Abstract
Introduction
The role of oscillometry in interstitial lung disease (ILD) is still unclear. The main objective of our study was to describe the parameters determined by oscillometry in these patients and compare them with those obtained in conventional respiratory function tests.
Methods
This was a cross-sectional observational study. Patients with no respiratory disease and patients being followed up for ILD in the specialist unit of Hospital Universitario de Getafe, Madrid were included.
Results
A total of 45 individuals were analyzed. Fifteen had no respiratory disease, 15 were ILD patients with mild functional impairment, and another 15 were ILD patients with severe impairment. None of the participants had an obstructive pattern on spirometry.
Comparison between the three groups showed statistically significant differences in the values of R5-19, reactance at 5 Hz and reactance at 11 Hz. No differences were observed between the three groups in Delta Xrs. The study showed a strong correlation between total and inspiratory reactance at 5 Hz and forced vital capacity and diffusing capacity for carbon monoxide.
Conclusions
Our results suggest that the findings in ILD are characteristic of this disease and that they differ from those found in other diseases such as chronic obstructive pulmonary disease. It also seems that there are differences according to the degree of functional impairment of the patients. The results show a strong correlation with standard pulmonary function tests, so oscillometry could be a useful tool in patients with ILD who are unable to perform it, and could provide additional information.
Keywords: Lung function, ILD, Physiology, Reactance
Abstract
Introducción
El papel de la oscilometría en la enfermedad pulmonar intersticial difusa (EPID) aún no está claro. El objetivo principal de nuestro estudio fue describir los parámetros determinados por oscilometría en estos pacientes y compararlos con los obtenidos en las pruebas de función respiratoria convencionales.
Métodos
Se trata de un estudio observacional transversal. Se incluyeron tanto pacientes sin enfermedad respiratoria como pacientes con EPID en seguimiento en la unidad especializada del Hospital Universitario de Getafe, Madrid.
Resultados
Se analizaron un total de 45 individuos. Quince no tenían ninguna enfermedad respiratoria, 15 eran pacientes con EPID y deterioro funcional leve, y otros 15 eran pacientes con EPID con deterioro grave. Ninguno de los participantes tenía un patrón obstructivo en la espirometría.
La comparación entre los tres grupos mostró diferencias estadísticamente significativas en los valores de R5-19, Reactancia a 5 Hz y Reactancia a 11 Hz. No se observaron diferencias entre los tres grupos en Delta Xrs. El estudio mostró una fuerte correlación entre la reactancia total e inspiratoria a 5 Hz y la capacidad vital forzada y capacidad de difusión del monóxido de carbono.
Conclusiones
Nuestros resultados sugieren que los hallazgos en EPID son característicos de esta enfermedad y que difieren de los que se encuentran en otras enfermedades como la enfermedad pulmonar obstructiva crónica. También parece que existen diferencias según el grado de deterioro funcional de los pacientes. Los resultados muestran una fuerte correlación con las pruebas estándar de función pulmonar, por lo que la oscilometría podría ser una herramienta útil en pacientes con EPID que no pueden realizarla, y podría aportar información adicional.
Palabras clave: Función pulmonar, EPID, Fisiología, Reactancia
Introduction
Respiratory function tests are key in routine clinical practice to rule out respiratory diseases. Oscillometry is a non-invasive technique, first described in 1953 by DuBois1 for measuring the mechanical properties of the respiratory system. It involves applying small pressure oscillations at different frequencies to the airway openings during quiet normal breathing. These pressures and airflow changes are analyzed by a pneumotachograph for subsequent interpretation of the results.
Until now, studies have focused primarily on children, due to the simplicity of the technique and the limited cooperation required. However, in recent years there has been an increased interest in the use of oscillometry in the adult population, especially in obstructive airway diseases.
Several studies have shown significant differences in lung function measured by oscillometry between patients with bronchial asthma or chronic obstructive pulmonary disease (COPD)2, 3 and healthy volunteers. However, further results are still needed before this technique can be incorporated into daily practice.4
Scant evidence is available on the use of this technique in interstitial lung disease (ILD). Existing data suggest that in these diseases, there is an increase in 5 Hz resistances and pulmonary reactance, reflecting total resistances and elastic properties, respectively.5, 6
These results focus mainly on idiopathic pulmonary fibrosis (IPF), the most widely studied disease, although they are also consistent with larger studies, such as that recently published by Liang et al.7 that compares both obstructive and restrictive lung diseases.
Another relevant finding in ILD is the difference between inspiratory and expiratory 5-Hz reactance. In these patients, a greater magnitude of X5 has been observed in inspiration than in expiration, which could help to differentiate it from obstructive airway diseases, since in COPD the expiratory X5 is increased, while in asthma no differences are observed between inspiration and expiration.8
Despite all this, oscillometry is still used more in research than in daily clinical practice, given the lack of cut-off points from which to determine the existence of disease and its severity.
The main objective of our study was to describe the parameters determined by oscillometry in diffuse interstitial diseases and determine any differences depending on the severity of the disease.
Material and methods
Study design and patients
This was a cross-sectional observational study conducted between August 1, 2021 and December 1, 2022.
Information was gathered from 45 patients: 15 had no respiratory disease (NRD) and 30 patients were being followed up for ILD in the specialist unit of the Hospital Universitario de Getafe, Madrid; half had mild functional impairment (MI) and half had severe functional impairment (SI). All patients who participated in the study were diagnosed according to current clinical practice guidelines, after being evaluated by a multidisciplinary committee.
The severity of functional impairment was established on the basis of forced vital capacity (FVC) and diffusing capacity for carbon monoxide (DLCO). The MI group had FVC and DLCO equal to or more than 70%, while the SI group had FVC less than 70% and/or DLCO less than 70%.
Patients who refused to participate in the study, patients with concomitant respiratory diseases that could interfere with the results (malignancies, heart failure, emphysema, chest wall deformity, neuromuscular diseases, etc.), and patients with obstructive pattern in simple spirometry were excluded.
Oscillometry was the first test to be conducted. It was performed in patients in a seated position who had abstained from smoking or sport in the previous 2 h. If simple spirometry, plethysmography and DLCO could not be performed on the same day, they were performed later, within a period not longer than one month. All tests were performed according to the current European Respiratory Society guidelines.9, 10, 11
The oscillometry device used was the Resmon Pro with a software version of V6.1.2, which performs the analysis at a frequency of 5, 11 and 19 Hz. SentrySuite™, version V2.21.7 software was used to perform simple spirometry, plethysmography and DLCO. A minimum of three tests were performed per patient, in compliance with European reproducibility and quality criteria.
All subjects were given an adequate explanation of the study and provided written informed consent. This study was conducted in accordance with the International Conference on Harmonization on Good Clinical Practice and the Declaration of Helsinki (2008), and approval was granted by the institutional research ethics committee of Hospital Universitario de Getafe (approval number: CEIm21-36).
Study variables
Anthropometric and clinical data (body mass index, smoking history, dyspnea, arterial hypertension, dyslipidemia and diabetes mellitus) were extracted from patients’ electronic medical records. All patients, including the non-respiratory disease control group, had to have undergone a high-resolution computed tomography of the chest (HRCT), for reasons unrelated to the study, during the previous year.
Respiratory impedance was measured in all patients using oscillometry.
Resistances and reactances were measured at all frequencies, both in inspiration and expiration. The Resmon Pro V3 also has a patented index, called Delta Xrs, which consists of the difference between the reactance at 5 Hz in expiration and inspiration, which helps to determine the presence of airflow limitation in expiration.
The R5-19 parameter refers to the resistance of the small airways, since it is total resistance (R5 Hz) minus resistance at 19 Hz.
Our device does not calculate resonance frequency or reactance area.
Statistical analysis
Statistical analyses were performed using IBM SPSS v 21. The absolute and % predicted values are presented as mean ± SD or median and IQR, as appropriate.
A significance level of 0.05 was established. For continuous variables with a normal distribution, ANOVA was used. For variables without a normal distribution, the Kruskal–Wallis hypothesis test was used. The chi-square test was used for the analysis of contingency tables and for the comparison of proportions and/or frequency distributions.
Results
Characteristics of the study population
A summary of the characteristics of the patients in each group is shown in Table 1.
Table 1.
Main patient characteristics. The table shows the data presented as mean ± SD or median and [interquartile range], as appropriate.
| NRD (n = 15) |
MI (n = 15) |
SI (n = 15) |
p-Value | |
|---|---|---|---|---|
| Sex: male/female | 4/11 | 12/3 | 11/4 | 0.01 |
| Age (years) | 57 ± 9.7 | 68.9 ± 7.1 | 65.2 ± 9.4 | 0.00 |
| Smoking pack-years | 20 [15] | 30 [25] | 25 [20] | 0.743 |
| BMI, kg/m2 | 28 [5.7] | 28 [5] | 28.8 [5.9] | 0.981 |
|
mMRC dyspnea scale | ||||
| 0 (n) | 15 | 0 | 0 | 0.00 |
| 1 (n) | 0 | 13 | 4 | |
| 2 (n) | 0 | 1 | 3 | |
| 3 (n) | 0 | 0 | 6 | |
| 4 (n) | 0 | 0 | 1 | |
BMI: body mass index; MI: mild functional impairment; mMRC: modified Medical Research Council; NRD: non-respiratory disease control group; SI: severe functional impairment. The p-value was calculated with the chi-squared test, Student's t-test, Mann–Whitney U test or Fisher's exact test as appropriate (normal distribution or not).
Seven patients in the MI group had a diagnosis of IPF, 3 indeterminate ILD, 2 interstitial lung abnormalities (ILA), 1 smoking-related interstitial fibrosis (SRIF), 1 organizing pneumonia (NO), and 1 interstitial lung disease post-COVID.
On HRCT, 6 patients in this group showed a reticular interstitial pattern with peripheral subpleural distribution, 3 interstitial thickening and 6 had a fibrotic pattern (with honeycombing, traction bronchiectasis and lung volume loss).
Six patients in the SI group had a diagnosis of progressive pulmonary fibrosis (PPF), 4 IPF, 2 non-progressive fibrotic hypersensitivity pneumonitis (HP), 2 indeterminate ILD, and 1 pulmonary Langerhans cell histiocytosis.
On HRCT, all patients in this group had a fibrotic pattern, except for 1 patient who had a predominantly cystic pattern.
Conventional pulmonary function test and oscillometry results
None of the participants had an obstructive pattern on spirometry. The mild and severe groups were differentiated on the basis of FVC and DLCO values.
The MI group had FVC and DLCO equal to or greater than 70%, while the SI group had FVC less than 70% and/or DLCO less than 70%.
Table 2 shows the spirometry, plethysmography and DLCO results in the different groups.
Table 2.
Conventional PFT results. The table shows the data presented as mean ± SD. The p-value was calculated with the chi-squared test, Student's t-test or Mann–Whitney U as appropriate (normal distribution or not).
| NRD (n = 15) |
MI (n = 15) |
SI (n = 15) |
p-Value | |
|---|---|---|---|---|
| FVC (mL) | 3581 ± 659 | 3228 ± 715 | 2229 ± 536 | 0.00 |
| FVC (%) | 109 ± 15.3 | 96 ± 15.7 | 64.4 ± 10.8 | 0.00 |
| FEV1/FVC (%) | 78 ± 3 | 80 ± 7.7 | 78 ± 4.6 | 0.638 |
| TLC (L) | 5.4 ± 1.1 | 5.2 ± 1.1 | 3.5 ± 0.6 | 0.004 |
| TLC (%) | 105.3 ± 14.6 | 90.5 ± 11.2 | 59.9 ± 6.9 | 0.00 |
| RV (L) | 2.1 ± 0.6 | 2 ± 0.5 | 1.4 ± 0.3 | 0.030 |
| RV (%) | 111 ± 16 | 84 ± 19 | 58 ± 24 | 0.00 |
| RV/TLC (%) | 98 ± 14.6 | 93 ± 14.3 | 100 ± 22.2 | 0.371 |
| DLCO (mmol/(min kPa)) | 8.1 ± 1.5 | 6.5 ± 1.3 | 3.9 ± 1.1 | 0.00 |
| DLCO (%) | 105 ± 17 | 84.8 ± 11.8 | 48.7 ± 10.5 | 0.00 |
DLCO: diffusing capacity for carbon monoxide; FEV1: forced expiratory volume in the first second; FVC: forced vital capacity; MI: mild functional impairment; NRD: non-respiratory disease control group; RV: residual volume; SI: severe functional impairment; TLC: total lung capacity.
Oscillometry results are shown in Table 3.
Table 3.
Oscillometry results in the three different groups. The table shows the data presented as mean ± SD or median and [interquartile range], as appropriate. The p-value was calculated with the ANOVA or Kruskal–Wallis test, as appropriate (normal distribution or not).
| NRD (n = 15) |
MI (n = 15) |
SI (n = 15) |
p-Value | |
|---|---|---|---|---|
| R5 Hz (cmH2O/(L/S)) | 2.7 ± 1 | 3.3 ± 0.9 | 3.4 ± 1.3 | 0.198 |
| R5 Hz (%) | 79.9 [47.6] | 87.3 [28.3] | 101.8 [50.4] | 0.110 |
| R11 Hz (cmH2O/(L/S)) | 2.6 ± 0.9 | 3 ± 0.7 | 2.9 ± 1 | 0.470 |
| R11 Hz (%) | 84.7 [38.2] | 90.4 [17.1] | 98.7 [39.9] | 0.204 |
| R19 Hz (cmH2O/(L/S)) | 2.6 ± 0.9 | 2.6 ± 0.6 | 2.6 ± 0.8 | 0.978 |
| R19 Hz (%) | 84.6 [41.4] | 82.9 [8] | 86.6 [27.8] | 0.505 |
| R5_19 | 0.1 [0.5] | 0.5 [0.6] | 0.9 [0.6] | <0.001 |
| Delta Xrs | −0.1 [0.4] | 0.2 [0.7] | −0.1 [0.7] | 0.09 |
| Inspiratory X5 Hz (cmH2O/(L/S)) | −0.7 ± 0.4 | −1.2 ± 0.6 | −1.8 ± 0.4 | <0.001 |
| Expiratory X5 Hz (cmH2O/(L/S)) | −0.8 [0.5] | −1.2 [1] | −1.9 [1] | <0.001 |
| Total X5 Hz (cmH2O/(L/S)) | −0.7 ± 0.4 | −1.3 ± 0.6 | −1.9 ± 0.8 | <0.001 |
| Inspiratory X5 Hz (%) | 53.3 [30.4] | 85.1 [77.6] | 132.9 [30.5] | <0.001 |
| Expiratory X5 Hz (%) | 50 [33.1] | 98.2 [63.1] | 112.8 [116.4] | <0.001 |
| Total X5 Hz (%) | 51 ± 26.8 | 92.4 ± 41.6 | 146.1 ± 48.8 | <0.001 |
| Inspiratory X11 Hz (cmH2O/(L/S)) | 0 [0.1] | −0.5 [0.4] | −0.8 [0.5] | <0.001 |
| Expiratory X11 Hz (cmH2O/(L/S)) | −0.1 [0.4] | −0.7 [1.1] | −0.5 [0.9] | <0.001 |
| Total X11 Hz (cmH2O/(L/S)) | −0.1 [0.3] | −0.7 [0.7] | −1 [0.8] | <0.001 |
| Inspiratory X11 Hz (%) | 7.1 [69.6] | 241.6 [132.1] | 376 [450.4] | 0.002 |
| Expiratory X11 Hz (%) | 48.7 [237.9] | 299.8 [319.8] | 221.6 [777.4] | 0.001 |
| Total X11 Hz (%) | 29.4 [134.3] | 280.2 [223.5] | 295.7 [604.5] | <0.001 |
MI: mild functional impairment; NRD: non-respiratory disease control group; R: resistance; SI: severe functional impairment; X: reactance.
Relationship between oscillometry and conventional PFTs
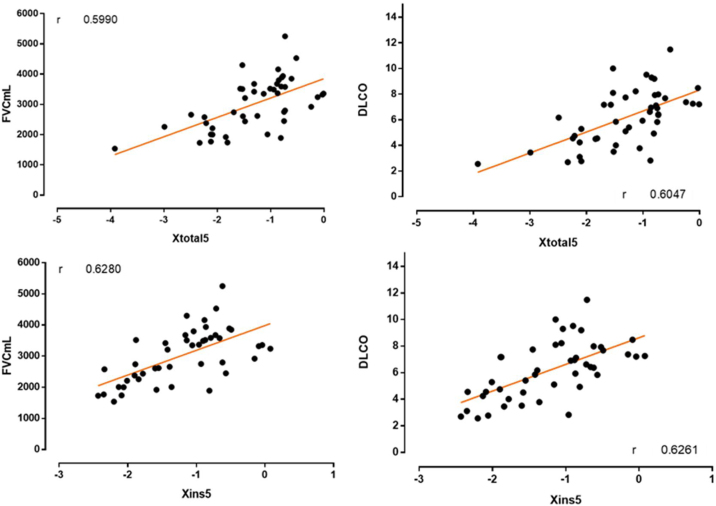
The correlation coefficient between DLCO and FVC values with respect to total and inspiratory X5 Hz, can be seen in Fig. 1. There is a close correlation (0.6 < r < 0.8) between DLCO and total X5 Hz, and between inspiratory X5 Hz and both FVC and DLCO.
Fig. 1.
Correlation coefficient between DLCO and FVC values with respect to total and inspiratory X5 Hz.
Discussion
To the best of our knowledge, this is the first Spanish study on the use of oscillometry in ILD. It highlights three main findings regarding the usefulness of this technique in this group of patients: (1) statistically significant differences in reactance were observed at both 5 Hz and 11 Hz in the study population; (2) statistically significant differences in the R5-19 values were observed between the groups analyzed; and (3) a strong correlation was observed between the values of the conventional pulmonary function tests, such as FVC and DLCO, with reactance, both total and inspiratory, at 5 Hz.
Pulmonary reactance represents the reactive component of respiratory impedance and, at frequencies lower than resonance figures, has always been related to pulmonary elastance. One of the first articles that analyzed Xrs measured by oscillometry in ILD was published by Van Noord et al.12 in 1989. Already then, this parameter was seen to be altered in these patients, although it did not seem to be specific to these diseases. A single value alone does not indicate a disease; the entire result must be evaluated as a whole.
Other more recent articles have supported this finding, such as that published by Liang et al.,7 which compares the results obtained by oscillometry in different diseases. They also describe Xrs alterations in ILD patients, although they observed even more negative values in obstructive airway diseases, suggesting that X5 Hz is more sensitive to changes in airway obstruction, due to small airway involvement, than to lung elasticity.
Our results show statistically significant differences in Xrs in the three groups. In fact, this seems to be related to the severity of the functional impairment, since in severe patients, we observed more negative mean values than in those with mild impairment.
Most publications to date refer to reactance at 5 Hz. However, our equipment also measures X at 11 Hz, which is also significantly impaired. This implies more negative values for all the parameters of the reactance curve and, therefore, an increase in the reactance area in these patients.
In our study, we did not observe statistically significant differences in the measurement of Delta Xrs. This suggests that these patients have no expiratory airflow limitation, which could be the key to differentiating it from COPD, a disease characterized by this finding.13
A remarkable aspect of our study is that we were able to describe and measure inspiratory and expiratory reactance separately, not only the total figures. Sugiyama et al.8 attempted to describe the main differences between the two phases of respiration. They found that the magnitudes of expiratory X5 were greater than those of inspiratory X5 in patients with COPD, whereas this situation was reversed in patients with ILD. In our study, we corroborated this finding in the group with severe impairment but not in the group with mild impairment.
Another parameter found to be significantly altered in our study is R5-19, reflecting small airway resistance. The existence or absence of altered resistance in ILD has been studied previously. Mikamo et al.14 conducted an interesting study aimed at analyzing the small airway in this group of patients. They divided the study population in two: patients with ILD and small airway involvement (mosaic pattern, air trapping and centrilobular nodules) and patients with ILD but without small airway involvement. They observed that the R5-20 parameter was significantly altered in the first group, as is the case in our study when comparing ILD patients with healthy controls. However, in our case these findings were not specified on CT, but the result is in line with other researchers who have evaluated oscillometry in ILD.
Another recent publication by Panagopoulos et al.15 evaluated the small airway in patients with scleroderma, with and without ILD, finding a significant increase in R5-20 in the first group.
This result is in agreement with that of the abovementioned registry published by Liang et al.,7 which highlights the increase in resistance, mainly at the expense of the small airways. This fact is mainly related to TLC: the lower the TLC, the higher the peripheral resistances, which would explain our finding. The R5-20 parameter increases as does the severity of respiratory functional impairment.
Our results also show a progressive increase in the mean values of R5 and R11 Hz as severity increases, although the differences were not statistically significant. This is not the case at R19 Hz, which helps to differentiate ILD from COPD, and usually presents alterations at all frequencies.16
The most studied disease among the ILD is IPF, and the updated guideline on IPF and PPF has recently been published.17 The importance of pulmonary function in diagnosis and treatment is clear in these recommendations. Indeed, the diagnostic criteria for PPF, in addition to radiological and clinical criteria, include a 5% absolute decline in FVC in 1 year and a 10% decline in DLCO in the same period. These parameters are also used in clinical trials as the main objective to monitor the usefulness of new drugs, as was the case in the INBUILD18 study or the study conducted by Maher et al.19
The results of our study show a strong correlation between FVC and DLCO values and total and inspiratory 5 Hz reactance and open a door to further research in this area. This would support the usefulness of oscillometry as an additional tool for the evaluation of this patient profile, although further studies in this regard are required.
Adding oscillometry to routine clinical practice would provide important benefits, including more detailed information on patient function (differentiating between resistance and reactance at different levels of the airway) and improved comfort. In patients with severe impairment, it is sometimes impossible to perform spirometry or diffusion, given their limited functional capacity. Instead, oscillometry can be performed at tidal volume, without any additional effort, and completed in less than a minute.20
The differences observed in our study between the different study groups also support the potential usefulness of oscillometry in the classification of severity. This could be further investigated to determine if oscillometry detects changes more sensitively than spirometry, so that it could be used for disease screening. It has already been studied with promising results.21
This study may have some limitations. The primary limitation to the generalization of these results is the small sample size, as it was conducted in a single center. Furthermore, the patients were analyzed according to the severity of their functional impairment with no differentiation in terms of underlying disease, which may have an influence, given that ILD are very heterogeneous.
In conclusion, this study analyzes the correlation between conventional functional values and oscillometry, with positive results. This is the first study conducted on ILD in Spain: most of the studies conducted to date focus on the Asian population. It is an accessible and easy-to-use tool that is much better tolerated by patients with severe involvement. Studies such as ours underline the need for more studies in this area with larger sample sizes and a multicenter design.
Funding
The authors have no funding information to declare.
Authors’ contributions
Cristina Matesanz-López has participated in the conception of the study, its design, data acquisition, analysis and interpretation, writing of the same.
Beatriz Raboso-Moreno has participated in data acquisition.
Leonardo Ernesto Saldaña-Pérez has participated in the study design, analysis and interpretation.
María Jesús Rodríguez-Nieto has participated in the analysis and interpretation of the study.
María Teresa Río-Ramírez has participated in the analysis and interpretation of the study.
All authors contributed equally to conceptualization, writing of original draft and writing and review of the final version of the manuscript.
Conflicts of interest
The authors have no conflict of interest to declare.
References
- 1.Desiraju K., Agrawal A. Impulse oscillometry: the state-of-the-art for lung function testing. Lung India. 2016;33:410. doi: 10.4103/0970-2113.184875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaminsky D.A., Simpson S.J., Berger K.I., Calverley P., de Melo P.L., Dandurand R., et al. Clinical significance and applications of oscillometry. Eur Respir Rev. 2022;31:210208. doi: 10.1183/16000617.0208-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crim C., Celli B., Edwards L.D., Wouters E., Coxson H.O., Tal-Singer R., et al. Respiratory system impedance with impulse oscillometry in healthy and COPD subjects: ECLIPSE baseline results. Respir Med. 2011;105:1069–1078. doi: 10.1016/j.rmed.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 4.Johansson H., Wollmer P., Sundström J., Janson C., Malinovschi A. Bronchodilator response in FOT parameters in middle-aged adults from SCAPIS: normal values and relationship to asthma and wheezing. Eur Respir J. 2021;58:2100229. doi: 10.1183/13993003.00229-2021. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto Y., Miki K., Tsujino K., Kuge T., Okabe F., Kawasaki T., et al. Oscillometry and computed tomography findings in patients with idiopathic pulmonary fibrosis. ERJ Open Res. 2020;6:00391–2020. doi: 10.1183/23120541.00391-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu J.K.Y., Ma J., Nguyen L., Dehaas E.L., Vasileva A., Chang E., et al. Correlation of respiratory oscillometry with CT image analysis in a prospective cohort of idiopathic pulmonary fibrosis. BMJ Open Respir Res. 2022;9:e001163. doi: 10.1136/bmjresp-2021-001163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liang X., Zheng J., Gao Y., Zhang Z., Han W., Du J., et al. Clinical application of oscillometry in respiratory diseases: an impulse oscillometry registry. ERJ Open Res. 2022;8:00080–2022. doi: 10.1183/23120541.00080-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sugiyama A., Hattori N., Haruta Y., Nakamura I., Nakagawa M., Miyamoto S., et al. Characteristics of inspiratory and expiratory reactance in interstitial lung disease. Respir Med. 2013;107:875–882. doi: 10.1016/j.rmed.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 9.King G.G., Bates J., Berger K.I., Calverley P., de Melo P.L., Dellacà R.L., et al. Technical standards for respiratory oscillometry. Eur Respir J. 2020;55:1900753. doi: 10.1183/13993003.00753-2019. [DOI] [PubMed] [Google Scholar]
- 10.Graham B.L., Steenbruggen I., Miller M.R., Barjaktarevic I.Z., Cooper B.G., Hall G.L., et al. Standardization of spirometry 2019 update. An official American Thoracic Society and European Respiratory Society Technical Statement. Am J Respir Crit Care Med. 2019;200:e70–e88. doi: 10.1164/rccm.201908-1590ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall G.L., Filipow N., Ruppel G., Okitika T., Thompson B., Kirkby J., et al. Official ERS technical standard: global lung function initiative reference values for static lung volumes in individuals of European ancestry. Eur Respir J. 2021;57:2000289. doi: 10.1183/13993003.00289-2020. [DOI] [PubMed] [Google Scholar]
- 12.Van Noord J.A., Clément J., Cauberghs M., et al. Total respiratory resistance and reactance in patients with diffuse interstitial lung disease. Eur Respir J. 1989;2:846–852. [PubMed] [Google Scholar]
- 13.Dellacà R.L., Duffy N., Pompilio P.P., Aliverti A., Koulouris N.G., Pedotti A., et al. Expiratory flow limitation detected by forced oscillation and negative expiratory pressure. Eur Respir J. 2007;29:363–374. doi: 10.1183/09031936.00038006. [DOI] [PubMed] [Google Scholar]
- 14.Mikamo M., Fujisawa T., Oyama Y., Kono M., Enomoto N., Nakamura Y., et al. Clinical significance of forced oscillation technique for evaluation of small airway disease in interstitial lung diseases. Lung. 2016;194:975–983. doi: 10.1007/s00408-016-9949-1. [DOI] [PubMed] [Google Scholar]
- 15.Panagopoulos P.K., Goules A.V., Georgakopoulou V.E., Kallianos A., Chatzinikita E., Pezoulas V.C., et al. Small airways dysfunction in patients with systemic sclerosis and interstitial lung disease. Front Med (Lausanne) 2022;9:1016898. doi: 10.3389/fmed.2022.1016898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Porojan-Suppini N., Fira-Mladinescu O., Marc M., Tudorache E., Oancea C. Lung function assessment by impulse oscillometry in adults. Ther Clin Risk Manag. 2020;16:1139–1150. doi: 10.2147/TCRM.S275920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raghu G., Remy-Jardin M., Richeldi L., Thomson C.C., Inoue Y., Johkoh T., et al. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med. 2022;205:e18–e47. doi: 10.1164/rccm.202202-0399ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wollin L., Distler J.H.W., Redente E.F., Riches D.W.H., Stowasser S., Schlenker-Herceg R., et al. Potential of nintedanib in treatment of progressive fibrosing interstitial lung diseases. Eur Respir J. 2019;54:1900161. doi: 10.1183/13993003.00161-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maher T.M., Corte T.J., Fischer A., Kreuter M., Lederer D.J., Molina-Molina M., et al. Pirfenidone in patients with unclassifiable progressive fibrosing interstitial lung disease: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir Med. 2020;8:147–157. doi: 10.1016/S2213-2600(19)30341-8. [DOI] [PubMed] [Google Scholar]
- 20.Lundblad L.K.A., Robichaud A. Oscillometry of the respiratory system: a translational opportunity not to be missed. Am J Physiol Lung Cell Mol Physiol. 2021;320:L1038–L1056. doi: 10.1152/ajplung.00222.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elkolaly R.M., Ganna S.A., Nada D.W., Elnaggar M.H. Impulse oscillometry, an aid or a substitute? Egypt J Bronchol. 2019;13:416–423. [Google Scholar]