Abstract
Objective
The purpose of this investigation was to elucidate the relationship between the zinc and iron intake and the advancement of subchondral sclerosis among patients with osteoarthritis (OA). The goal was to establish personalized, nutritionally-informed strategies designed to retard the progression of subchondral sclerosis and conserve joint structure.
Methods
For the purposes of this research, we derived data from the Bone Ancillary Study (BAS), a constituent study of the Osteoarthritis Initiative (OAI). The intake of zinc and iron was evaluated via a food frequency questionnaire. Magnetic Resonance Imaging trabecular morphometry was employed to ascertain the microarchitecture of the subchondral bone. For the analysis of collected data, we employed logistic regression along with generalized additive models (GAMs).
Results
The participant cohort was comprised of 474 OA patients (216 females, 258 males, mean [SD] age 64.1[9.2]). Notably, an increment in zinc consumption was linked with a significantly reduced likelihood of deterioration in Tb.N (OR = 0.967, 95 % CI, 0.939–0.996, P-value = 0.026), Tb.Th (OR = 0.958, 95 % CI, 0.929–0.989, P-value = 0.008), and Tb.Sp (OR = 0.967, 95 % CI, 0.939–0.996, P-value = 0.013). An elevation in iron intake seemed to enhance the risk of subchondral sclerosis, as indicated by the GAM. Subgroup analysis revealed an interaction between the effectiveness of zinc intake and factors such as gender, age, radiographic severity, and macronutrient consumption. An increased intake of calcium amplified the beneficial impact of zinc on subchondral sclerosis.
Conclusions
Our findings indicate a positive association between elevated zinc intake and a slowdown in the progression of subchondral sclerosis in OA patients, notably among females, middle-aged individuals, and those with higher calcium and magnesium intake. Conversely, a higher iron intake might intensify subchondral sclerosis. These results suggest that personalized, diet-based interventions focusing on zinc consumption, in tandem with adequate calcium intake, could potentially decelerate the progression of subchondral sclerosis in individuals afflicted with OA.
Keywords: Knee osteoarthritis, Subchondral sclerosis, Zinc intake, Iron intake, Subchondral bone morphometry
1. Introduction
Osteoarthritis (OA) stands as the predominant joint disorder extensively observed within the United States, manifesting through the degradation of articular cartilage, synovitis, and consequential alterations in subchondral bone [1]. This condition significantly contributes to the experience of pain and disability, resulting in a considerable decline in overall quality of life and imposing a substantial socioeconomic burden [2].
Subchondral bone, situated distal to calcified cartilage, assumes a pivotal role in the pathogenesis of OA [3]. Notably, subchondral sclerosis has firmly established itself as a highly sensitive biological marker and distinctive characteristic of the disease, strongly linked to the progression and exacerbation of OA symptoms [4,5]. The intricate interplay of prolonged and excessive mechanical loading, in conjunction with multiple contributing factors, triggers a complex array of subchondral bone damage and repair mechanisms [6]. These mechanisms entail aberrant responses and activation of osteoblasts, osteoclasts, macrophages, mesenchymal stem cells, as well as the intricate orchestration of chemical signaling [7]. As a consequence, the development of subchondral bone lesions and, in advanced cases, subchondral sclerosis occurs [8]. Remarkably, subchondral sclerosis commonly presents with an increase in bone volume fraction (BV/TV), trabecular thickness (Tb.Th), and trabecular number (Tb.N), coupled with a decrease in trabecular separation (Tb.Sp) [9]. These alterations further illuminate the intricate nature of subchondral sclerosis and its significant impact on the microarchitecture of bone tissue [10].
Throughout the protracted process of remodeling, the subchondral bone demonstrates adaptability to specific concentrations of trace elements [11,12]. Any disturbances in trace element levels, characterized by excess or deficiency within the joint, possess the potential to impair joint system functionality and predispose individuals to OA [13]. Of particular interest among trace elements, zinc [14] and iron [15] assume critical roles in bone metabolism, bone homeostasis, and the pathogenesis of OA [16]. Nonetheless, the precise extent to which these elements influence the progression of subchondral osteosclerosis in OA remains unclear.
Given the potential associations suggested by previous studies, the aim of this study was to investigate the relationship between zinc and iron intake and subchondral sclerosis in older adults with OA using a large North American-based population-based cohort study.
2. Methods
2.1. Study design
Our study was designed as a prospective cohort study. The Institutional Review Boards of the University of California, San Francisco, as well as the four clinical centers of the Osteoarthritis Initiative (OAI) — namely, the University of Pittsburgh, Ohio State University, University of Maryland, Baltimore, and Memorial Hospital of Rhode Island — have granted approval for the OAI. All enrollees willingly provided informed consent prior to their participation in the study. Furthermore, the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) under the auspices of the National Institutes of Health (NIH) commissioned an independent Observational Study Monitoring Board (OSMB) to supervise the OAI study.
2.2. Participants
The Bone Ancillary Study (BAS), a subsidiary study under the primary project of the Osteoarthritis Initiative (OAI), served as the source of the subchondral bone microarchitecture data pertinent to our investigation. The BAS comprised 629 participants who underwent MRI scanning, which was specifically optimized for the assessment of subchondral bone in conjunction with the standard OAI MRI. All the participants of the BAS were also members of the progression sub-cohort, which included individuals exhibiting both frequent knee symptoms and radiographic osteoarthritis (OA) in at least one knee at the inception of the OAI [17].
For inclusion in the progression sub-cohort, two concurrent criteria needed to be present in at least one knee during the baseline evaluation. The first criterion was the frequent occurrence of knee symptoms, defined as the experience of pain, aching, or stiffness on most days of a month over the past year. The second criterion was the radiographic evidence of OA, defined as the presence of definite tibiofemoral osteophytes with an Osteoarthritis Research Society International (OARSI) atlas grade of ≥1, as observed on X-ray images [18]. Our analysis exclusively incorporated subjects who underwent subchondral bone microarchitecture measurements of the right knee at the 30th month (or 36th month) and the 48th month during the OAI follow-up.
2.3. Exposures: zinc and iron intake
Dietary information was obtained during the baseline assessment utilizing the Block Brief 2000 food frequency questionnaire (FFQ), encompassing a comprehensive dataset of 72 commonly consumed food items [1]. For each item, the participants’ typical food and average consumption over the past year were assessed according to the predetermined categories, which ranged from “never” to “every day” [19]. Food-consumption data were converted to minerals, and the other nutrient intakes were calculated by using the nutrient-composition values developed in the Second National Health and Nutrition Examination Survey [20]. In addition, in order to assess the mineral intake from nutritional supplements, specific sections of the FFQ were used to record the average daily amount of 30 days of a vitamin/supplement-combination intake. Zinc and iron intake were independently computed for the OA Initiative (OAI) participants, relying on self-reported dietary sources and supplements, employing NutritionQuest, the manufacturer of the Block Brief 2000 FFQ [2]. Caloric adjustments were made to ensure accurate estimations [21].
2.4. Measurement of MRI trabecular morphometry
The assessment procedure for the research subjects incorporated the use of a coronal-oblique, three-dimensional fast imaging sequence with steady-state free precession (FISP) MRI [22]. This sequence was specifically optimized to enhance the visibility of the subchondral trabecular bone [23]. Supplementary Table S1 contains the comprehensive parameters utilized for this imaging technique. It is noteworthy that FISP acquisition methodologies underscore the disparities in magnetic susceptibility and chemical shifts between marrow fat and trabeculae.
Subchondral bone morphometry was measured by the established software calcDCN (University of California, San Francisco). A rectangular region of interest (ROI) was strategically situated proximally in the medial tibia, adjacent to the articular cartilage. The ROI dimensions, with a height of 3.75 mm and a width varying from 14 to 17 mm based on knee size, were then analyzed. This yielded measurements of apparent total bone volume fraction (BVF), apparent trabecular thickness (Tb.Th), apparent trabecular number (Tb.N), and apparent trabecular spacing (Tb.Sp). The BVF was computed as the percentage of pixels comprising the bone signal void over the total pixel count in the ROI. The Tb.Th calculation involved acquiring the mean intercept length of all angles through a given image in millimeters. The Tb.N was derived by dividing BVF by Tb.Th, while Tb.Sp was computed by (1/Tb.N)–Tb.Th [17]. This measurement focused exclusively on the medial compartment due to the higher prevalence of medial tibiofemoral OA compared to the lateral counterpart. The subchondral bone morphometry was subsequently calculated based on 20 consecutive MRI slices centered within the joint [24,25].
2.5. Clinical variables collected
Various pertinent covariates were considered for this study. For the assessment of OA, the included covariates comprised age, sex, race, body mass index (BMI), Kellgren-Lawrence (KL) grade, and physical activity scale for the elderly (PASE) score. The PASE scale has demonstrated substantial structural validity and retest reliability in evaluating activities that elderly individuals typically participate in during the preceding week. The PASE scale assesses familial, professional, and leisure sports activities within a 7-day period, generating a comprehensive score for a single sports activity that ranges from 0 to 400. A higher score corresponds to increased sports activities. For bone measurements, the covariates incorporated alcohol use, smoking status, history of bone fracture after the age of 45, history of hip fracture, history of vertebral/spine fracture, history of oral corticosteroid medication use, history of bisphosphonate intake [26], intact parathyroid hormone (PTH) level [27], serum 25-vitamin D level and calcium and magnesium intake.
2.6. Statistical analysis
The reliable change index (RCI) method was used to evaluate the variations in subchondral bone morphometry over the first and second years of the study. For BV/TV, Tb.N and Tb.Th, any RCI ≤ −1.96 (confidence interval (CI) of 95 %) was considered as worsening change; For Tb.Sp,any RCI≥1.96 (confidence interval (CI) of 95 %) was considered as worsening change [28]. Descriptive statistics were analyzed through the utilization of ANOVA and Chi-square tests. Generalized logistic regression and generalized additive models (GAMs) were used to explore the association between zinc and iron intake and deterioration of subchondral bone morphometry. The GAM includes a smoothing function that treats the smoothed term as a random effect, permitting the fitting of various nonlinear effects such as random effects, nonlinear interactions, and other nonlinear effects into a framework by adjusting the spline function. A two-tailed p-value of <0.05 was considered significant. Additionally, further subgroup analyses were conducted, and all statistical analyses were executed using R version 4.2.0.
3. Results
3.1. Characteristics of study sample
In the BAS, a cohort of 629 subjects was initially enrolled. 23 subjects who solely underwent microarchitecture analysis of the left knee were excluded from the analysis. An additional 116 subjects lacked baseline subchondral bone microarchitecture data, and nine subjects experienced subsequent loss of subchondral bone microarchitecture data, resulting in their exclusion from the study. After excluding participants with missing Kellgren-Lawrence (KL) grade data, a final sample of 474 subjects was included for analysis in our analysis (Fig. 1). There are no missing values for the variables in our final sample.
Fig. 1.
Study diagram. KL - Kellgren and Lawrence score.
Table 1 presents the baseline characteristics according to quartiles of zinc and iron consumption. The findings indicate that individuals with higher intakes of zinc and iron exhibit several distinguishing features. Specifically, this group tends to be older, predominantly female, and displays elevated levels of serum 25-hydroxyvitamin D, along with reduced concentrations of PTH. Furthermore, they demonstrate a higher intake of macronutrients such as calcium, magnesium, and phosphorus compared to those with lower zinc and iron consumption. In the one-year follow-up, a noteworthy degradation of tibial subchondral trabecular structures was observed in approximately 30–40 % of OA patients. Moreover, it was found that patients with higher zinc intake exhibited a significantly lower rate of deterioration in their tibial subchondral trabeculae compared to those with lower zinc intake.
Table 1.
Baseline characteristics of participants (N = 474).
| Total sample N = 474 | ZINC quartile |
P-value | IRON quartile |
P-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 N = 119 | Q2 N = 118 | Q3 N = 118 | Q4 N = 119 | Q1 N = 119 | Q2 N = 118 | Q3 N = 118 | Q4 N = 119 | ||||
| Age, Mean (SD) | 64.1 ± 9.2 | 63.5 ± 9.8 | 62.0 ± 8.8 | 65.7 ± 9.3 | 65.4 ± 8.6 | 0.006 | 62.4 ± 9.4 | 63.1 ± 9.5 | 66.6 ± 8.4 | 64.5 ± 9.0 | 0.003 |
| Gender, n (%) | <0.001 | <0.001 | |||||||||
| Male | 258 (54.4 %) | 72 (60.5 %) | 81 (68.6 %) | 61 (51.7 %) | 44 (37.0 %) | 81 (68.1 %) | 78 (66.1 %) | 60 (50.8 %) | 39 (32.8 %) | ||
| Female | 216 (45.6 %) | 47 (39.5 %) | 37 (31.4 %) | 57 (48.3 %) | 75 (63.0 %) | 38 (31.9 %) | 40 (33.9 %) | 58 (49.2 %) | 80 (67.2 %) | ||
| Race, n (%) | 0.001 | 0.049 | |||||||||
| Other | 11 (2.3 %) | 2 (1.7 %) | 2 (1.7 %) | 3 (2.5 %) | 4 (3.4 %) | 3 (2.5 %) | 2 (1.7 %) | 3 (2.5 %) | 3 (2.5 %) | ||
| White | 353 (74.5 %) | 72 (60.5 %) | 90 (76.3 %) | 101 (85.6 %) | 90 (75.6 %) | 82 (68.9 %) | 87 (73.7 %) | 102 (86.4 %) | 82 (68.9 %) | ||
| Black | 106 (22.4 %) | 44 (37.0 %) | 26 (22.0 %) | 12 (10.2 %) | 24 (20.2 %) | 34 (28.6 %) | 27 (22.9 %) | 12 (10.2 %) | 33 (27.7 %) | ||
| Asian | 4 (0.8 %) | 1 (0.8 %) | 0 (0.0 %) | 2 (1.7 %) | 1 (0.8 %) | 0 (0.0 %) | 2 (1.7 %) | 1 (0.8 %) | 1 (0.8 %) | ||
| BMI (kg/m2) | 29.5 ± 4.6 | 30.1 ± 4.8 | 30.0 ± 4.5 | 28.7 ± 4.6 | 29.4 ± 4.5 | 0.101 | 30.0 ± 4.7 | 29.8 ± 4.4 | 28.9 ± 4.6 | 29.4 ± 4.7 | 0.271 |
| PASE score, Mean (SD) | 156.7 ± 83.5 | 147.4 ± 80.5 | 178.4 ± 87.6 | 153.0 ± 81.9 | 148.5 ± 81.2 | 0.013 | 160.0 ± 84.4 | 167.0 ± 87.4 | 150.4 ± 79.7 | 149.5 ± 82.2 | 0.318 |
| Smoking status, n (%) | 0.365 | 0.592 | |||||||||
| Never | 258 (54.4 %) | 73 (61.3 %) | 60 (50.8 %) | 62 (52.5 %) | 63 (52.9 %) | 69 (58.0 %) | 59 (50.0 %) | 67 (56.8 %) | 63 (52.9 %) | ||
| Current/Former | 216 (45.6 %) | 46 (38.7 %) | 58 (49.2 %) | 56 (47.5 %) | 56 (47.1 %) | 50 (42.0 %) | 59 (50.0 %) | 51 (43.2 %) | 56 (47.1 %) | ||
| Alcohol consumption, n (%) | 0.135 | 0.073 | |||||||||
| <1 drink/week | 247 (52.1 %) | 72 (60.5 %) | 55 (46.6 %) | 54 (45.8 %) | 66 (55.5 %) | 60 (50.4 %) | 63 (53.4 %) | 50 (42.4 %) | 74 (62.2 %) | ||
| 1-7 drinks/week | 159 (33.5 %) | 29 (24.4 %) | 43 (36.4 %) | 48 (40.7 %) | 39 (32.8 %) | 38 (31.9 %) | 39 (33.1 %) | 47 (39.8 %) | 35 (29.4 %) | ||
| >8 drinks/week | 68 (14.3 %) | 18 (15.1 %) | 20 (16.9 %) | 16 (13.6 %) | 14 (11.8 %) | 21 (17.6 %) | 16 (13.6 %) | 21 (17.8 %) | 10 (8.4 %) | ||
| Radiographic KL grade | 0.136 | 0.605 | |||||||||
| 0 | 66 (13.9 %) | 19 (16.0 %) | 11 (9.3 %) | 22 (18.6 %) | 14 (11.8 %) | 15 (12.6 %) | 18 (15.3 %) | 19 (16.1 %) | 14 (11.8 %) | ||
| 1 | 73 (15.4 %) | 13 (10.9 %) | 19 (16.1 %) | 22 (18.6 %) | 19 (16.0 %) | 15 (12.6 %) | 21 (17.8 %) | 18 (15.3 %) | 19 (16.0 %) | ||
| 2 | 151 (31.9 %) | 35 (29.4 %) | 49 (41.5 %) | 30 (25.4 %) | 37 (31.1 %) | 34 (28.6 %) | 42 (35.6 %) | 33 (28.0 %) | 42 (35.3 %) | ||
| 3 | 141 (29.7 %) | 38 (31.9 %) | 28 (23.7 %) | 33 (28.0 %) | 42 (35.3 %) | 43 (36.1 %) | 26 (22.0 %) | 35 (29.7 %) | 37 (31.1 %) | ||
| 4 | 43 (9.1 %) | 14 (11.8 %) | 11 (9.3 %) | 11 (9.3 %) | 7 (5.9 %) | 12 (10.1 %) | 11 (9.3 %) | 13 (11.0 %) | 7 (5.9 %) | ||
| Any fracture after age 45 | 0.904 | 0.331 | |||||||||
| No | 402 (84.8 %) | 103 (86.6 %) | 100 (84.7 %) | 98 (83.1 %) | 101 (84.9 %) | 98 (82.4 %) | 106 (89.8 %) | 100 (84.7 %) | 98 (82.4 %) | ||
| Yes | 72 (15.2 %) | 16 (13.4 %) | 18 (15.3 %) | 20 (16.9 %) | 18 (15.1 %) | 21 (17.6 %) | 12 (10.2 %) | 18 (15.3 %) | 21 (17.6 %) | ||
| Hip fracture | 0.258 | 0.261 | |||||||||
| No | 470 (99.2 %) | 119 (100.0 %) | 116 (98.3 %) | 118 (100.0 %) | 117 (98.3 %) | 117 (98.3 %) | 118 (100.0 %) | 118 (100.0 %) | 117 (98.3 %) | ||
| Yes | 4 (0.8 %) | 0 (0.0 %) | 2 (1.7 %) | 0 (0.0 %) | 2 (1.7 %) | 2 (1.7 %) | 0 (0.0 %) | 0 (0.0 %) | 2 (1.7 %) | ||
| Vertebrae/spine fracture | 0.796 | 0.39 | |||||||||
| No | 466 (98.3 %) | 117 (98.3 %) | 115 (97.5 %) | 117 (99.2 %) | 117 (98.3 %) | 115 (96.6 %) | 117 (99.2 %) | 117 (99.2 %) | 117 (98.3 %) | ||
| Yes | 8 (1.7 %) | 2 (1.7 %) | 3 (2.5 %) | 1 (0.8 %) | 2 (1.7 %) | 4 (3.4 %) | 1 (0.8 %) | 1 (0.8 %) | 2 (1.7 %) | ||
| History of Bisphosphonates | 0.004 | <0.001 | |||||||||
| No | 427 (90.3 %) | 109 (91.6 %) | 115 (97.5 %) | 104 (88.1 %) | 99 (83.9 %) | 112 (94.1 %) | 114 (96.6 %) | 104 (88.1 %) | 97 (82.2 %) | ||
| Yes | 46 (9.7 %) | 10 (8.4 %) | 3 (2.5 %) | 14 (11.9 %) | 19 (16.1 %) | 7 (5.9 %) | 4 (3.4 %) | 14 (11.9 %) | 21 (17.8 %) | ||
| History of Oral Corticosteroids | 0.404 | 0.081 | |||||||||
| No | 459 (96.8 %) | 114 (95.8 %) | 114 (96.6 %) | 117 (99.2 %) | 114 (95.8 %) | 113 (95.0 %) | 115 (97.5 %) | 118 (100.0 %) | 113 (95.0 %) | ||
| Yes | 15 (3.2 %) | 5 (4.2 %) | 4 (3.4 %) | 1 (0.8 %) | 5 (4.2 %) | 6 (5.0 %) | 3 (2.5 %) | 0 (0.0 %) | 6 (5.0 %) | ||
| BV/TV | 0.603 | 0.404 | |||||||||
| No | 306 (64.6 %) | 78 (65.5 %) | 72 (61.0 %) | 74 (62.7 %) | 82 (68.9 %) | 80 (67.2 %) | 76 (64.4 %) | 69 (58.5 %) | 81 (68.1 %) | ||
| Yes | 168 (35.4 %) | 41 (34.5 %) | 46 (39.0 %) | 44 (37.3 %) | 37 (31.1 %) | 39 (32.8 %) | 42 (35.6 %) | 49 (41.5 %) | 38 (31.9 %) | ||
| Tb.N | 0.496 | 0.953 | |||||||||
| No | 299 (63.1 %) | 71 (59.7 %) | 71 (60.2 %) | 76 (64.4 %) | 81 (68.1 %) | 75 (63.0 %) | 76 (64.4 %) | 72 (61.0 %) | 76 (63.9 %) | ||
| Yes | 175 (36.9 %) | 48 (40.3 %) | 47 (39.8 %) | 42 (35.6 %) | 38 (31.9 %) | 44 (37.0 %) | 42 (35.6 %) | 46 (39.0 %) | 43 (36.1 %) | ||
| Tb.Th | 0.309 | 0.295 | |||||||||
| No | 281 (59.3 %) | 70 (58.8 %) | 66 (55.9 %) | 66 (55.9 %) | 79 (66.4 %) | 76 (63.9 %) | 69 (58.5 %) | 62 (52.5 %) | 74 (62.2 %) | ||
| Yes | 193 (40.7 %) | 49 (41.2 %) | 52 (44.1 %) | 52 (44.1 %) | 40 (33.6 %) | 43 (36.1 %) | 49 (41.5 %) | 56 (47.5 %) | 45 (37.8 %) | ||
| Tb.Sp | 0.628 | 0.516 | |||||||||
| No | 335 (70.7 %) | 79 (66.4 %) | 83 (70.3 %) | 87 (73.7 %) | 86 (72.3 %) | 90 (75.6 %) | 82 (69.5 %) | 79 (66.9 %) | 84 (70.6 %) | ||
| Yes | 139 (29.3 %) | 40 (33.6 %) | 35 (29.7 %) | 31 (26.3 %) | 33 (27.7 %) | 29 (24.4 %) | 36 (30.5 %) | 39 (33.1 %) | 35 (29.4 %) | ||
| Intact parathyroid hormone level (pg/mL) | 55.0 ± 27.5 | 57.5 ± 26.0 | 59.2 ± 29.7 | 52.3 ± 25.7 | 50.9 ± 27.8 | 0.052 | 56.6 ± 23.9 | 59.0 ± 30.4 | 50.5 ± 24.3 | 53.8 ± 30.1 | 0.096 |
| Serum 25-vitamin D level (ng/mL) | 26.3 ± 10.4 | 21.4 ± 9.3 | 24.4 ± 9.7 | 30.1 ± 9.6 | 29.3 ± 10.6 | <0.001 | 22.4 ± 10.1 | 24.3 ± 9.3 | 30.4 ± 10.7 | 28.0 ± 9.6 | <0.001 |
| Calcium intake adjusted by calorie (mg/1000 kcal) | 801.5 ± 518.4 | 545.6 ± 384.8 | 620.6 ± 365.7 | 815.4 ± 373.8 | 1222.9 ± 616.2 | <0.001 | 557.4 ± 427.9 | 639.6 ± 353.2 | 838.8 ± 384.9 | 1169.1 ± 632.0 | <0.001 |
| Magnesium intake adjusted by calorie (mg/1000 kcal) | 210.6 ± 72.5 | 144.2 ± 44.7 | 180.4 ± 42.1 | 237.7 ± 45.2 | 280.2 ± 65.7 | <0.001 | 141.5 ± 40.9 | 184.2 ± 41.8 | 234.5 ± 46.0 | 282.3 ± 65.6 | <0.001 |
Q1–Q4 – calorie-adjusted quartiles of total zinc and iron intake from diet and supplements, based on the FFQ at baseline. Quartile ranges for zinc (mg of zinc per day per 1000 kcal): Q1 < 5.9, Q2 5.9–12.2, Q3 12.2–18.4, Q4 > 18.4. Quartile ranges for iron (mg of iron per day per 1000 kcal) Q1 < 7.4, Q2 7.4–15.3, Q3 15.3–22.3, Q4 > 22.3.
SD – standard deviation. OA – osteoarthritis. KL – Kellgren and Lawrence score. PASE – Physical Activity Scale for the Elderly.
3.2. Association between the sclerosis of tibial subchondral bone and zinc and iron intake
Table 2 presents the findings indicating that increased zinc intake has a significant protective effect against tibial subchondral bone deterioration. This association remains statistically significant even after adjusting for all relevant covariates. Specifically, increasing zinc intake was associated with a significantly lower likelihood of worsening Tb.N (OR, 0.967, 95 % CI, 0.939–0.996), Tb.Th (OR, 0.958, 95 % CI, 0.929–0.989), and Tb.Sp (OR, 0.967, 95 % CI, 0.939–0.996). Additionally, logistic regression analysis reveals that increasing iron intake does not appear to reduce the likelihood of sclerosis in the tibial subchondral bone.
Table 2.
Zinc and iron intake-attributed differences in likelihood of progression of tibial trabecular sclerosis in OA knees.
| Exposure | Unadjusted estimate: β (95%CI) P value | Adjusted estimate*: β (95%CI) P value |
|---|---|---|
| Zinc | ||
| BV/TV | 0.988 (0.969, 1.008) 0.24010 | 0.983 (0.957, 1.011) 0.23136 |
| Tb.N | 0.980 (0.960, 1.000) 0.04976 | 0.967 (0.939, 0.996) 0.02632 |
| Tb.Th | 0.981 (0.961, 1.000) 0.04990 | 0.958 (0.929, 0.989) 0.00767 |
| Tb.Sp | 0.986 (0.966, 1.007) 0.20417 | 0.959 (0.928, 0.991) 0.01319 |
| Iron | ||
| BV/TV | 1.001 (0.987, 1.016) 0.85808 | 1.007 (0.988, 1.026) 0.49803 |
| Tb.N | 1.005 (0.991, 1.020) 0.48708 | 1.011 (0.992, 1.030) 0.26524 |
| Tb.Th | 1.000 (0.985, 1.014) 0.96539 | 1.000 (0.981, 1.019) 0.96010 |
| Tb.Sp | 1.012 (0.997, 1.027) 0.13143 | 1.009 (0.989, 1.028) 0.37549 |
Estimates represent a mean difference in the likelihood of progression of subchondral sclerosis for every additional 1 mg/1000 kcal of zinc or iron intake.
*Adjusted for age, sex, race, BMI, Kellgren-Lawrence (KL) grade, and PASE score, alcohol use, smoking status, history of bone fracture after the age of 45, history of hip fracture, history of vertebral/spine fracture, history of oral corticosteroid medication use, history of bisphosphonate intake, intact parathyroid hormone level, serum 25-vitamin D level and calcium and magnesium intake.
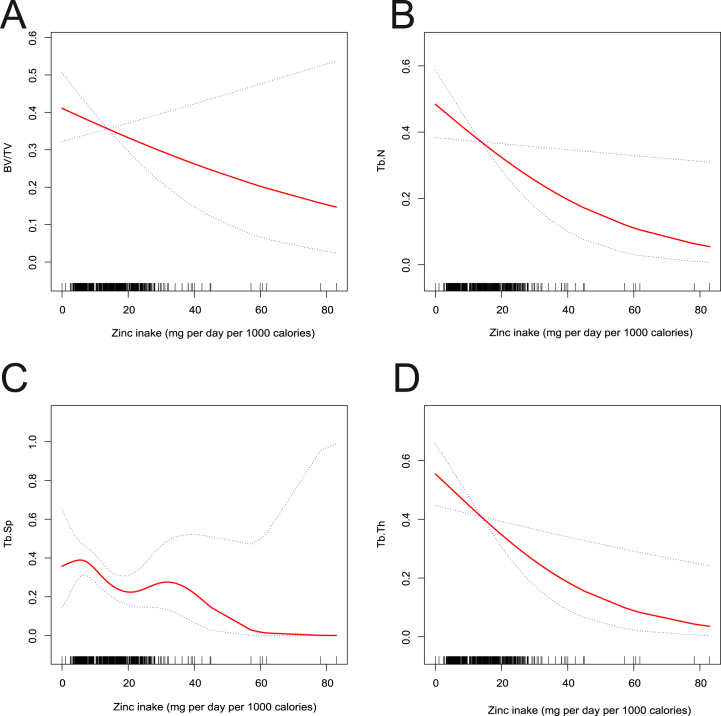
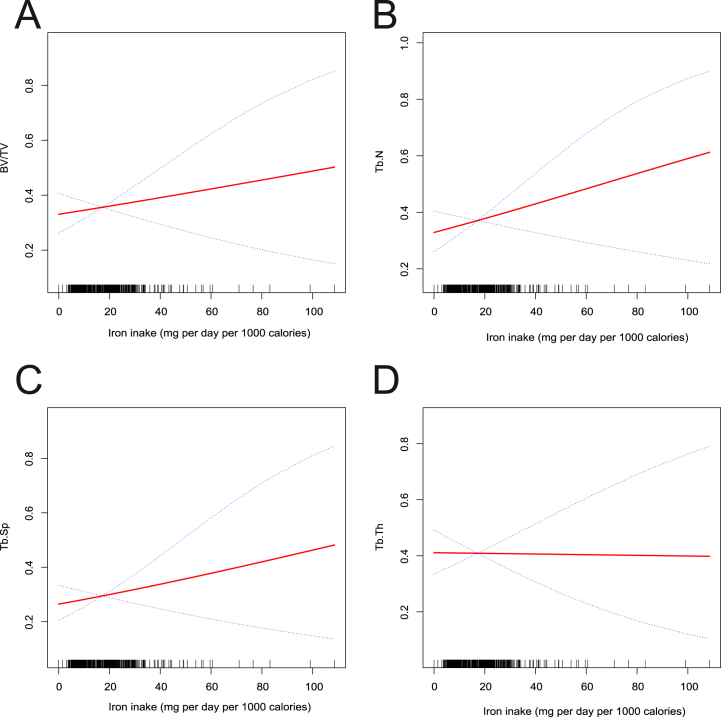
To delve deeper into the intricate relationship between zinc, iron intake, and tibial subchondral sclerosis, we employed a generalized additive model with smoothing curve fitting. Fig. 2 illustrates that increased zinc intake significantly lowers the likelihood of deterioration in Tb.N (Fig. 2(B)), Tb.Sp (Fig. 2(C)), Tb.Th (Fig. 2(D)), and to some extent, BV/TV (Fig. 2(A)). These results align with the findings obtained from logistic regression analysis. Conversely, Fig. 3 demonstrates that higher iron intake moderately accelerates the likelihood of deterioration in BV/TV (Fig. 3(A)), Tb.N (Fig. 3(B)), and Tb.Sp (Fig. 3(C)), but this trend was not significant for Tb.Th (Fig. 3(D)).
Fig. 2.
Fitted smoothing curves based on generalized additive models with the x-axis representing zinc intake and the y-axis representing the likelihood of progression of tibial trabecular sclerosis. (A) BV/TV - Bone volume fraction, (B) Tb.N - Trabecular number, (C) Tb.Sp - Trabecular separation, (D) Tb.Th - Trabecular thickness.
Fig. 3.
Fitted smoothing curves based on generalized additive models with the x-axis representing iron intake and the y-axis representing the likelihood of progression of tibial trabecular sclerosis. (A) BV/TV - Bone volume fraction, (B) Tb.N - Trabecular number, (C) Tb.Sp - Trabecular separation, (D) Tb.Th - Trabecular thickness.
3.3. Further subgroup analysis
In order to customize the nutritional treatment strategy for OA across different populations, a subgroup analysis was conducted by stratifying the study population based on sex, age (65 years), radiographic severity (KL grade), and macronutrient intake, with a particular focus on calcium and magnesium, which have a strong association with bone metabolism [29]. This approach aimed to identify specific dietary interventions that would be most effective for each subgroup.
The findings presented in Table 3 reveal the outcomes of the interaction analysis performed within the framework of subgroup stratification. It is observed that the calculated probability (P) values for interaction, when stratified by variables such as sex, age, imaging severity, and magnesium intake, all exceeded the critical threshold of 0.05. However, in the case of calcium intake, the P values for interaction pertaining to Tb.N and Tb.Sp were found to be less than 0.05. This significant difference suggests that among individuals with higher calcium intake, the beneficial impact of zinc intake becomes more pronounced. Consequently, it can be surmised that the effectiveness of zinc supplementation in mitigating the progression of subchondral sclerosis is contingent, to some extent, upon a heightened intake of calcium.
Table 3.
Subgroup analysis and interaction test.
| Middle-aged | Elderly | P for interaction | |
|---|---|---|---|
| For BVF1 | 0.976 (0.944, 1.009) 0.1543 | 0.996 (0.957, 1.036) 0.8338 | 0.3758 |
| For TRN1 | 0.961 (0.927, 0.996) 0.0313 | 0.980 (0.941, 1.021) 0.3365 | 0.3928 |
| For TRTH1 | 0.962 (0.929, 0.996) 0.0277 | 0.947 (0.906, 0.990) 0.0165 | 0.5253 |
| For TRSP1 | 0.962 (0.929, 0.997) 0.0343 | 0.945 (0.899, 0.994) 0.0281 | 0.4851 |
|
Male |
Female |
||
| For BVF1 | 0.996 (0.960, 1.032) 0.8062 | 0.971 (0.936, 1.007) 0.1183 | 0.2692 |
| For TRN1 | 0.963 (0.925, 1.003) 0.0665 | 0.969 (0.935, 1.004) 0.0832 | 0.7853 |
| For TRTH1 | 0.957 (0.918, 0.997) 0.0334 | 0.956 (0.920, 0.994) 0.0226 | 0.9922 |
| For TRSP1 | 0.969 (0.928, 1.013) 0.1651 | 0.952 (0.914, 0.991) 0.0176 | 0.4716 |
|
KL grade: 0,1 |
KL grade: 2,3,4 |
||
| For BVF1 | 0.969 (0.920, 1.020) 0.2280 | 0.986 (0.958, 1.016) 0.3555 | 0.4862 |
| For TRN1 | 0.960 (0.913, 1.009) 0.1108 | 0.969 (0.939, 1.001) 0.0540 | 0.7052 |
| For TRTH1 | 0.962 (0.915, 1.012) 0.1311 | 0.955 (0.923, 0.988) 0.0084 | 0.7836 |
| For TRSP1 | 0.954 (0.903, 1.008) 0.0934 | 0.960 (0.927, 0.995) 0.0255 | 0.8129 |
|
Low calcium intake |
High calcium intake |
||
| For BVF1 | 1.011 (0.968, 1.057) 0.6222 | 0.971 (0.939, 1.005) 0.0917 | 0.1119 |
| For TRN1 | 1.003 (0.959, 1.049) 0.8980 | 0.949 (0.913, 0.986) 0.0076 | 0.0333 |
| For TRTH1 | 0.978 (0.933, 1.024) 0.3449 | 0.946 (0.911, 0.983) 0.0048 | 0.2207 |
| For TRSP1 | 1.006 (0.959, 1.056) 0.7991 | 0.935 (0.894, 0.977) 0.0030 | 0.01 |
|
Low magnesium intake |
High magnesium intake |
||
| For BVF1 | 1.004 (0.966, 1.044) 0.8232 | 0.969 (0.932, 1.007) 0.1062 | 0.1713 |
| For TRN1 | 0.962 (0.918, 1.009) 0.1126 | 0.971 (0.937, 1.007) 0.1158 | 0.7517 |
| For TRTH1 | 0.979 (0.939, 1.020) 0.3070 | 0.942 (0.901, 0.984) 0.0076 | 0.186 |
| For TRSP1 | 0.968 (0.920, 1.019) 0.2124 | 0.957 (0.918, 0.997) 0.0343 | 0.7087 |
Adjusted for age, sex, race, BMI, Kellgren-Lawrence (KL) grade, and PASE score, alcohol use, smoking status, history of bone fracture after the age of 45, history of hip fracture, history of vertebral/spine fracture, history of oral corticosteroid medication use, history of bisphosphonate intake, intact parathyroid hormone level, serum 25-vitamin D level and calcium and magnesium intake.
In order to examine the impact of zinc and iron intake on various subgroups within the population, a stratified analysis utilizing curve-fitting techniques was conducted. Supplementary Fig. S1 illustrates the findings, indicating that heightened zinc consumption among women leads to a more substantial postponement of subchondral bone deterioration compared to men. This effect is evident in the parameters BV/TV, Tb.Th, Tb.N, and Tb.Sp. Furthermore, when considering age and imaging-based OA progression, it was observed that increased zinc intake offers a more consistent and enduring benefit in middle-aged patients and those with more advanced imaging OA progression (Supplementary Figs. S2 and 3). Notably, similar to the outcomes of the interaction analysis, patients with OA who have higher calcium and magnesium consumption experience a greater delay in subchondral sclerosis as a result of increased zinc intake (Supplementary Figs. S4 and 5).
A stratified generalized additive model was employed to investigate the impact of iron intake. Supplementary Figs. S1 and 2 provides a visual representation of the results, indicating that men and middle-aged individuals may experience a heightened acceleration of subchondral sclerosis in response to excessive iron intake, in comparison to women and older populations. Moreover, among individuals with less progressive OA, excessive iron intake may further exacerbate the progression of subchondral bone sclerosis (Supplementary Fig. S3). Additionally, as macronutrient intake, including calcium and magnesium, plays a significant role in OA, it was utilized as a stratification factor. The analysis revealed that increased iron intake led to an acceleration of subchondral sclerosis in individuals with lower magnesium intake. However, no significant difference was observed between the groups with higher and lower calcium intake (Supplementary Figs. S4 and 5).
4. Discussion
Our investigation, grounded in an extensive cohort study of OA, presents novel insights into the significant delay in the progression of osteoarthritic subchondral sclerosis as a result of increased zinc intake. This delay is particularly marked in female and middle-aged patients, individuals with higher calcium and magnesium intake, and patients with more severe OA as determined by imaging. On the contrary, increased iron intake intensifies osteoarthritic subchondral sclerosis to a certain degree. Additionally, interaction studies from subgroup analysis propose that the efficacy of zinc in decelerating the progression of subchondral sclerosis largely depends on adequate calcium intake.
The "osteoarticular junction," a tight functional unit comprising articular cartilage and subchondral bone, is a site of significant biomechanical and biochemical interactions [30]. Pathological changes, such as microfractures from biomechanical overloading, often start in the subchondral bone, triggering a remodeling process that increases bone stiffness and accelerates cartilage degeneration [31]. Unlike the slow-metabolizing cartilage, the bone tissue, with its high metabolic activity, is capable of regeneration and remodeling [32]. Thus, investigating micronutrients, like zinc, that influence subchondral bone remodeling in OA could reveal potential intervention targets.
Elevated zinc intake's ability to resist subchondral bone changes is noteworthy. Zinc, predominantly present at the mineralization front of articular cartilage, particularly in the tidemark region, is closely linked to metabolic activity there [33]. It provides several benefits such as enhancing chondrocyte survival via the direct activation of the PI3K-Akt signaling pathway [34], thus boosting matrix synthesis [35]. Zinc also encourages chondrocyte proliferation, aids in mesenchymal stem cell differentiation into chondrocytes, and stimulates cartilage growth and maturation [36]. Its activation of internal antioxidant defense systems inhibits proinflammatory cytokines' secretion and reduces matrix metalloproteinases production within chondrocytes [37]. Zinc plays a key role in bone development and growth by activating proteins involved in bone homeostasis [38], using zinc fingers or clusters, via 14 Zrt- and Irt-like protein (ZIP) family members and 10 zinc transporter (ZnT) family members, to maintain bone homeostasis [39]. This dual modulatory action on both joint and bone could be a critical mechanism to hinder the progression of subchondral bone lesions.
Iron overload often exacerbates subchondral bone lesions in OA by increasing oxidative capacity and pro-inflammatory cytokines, leading to joint damage [40]. It induces chondrocyte apoptosis and matrix destruction [41], reduces the expression of certain transcripts, and promotes osteoclast differentiation, all contributing to an OA phenotype [42]. Additionally, it disrupts the balance between osteoblasts and osteoclasts by inhibiting osteogenic differentiation and inducing autophagy and apoptosis in osteoblasts [43]. The remodeling effect of subchondral bone thus occurs via multiple mechanisms.
Treatment paradigms for OA predominantly focus on the reduction of pain and inflammation related to disease activity. However, pain medications fail to prevent damage to cartilage and associated joint structures, or inhibit further disease progression [44]. Over time, analgesic treatments lose their efficacy and necessitate increased dosages, leading to heightened toxicity and side effects [45]. Large-scale studies demonstrate that the usage of painkillers such as tramadol significantly diminishes patients' life expectancy [46]. Nutritional therapy, due to its role in inflammatory, immune, and oxidative processes, along with the safety of nutritional interventions, has yielded promising results [47]. Our study corroborates those dietary interventions with iron and zinc can defer the onset of subchondral osteosclerosis in OA to a certain degree, thereby decelerating the progression of OA.
Additional stratification studies have the potential to identify sensitive populations for nutritional interventions, thus enhancing efficacy. Our stratified subgroup analysis illustrates that increased zinc intake may yield substantial benefits, particularly among individuals with more severe OA, as verified by imaging, and those with a higher calcium intake. The statistical significance of interaction test results relating to calcium intake underscores the necessity of appropriate calcium supplementation when implementing zinc supplementation as a preventive or therapeutic strategy aimed at slowing the progression of subchondral bone lesions in OA. According to our stratified analysis, the aggravation of subchondral sclerosis due to excessive iron intake is more noticeable in men and middle-aged individuals than in women and the elderly demographic. In individuals with less severe OA, as determined via imaging, excess iron may hasten the progression of subchondral sclerosis. Further experimental verification and interpretation are necessary to clarify the specific biological mechanisms underpinning these observations.
Our study boasts several strengths. Primarily, the Osteoarthritis Initiative (OAI) cohort, a comprehensive and well-established OA cohort, offers stringent questionnaire data standards alongside reliable follow-up radiographic data. Secondly, considering that subchondral bone trabeculae remodel under increased loading, the measurement of subchondral bone microarchitecture using Fast Imaging with Steady-state Precession (FISP) MRI incorporated in this study serves as an optimal indicator for monitoring subchondral bone lesions in OA. Lastly, our study employed the statistically robust and highly flexible Generalized Additive Model (GAM), thereby ensuring optimized data utilization and enhanced robustness of results.
Iron and zinc, as materials for orthopedic implants, have attracted considerable interest in both existing and future-generation alloys. The current study furnishes compelling nutritional evidence underscoring the critical protective role of zinc in preserving articular subchondral bone. The utilization of the extensive and reliable OAI cohort has ensured a robust dataset comprising both radiographic and questionnaire data, supplemented by long-term follow-up. Precise identification of subchondral bone lesions was achieved through the application of optimized MRI sequences targeting the subchondral trabeculae, which show heightened sensitivity in lesion detection. Furthermore, stratifying the study population into subgroups facilitated the identification of individuals exhibiting the most favorable response, paving the way for the development of personalized dietary treatment strategies [48].
The current study provides compelling nutritional evidence that underscores the crucial protective role of zinc in preserving articular subchondral bone. Moreover, the utilization of the well-established and substantial OA Initiative (OAI) cohort has ensured a robust and reliable dataset encompassing both radiographic and questionnaire data, supplemented by long-term follow-up. Precise measurements of subchondral bone lesions were achieved through the application of optimized MRI sequences targeting the subchondral trabeculae, which exhibit heightened sensitivity in their identification. Furthermore, the stratification of the study population into subgroups facilitated the identification of individuals displaying the most favorable response, enabling the development of personalized dietary treatment strategies.
Nevertheless, it is important to acknowledge the limitations of our study. Firstly, dietary estimates derived from the FFQ, although validated, rely on self-reported data, which may introduce inherent bias. Secondly, the role of iron in subchondral bone remodeling warrants further exploration, restricted by the constraints of follow-up duration and sample size. Thirdly, being a secondary study based on the OAI database, precise sample size calculations were not conducted, and selection of the included population was not feasible. Despite these constraints, previous studies have demonstrated considerable reliability in analyzing the population data of OA patients included in the Bone Ancillary Study (BAS) project for OAI [17,23]. Lastly, extrapolation of our findings to other populations should be approached with caution, as the OAI cohort comprises data from an adult cohort in the United States. Therefore, validation of our results through additional investigations involving diverse cohorts or clinical trials will be indispensable in the future.
5. Conclusion
In summary, our study findings provide evidence of the significant protective effect of increased zinc intake against tibial subchondral bone deterioration in individuals with OA. Conversely, higher iron intake is associated with a moderate acceleration of subchondral bone deterioration. Subgroup analysis reveals that the beneficial impact of zinc intake is particularly prominent in women, middle-aged patients, and those with advanced OA. Moreover, calcium intake demonstrates an interactive effect with zinc, suggesting a potential synergistic relationship. It is noteworthy that excessive iron intake exacerbates subchondral sclerosis, especially in men, middle-aged individuals, and those with less progressive OA. These findings emphasize the need for further research to validate the results and explore personalized dietary interventions in this context.
Ethics approval and consent to participate
The Institutional Review Boards of the University of California, San Francisco, as well as the four clinical centers of the Osteoarthritis Initiative (OAI) — namely, the University of Pittsburgh, Ohio State University, University of Maryland, Baltimore, and Memorial Hospital of Rhode Island — have granted approval for the OAI. All participants provided written informed consent before participating.
Role of funding source.
This study was supported by Non-profit Central Research Institute Fund of Beijing Hospital (BJ-2018-088) and National Key R&D Program of China (No. 2020YFC2008500).
The study and image acquisition were funded by the OAI, a public-private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This manuscript was prepared using an OAI public use dataset and does not necessarily reflect the opinions or views of the OAI investigators, the NIH, or the private funding partners. None of the study sponsors had any role in data collection, storage or analysis, in manuscript writing, or the decision to publish this manuscript.
Consent for publication
All participants provided written informed consent before participation.
Data availability statement
Data will be made available on request and the data used in this study can be downloaded for free in OAI.
CRediT authorship contribution statement
Zitian Zheng: Writing – review & editing, Writing – original draft, Validation, Software, Methodology, Investigation, Data curation, Conceptualization. Huanhuan Luo: Formal analysis, Data curation. Chao Sun: Validation, Supervision. Qingyun Xue: Writing – review & editing, Writing – original draft, Visualization, Supervision, Software.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank the OAI investigators, clinical staff, and OAI participants at each of the OAI clinical centers for their contributions in acquiring the publicly available clinical and imaging data.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e22046.
Abbreviations
- OAI
Osteoarthritis Initiative
- OA
Osteoarthritis KL Kellgren-Lawrence BAS Bone Ancillary Study
- MRI
Magnetic Resonance Imaging
- FFQ
Food Frequency Questionnaire
- BV/TV
Bone Volume Fraction
- Tb.Th
Trabecular Thickness
- Tb.N
Trabecular Number
- Tb.Sp
Trabecular Separation
- OARSI
Osteoarthritis Research Society International atlas grade
- FFQ
Food Frequency Questionnaire
- FISP
Fast Imaging with Steady-state Free Precession
- ROI
Region of Interest
- PASE
Physical Activity Scale for the Elderly
- BMI
Body Mass Index
- PTH
Parathyroid hormone
- RCI
Reliable Change Index
- CI
Confidence Interval
- ZIP
Zrt- and Irt-like protein
- ZnT
Zinc transporter
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Mandl L.A. Osteoarthritis year in review 2018: clinical. Osteoarthritis Cartilage. 2019;27(3):359–364. doi: 10.1016/j.joca.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Litwic A., Edwards M.H., Dennison E.M., Cooper C. Epidemiology and burden of osteoarthritis. Br. Med. Bull. 2013;105:185–199. doi: 10.1093/bmb/lds038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li G., Yin J., Gao J., Cheng T.S., Pavlos N.J., Zhang C., Zheng M.H. Subchondral bone in osteoarthritis: insight into risk factors and microstructural changes. Arthritis Res. Ther. 2013;15(6):223. doi: 10.1186/ar4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu W., Chen Y., Dou C., Dong S. Microenvironment in subchondral bone: predominant regulator for the treatment of osteoarthritis. Ann. Rheum. Dis. 2021;80(4):413–422. doi: 10.1136/annrheumdis-2020-218089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu S., Zhu J., Zhen G., Hu Y., An S., Li Y., Zheng Q., Chen Z., Yang Y., Wan M., et al. Subchondral bone osteoclasts induce sensory innervation and osteoarthritis pain. J. Clin. Invest. 2019;129(3):1076–1093. doi: 10.1172/JCI121561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vincent T.L. Mechanoflammation in osteoarthritis pathogenesis. Semin. Arthritis Rheum. 2019;49(3s):S36–s38. doi: 10.1016/j.semarthrit.2019.09.018. [DOI] [PubMed] [Google Scholar]
- 7.Henrotin Y., Pesesse L., Sanchez C. Subchondral bone and osteoarthritis: biological and cellular aspects. Osteoporos. Int. 2012;23(Suppl 8):S847–S851. doi: 10.1007/s00198-012-2162-z. [DOI] [PubMed] [Google Scholar]
- 8.Kon E., Ronga M., Filardo G., Farr J., Madry H., Milano G., Andriolo L., Shabshin N. Bone marrow lesions and subchondral bone pathology of the knee. Knee Surg. Sports Traumatol. Arthrosc. 2016;24(6):1797–1814. doi: 10.1007/s00167-016-4113-2. [DOI] [PubMed] [Google Scholar]
- 9.Li M., Zeng Y., Nie Y., Wu Y., Liu Y., Wu L., Shen B. Do knee-straining activities influence the subchondral bone microarchitecture and accelerate knee osteoarthritis progression? Am. J. Phys. Med. Rehabil. 2022;101(11) doi: 10.1097/PHM.0000000000001958. [DOI] [PubMed] [Google Scholar]
- 10.Driban J.B., Tassinari A., Lo G.H., Price L.L., Schneider E., Lynch J.A., Eaton C.B., McAlindon T.E. Bone marrow lesions are associated with altered trabecular morphometry. Osteoarthritis Cartilage. 2012;20(12):1519–1526. doi: 10.1016/j.joca.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li G., Cheng T., Yu X. The impact of trace elements on osteoarthritis. Front. Med. 2021;8 doi: 10.3389/fmed.2021.771297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martínez-Nava G.A., Mendoza-Soto L., Fernández-Torres J., Zamudio-Cuevas Y., Reyes-Hinojosa D., Plata-Rodríguez R., Olivos-Meza A., Ruíz-Huerta E.A., Armienta-Hernández M.A., Hernández-Álvarez E., et al. Effect of cadmium on the concentration of essential metals in a human chondrocyte micromass culture. J. Trace Elem. Med. Biol. 2020;62 doi: 10.1016/j.jtemb.2020.126614. [DOI] [PubMed] [Google Scholar]
- 13.Żaneta C., Danuta K.B., Natalia Ł A., Karolina K., Maciej K., Paweł Z., Patrycja K., Aleksandra S., Iwona R. Concentration of selected elements in the infrapatellar fat pad of patients with a history of total knee arthroplasty. Int. J. Environ. Res. Publ. Health. 2019;16(10) doi: 10.3390/ijerph16101734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang T., Yan G., Guan M. Zinc homeostasis in bone: zinc transporters and bone diseases. Int. J. Mol. Sci. 2020;21(4) doi: 10.3390/ijms21041236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun K., Guo Z., Hou L., Xu J., Du T., Xu T., Guo F. Iron homeostasis in arthropathies: from pathogenesis to therapeutic potential. Ageing Res. Rev. 2021;72 doi: 10.1016/j.arr.2021.101481. [DOI] [PubMed] [Google Scholar]
- 16.Hunter D.J., Spector T.D. The role of bone metabolism in osteoarthritis. Curr. Rheumatol. Rep. 2003;5(1):15–19. doi: 10.1007/s11926-003-0078-5. [DOI] [PubMed] [Google Scholar]
- 17.Lo G.H., Merchant M.G., Driban J.B., Duryea J., Price L.L., Eaton C.B., McAlindon T.E. Knee alignment is quantitatively related to periarticular bone morphometry and density, especially in patients with osteoarthritis. Arthritis Rheumatol. 2018;70(2):212–221. doi: 10.1002/art.40325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunter D.J., Niu J., Zhang Y., Totterman S., Tamez J., Dabrowski C., Davies R., Le Graverand M.P., Luchi M., Tymofyeyev Y., et al. Change in cartilage morphometry: a sample of the progression cohort of the Osteoarthritis Initiative. Ann. Rheum. Dis. 2009;68(3):349–356. doi: 10.1136/ard.2007.082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Block G., Hartman A.M., Naughton D. A reduced dietary questionnaire: development and validation. Epidemiology. 1990;1(1):58–64. doi: 10.1097/00001648-199001000-00013. [DOI] [PubMed] [Google Scholar]
- 20.Block G., Hartman A.M., Dresser C.M., Carroll M.D., Gannon J., Gardner L. A data-based approach to diet questionnaire design and testing. Am. J. Epidemiol. 1986;124(3):453–469. doi: 10.1093/oxfordjournals.aje.a114416. [DOI] [PubMed] [Google Scholar]
- 21.Shmagel A., Onizuka N., Langsetmo L., Vo T., Foley R., Ensrud K., Valen P. Low magnesium intake is associated with increased knee pain in subjects with radiographic knee osteoarthritis: data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2018;26(5):651–658. doi: 10.1016/j.joca.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Schneider E., Lo G.H., Sloane G., Fanella L., Hunter D.J., Eaton C.B., McAlindon T.E. Magnetic resonance imaging evaluation of weight-bearing subchondral trabecular bone in the knee. Skeletal Radiol. 2011;40(1):95–103. doi: 10.1007/s00256-010-0943-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li M., Nie Y., Zeng Y., Wu Y., Liu Y., Wu L., Xu J., Shen B. Does bisphosphonate increase the sclerosis of tibial subchondral bone in the progression of knee osteoarthritis-A propensity score matching cohort study based on osteoarthritis initiative. Front. Med. 2021;8 doi: 10.3389/fmed.2021.781219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacKay J.W., Kapoor G., Driban J.B., Lo G.H., McAlindon T.E., Toms A.P., McCaskie A.W., Gilbert F.J. Association of subchondral bone texture on magnetic resonance imaging with radiographic knee osteoarthritis progression: data from the Osteoarthritis Initiative Bone Ancillary Study. Eur. Radiol. 2018;28(11):4687–4695. doi: 10.1007/s00330-018-5444-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lo G.H., Tassinari A.M., Driban J.B., Price L.L., Schneider E., Majumdar S., McAlindon T.E. Cross-sectional DXA and MR measures of tibial periarticular bone associate with radiographic knee osteoarthritis severity. Osteoarthritis Cartilage. 2012;20(7):686–693. doi: 10.1016/j.joca.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haj-Mirzaian A., Guermazi A., Roemer F.W., Bowes M.A., Conaghan P.G., Demehri S. Bisphosphonates intake and its association with changes of periarticular bone area and three-dimensional shape: data from the Osteoarthritis Initiative (OAI) Osteoarthritis Cartilage. 2018;26(4):564–568. doi: 10.1016/j.joca.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 27.Sun Q., Zhen G., Li T.P., Guo Q., Li Y., Su W., Xue P., Wang X., Wan M., Guan Y., et al. Parathyroid hormone attenuates osteoarthritis pain by remodeling subchondral bone in mice. Elife. 2021;10 doi: 10.7554/eLife.66532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haj-Mirzaian A., Guermazi A., Hafezi-Nejad N., Sereni C., Hakky M., Hunter D.J., Zikria B., Roemer F.W., Demehri S. Superolateral Hoffa's fat pad (SHFP) oedema and patellar cartilage volume loss: quantitative analysis using longitudinal data from the Foundation for the National Institute of Health (FNIH) Osteoarthritis Biomarkers Consortium. Eur. Radiol. 2018;28(10):4134–4145. doi: 10.1007/s00330-018-5334-1. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y., Chen T., Luo P., Li S., Zhu J., Xue S., Cao P., Zhu Z., Li J., Wang X., et al. Associations of dietary macroelements with knee joint structures, symptoms, quality of life, and comorbid conditions in people with symptomatic knee osteoarthritis. Nutrients. 2022;14(17) doi: 10.3390/nu14173576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldring S.R., Goldring M.B. Changes in the osteochondral unit during osteoarthritis: structure, function and cartilage-bone crosstalk. Nat. Rev. Rheumatol. 2016;12(11):632–644. doi: 10.1038/nrrheum.2016.148. [DOI] [PubMed] [Google Scholar]
- 31.Zhang L.Z., Zheng H.A., Jiang Y., Tu Y.H., Jiang P.H., Yang A.L. Mechanical and biologic link between cartilage and subchondral bone in osteoarthritis. Arthritis Care Res. 2012;64(7):960–967. doi: 10.1002/acr.21640. [DOI] [PubMed] [Google Scholar]
- 32.Holzer L.A., Kraiger M., Talakic E., Fritz G.A., Avian A., Hofmeister A., Leithner A., Holzer G. Microstructural analysis of subchondral bone in knee osteoarthritis. Osteoporos. Int. 2020;31(10):2037–2045. doi: 10.1007/s00198-020-05461-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roschger A., Hofstaetter J.G., Pemmer B., Zoeger N., Wobrauschek P., Falkenberg G., Simon R., Berzlanovich A., Thaler H.W., Roschger P., et al. Differential accumulation of lead and zinc in double-tidemarks of articular cartilage. Osteoarthritis Cartilage. 2013;21(11):1707–1715. doi: 10.1016/j.joca.2013.06.029. [DOI] [PubMed] [Google Scholar]
- 34.Yang Y., Wang Y., Zhao M., Jia H., Li B., Xing D. Tormentic acid inhibits IL-1β-induced chondrocyte apoptosis by activating the PI3K/Akt signaling pathway. Mol. Med. Rep. 2018;17(3):4753–4758. doi: 10.3892/mmr.2018.8425. [DOI] [PubMed] [Google Scholar]
- 35.Brodziak-Dopierała B., Kwapuliński J., Sobczyk K., Wiechuła D. The content of manganese and iron in hip joint tissue. J. Trace Elem. Med. Biol. 2013;27(3):208–212. doi: 10.1016/j.jtemb.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 36.Huang T.C., Chang W.T., Hu Y.C., Hsieh B.S., Cheng H.L., Yen J.H., Chiu P.R., Chang K.L. Zinc protects articular chondrocytes through changes in nrf2-mediated antioxidants, cytokines and matrix metalloproteinases. Nutrients. 2018;10(4) doi: 10.3390/nu10040471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mariani E., Mangialasche F., Feliziani F.T., Cecchetti R., Malavolta M., Bastiani P., Baglioni M., Dedoussis G., Fulop T., Herbein G., et al. Effects of zinc supplementation on antioxidant enzyme activities in healthy old subjects. Exp. Gerontol. 2008;43(5):445–451. doi: 10.1016/j.exger.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 38.Rył A., Miazgowski T., Szylińska A., Turoń-Skrzypińska A., Jurewicz A., Bohatyrewicz A., Rotter I. Bone Health in aging men: does zinc and cuprum level matter? Biomolecules. 2021;11(2) doi: 10.3390/biom11020237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kambe T., Tsuji T., Hashimoto A., Itsumura N. The physiological, biochemical, and molecular roles of zinc transporters in zinc homeostasis and metabolism. Physiol. Rev. 2015;95(3):749–784. doi: 10.1152/physrev.00035.2014. [DOI] [PubMed] [Google Scholar]
- 40.Tchetina E.V., Markova G.A., Poole A.R., Zukor D.J., Antoniou J., Makarov S.A., Kuzin A.N. Deferoxamine suppresses collagen cleavage and protease, cytokine, and COL10A1 expression and upregulates AMPK and krebs cycle genes in human osteoarthritic cartilage. Internet J. Rheumatol. 2016;2016 doi: 10.1155/2016/6432867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simão M., Gavaia P.J., Camacho A., Porto G., Pinto I.J., Ea H.K., Cancela M.L. Intracellular iron uptake is favored in Hfe-KO mouse primary chondrocytes mimicking an osteoarthritis-related phenotype. Biofactors. 2019;45(4):583–597. doi: 10.1002/biof.1520. [DOI] [PubMed] [Google Scholar]
- 42.Burton L.H., Radakovich L.B., Marolf A.J., Santangelo K.S. Systemic iron overload exacerbates osteoarthritis in the strain 13 Guinea pig. Osteoarthritis Cartilage. 2020;28(9):1265–1275. doi: 10.1016/j.joca.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang J., Dong D., Luo X., Zhou J., Shang P., Zhang H. Iron overload-induced osteocyte apoptosis stimulates osteoclast differentiation through increasing osteocytic RANKL production in vitro. Calcif. Tissue Int. 2020;107(5):499–509. doi: 10.1007/s00223-020-00735-x. [DOI] [PubMed] [Google Scholar]
- 44.Felson D. Tramadol and mortality in patients with osteoarthritis. JAMA. 2019;322(5):465–466. doi: 10.1001/jama.2019.7216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bodden J., Joseph G.B., Schirò S., Lynch J.A., Lane N.E., McCulloch C.E., Nevitt M.C., Link T.M. Opioid users show worse baseline knee osteoarthritis and faster progression of degenerative changes: a retrospective case-control study based on data from the Osteoarthritis Initiative (OAI) Arthritis Res. Ther. 2021;23(1):146. doi: 10.1186/s13075-021-02524-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xie J., Strauss V.Y., Martinez-Laguna D., Carbonell-Abella C., Diez-Perez A., Nogues X., Collins G.S., Khalid S., Delmestri A., Turkiewicz A., et al. Association of tramadol vs codeine prescription dispensation with mortality and other adverse clinical outcomes. JAMA. 2021;326(15):1504–1515. doi: 10.1001/jama.2021.15255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Farì G., Megna M., Scacco S., Ranieri M., Raele M.V., Chiaia Noya E., Macchiarola D., Bianchi F.P., Carati D., Panico S., et al. Hemp seed oil in association with β-caryophyllene, myrcene and ginger extract as a nutraceutical integration in knee osteoarthritis: a double-blind prospective case-control study. Medicina (Kaunas) 2023;59(2) doi: 10.3390/medicina59020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Su Y., Cockerill I., Wang Y., Qin Y.X., Chang L., Zheng Y., Zhu D. Zinc-based biomaterials for regeneration and therapy. Trends Biotechnol. 2019;37(4):428–441. doi: 10.1016/j.tibtech.2018.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request and the data used in this study can be downloaded for free in OAI.