Graphical abstract
Keywords: Bio-degumming, Trypsinogen, Silk fiber material, Silkworm, Transgenic technology
Highlights
-
•
Genetic creation of transgenic silkworm which can produce silk containing trypsinogen.
-
•
This genetic-engineered silk can self-degummed in water or under mild condition.
-
•
The self-degummed silk can obtain silk fibroin fiber with cleaner, larger equivalent radius and better mechanical properties.
-
•
The self-degummed sericin is easier to recover and has the biological function of promoting cell proliferation.
-
•
Providing an eco-friendly, energy-saving and sustainable development strategy for reducing the environmental burden brought by the silk industry.
Abstract
Introduction
Conventional hot-alkaline cocoon degumming techniques greatly weaken the physicochemical and mechanical properties of silk fibroin fiber, thus affecting the quality of silk fabric. Moreover, it causes massive energy waste and serious environmental pollution.
Objective
This study aims to establish a novel cocoon self-degumming method by genetic modification of silkworm varieties and silk fibers.
Methods
The self-degummed cocoon material was generated by specifically overexpressing trypsinogen protein in the sericin layer of silk thread; the effect of cocoon self-degumming method was evaluated by the degumming rate of sericin protein, the cleanliness and equivalent diameter of silk fibroin fiber; the basic characteristics of silk fibroin fiber degummed by cocoon self-degumming method and conventional hot-alkaline degumming technique were determined by electron microscopy, Fourier infrared spectroscopy, X-ray diffraction and tensile tests; the composition and biological activity of degummed sericin protein was respectively analyzed by liquid chromatograph-mass spectrometry and cytological experiments.
Results
The genetically engineered self-degumming cocoon containing trypsinogen protein was successfully created, and the content of trypsinogen protein in silk was 47.14 ± 0.90 mg/g. The sericin protein in the self-degumming cocoon was removed out in water or 1 mM Tris-HCl buffer (pH = 8.0). Compared to alkaline-degummed silk fibroin, self-degummed silk fibroin had better cleanliness, thicker equivalent diameter, more complete silk structure and better mechanical property. In addition, sericin protein degummed from self-degumming cocoons significantly promoted cell proliferation and caused no obvious cytotoxicity.
Conclusion
Compared to conventional hot-alkaline degumming technique, the cocoon self-degumming method by genetically overexpressing trypsinogen protein in sericin layer of silk thread can self-degummed in a mild degumming condition, and gain silk fiber with better quality and more biologically active sericin protein products. This strategy can not only reduce the environmental impact, but also generate greater economic value, which will accelerate its application in the silk and pharmaceutical industries.
Introduction
As a major natural protein fiber with unique qualities, including aesthetic appeal, fineness, comfortable touch, natural luster, and toughness, silk is an essential fibrous raw material worldwide. Being produced in more than 20 countries, including China, India, Uzbekistan, Thailand, and Brazil, silk has been deeply integrated into local economies and the daily lives of millions of people [1]. However, the detrimental impact of the outdated silk degumming process on the environment has drawn concern from global environmental activists [2]. Under the pressure of environmental protection, most enterprises have accelerated their withdrawal from the raw silk market. As a result, the silk industry is experiencing a sharp decline.
The common cocoon degumming involves bathing the silk fiber in boiling alkaline solution containing 0.1– 0.5 % Na2CO3 (w/v). This alkaline degumming method (ADM) is simple, fast, inexpensive, and completely removes sericin protein in the outer layer of the silk thread [3]. However, it is a water- and energy-consuming process. Moreover, ADM reportedly destroys the physical structure and reduces the mechanical properties of silk fiber [4]. Currently, the global silk industry yields at least 1.97 × 108 tons of wastewater and consumes 3.28 × 108 kWh of power every year [5], [6]. In China, the major producer of silk, more than $100 million is spent annually to reduce chemical oxygen demand, biochemical oxygen demand, and other pollutants in the outlet water to meet global emission standards (data provided by Zhejiang Misai Silk Co., ltd.).
Compared with traditional ADM, the enzymatic degumming method (EDM) is an improvement. Not only can it completely and quickly remove sericin protein, but also it can reduces damage to the integrity and mechanical properties of silk [7]. In addition, EDM efficiently reduces environmental pollution as well as water and energy consumption, making it a more sustainable cocoon degumming technology [8]. EDM is a complex process that centers on accelerating protein degradation through proteolytic enzymes, typically in mild conditions. These proteolytic enzymes include acidic (cellulase and pectate lyase), neutral (papain and catalase), and alkaline enzymes (keratinase and protease K) [9], [10]. EDM has been widely utilized to de-size cotton and bleach wool in industrial textile processes [4], [11]. In addition, researchers have successfully applied this method to cocoon degumming on a small-scale using cocoonase collected from tasar silkworm in vitro and commercial papain extracted from carieapapaya [12], [13]. Both enzymes efficiently removed the sericin protein layer of the silk thread in mild conditions and could better maintain the natural color, smoothness, luster, physical structure, and tensile strength of the silk fibroin fiber than the alkali-degummed silk fibroin fiber (ADSF). However, EDM is yet to be applied in large-scale processes of the textile industry for multiple reasons. First, it involves the consumption of large amounts of enzymes in a market with an insufficient supply. Second, the price of enzymes is higher than that of chemical reagents. Finally, establishment of the EDM system involves regulation of temperature and pH of the reaction buffer, and does not have sufficient competitive advantages relate to energy and chemical reagent consumption compared with traditional methods. Therefore, whether exogenous proteases can be simultaneously synthesized with silk protein in the silk gland to generate a self-degumming silk material (SDSM) is key factor to the large-scale application of EDM.
With the rapid development of silkworm genetic manipulation technologies, artificially optimized target genes can be efficiently and easily expressed through various tissue-specific genetic modification systems such as the sericin-1 (Ser1) and fibroin-heavy-chain/fibroin-light-chain (FibH/FibL) expression systems in the silk gland and their translated protein exhibit their biological activities [14], [15], [16]. This suggests that these systems could be used to express target protease in silk thread and create a novel SDSM. For suitable protease competitors, the ∼ 23 kDa serine protease porcine trypsin (TRY) is an attractive alternative for silk enzymatic degumming. In mammals, it can efficiently regulate other protein digesting enzymes and digest proteins into smaller peptides or amino acids by hydrolyzing the peptide bonds at the C-terminals of lysine and arginine during digestion [17], [18]. These reactions occur after the inhibitory peptides (FPTDDDDK) is removed from its precursor trypsinogen (TRYP) through auto-activation, self-hydrolyzing, or allo-hydrolyzing by enterokinase [19], [20], [21]. It is important to note that TRY only hydrolyzes the sericin layer but not the fibroin layer of silk thread. This is because there are few cleavage sites of TRY in fibroin, whereas they are abundant in sericin protein [12], [22].
The present study aimed to develop a novel strategy for auto-eliminating the sericin layer of cocoon by genetically creating an SDSM that contains porcine TRYP protein in the sericin layer. Our results showed that SDSM can efficiently remove the sericin protein layer in mild conditions compared to ADM. In addition, the self-degummed silk fibroin fiber (SDSF) has better physical structure and mechanical properties than ADSF. The sericin peptides removed from SDSM have an enhanced cell proliferation ability and good biosafety, suggesting that SDSM has a promising application in the textile industry and the medical field.
Materials and methods
Vector construction and generation of transgenic silkworms
The transgenic vector was constructed as previously described, with some modifications [23]. Briefly, the optimized porcine TRYP coding sequence was synthesized by Genscript Biotechnology Co., ltd (Nanjing, China) and inserted into the shuttle vector containing a nuclear polyhedrosis virus enhancer (Hr3), Ser1 gene promoter, and the polyadenylation signal of the Ser1 gene (Ser1PA). Subsequently, the fragment of the expression cassette [hr3-Ser1P-TRYP-Ser1PA] was amplified from the vector and inserted into the AscI-treated vector pBac[3xp3-EGFP-Sv40] using the Pro Ligation-Free Cloning kit (Abm, China) to generate the final transgenic expression vector pBac[3xp3-EGFP-Sv40; Hr3-Ser1P-TRYP-Ser1PA] (pBac-TRYP).
The transgenic silkworm germline was transformed as previously described [24]. Briefly, pBac-TRYP plasmids mixed with an Hsp70-PIG helper at a ratio of 1:1 (w/w) were microinjected into 400 non-diapause embryos of the silkworm D9L strain within 2 h after oviposition. G1 silkworms were obtained after the sib-mating of moths metamorphosed from the G0-hatched silkworms. Finally, positive transgenic lines with green fluorescence in the eyes were screened using the Olympus SZX12 fluorescence stereomicroscope (Olympus, Japan).
Insertion site identification
To identify the insertion site of the transgene in the genome, inverse PCR was performed as our previous report [25]. Briefly, genomic DNA was extracted from transgenic moths using an Omega tissue DNA kit (Omega Bio-tek, Norcross, GA, USA) and treated with restriction endonuclease HaeIII (New England Biolabs, Ipswich, MA, USA) at 37 °C overnight. It was subsequently recycled using an OMEGA Gel Extraction Kit (Omega Bio-tek). Then, 1 μg DNA was cyclized with T4 ligase as the template to perform inverse PCR. Finally, the DNA product containing the insertion site information was recycled and cloned into a T5-Zero plasmid (TransGen, China) for sequencing. The primers used are listed in Table 2 and the sequencing results were analyzed using SilkDB 3.0 (https://silkdb.bioinfotoolkits.net/).
Table 2.
Primers used in this study.
| Name | The forward primer | The reverse primer |
|---|---|---|
| 1pBac-L | 5′-ATCAGTGACACTTACCGCTTGAGA-3′ | 5′-TGACGACTTGTTGGTGAGGATTCT-3′ |
| 2pBac-R | 5′-TACGCAATGATTATCTTTAACGTA −3′ | 5′-GGGGTCCGTCAAAACAAAACATC −3′ |
pBac-L means the left arm of piggyBac transposon, and 2pBac-R means the right arm of piggyBac transposon.
Calculation of degumming rate
Degumming experiments were divided into three groups: wild type (WT), SDM and ADM. In the WT group, WT cocoons were cut into 3 mg slices and transferred into a 1.5 mL centrifugal tube with 150 μL reaction buffer (1 mM Tris-HCl, pH = 8.0). This step was followed by incubation at 37 °C for 24 h. The degumming experiment conducted in the SDM and ADM groups slightly varied from the WT group. WT cocoons was replaced with transgenic TRYP cocoons in the SDM group, whereas silk was degummed using a 0.5 % Na2CO3 solution at 100 °C for 45 min in the ADM group. After treatment, the residual silk fibroin was rinsed three times with distilled water (ddH2O) and air-dried before weighing. The degumming rate was then calculated as follows:
SDS-PAGE analysis
To identify TRYP in transgenic silk, total sericin protein was extracted from TRYP cocoons using 8 M urea solution at 60 °C for 30 min, and the supernatant was collected through centrifugation at 1.34 × 104 rpm for 10 min. After determining the protein concentration using a BCA protein concentration assay kit (Beyotime, China), 20 μg total protein was used in the SDS-PAGE experiment and Coomassie brilliant blue G250 staining assay. The SDS-PAGE gel used in this study is a commercial polyacrylamide gel with the gradient from 4 % to 20 % (Gencript, China).
Western blot analysis
To calculate the content of TRYP in transgenic silk, PierceTM Trypsin protein (Thermo Fisher Scientific, Waltham, MA USA) was used as a standard. First, 20 μg sericin and standard with gradient concentration were loaded in different loading holes, separated using a 4–20 % gel, and then transferred to a polyvinylidene fluoride membrane (Suez (GE), Feasterville-Trevose, PA, USA) for western blot analysis. Next, the membrane was blocked with QuickBlock™ Western solution (Beyotime, China) and incubated with anti-TRY primary antibody (Cloud-clone, China). The membrane was then washed with 1 × TBST and incubated with goat anti-rabbit secondary IgG conjugated with horseradish peroxidase (Beyotime, China) followed by washing with 1 × TBST. Finally, the TRYP signal was detected using a BeyoECL Moon kit (Beyotime, China) and immediately recorded using a Champchemi 610 plus chemiluminescence imaging system (Clinx, China).
To confirm TRYP auto-activation, 20 μg sericin extracted through 8 M urea or reaction buffer and 1 μg standard were added in different loading holes, separated using SDS-PAGE, and then analyzed via western blotting. The primary antibodies used in this experiment were anti-TRY and anti-DDDDK antibodies (ABclonal, China), and all antibodies were diluted with 1 × TBST at a ratio of 1:10000.
Silver staining
Sericin samples (20 μg) collected from the WT, SDM, and ADM groups were separated using a 4–20 % gel, immobilized in a stationary solution (50 mL methanol, 12 mL acetic acid, 50 μL formaldehyde, and 38 mL ddH2O for 30 min, and washed with washing buffer (50 mL methanol and 50 mL ddH20) twice. After fixing in a fixative solution (0.02 g sodium thiosulfate and 100 mL ddH2O) for 2 min, the gel was washed with ddH2O three times and stained with a silver staining solution (0.2 g silver nitrate, 75 μL formaldehyde, and 100 mL ddH2O) for 30 min. Subsequently, the gel was dyed in developing solution (6 g sodium carbonate, 50 μL formaldehyde, 0.001 g sodium thiosulfate, and 100 mL ddH2O) and the process was stopped using a termination buffer (50 mL methanol, 12 mL acetic acid, and 38 mL ddH2O). The protein bands were then visualized. All reagents used in this experiment were purchased from Sangon Biotech Co., ltd (Shanghai, China).
Microscopic observation of silk and calculation of equivalent diameter
Micromorphology of silk in the WT, SDM and ADM groups was observed via scanning electron microscopy (Hitachi, Japan). Subsequently, the silk was crosscut into 4-mm sections using a freezing microtome (CRYOSTAR VX500, Thermo Fisher) to record the cross-sectional fluorescent photos and measure the average long axis of the cross-section area. Fifty cross-sectional areas were randomly selected to investigate the distribution of diameter and compute its equivalent diameter. The equivalent diameter of silk fibroin was converted from the cross-section area using the following formula:
Tensile test
To test the tensile force on a DMA Q800 dynamic mechanical analyzer (TA Universal Analysis, New Castle, DE, USA), 25 ± 5 pre- or post-treatment single silk in the WT, SDM, and ADM groups were selected at random. The experimental conditions were as follows: initial length, 12 mm; stretching speed, 1.2 mm per min; ambient temperature, 26 °C; and relative humidity, 60 %.
Liquid chromatograph-mass spectrometry (LC-MS) analysis
A total of 150 μL supernatant in the three groups was transferred into an ultrafiltration tube with a 10 kDa nominal molecular weight limit (Meck, Germany) for centrifugation at 3000 × g to collect the first filtrate. Next, 150 μL 9.3 M lithium bromide (Sigma-Aldrich, St. Louis, MO USA) solution was added to the remaining proteins in the ultrafiltration tube and incubated at 4 °C overnight. The samples were subsequently washed with 50 mM urea and digested with TRY (Thermo Fisher) at 37 °C for 12 h. The second filtrate was obtained using the same method and mixed with the first filtrate to dry in a vacuum freeze drier. Then, the samples were dissolved in 0.1 % (w/v) trifluoroacetic acid, desalted using a desalination column (Suez (GE)), dried in a vacuum freeze drier, and dissolved in 0.1 % (v/v) formic acid solution. Finally, LC-MS analysis was conducted using a Thermo Scientific™ Liquid chromatograph-mass spectrometer (Thermo Fisher), and the data were processed using Byonic v2.16.11 (Protein Metrics) software.
ATR-FTIR and XRD analysis
Infrared spectroscopy was conducted as previously described, with some modifications [26]. Briefly, silk fibroin fiber collected from the SDM and ADM groups was cleaned with ddH2O, air-dried, and milled into powder using a 6875 Freezer/Mill® High-Capacity Cryogenic Grinder (SPEX SamplePrep, Metuchen, NJ, USA). The silk power was added to an equal volume of potassium bromide (Sigma), and the mixture was pressed into thin sheets for detection. The spectra signal of samples between 1000 and 4000 cm−1 and at a resolution of 0.25 cm−1 with 1024 scans was read using a Thermo Scientific Nicolet iN10 instrument, and data were processed using the OMNIC 9 software.
For XRD analysis, silk power was filled up to the sample pool and scanned from 5.0° to 55.0° (2θ) at 2.0°/min using the X’Pert3 Powder X-ray diffractometer (PANalytical, Netherlands). Data were processed using the MDI JADE 6.5 software.
Determination of SDSM sericin biological activity
Ten grams of SDSM was directly immersed into 500 mL reaction buffer for 24 h. Then, the supernatant was collected through centrifugation at 1.34 × 104 rpm, heated to 100 °C for 15 min to inactivate TRY, concentrated, and desalted using a JLKJQXL6881 membrane system (Guidling, China), and freeze-dried for storage at 4 °C.
For the cell proliferation assay, mouse fibroblasts (NIH/3T3) were seeded in a 96-well plate at 2 × 104 cells/well and cultured with 100 μL Dulbecco’s Modified Eagle Medium (DMEM, Gibco, Waltham, MA, USA) that was supplemented with 10 % fetal bovine serum (v/v) (FBS, Gibco) and 2 μg SDSM sericin. After 24 and 48 h, cells were counted using the CCK-8 Cell Counting Kit (Beyotime, China), and cell viability was determined using a LIVE&DEAD™ Viability/Cytotoxicity Kit (Thermo Fisher). In addition, the DNA replication activity of cells was detected through a Click-iT™ Plus EdU Cell Proliferation Kit for Imaging (Thermo Fisher).
For cellular inflammation analysis, mouse macrophages (RAW 264.7) were seeded in a 96-well plate at 2 × 104 cells /well and cultured with 100 μL Roswell Park Memorial Institute 1640 (RPMI) medium containing 10 % FBS (v/v) + 500 ng lipopolysaccharide (LPS, Sigma), or 10 % FBS (v/v) + 1 or 2 μg SDSM sericin. After 24 h, the medium was collected to test the concentration of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) using a Mouse TNF-α or IL-6 ELISA Kit (Meimian, China). The cells were treated with 0.1 μM calcein-AM to observe their morphology through an EVOS FL AUTO fluorescence microscope (Thermo Fisher) or lysed with 100 μL cell-lysate RIPA (Beyotime, China) to detect the expression of nitric oxide synthase (iNos) through western blotting. These detections were carried out according to the manufacturers’ specifications.
Statistical analysis
Data are shown as the mean ± standard error of the mean (SEM, error bar). Statistical analysis was conducted using a Student’s t-test and p < 0.05 was considered statistically significant.
Results
Genetic creation of TRYP silkworm strain and expression of TRYP
To generate transgenic TRYP silkworms and SDSM, expression vector pBac-TRYP (Fig. 1A) containing the optimized porcine TRYP that is controlled by the Hr3 enhancer, Ser1 promoter, and Ser1PA terminator [23], was constructed and microinjected into the non-diapause eggs of the D9L strain, which owes a clear genomic sequencing information and its embryos is conducive to screen transgenic positive individuals. In total, 400 non-diapause eggs were microinjected and 135 were hatched (33.75 %) and carefully reared to produce offspring through sibling crossing. In the first generation of offspring (G1), five (33.3 %) positive broods were gained by screening green fluorescence in the eyes of the embryo, pupa, and adult (Fig. 1B and Table1), indicating that transgenic TRYP silkworm can secrete SDM silk thread. The cocoon was successfully constructed, and TRYP was located at 6,584,102 bases of chromosome 25 (Fig. 1H). Thereafter, expression of TRYP in the SDM cocoons was analyzed. A specific band (25 kDa) in the transgenic cocoon with a higher molecular weight than the natural TRYP protein was successfully detected during SDS-PAGE and western blotting (Fig. 1D). Moreover, the highest TRYP content was 47.14 ± 0.90 mg/g in the transgenic cocoon of line 5 (Fig. S1). Furthermore, immunohistochemistry assay results indicated the TYRP was well-mixed with sericin protein in the outer layer of the SDM silk thread (Fig. 1E). In addition, compared to WT silkworm, the embryos, larvae, cocoon shape and weight, pupa, and moth of TRYP silkworm had no obvious difference (Fig.S2). And SDM silk thread had the similar average equivalent diameter, mechanical properties, and secondary structure to the WT silk thread (Fig. S2).
Fig. 1.
Establishment and identification of transgenic TRYP silkworm. (A) A sketch of the pBac-TRYP transgenic vector. (B) Fluorescent images of the transgenic silkworm in the embryo (a, b), pupa (c, d), and moth (e, f) (scale bar, 200 μm). (C) The amino acid sequence of porcine TRYP. FPTDDDDK (green) is the inhibitory peptide of TRYP. ((D) SDS-PAGE and western blotting analysis of recombinant TRYP protein in cocoons. The band of recombinant TRYP is marked with a red asterisk. (E) Fluoroimmunoassay results of raw silk sections (scale bar, 5 μm). (F) Digestion of the TRYP transgenic silkworm genome. (G) Amplification of genomic DNA fragments linked to the left (L) or right (R) arm of the piggyBac transposon. (H) The insertion site of TRYP in the transgenic silkworm genome. (I) TTAA, the target sequence specifically recognized by piggyBac transposase, is highlighted in red.
Table 1.
Statistics of transgenic vector microinjection.
| Vector | Strain | Injected eggs | Hacthed eggs | G1 broods | Positive brood (%) |
|---|---|---|---|---|---|
| pBac-TRYP | D9L | 400 | 135 | 15 | 5 (33.31) |
Positive rate of transgenic silkworm strain relative to G1 generation broods.
SDSM can efficiently and automatically remove its sericin layer of silk thread
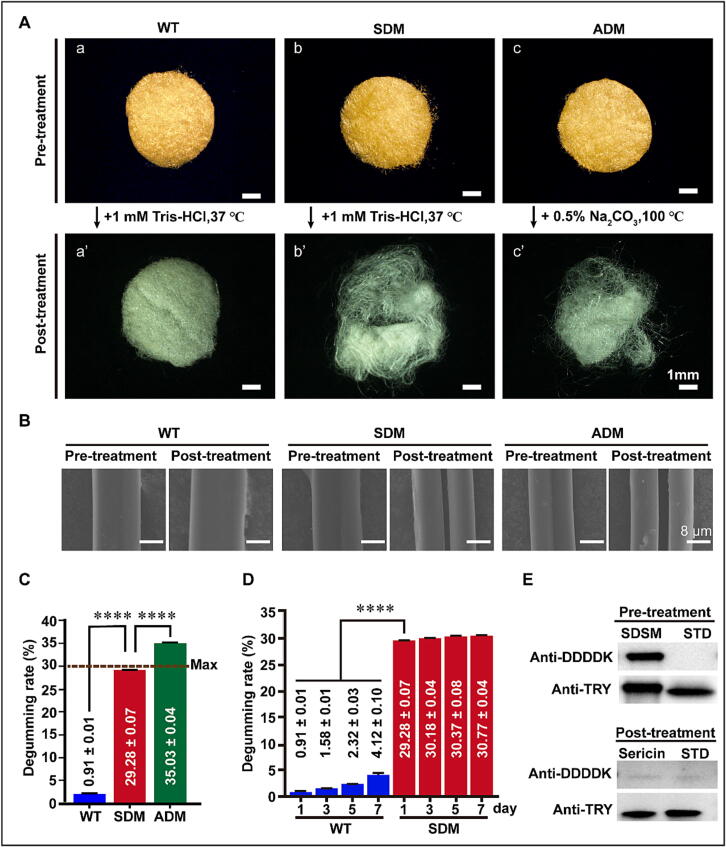
To analyze the characteristics of SDSM, SDM and WT cocoons were immersed in reaction buffer (1 mM Tris-HCl, pH = 8.0) and their morphological change was observed via optical microscopy, which revealed a color fade in all cocoon slices. However, only the SDM and ADM cocoon slices loosened (Fig. 2A, a-a’, b-b’, c-c’), and their silk thread were completely divided into two monofilaments compared to WT silk (Fig. 2B). Moreover, western blotting analysis of SDM sericin showed that the amino acid sequence FPTDDDDK at the N-terminal of TRYP disappeared in the reaction buffer but was retained in 8 M urea solution (Fig. 1C and Fig. 2E). Furthermore, the degumming rate of SDSM (29.28 ± 0.07 %) was significantly higher than that of WT silk (0.91 ± 0.01 %) but lower compared to the ADM group (35.03 ± 0.04 %). This degumming rate was maintained at approximately 30 % for one week (Fig. 2C and Fig. 2D).
Fig. 2.
SDSM automatically removes its sericin layer. (A) Changes in cocoon slice appearance between pre- and post-treatment (scale bar, 1 mm). (B) Changes in silk thread appearance between pre- and post-treatment (scale bar, 8 μm). (C) Calculation of the degumming rate. The maximum sericin content of silk reported in previous works is labelled with a dotted line [40]. (D) Degumming rate of SDM in a week. (E) Determination of the activated TRYP. Full-length TRYP could be recognized by the anti-DDDDK and anti-TRY primary antibody, whereas TRY was only detected by the anti-TRY antibody. Experiments in this part were independently performed in triplicates, and data are expressed as the mean ± standard error of the mean (SEM, error bar). For the significance test: *P < 0.05, **P < 0.01, ***P < 0.001, **** P < 0.0001, versus WT.
SDSF has better micromorphology, structural integrity, and mechanical properties
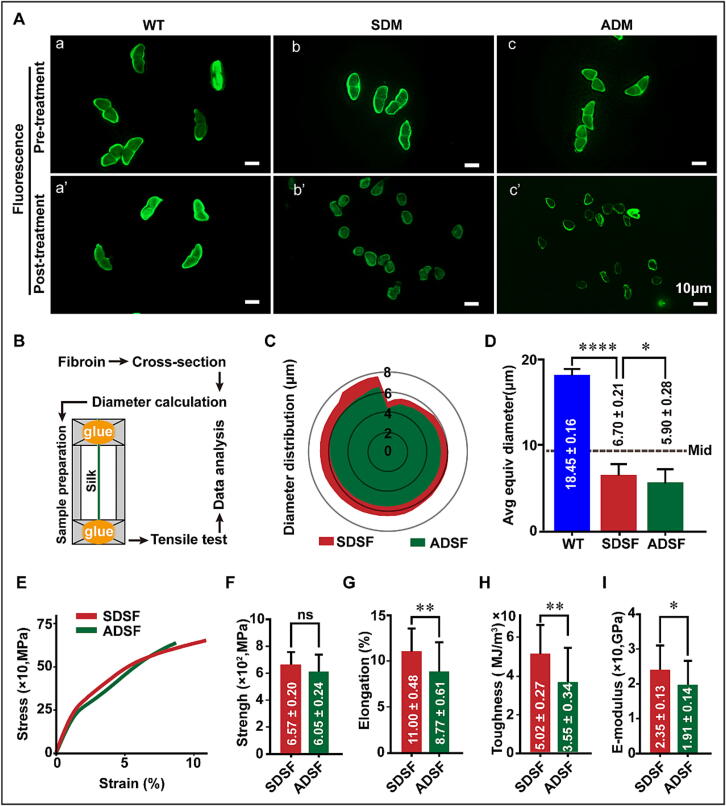
The basic characteristics of SDSF and ADSF were analyzed. SEM results indicated that SDSF was smoother than ADSF (Fig. 2B, Fig. 3A, b’ and c’). However, FTIR results showed highly similar absorption spectra of SDSF and ADSF from 1750 cm to 1 to 1150 cm-1 (Fig. 3B). In addition, analysis of the amide I bands revealed the contents of the β-sheet and α-helix in SDSF to be 34.33 ± 0.24 % and 23.68 ± 0.10 %, respectively. This is significantly higher than the respective 32.63 ± 0.36 % and 22.81 ± 0.10 % observed in the β-sheet and α-helix content of ADSF (Fig. 3D and Table 3). The X-ray results showed that the crystallinity content of SDSF was 52.41 ± 0.08 %, which was higher than that of ADSF (50.24 ± 0.08 %) (Fig. 3G). Moreover, mechanical property test results of the two silk fibers showed that the elongation, toughness, and elastic modulus of SDSF were 657.0 ± 20.0 MPa, 11.00 ± 0.48 %, 50.2 ± 2.7 MJ/m3, and 23.5 ± 1.3 Gpa, respectively. These were significantly higher than the 8.77 ± 0.11 %, 35.5 ± 3.4 MJ/m3, and 19.1 ± 1.4 Gpa observed in ADSF, except for strength (Fig. 4F–I). These results suggest that SDSF has better micromorphology, crystal structure, and mechanical properties than ADSF. Fluorescence observation of the cross-sections of SDSF and ADSF showed that the diameter of these silk fibers was mainly distributed in the 5–8 μm region, and the average equivalent diameter of SDSF was relatively larger (Fig. 4C and D).
Fig. 3.
Analysis of the basic characteristics of SDSF. (A) SEM images of pre- and post-treated silk (scale bar, 60 μm). (B) ATR-FTIR spectra of SDSF and ADSF from 1750 cm−1 to 1150 cm−1. The absorption from 1700 cm−1 to 1600 cm−1 represents amide I. (C) Deconvolution of the amide I band. The absorption at 1620 cm−1, 1639 cm−1, 1660 cm−1 and 1686 cm−1 was attributed to the β-sheet, random coil, α-helix, and β-turn, respectively. (D) Secondary structure content (%) of SDSF and ADSF. (E) X-ray diffraction patterns of SDSF and ADSF. (J) Deconvolution of X-ray diffraction patterns. Diffraction peaks were calculated as the sum of five Gaussians, three crystalline peaks (Cry-Peak, red), and two amorphous peaks (Amo-Peak, green). The area of peaks located at 9.0°, 20.5°, and 24.5° represents the content of crystallinity. (K) Crystallinity of silk in SDSF and ADSF. The number of independent experimental repetitions during ATR-FITR and XRD analysis was 3 and 3. Data are presented as the mean ± SEM (error bar). For the significance test: *P < 0.05, **P < 0.01, versus ADSF.
Table 3.
Secondary structure content of silk fibroin fiber.
| Group | β-sheet | random coil | α-helix | β-turn |
|---|---|---|---|---|
| SDSF | 134.33 ± 0.24* | 29.54 ± 0.20*** | 23.68 ± 0.10** | 12.45 ± 0.14 ns |
| ADSF | 32.63 ± 0.36 | 32.43 ± 0.24 | 22.81 ± 0.10 | 12.13 ± 0.13 |
For the significant test:*p < 0.05, **p < 0.01, ***p < 0.001, versus ADSF.
Fig. 4.
Comparison of the diameter and mechanical properties of SDSF and ADSF. (A) Fluorescence images of pre- and post-treated silk cross-sections (scale bar, 10 μm). (B) Preparation for the tensile test. (C) A radar distribution image of the silk fibroin fiber diameter. (D) The average equivalent diameter of silk fibroin fiber. The bisector line of average equivalent diameter in WT silk is highlighted in gray and marked as a Mid. (E) Stress-stain curve. (F) Strength. (G) Elongation. (H) Toughness. (I) Elastic modulus. The number of independent experimental repetitions in the tensile test was 25 ± 5. Data are presented as the mean ± SEM (error bar). For the significance test: *P < 0.05, **P < 0.01, versus ADSF.
SDM better remains the molecule structure of fibroin protein
Since the physical and chemical performances of silk fiber is closely related to silk fibroin molecule structure, whether SDM destroys its structure was determined by analyzing the constituents of sericin and fibroin collected from SDM and ADM cocoons slice. The SDS-PAGE results showed that the main components of silk fibroin in ADM, especially FibH, were degraded more than that in SDM (Fig. 5D). The LC-MS results showed that both sericin samples were classified into seven categories according to their functions (Fig. 5B), which mainly include enzyme, protease inhibitor, adhesion protein, sericin, and fibroin. Moreover, the order of total protein quantity was ADSM > SDSM > WT (Fig. 5A). Fibroin proteins, including FibH and FibL, were successfully detected in the SDM and ADM groups, and their contents in the ADM group were significantly higher than those in the SDM group (Fig. 5C). Moreover, the P25 protein was only detected in the ADM group (Fig. 5C). To determine the effect of SDM on FibH, its peptides were aligned with the original sequence of FibH to ascertain the N/C-terminal cleavage sites. The cleavage sites of FibH in SDM silk were at K104 and R5232, while those kept unknown in ADM silk (Fig. 5E, a–d). Therefore, we can conclude that SDSF has better micromorphology, structure integrity, and mechanical properties.
Fig. 5.
Proteomic analysis of sericin in different degumming solutions. (A) A Venn diagram of proteins identified in each group. (B) Classification of proteins in the degumming solutions of SDM and ADM. (C) Abundance of fibroin-related proteins. (D) SDS-PAGE analysis of silk fibroin fiber in SDM and ADM group. (E) Effects of SDM and ADM on the FibH chain. (a) The cleavage sites of FibH after treatment in SDM. The N-amorphous domain, crystalline regions (Cry-regions), amorphous regions (Amo-regions), and C-amorphous domain are represented by yellow, dark-blue, blue-green, and gray, respectively. The inferred changes of the FibH protein in WT, SDM, and ADM silk are displayed in (b), (c), and (d). All experiments were performed in triplicates, and data are presented as the mean ± SEM (error bar). For the significance test: *P < 0.05, **P < 0.01, versus SDM.
The SDM sericin peptide has cell proliferation activity and biosafety
SDS-PAGE results showed that the molecular weight of sericin mainly concentrated in the interval was < 25 kDa (Fig. 6A). Small sericin peptides can efficiently support cell attachment and proliferation [27], [28]. Thus, the cell proliferation activity and biosafety of sericin recovered from the degumming buffer of SDM were carefully examined. When NIH/3T3 cells were cultured in 2.5 % FBS-DMEM medium containing 20 μg/mL sericin peptide for 1–2 days, the live and D = dead cell staining results showed that the number of live cells with green fluorescence in the SDM group was significantly higher than that of the Null group (Fig. 6B). Moreover, the EdU assay results showed that the quantity of proliferating cells with red fluorescent signal in the SDM group was higher (Fig. 6C). Similarly, the CCK-8 assays showed that the cell number in the SDM group increased significantly compared to that in the WT group (Fig. 6D). Our SDM sericin peptide analysis strongly indicated that the peptide has low cell toxicity and improved cell proliferation activity. Moreover, the sericin peptide of SDM was co-cultured with RAW 264.7 cells to determine whether it induces severe cellular inflammation. Compared to the positive LPS group, 10 and 20 μg/mL SDM sericin peptide in the cell medium induced no obvious morphological variation (Fig. 6E). However, an increase in iNOS expression was observed, and the main inflammatory cytokines including TNF-α and IL-6 were detected in the cell medium of the LPS group (Fig. 6E–H).
Fig. 6.
Analysis of SDM sericin biological activity in vitro. (A) Silver staining of degummed sericin in the WT (1), SDM (2), and ADM (3) groups. The main degraded products of SDSM were highlighted through a red dashed rectangle. (B) Live and Dead staining of NIH/3T3 cells. (C) EdU assay of NIH/3T3 cells. (D) CCK-8 assay of NIH/3T3 cells. (E) Morphological changes of Raw264.7 cells after treatment. Differentiated macrophages are marked with white arrows. (F) Western blotting analysis of iNos expression. (G) (H) Elisa assay of cytokines TNF-α and IL6. All experiments were performed in three biological duplications, and data are presented as the mean ± SEM (error bar). For the significance test: *P < 0.05, ****P < 0.0001, versus Null group.
Discussion
With hot-air cocoon drying, high-temperature degumming, and silk reeling, weaving, printing, and dyeing as the main industrial processes, the silk industry has adverse environmental impacts[29]. Degumming has the greatest environmental effect of all these processes. It is commonly performed using ADM, which consumes many resources and produces a large amount of wastewater[30]. The high resource consumption and environmental protection costs have severely hindered the sustainable development of the silk industry. Thus, the urgent development of new environmentally friendly and energy-efficient degumming technologies will be essential to the growth of this industry.
The preferred method for reducing energy consumption and increasing energy efficiency during degumming is the optimization of sericin degumming parameters of the ADM, including the degumming temperature, bathing time, and concentration of alkali reagent Na2CO3 [3], [31]. However, this method cannot fundamentally solve the problem. As such, much attention has been paid to the exploration of EDM, which is highly specific, environmentally friendly, and energy-efficient compared with ADM [2], [29]. Moreover, EMD protects the innate properties of silk fibroin, including strong mechanical performance, smooth surface, and elegant luster [12], [13]. However, this method has no competitive advantage in the cost of raw silk production, restricting its popularization in the silk industry [32].
Silk proteins, including fibroin, sericin, protease, and protease inhibitor, are synthesized in the silk gland in little time and secreted to the silk gland lumen for the formation of cocoons [33]. This characteristic constitutes the biological basis for silk production and proves that silk gland organs have high-efficiency protein synthesis abilities. These abilities have led to the exploration of the silk gland as an efficient bioreactor in the production of heterologous medicinal proteins [16]. Nearly 100 kinds of functional proteins, including human cytokines, antibodies, and functional subunits, have been produced in the silk gland, although they rarely have practical applications [34]. However, the market potential of the silk gland as a bioreactor cannot be underestimated. Based on this premise, we envisaged that sericin was simultaneously synthesized with exogenous proteolytic enzymes in the silk gland and naturally well-mixed, followed by the formation of cocoons. Subsequently, the proteolytic enzymes were released to degum the sericin protein into peptides during degumming, causing reeling of the silk fibroin. Based on this assumption, we believe that the difficulties encountered in the application of EDM will be virtually solved.
The realization of the above idea strongly relies on the following premise: First, proteolytic enzymes can be synthesized in the silk gland and secreted into the sericin layer. Second, that proteolytic enzymes remain inactive during cocoon formation but have biological activity during sericin removal. Finally, proteolytic enzymes can specifically remove sericin and cause less or no damage to silk fibroin. Therefore, the selection of enzymes could determine the success or failure of our hypothesis. TRYP was a good candidate for our experiments because it meets the three demanding conditions stated above. Porcine TRYP can be activated in three ways: auto-activation in which TRYP is converted to TRY under appropriate pH conditions; self-hydrolyzing where TRYP is converted to TRY through the hydrolysis of another TRY molecule; and allo-hydrolyzing in which TRYP is converted to TRY through the hydrolysis of enterokinase [35], [36]. These activation modes have the same mechanism; in all three, the cleavage of the peptide bond between lysine6 and isoleucine7 at the C-terminal of the TRYP inhibitory peptide (FPTDDDDK) occurs under suitable pH conditions and then converts inactive TRYP into active TRY [20].
In the present study, we recommend a novel strategy for sericin self-degumming from silk thread. Furthermore, we integrated porcine TRYP into silk materials through the genetic engineering of silk thread using a Ser-1-based silkworm exogenous gene-specific expression technique [23]. Our results show that in an accelerated reaction system (1 mM Tris-HCl, pH = 8.0, 37 °C), the sericin-degumming rate of genetic engineered SDSM reached 29.28 ± 0.07 % in 24 h and was slightly lower than that of traditional ADM (35.03 ± 0.07 %). Importantly, the intensity of FibH protein in the SDM sericin is only 0.75 ± 0.07 × 109, much lower than 2.24 ± 0.21 × 109 in ADM sericin, and the same happens to the other two main components of silk fibroin, FibL and P25. Thus, we think that SDSF suffer less damage during degumming, and more α-helix and β-sheet conformations are retained to form better secondary and crystal structure, resulting in better mechanical properties. Moreover, compared to WT cocoon, the cocoon weight, hole silk diameter and secondary structure of TRYP cocoon were found to be highly similar. In addition, SDSF has a larger radius, better cleanliness and excellent mechanical properties, thus, we speculate that SDSF and ADSF will exhibit similar silk-reeling properties in silk industry. To summarize, we think that SDM is an attractive alternative to conventional ADM.
Conditions such as temperature and pH can determine the consumption of energy and chemical reagents in the degumming system. Traditional ADM utilizes high concentrations of alkaline solutions at high temperatures, resulting in high energy and chemical reagent consumption and environmental pollution[37]. In the current study, SDSM could be self-degummed in water at room temperature after one week (data not showed). To accelerate the degumming process, we only adjusted the reaction buffer temperature to 37 °C and pH to 8. This SDM will greatly reduce the costs of EDM, energy consumption, and wastewater production, and consequently protect the environment. We speculated the degumming mechanism to be as follows: once SDSM is immersed in the reaction buffer, TRYP that is located at the outermost sericin layer dissolves into the buffer, self-activates, and converts into active TRY. Then active TRY degrades sericin in SDSM to release more TRYP, producing more active TRY through self-hydrolyzing to accelerate the degradation of sericin. Finally, after sericin is completely degraded and removed by TRY, the degumming process ends, as TRY hardly reacts with fibroin.
Sericin is an excellent natural substance for the fabrication of various biomaterials owing to its good biocompatibility, biodegradability, and low immunogenicity [38]. Compared to the huge cost of sericin recovery from the waste alkali buffer in conventional ADM, sericin degraded by SDM is relatively easier and inexpensive to extract [30]. Our results show that this protein not only has improved cell proliferation activity but also causes no obvious cellular inflammation. These results suggest that SDM can be used to fabricate various sericin-based biomaterials or medical products with anti-bacterial, anti-fungal, anti-inflammatory, and anti-tumor activities. For example, human lactoferrin, which has antibacterial and anti-inflammatory activities after being degraded by TRY, can be co-expressed with TRYP in the silk thread [39]. Thus, sericin-based biomaterials with multiple biological functions can be easily fabricated though the self-degumming process of SDM.
Conclusion
Based on the genetic manipulation technology of silkworms, we developed new engineered silkworm cocoon that can be self-degummed under mild conditions. After self-degumming, it not only produce good fibroin fiber material but also active sericin ingredients for the development of functional sericin-based biomaterials. This strategy can effectively reduce the impact of the degumming process on the environment, generate greater economic value, and further accelerate the application of SDSM in the silk industry.
Compliance with ethics requirement
This paper does not contain any studies with human participants or animals by any of the authors.
CRediT authorship contribution statement
Riyuan Wang: Conceptualization, Methodology, Software, Visualization, Investigation, Writing – original draft. Yuancheng Wang: Software, Formal analysis, Visualization, Writing – original draft, Writing – review & editing, Funding acquisition. Jianxin Song: Validation, Resources, Methodology, Investigation, Data curation, Writing – original draft. Chi Tian: Resources, Methodology, Investigation, Data curation. Xinyuan Jing: Validation, Resources, Methodology, Investigation. Ping Zhao: Supervision, Project administration, Writing – review & editing. Qingyou Xia: Supervision, Writing – review & editing, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank Zhu zhengxian, the manager of China Zhejiang misai silk Co., Ltd, for providing data related to silk production. This work was supported by the Key Program of the National Natural Science Foundation of China (32030103), National Natural Science Foundation of China (32000941), and Fundamental Research Funds for the Central Universities (SWU120081).
Footnotes
Peer review under responsibility of Cairo University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2022.12.005.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Wee, R.Y. World Leaders In Silk Production. WordAtlas 2017; Available from: www.worldatlas.com/articles/world-leaders-in-silk-production.html (accessed on June 21, 2022).
- 2.Zhu L., et al. Recent advances in environmentally friendly and green degumming processes of silk for textile and non-textile applications. Polymers (Basel) 2022;14(4) doi: 10.3390/polym14040659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bucciarelli A., et al. A design of experiment rational optimization of the degumming process and its impact on the silk fibroin properties. ACS Biomater Sci Eng. 2021;7(4):1374–1393. doi: 10.1021/acsbiomaterials.0c01657. [DOI] [PubMed] [Google Scholar]
- 4.Toprak T., Akgun M., Anis P. Effects of environmentally friendly degumming methods on some surface properties, physical performances and dyeing behaviour of silk fabrics. Industria Textila. 2020;71(4):380–387. [Google Scholar]
- 5.Popescu A., Stoian E., Serban V. Trens in the world production of natural fibers of animal origin- silk and wool. Scient Pap-Ser Manage Econ Eng Agric Rural Develop. 2019;19(4):273–288. [Google Scholar]
- 6.Baumwollbörse, B. Forecast world fibre production. Discover Natural Fibres Initiative - DNFI 2019; Available from: www.textile-network.com/en/Technical-Textiles/Fasern-Garne/Forecast-world-fibre-production (accessed on June 21,2022).
- 7.Gulrajani M.L., et al. Degumming of silk with different protease enzymes. Indian J Fibre Text Res. 1996;21(4):270–275. [Google Scholar]
- 8.More S.V. Proteases for degumming: a novel, green and ecofriendly way to quality silk production. Int J Curr Res. 2015;7(11):23032–23038. [Google Scholar]
- 9.Yushkova E.D., et al. Application of immobilized enzymes in food industry. J Agric Food Chem. 2019;67(42):11553–11567. doi: 10.1021/acs.jafc.9b04385. [DOI] [PubMed] [Google Scholar]
- 10.Kabir, S.M.M. and J. Koh, Sustainable Textile Processing by Enzyme Applications. 2021: Biodegradation [Working Title].
- 11.Naveed M., et al. Protease-a versatile and ecofriendly biocatalyst with multi-industrial applications: an updated review. Catal Lett. 2021;151(2):307–323. [Google Scholar]
- 12.Anand P., Pandey J.P., Pandey D.M. Study on cocoonase, sericin, and degumming of silk cocoon: computational and experimental. J Genet Eng Biotechnol. 2021;19(1):32. doi: 10.1186/s43141-021-00125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng Y., et al. High molecular weight silk fibroin prepared by papain degumming. Polymers (Basel) 2020;12(9) doi: 10.3390/polym12092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Itoh K., et al. Recent progress in development of transgenic silkworms overexpressing recombinant human proteins with therapeutic potential in silk glands. Drug Discov Ther. 2016;10(1):34–39. doi: 10.5582/ddt.2016.01024. [DOI] [PubMed] [Google Scholar]
- 15.Wang F., et al. Large-scale production of bioactive recombinant human acidic fibroblast growth factor in transgenic silkworm cocoons. Sci Rep. 2015;5:16323. doi: 10.1038/srep16323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tamura T., et al. Germline transformation of the silkworm Bombyx mori L. using a piggyBac transposon-derived vector. Nat Biotechnol. 2000;18(1):81–84. doi: 10.1038/71978. [DOI] [PubMed] [Google Scholar]
- 17.Ru B.G., et al. Active products of porcine trypsin after autolysis. Sci Sin. 1980;23(11):1453–1460. [PubMed] [Google Scholar]
- 18.Zhang Y.F., et al. High-yield secretory production of stable, active trypsin through engineering of the N-terminal peptide and self-degradation sites in Pichia pastoris. Bioresour Technol. 2018;247:81–87. doi: 10.1016/j.biortech.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 19.Weil L., Timasheff S.N. Enzymic activity of trypsin autolysis products. Arch Biochem Biophys. 1966;116(1–3):p. 252-+. doi: 10.1016/0003-9861(66)90030-0. [DOI] [PubMed] [Google Scholar]
- 20.Roach J.C., et al. The molecular evolution of the vertebrate trypsinogens. J Mol Evol. 1997;45(6):640–652. doi: 10.1007/pl00006268. [DOI] [PubMed] [Google Scholar]
- 21.Chao L., Liener I.E. Autoactivation of porcine trypsinogen in the presence and absence of calcium. Biochim Biophys Acta. 1965;96:508–516. doi: 10.1016/0005-2787(65)90567-8. [DOI] [PubMed] [Google Scholar]
- 22.Omar A., et al. Effects of trypsin-induced limited hydrolysis on the structural, functional, and bioactive properties of sericin. RSC Adv. 2021;11(41):25431–25440. doi: 10.1039/d1ra03772b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang F., et al. An optimized sericin-1 expression system for mass-producing recombinant proteins in the middle silk glands of transgenic silkworms. Transgenic Res. 2013;22(5):925–938. doi: 10.1007/s11248-013-9695-6. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y.C., et al. Transdermal peptide conjugated to human connective tissue growth factor with enhanced cell proliferation and hyaluronic acid synthesis activities produced by a silkworm silk gland bioreactor. Appl Microbiol Biotechnol. 2020;104(23):9979–9990. doi: 10.1007/s00253-020-10836-0. [DOI] [PubMed] [Google Scholar]
- 25.Xu S., et al. A silkworm based silk gland bioreactor for high-efficiency production of recombinant human lactoferrin with antibacterial and anti-inflammatory activities. J Biol Eng. 2019;13 doi: 10.1186/s13036-019-0186-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peng Z., et al. Structural and mechanical properties of silk from different instars of Bombyx mori. Biomacromolecules. 2019;20(3):1203–1216. doi: 10.1021/acs.biomac.8b01576. [DOI] [PubMed] [Google Scholar]
- 27.Terada S., et al. Sericin, a protein derived from silkworms, accelerates the proliferation of several mammalian cell lines including a hybridoma. Cytotechnology. 2002;40(1–3):3–12. doi: 10.1023/A:1023993400608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takahashi M., et al. The silk protein, sericin, protects against cell death caused by acute serum deprivation in insect cell culture. Biotechnol Lett. 2003;25(21):1805–1809. doi: 10.1023/a:1026284620236. [DOI] [PubMed] [Google Scholar]
- 29.DeBari M.K., et al. Silk fibroin as a green material. ACS Biomater Sci Eng. 2021;7(8):3530–3544. doi: 10.1021/acsbiomaterials.1c00493. [DOI] [PubMed] [Google Scholar]
- 30.Vaithanomsat P., Kitpreechavanich V. Sericin separation from silk degumming wastewater. Sep Purif Technol. 2008;59(2):129–133. [Google Scholar]
- 31.Bucciarelli A., et al. Tidy dataset of the experimental design of the optimization of the alkali degumming process of Bombyx mori silk. Data Brief. 2021;38 doi: 10.1016/j.dib.2021.107294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marcus-Sekura C., et al. Evaluation of the human host range of bovine and porcine viruses that may contaminate bovine serum and porcine trypsin used in the manufacture of biological products. Biologicals. 2011;39(6):359–369. doi: 10.1016/j.biologicals.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dong Z., et al. Comparative proteomics reveal diverse functions and dynamic changes of Bombyx mori silk proteins spun from different development stages. J Proteome Res. 2013;12(11):5213–5222. doi: 10.1021/pr4005772. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y., et al. Genetic fabrication of functional silk mats with improved cell proliferation activity for medical applications. Biomater Sci. 2019;7(11):4536–4546. doi: 10.1039/c9bm01285k. [DOI] [PubMed] [Google Scholar]
- 35.Shu M., et al. Expression, activation and characterization of porcine trypsin in Pichia pastoris GS115. Protein Expr Purif. 2015;114:149–155. doi: 10.1016/j.pep.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y., et al. Improved production of active streptomyces griseus trypsin with a novel auto-catalyzed strategy. Sci Rep. 2016;6:23158. doi: 10.1038/srep23158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qian, C., Recent Developments in Silk Degumming with Enzyme at Abroad. China Textile Leader, 2004.
- 38.Shitole M., et al. Pharmaceutical applications of silk sericin. Ann Pharm Fr. 2020;78(6):469–486. doi: 10.1016/j.pharma.2020.06.005. [DOI] [PubMed] [Google Scholar]
- 39.Wang B., et al. Lactoferrin: structure, function, denaturation and digestion. Crit Rev Food Sci Nutr. 2019;59(4):580–596. doi: 10.1080/10408398.2017.1381583. [DOI] [PubMed] [Google Scholar]
- 40.Takasu Y., Yamada H., Tsubouchi K. Extraction and chromatographic analysis of cocoon sericin of the silkworm, Bombyx mori. J Insect Biotechnol Sericol. 2002;3 doi: 10.1271/bbb.66.2715. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.