness after catheterization, GBS also need to be considered as one of the probable causes.
In conclusion, this is the first case of GBS occurrence in a patient after cardiac catheterization, to the best of our knowledge. Catheterization such as PCI and TAVI may have a potential risk for the occurrence of GBS and early diagnosis and prompt treatment are imperative to reduce mortality.
The following are the supplementary data related to this article.
Nerve conduction study.
Comparison of characteristics [10].
Final Angiography showed no obvious complication.
Sources of funding
None.
Patient permission
The patient provided consent for the publication.
Permissions information
The authors do hereby declare that all figures in the manuscript are entirely original and do not require reprint permission.
Declaration of competing interest
Dr. Asami is a clinical proctor for Medtronic and he reports having received remuneration from Medtronic, Edwards Lifesciences, Boston Scientific, Abbott Medical, and Canon Medical Systems.
Dr. Tanabe is a clinical proctor for Edwards Lifesciences and he receives remuneration from Medtronic, Edwards Lifesciences, Boston Scientific, Abbott Medical, Japan Lifeline, and Orbusneich.
Acknowledgements
None.
References
- 1.Gong Q., Liu S., Liu Y., Yao J., Fu X., Xiao Z., et al. Guillain-Barré syndrome triggered by surgery in a Chinese population: a multicenter retrospective study. BMC Neurol. 2021;21(1) doi: 10.1186/s12883-021-02067-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Den Berg B., Walgaard C., Drenthen J., Fokke C., Jacobs B.C., Van Doorn P.A. Guillain–Barré syndrome: pathogenesis, diagnosis, treatment and prognosis. Nat Rev Neurol. 2014;10(8):469–482. doi: 10.1038/nrneurol.2014.121. [DOI] [PubMed] [Google Scholar]
- 3.Gensicke H., Datta A.N., Dill P., Schindler C., Fischer D. Increased incidence of Guillain–Barré syndrome after surgery. Eur J Neurol. 2012;19(9):1239–1244. doi: 10.1111/j.1468-1331.2012.03730.x. [DOI] [PubMed] [Google Scholar]
- 4.Steiner I., Argov Z., Cahan C., Abramsky O. Guillain-Barré syndrome after epidural anesthesia: direct nerve root damage may trigger disease. Neurology. 1985;35(10):1473–1475. doi: 10.1212/wnl.35.10.1473. [DOI] [PubMed] [Google Scholar]
- 5.Yang B., Lian Y., Liu Y., Wu B.-Y., Duan R.-S. A retrospective analysis of possible triggers of Guillain-Barre syndrome. J Neuroimmunol. 2016;293:17–21. doi: 10.1016/j.jneuroim.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Zhong Y.X., Lu G.F., Chen X.L., Cao F. Postoperative Guillain-Barré syndrome, a neurologic complication that must not be overlooked: a literature review. World Neurosurg. 2019;128:347–353. doi: 10.1016/j.wneu.2019.04.239. [DOI] [PubMed] [Google Scholar]
- 7.Cingoz F., Tavlasoglu M., Kurkluoglu M., Sahin M.A. Guillain–Barré syndrome after coronary artery bypass surgery. Interact Cardiovasc Thorac Surg. 2012;15(5):918–919. doi: 10.1093/icvts/ivs367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armijo G., Nombela-Franco L., Tirado-Conte G. Cerebrovascular events after transcatheter aortic valve implantation. Front Cardiovasc Med. 2018;5:104. doi: 10.3389/fcvm.2018.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hogan B.V., Peter M.B., Shenoy H.G., Horgan K., Hughes T.A. Surgery induced immunosuppression. Surgeon. 2011;9(1):38–43. doi: 10.1016/j.surge.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 10.Bao L., Chen X., Li Q., Zhang R., Shi H., Cui G. Surgery and Guillain-Barré syndrome: a single-center retrospective study focused on clinical and electrophysiological subtypes. Neuropsychiatr Dis Treat. 2020;16:969–974. doi: 10.2147/NDT.S241128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Nerve conduction study.
Comparison of characteristics [10].
Final Angiography showed no obvious complication.