Graphical abstract
Keywords: Hotspot, Crossovers, Recombination, Wheat, DNA methylation, Ph1 locus
Highlights
-
•
Plant recombination sites were determined to as little as 19–593 bp.
-
•
The open chromatin and DNA hypomethylation were found associated with CO except of CHH methylation.
-
•
A new method (ddPing) for efficient assessment of CO frequency in large gamete populations was developed.
-
•
The Ph1 locus does not affects all recombination sites in wheat.
-
•
The results are implying additional independent pathway in wheat recombination control.
Abstract
Introduction
Meiotic recombination is one of the most important processes of evolution and adaptation to environmental conditions. Even though there is substantial knowledge about proteins involved in the process, targeting specific DNA loci by the recombination machinery is not well understood.
Objectives
This study aims to investigate a wheat recombination hotspot (H1) in comparison with a “regular” recombination site (Rec7) on the sequence and epigenetic level in conditions with functional and non-functional Ph1 locus.
Methods
The DNA sequence, methylation pattern, and recombination frequency were analyzed for the H1 and Rec7 in three mapping populations derived by crossing introgressive wheat line 8.1 with cv. Chinese Spring (with Ph1 and ph1 alleles) and cv. Tähti.
Results
The H1 and Rec7 loci are 1.586 kb and 2.538 kb long, respectively. High-density mapping allowed to delimit the Rec7 and H1 to 19 and 574 bp and 593 and 571 bp CO sites, respectively. A new method (ddPing) allowed screening recombination frequency in almost 66 thousand gametes. The screening revealed a 5.94-fold higher recombination frequency at the H1 compared to the Rec7. The H1 was also found out of the Ph1 control, similarly as gamete distortion. The recombination was strongly affected by larger genomic rearrangements but not by the SNP proximity. Moreover, chromatin markers for open chromatin and DNA hypomethylation were found associated with crossover occurrence except for the CHH methylation.
Conclusion
Our results, for the first time, allowed study of wheat recombination directly on sequence, shed new light on chromatin landmarks associated with particular recombination sites, and deepened knowledge about role of the Ph1 locus in control of wheat recombination processes. The results are suggesting more than one recombination control pathway. Understanding this phenomenon may become a base for more efficient wheat genome manipulation, gene pool enrichment, breeding, and study processes of recombination itself.
Introduction
Meiotic recombination plays a crucial role in the evolution of species and the generation of new haplotypes. Recombination is initiated by the formation of DNA double-strand breaks (DSBs) at the onset of the first meiotic division. However, only a fraction of DSBs is processed into chromosome recombination known as crossovers (COs). The COs are necessary for accurate segregation, and the development of balanced gametes [1]. Lack of the formation of at least one CO per chromosome pair leads to chromosome nondisjunction and aneuploidy [2]. Thus, there is a requirement for an ‘obligate CO’ per chromosome pair and 1–3 COs per chromosome pair in most eukaryotes can be observed [3]. Many studies have demonstrated that COs are not evenly distributed along the chromosome arms [4], [5], [6]. In cereals, COs concentrate in centromere-distal euchromatic chromosome regions with high gene density, while centromere-proximal regions are usually poor in COs. At the genomic scale, COs in plants were identified near promoters and terminators of genes in regions where DNA is accessible [7], [8], [9], [10], [11]. Other CO determinants include transcription factors, histone modifications, and chromatin methylation [6]. In some species, narrow regions characterized by high recombination frequencies determined as recombination hotspots were identified suggesting the presence of markers for preferential COs occurrence [12], [13]. In Arabidopsis and maize genomes domains with high CO rates (hot regions) alternate with domains with lower CO rates (cold regions). So far, relatively small (2.5 kb) recombination hotspots were determined for plants with small or moderate genomes as Arabidopsis thaliana and Zea mays with DNA repeat content of 30 % and 50 %, respectively [14]. However, a greater (23 kb) recombination hotspot was reported for bread wheat (Triticum aestivum L.) [15], [16] within a large and complex genome with more than 80 % of repetitive DNA [17]. Recombination hotspots in all eukaryotes are often associated with open chromatin. However, particular sequence, chromatin accessibility, and higher-order genome organization of the hotspots remain unclear, especially in plants [6], [8], [13].
The bread wheat is an allohexaploid species composed of three homoeologous genomes (AABBDD, 2n = 42) derived from three diploid ancestors. Despite the homoeology between chromosomes of particular genomes, wheat behaves as a diploid during meiosis, as each chromosome pair recombine only with its true homologue. This behavior is controlled by the Pairing homoeologous 1 (Ph1) locus localized on chromosome 5B [18], [19]. The Ph1 locus is associated with a group of kinase-like genes [20]. The exact mechanism of the Ph1 function has not been deciphered yet. The locus appears to impose a very high DNA homology requirement on CO, affecting not only homoeologous but also homologous chromosomes, and it appears to be dosage-dependent [21]. When the Ph1 locus is active, synapses occur only between homologous chromosomes at the onset of meiosis, and not all of these synapses result in COs. However, inactivation of the Ph1 locus results in the formation of a larger number of synapses and COs even between homoeologous chromosomes. A single Ph1 deletion mutant (CS ph1b) generated by Sears [22] in wheat variety Chinese Spring (CS) has been used worldwide in research and breeding programs to introgress chromatin of wild relatives into wheat [23], [24].
In bread wheat, more than 80 % of the CO events occur in less than a quarter of the genome [25], [26]. In plant genomes, including wheat, recombination rates increase from centromeres to telomeres although with different degrees of intensity [27], [28], [29] and are often linked with gene regions [30]. Genome recombination mediated through meiotic COs is a crucial tool for gene or allele reshuffling prerequisite for adaptation to new conditions. Significant progress in the understanding of meiotic progression and recombination has been reported for model organisms, i.e., yeasts or A. thaliana, and even some crop species with less complex genomes, e.g., Oryza sativa and Z. mays [9], [31], [32], [33]. Even though the recombination processes are relatively conserved, substantial differences between species are observed [6]. This creates demand for studying the COs directly in important crops like Hordeum vulgare or T. aestivum.
Here, we report identification and characterization of a recombination hotspot in bread wheat within a genomic region introgressed from the T. militinae. The introgressive region is recombining and is a source of a high-level nucleotide polymorphism, which allowed identification and analysis of the recombination hotspot with unprecedented resolution. The study is focused on the determination of DNA sequence and epigenetic features directing the COs to the hotspot and its comparison to the “regular” recombination site under altered influence of the Ph1 locus.
Material and methods
Plant materials
Mapping populations used for the study of a hotspot and “regular” recombination region were initially created to clone gene/genes in the adult plant powdery mildew resistance locus QPm.tut-4A [34]. The first mapping population (Ph1) was derived from a cross between wheat cv. Chinese Spring (CS) and introgressive wheat line 8.1 (cv. Tähti with introgressed T. militinae genome segments, Jakobson et al. [35]). The second mapping population (ph1) was obtained by crossing wheat cv. CS mutant ph1b [22] with T312.30.38.16, a derivative of introgressive line 8.1 with only 4A T. militinae segment harboring the QPm.tut-4A locus. The third mapping population was derived from a cross of wheat cv. Tähti and line 8.1 [36]. Following wheat lines were used as controls: the aneuploid stocks of cv. CS nullisomic 4A-tetrasomic 4B and nullisomic 4A-tetrasomic 4D lines, provided by the National BioResource Centre (Kyoto, Japan), the T. militinae Zhuk. et Migush (TM) accession no. K-46007 (the donor of introgressive regions) was provided by the N.I.Vavilov Institute of Plant Industry (St. Petersburg, Russia), a doubled haploid line (DH81) carrying the same T. militinae translocations which determined the powdery mildew resistance as line 8.1 (8.1) [36] as well as commercial cv. Tähti (TAT). For recombination frequency assessment, twelve recombinant lines from Ph1 and ph1 mapping populations, with recombination events in the H1 and Rec7 regions, were selected (Table 1).
Table 1.
List of lines with identified recombinations within H1 (hotspot, owm110-owm111) and Rec7 (owm103-owm110) regions. A = Chinese Spring allele, B = T. militinae allele.
| Name | Mapping population | owm103 | owm110 | owm111 | CO Region |
|---|---|---|---|---|---|
| 76.14.55.94 | Ph1 | B | B | A | H1 |
| 76.14.45.1 | Ph1 | A | A | B | H1 |
| 76.14.55.9.41 | Ph1 | A | A | B | H1 |
| 76.14.55.4.154 | Ph1 | A | A | B | H1 |
| 88.11 | Ph1 | B | B | A | H1 |
| 96.14.5.9.177 | Ph1 | B | B | A | H1 |
| 76.14.52.101 | Ph1 | B | A | A | Rec7 |
| 22.18 | ph1 | B | B | A | H1 |
| 22.83 | ph1 | B | B | A | H1 |
| 86.13 | ph1 | H | H | B | H1 |
| 87.45 | ph1 | H | H | A | H1 |
| 27.16 | ph1 | B | H | H | Rec7 |
Primer design and verification
The target sequences for the design of new primers were selected using the CS 4AL and 4AL-TM chromosome-specific sequences [37], [38] and the cv. CS reference sequence [17]. The polymorphism was identified by comparing the CS sequence with the 4AL-TM chromosome-specific survey sequence using the BlastN algorithm [39]. Only sequences with greater than 95 % identity were considered for marker development, and those containing indels were preferred. If no indel was available, a cleaved amplified polymorphic sequence approach [40] was tested. The SNPs (single nucleotide polymorphisms) genotyped by sequencing were the least preferred. PCR primers were designed using Primer3 software [41]. All primer pairs were tested for specificity using DNA of the CS, DH81, H (CS/DH81 in 1:1 mixture representing derivative heterozygote), nullisomic 4A-tetrasomic 4B, and nullisomic 4A-tetrasomic 4D, and DNA from flow-sorted chromosome arms 4AL-CS and 4AL-TM amplified according to Šimková et al. [42]. Amplicon sequencing was done by the ABI 3730xl gene analyzer using BigDye terminator v3.1 according to the manufacturer’s instructions (Applied Biosystems, USA). DNA sequences were analyzed using the Geneious Prime Software (version 2020.2.4).
High-density mapping
DNA was extracted according to Ivaničová et al. [43] from 100 mg lyophilized leaf segments disrupted with two 5 mm glass beads in sealed 96-well 2 ml plates using Retsch MM301 mill at 27 Hz for 4 min. Lysis was performed in 1 ml of lysis buffer pH 8.0 (500 mM NaCl, 100 mM Tris, 50 mM EDTA, 5 g/l sodium bisulfite, 0.1 % 2-mercaptoethanol, 0.1 % ascorbic acid, and 0.1 % RNAse) at 65 °C for 45 min. Subsequently, plates were centrifuged at 1300 × g for 10 min at 4 °C. DNA purification was done at a Biomek® NXP automated workstation (Beckman Coulter Life Sciences, USA). A 100 µl of lysate was transferred to a new PCR plate and mixed with 70 µl of isopropanol and 5 µl of Agencourt® Genfind® v2 magnetic beads (Beckman Coulter Life Sciences, USA) and incubated for 5 min at RT. Subsequent steps comprised 5 min incubation at the magnet, supernatant discarding, and three-time wash with 100 µl of 70 % ethanol. Elution was done in 100 µl of H20 with 10 min incubation at RT and 10 min incubation at the magnet. Eluted DNA was transferred to a new PCR plate.
PCR was done in 20 μl reaction mix comprising 0.01 % (w/v) o-cresolsulphonephtalein, 1.5 % (w/v) sucrose, 0.2 mM of each dNTP, 0.6 U of Taq DNA polymerase, 1 μM of each primer, 10 mM Tris-HCl, 50 mM KCl, 1.5 mM MgCl2 and 0.1 % (v/v) Triton X-100. 10–20 ng of genomic DNA or 5 ng of amplified chromosome-specific DNA was used as a template. The reaction conditions consisted of an initial denaturation step: 95 °C for 5 min, followed by 40 cycles of 95 °C for 30 s, optimized annealing temperature (Supplementary file S1) for 30 s, and 72 °C for 30 s per 500 bp of amplicon length; the reactions were completed with incubation at 72 °C for 5 min. In case of need for higher PCR specificity, a touchdown PCR (TD-PCR) was applied. The reaction conditions were: an initial denaturation step of 95 °C for 5 min, followed by 16 cycles of 95 °C for 30 s, 65 °C for 30 s with 0.7 °C decreases per cycle, and 72 °C for 50 s followed by 17 cycles of 95 °C for 30 s, 53 °C for 30 s and 72 °C for 50 s; the reactions were completed with an elongation step of 72 °C for 5 min. Cleaved amplified polymorphic sequence assays were completed with 2 h digestion using the appropriate restriction endonuclease, HindIII (owm103) and RsaI (owm110). The amplicons were electrophoretically separated on Mega Gel High Throughput Vertical System (C.B.S. Scientific, USA) using the 4 % non-denaturing polyacrylamide gels stained by ethidium bromide and visualized using the InGenius gel imaging system combined with GeneSnap Image Acquisition software version 7.12.06 (Syngene International Ltd., India).
Sequence analysis
To ensure that the sequences of the recombination hotspot H1 and the adjacent region Rec7 with recombination event are correctly assembled in the CS reference sequence and identify additional SNPs for more precise delimitation of the CO sites, both regions were divided into fragments up to 590 bp with new primers (Supplementary file S1). The amplicons were designed to overlap by 87–205 bp and were Sanger sequenced. Both regions were sequenced from all parental lines including CS, T. militinae, introgression line 8.1, and wheat cv. Tähti and the lines with recombination events in the H1 and Rec7 regions (Table 1). Sequences of the parental lines were compared with the non-redundant TREP nucleotide sequences database (release 16, https://wheat.pw.usda.gov/blast/) and NCBI EST database of Triticeae (ncbi.nlm.nih.gov) using BlastN [44] to verify the presence of transcribed and/or repetitive elements. De novo gene prediction was performed using FGENESH software (softberry.com) [45] using wheat as a specific gene-finding parameter. The sequence extraction of the recombination regions with the flanking regions from the CS reference sequence was performed using SAMtools 1.4 [46]. The GC content was analyzed using the GC Content Calculator (https://www.biologicscorp.com/tools/GCContent/) with a window size of 30. Sequences were aligned at the web based CLUSTALW tool (https://www.genome.jp/tools-bin/clustalw) using default settings.
DNA methylation status in the H1 and Rec7 regions
DNA methylation in the regions with recombination events was determined using the EZ DNA Methylation-Gold Kit (Zymo Research, USA). 400 ng of genomic DNA was bisulfite converted and purified according to the manufacturer's instruction (ver. 1.0.1). The DNA methylation was assessed in the recombination regions (H1 and Rec7) of the T. aestivum cv. CS, introgression line 8.1, T. militinae, cv. Tähti, and recombinant lines. For amplification of the bisulfite converted DNA, a set of new primers was designed (Supplementary file S1). The optimal annealing temperature was empirically optimized and 1 µl of CT-converted DNA was used as a template. The reaction conditions are as described above. The size of resulting amplicons was verified in 1 % agarose gel and Sanger sequenced as described above. Bisulfite sequencing analyses were performed with Kismeth [47], a web-based tool considering potential cytosine methylation in any sequence context (CG, CHG, and CHH) using default parameters.
Gamete collection and generative nuclei extraction
170 progenies of plants heterozygous in the QPm-tut.4A region from Ph1 and ph1 mapping populations were sown in the greenhouse. Plants were genotyped with owm110 and owm111 markers (Supplementary file S1), and only plants heterozygous in the tested regions were kept. Green and yellow-green anthers (250–300 per genotype) were collected in the 1.5 ml tube and suspended in 1000 µl of LB01 isolation buffer [48]. Subsequently, the suspension of anthers was vortexed for 3 min at 1500 rpm to release the pollen grains and centrifuged for 10 s at 3000 × g. Sedimented anthers were manually transferred to a petri dish, cut with a razor blade, and suspended in 1000 µl of fresh LB01 isolation buffer. Suspension of pollen grains released from chopped anthers was filtered to a new 1.5 ml tube using a 22 µm nylon mesh. Both pollen grains suspensions were homogenized by filling about 1/4 of the 1.5 ml tube with glass beads (425–600 µm) and vortexed for 1 min at maximum speed. Homogenates from both tubes were filtered through 22 µm nylon mesh to one tube, stained with DAPI (1.5 µg/ml), and used for flow sorting of haploid (1C DNA content) generative pollen nuclei. The nuclei flow sorting was done using BD FACSAria II high-speed flow sorter equipped with a 355 nm laser for DAPI excitation. As a reference for 1C and 2C nuclei from fresh leaves of wheat cv. CS was used. The 1C nuclei were collected in the 0.5 ml tubes with 40 µl sd H2O and stored at −80 °C for further analysis.
Recombination frequency assessment in the selected regions
DNA purification of flow-sorted nuclei was performed according to Šimková et al. [42]. The flow-sorted nuclei were treated with 60 ng/ml proteinase K (Roche, Basel, Switzerland) for 40 h at 50 °C, and DNA was purified using a Microcon YM-100 column (MilliporeSigma, Burlington, MA, USA). The DNA samples were eluted to 20 μl of sdH2O. Concentrations were measured using the Quant-iT PicoGreen assay (Invitrogen, Carlsbad, USA).
Comparison of recombination frequency in the “hotspot” H1 (delimited by flanking markers owm111 and owm110) and Rec7 “regular” (delimited by flanking markers owm110 and owm103) CO site was done using TaqMan probes specific for each flanking marker (Table 2). The probes were labeled with the FAM and VIC/HEX dyes for CS and TM variants, respectively (Table 2). The frequency of CO events was determined by a droplet digital PCR (ddPCR) set containing: QX200 Droplet Generator (Bio-Rad, Hercules, USA), C1000 Thermal Cycler (Bio-Rad, Hercules, USA), and QX200 Droplet Reader (Bio-Rad, Hercules, USA). Results were analyzed using QuantaSoft Software (version 1.7.4.0917) set for Copy Number Variation Analysis (Bio-Rad, Hercules, USA).
Table 2.
Sequences of primers and probes used for comparison of recombination frequency. The TaqMan probes were either labeled by FAM (T. aestivum cv. Chinese Spring [CS] variant) or VIC/HEX (T. militinae [TM] variant). Probes were quenched at their 3′end with QSY or 3IABkFQ with ZEN internal quencher.
| TaqMan probe | Marker | Sequence and modifications 5′–3′ |
|---|---|---|
| owm103_CS_FAM | owm103 | FAM/CTCAAGCTTCAGCGAAGTCGGC/QSY |
| owm103_TM_VIC | VIC/CTCAAGCGTCAGCGAAGTCGG/QSY | |
| owm110_CS_FAM | owm110 | FAM/CAAGTACAC/ZEN/TGAAAATCTAGGCATGTTTC/3IABkFQ |
| owm110_TM_HEX | HEX/CAAGTTCAC/ZEN/TGAAAATCTAGGCATGTTTC/3IABkFQ | |
| owm111_CS_FAM | owm111 | FAM/CTCCCGTGAGCATGCGTCC/QSY |
| owm111_TM_VIC | VIC/TCCCGGACCGTGGCATGC/QSY |
Each probe and combinations of probes were individually tested on parental DNAs, equal mixture of CS:TM (1:1) DNAs (artificial heterozygote), and also on DNA from a line from the cross of CS and 8.1 mapping population heterozygous for the tested region. As a negative control reaction without DNA was used. Performance of each probe combinations was tested on the same DNAs as before supplemented with DNAs from known homozygote recombinant lines for the region flanked by tested probes (Table 1). The saturation of the ddPCR reaction was tested by running it on different amounts of DNA from the recombinant line. DNA amount (sorted pollen nuclei) used for a single ddPCR reaction was optimized to 20 ng to prevent oversaturation of the reaction. The amount corresponds to 1200 copies of the wheat genome. Each DNA sample was analyzed in at least three technical replicates for both CO sites. The optimal annealing temperature for primers and probes was evaluated using genomic DNA of parental lines and known recombinants for both sites with ddPCR and the thermal gradient feature of Bio-Rad C1000 Thermal Cycler (Bio-Rad, Hercules, USA). The effectiveness of PCR reactions using designed primers and TaqMan probes was evaluated with RT-PCR using Bio-Rad CFX96 Real-Time PCR System (Bio-Rad, Hercules, USA). The ddPCR analyses were performed using ddPCR™ Supermix for Probes (no dUTP) (Bio-Rad, Hercules, USA) according to the manufacturer's instructions with 50 mM of each primer and 250 mM of each probe (in every experiment two sets of primers and four probes for each CO site were used) at 62 °C during the annealing/extension phase.
SNP distribution determination
SNP calling was done using the trimmed and quality-filtered 4AL-TM telosome chromosome-specific survey sequences [37] and the cv. CS reference genome sequence [17] of the 4AL chromosomal arm.
SNP distribution was analyzed using the GATK4 pipeline (https://gencore.bio.nyu.edu/variant-calling-pipeline-gatk4/). To reduce computational demands, the 4A reference sequence was truncated by the leading 622.5 Mb, and only the remaining 122 Mb were used for the next steps. The 4AL-TM reads were mapped to the reference using the BWA-MEM tool (https://bio-bwa.sourceforge.net/). The resulting aligned reads (Table 3) were sorted and duplicity entries and indels were removed. SNP variants were quality-filtered using GATK4 recommendations. Base quality score recalibration (BQSR) was performed to produce a high-confidence SNP dataset and SNP positions were adjusted to their original values (+600 Mb). These positions were used as input for the in-house-designed visualization tool.
Table 3.
Summary of sequence data for SNP distribution determination.
| Name | Reads | Reads aligned (%) | Read length | Reads duplicated (%) | Average coverage |
|---|---|---|---|---|---|
| 4AL-TM | 52,733,490 | 0.813649 | 99.86138 | 0.169107 | 26.7502 |
The distribution of SNPs was analyzed using the sliding-window approach. Due to a large amount of data, a javascript library, using AmCharts [https://www.amcharts.com/] tool for the dynamic chart visualization, allowing real-time adjustment of sliding-window parameters and re-computation of visualized data depending on the displayed position interval, was implemented. Dynamic parameter adjustment was used for an adaptation of sliding-window interval to selected chromosome range to prevent readability deterioration. The adjustment is based on two parameters, i: visible interval range (IS) and ii: number of points (POINTS) rendered in the final chart (maximum of 10,000). Sliding-window size (SW) is then computed as follows:
For each position (POS) in the IS interval (from left to right bound of IS with the step equal to STEP), a number of presented SNPs (TOTAL) in the corresponding interval was computed and then used as a point of the final chart with corresponding coordinates (x: POS; y: TOTAL).
The line chart was then supplemented by the two horizontal lines indicating a mean number of SNPs in the analyzed chromosomal region with a gap (695,000,000 and 698,364,950) in the expected break point between the T. aestivum and T. militinae chromosome segments as indicated by Abrouk et al. [37] to increase the mean calculation reliability.
Results
Analysis of the recombination sites sequences
During mapping of the powdery mildew resistance gene QPm-tut.4A (34), a region (Fig. 1), with six recombination events and flanked with markers owm111 and owm110 (Supplementary file S1) [34] in the Ph1 mapping population was identified. Since these COs occurred in a relatively narrow region of 1586 kb (Fig. 1B), it was designated as a recombination hotspot H1. To further saturate the region with markers and analyze DNA sequence composition in CS, TM, 8.1, TAT, and recombination lines, another eight additional primer pairs were designed (Supplementary file S1). We selected a region adjacent to H1, with only one recombination site in the Ph1 population for comparison. The region was labeled Rec7 and is flanked by owm110 and owm103 markers (spanning 2538 bp, Fig. 1B, Supplementary file S1). For a broader picture, a region of 8 kbp (4A: 718477000–718485000) harboring both recombination regions was extracted from the CS genome reference sequence and analyzed (Fig. 1). The mapping population derived from a cross of wheat cv. Tähti and line 8.1 was extended to 1111 lines but no recombination in the region owm82-gwm160 was observed. The owm82 and gwm160 [49] markers flank almost the whole T. militinae introgression in the 4A chromosome and were used for selection of lines with recombination events [34].
Fig. 1.
Characterization of the hotspot (H1) and Rec7 CO sites and their genomic properties. Physical maps showing: A) Position of two CO sites – Rec7 and H1 (hotspot) within the introgressed segment in chromosome 4A (TM segment is from 698 Mb till the end (744 Mb) of the 4A chromosome (Abrouk et al. 2017)); B) The 8 kb region with identified CO sites (blue) and about 2 kb flanking regions (yellow). Within the region, sites with CO events were delimited – Rec7 (“regular”, about 2.5 kb) flanked by markers owm103 and owm110 and H1 (about 1.7 kb) flanked by markers owm110 and owm111. The number of detected CO events is marked by asterisks: * (black) - CS × 8.1 Ph1 mapping population, * (red) - CS × 8.1 ph1 mapping population. The numbers below (in the colors of the blocks) represent the average percentage of GC content. Only the most relevant SNPs within the Rec7 and H1 delimiting the exact loci with recombinations are marked as dotted lines. Below the H1 is drawn an in silico predicted gene-like structure (pseudogene) which was not found to be transcribed; C) GC content based on a sliding window of 30 bp. The average wheat genome (CS) GC content is 48 % (red dotted line). GC content of some parts of the Rec7 was as low as 6 %; D, E, F) Graphs presenting the location of various types of methylated (colored bars above the x-axis) and unmethylated (colored bars at the bottom of the x-axis) C’s in the H1 and Rec7 region of the used parental lines: D) CS; E) 8.1; F) TM. The colors of the bars represent three types of methylation sites with sequences: red for CG (or CpG), green for CHG, and blue for CHH (where H corresponds to A, T, or C); G) Graphical expression of chromatin epigenetic marks possibly associated with CO formation in the whole 8 kb CS region acquired from the http://bioinfo.sibs.ac.cn/cs_epigenome. The H3K4me3, H3K9ac, H3K27ac, and DHS (Dnase hypersensitive site) are assumed to be associated with euchromatin, whereas H3K9me2 and H3K27me3 are associated with the constitutive and facultative heterochromatin. Positions of regions with COs are flanked by blue lines. Even the positions of the epigenetic determinants are derived from the leaf tissue and not from meiocytes, they are suggesting that both regions are part of euchromatin which is considered biologically active.
Sanger sequencing of the H1 and Rec7 regions from the recombinant lines (Table 1) of both CS-based mapping populations and the control lines (CS, TM, 8.1, TAT) verified the sequence composition and allowed more precise placement of the CO events (Supplementary file S2). However, PCR does not amplify the target sequences from TAT. Sequence analysis of the H1 region revealed two additional polymorphisms. One insertion of AG in 8.1/TM compared to CS at 1166 bp from the owm110 marker. The second SNP was nucleotide substitution (T(CS)/C(TM)) at 594 bp from the owm110 marker. The new polymorphisms divided the H1 region into three subregions of almost equal length (Fig. 1B). Sequences of ten recombinant lines (Table 1) of the Ph1 and ph1 populations revealed that the COs took place at only two subregions of the H1 region adjacent to the owm110 marker with equal frequency (Fig. 1B). Both regions of H1 have relatively uniform and similar GC content of 44.5 % (45.4 and 43.6 % of GC, for the 593 and 571 bp subregion, respectively). On the other hand, the third subregion of H1 with no recombination contains 59.6 % of GC.
A comparison of the Rec7 region between CS and line 8.1 provided 26 additional SNPs (Supplementary file S2). The SNPs allowed delimitation of the sites of CO events into two subregions of the Rec7 region (Fig. 1B). The first was a 19 bp subregion (36.8 % of GC) in a recombinant from the Ph1 mapping population. The second was the 574 bp subregion (30.8 % of GC) in a recombinant from the ph1 mapping population (Fig. 1B). Both regions and the interspacing part together comprise 30.3 % of GC only with some parts down to 6 % GC (Fig. 1C).
The whole analyzed sequence does not show any significant homology to repetitive elements, wheat ESTs, or confirmed protein-coding genes. However, de novo gene prediction identified the structure of a hypothetical protein coding gene overlapping the H1 site in the CS sequence (Fig. 1B). The gene has 129 amino acids (out of 453) of the first and the third exon 96 % identical to T. aestivum zinc finger MYM-type protein 1-like, XP_044357821.1. The remaining parts have no significant identity to any known protein. The shortest (the fourth) exon (98 bp) of the predicted gene overlaps with the 593 bp subregion of the H1 (Supplementary file S2) (Fig. 1B) and is rich in GC (58.2 %). In the T. militinae sequence, the gene was not predicted, and the expression of the gene was not confirmed by any known mRNA (NCBI, ncbi.nlm.nih.gov).
Methylation pattern in the recombination regions
The extent of methylation of DNA in the Rec7 and H1 region in CS (Fig. 1D), 8.1 (Fig. 1E), and TM (Fig. 1F) was analyzed to investigate the possible difference in the parental lines and methylation pattern divergence in the T. militinae chromatin in the genetic background of T. aestivum (introgression line 8.1) and the original genetic background (TM). The methylation pattern observed across the tested 4,290 bp region (owm103-owm111) was not even (Fig. 1D–F). H1 and Rec7 regions of CS were characterized by 11.55 % of methylated C’s comprising 45.81 % of CG's, 12.20 % of CHG’s, and 1.45 % of CHH’s. In introgression line 8.1, 16.51 % of methylated C’s, among which were 51.59 % of CG’s, 16.11 % of CHG’s, and 5.97 % of CHH’s were observed. The highest methylation level was identified in TM, 18.12 % of methylated C’s, comprising 55.31 % of CG’s, 18.33 % of CHG’s, and 6.77 % of CHH’s. It can be assumed that introgression line 8.1 has an intermediate level of overall methylation between CS and TM (Fig. 1D–F).
All three sub-regions of the H1 region are highly hypomethylated with similar methylation patterns at the edges of H1. The region close to the owm111 marker shows a high concentration of methylated C’s in all sequence contexts. Whereas the region around the owm110 marker shows low methylation which continues to the Rec7 region where high methylation is accompanied by high GC content at the edge of the 574 bp CO site. The recombination sites are again characterized by a low level of methylation. However, the 19 bp CO site overlaps with a high level of CHG and CHH methylation in the T. militinae chromatin (the same for 8.1 and TM), which is in contrast with CS chromatin (Fig. 1D-F). Methylation in all recombinant lines resembled the pattern of the introgression line 8.1. Decreasing level of methylation for the sequence at this region was observed as follows: CG > CHG > CHH. The H1 showed a similar pattern. The genome of the cv. Tähti showed indels within the tested region preventing sequencing with used primers.
CO sites and SNP distribution in T. Militinae introgression segment
The distal region of wheat chromosome 4AL (622.5–744.5 Mb) was analyzed to determine the distribution of high-confidence SNPs between CS and T. militinae (line 8.1, Fig. 2). The T. militinae segment was analyzed as 4AL-TM telosome created by crossing of the CS 4AL ditelosomic line with the 8.1 line and sequenced [37]. Exact positions of breakpoints between the TM introgression and crossover between the 4AL chromosome of cv. Tähti and CS are unknown. Thus, the terminal 122 Mb of the arm was selected for the analysis.
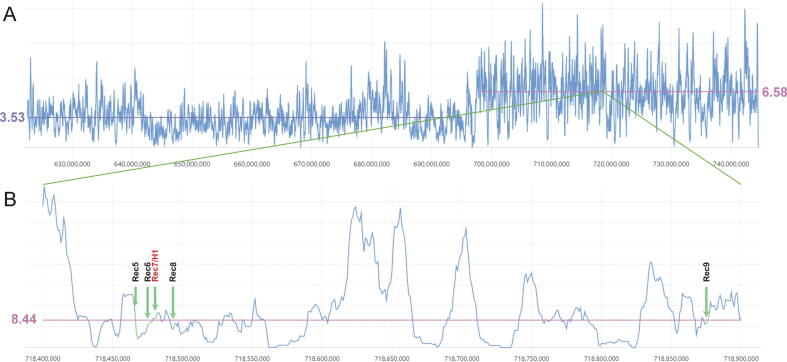
Fig. 2.
SNP density between CS and Tähti (both T. aestivum) and CS and T. militinae introgression segment: a) A SNP density (y-axis) in the 144.59 Mb of 4AL chromosome in comparison two T. aestivum cvs. (CS and Tähti) (left side) and between T. aestivum (CS) and T. militinae (TM) (right side). Violet and pink lines represent average SNP densities (SNPs/kb) determined between CS/Tähti and CS/TM chromatin, respectively. b) A detail of the 0.5 Mb region with six selected CO sites including the Rec7 and H1. Positions of the recombination sites (Rec5-Rec9) are indicated by arrows. The pink line represents the average level of SNP determined for the CS/TM segment.
For the T. aestivum segment (CS/Tähti, ∼622.5–695 Mb) 256,256 SNPs were identified with an average of 3.53 SNPs/kb, while for the TM segment (CS/TM, 698–744,5 Mb) 304,139 SNPs were identified with the average of 6.58 SNPs/kb (Fig. 2A). Besides the Rec7 and H1 (718,478,657–718,482,946), also additional recombination loci: Rec5, Rec6, Rec8 and Rec9 (718,465,781–718,468,941; 718,474,051–718,477,454; 718,492,302–718,494,113; 718,874,486–718,876,493) identified by Janáková et al. [34] do not deviate significantly from the average SNP density. The average SNP density for all tested CO sites is 8.32/kb, while for the whole region between Rec5 and Rec9 it is 8.44/kb (Fig. 2B).
Recombination frequency assessment
Out of 170 plants from the Ph1 and ph1 mapping populations, 68 plants heterozygous for the QPm-tut.4A region were identified. From pollen grains collected from the heterozygous plants 1,463,000 generative pollen nuclei were flow-sorted (about 16,000 to 128,000 nuclei per line).
For the Ph1 mapping population 52,800 gametes were analyzed, where 40 (Rf = 0.00076) and 256 (Rf = 0.0048) recombination events were identified for Rec7 and H1, respectively. Thus a 6.4-fold higher recombination frequency was observed at the H1 hotspot as compared to Rec7. From the ph1 mapping population, 13,200 gametes were tested to reveal 14 (Rec7) (Rf = 0.0011) and 65 (H1) (Rf = 0.0049) recombination events. In this case, the H1 region had a 4.64-fold higher recombination frequency. At Rec7 the absence of Ph1 significantly increased the CO frequency by 40 %, without any significant difference (1.6 %) observed for the H1.
We termed a new method for large-scale recombination frequency measurement as ddPCR Pollen genotyping (ddPing). It allows to distinguish the signal of each probe after the ddPCR. At the 2-D plots, signals were observed as separated clusters and enabled to determine the origin and even the order of recombined chromosome segments relative to the centromere (Fig. 3). This way a distortion in gamete frequency was identified. In the Ph1 mapping population, we identified 12 (Rec7) and 77 (H1) events of A(CS) × B(TM) recombination type (in all cases, chromatin proximal to the centromere is mentioned first) and 28 (Rec7) and 179 (H1) for B × A. For the ph1 mapping population 5 (Rec7) and 25 (H1), as well as 9 (Rec7) and 40 (H1) events of the A × B and B × A type of recombination were identified, respectively. There is a direct shift toward the B × A type of recombination (mean 68.5 % at Rec7, 68.2 % at the H1) in comparison to the A × B type of recombination (mean 31.5 % at Rec7, 31.8 % at H1) at both mapping populations. In the Ph1 mapping population, ratio of the A × B type to B × A type is the same at Rec7 and H1 (30:70) whereas in the ph1 mapping population there is a slight shift in this ratio at Rec7 (36:64) and H1 (38:62).
Fig. 3.
The scheme of ddPCR Pollen genotyping (ddPing) method for recombination frequency assessment. The method facilitates recombination frequency assessment in a large number of gametes at particular loci and combines the TaqMan probes designed from reliable flanking markers, droplet cluster identification (ddPCR), and classification of recombination events on flow-sorted pollen generative nuclei (red arrow). The data analysis revealed four possible situations: (A) probes for two flanking markers labeled by FAM for the T. aestivum cv. Chinese Spring chromatin (blue dots); (B) VIC/HEX for the T. militinae chromatin (green dots); (C) signals for all four probes for the particular recombination site at generative pollen nuclei collected from plant heterozygous for both tested loci. Dots (orange) in ovals correspond to the FAM and VIC/HEX double-positive droplets indicating recombination events. The position of the orange oval indicates the recombination where the CS chromatin is at the centromere side and TM chromatin at the telomere side. The purple oval indicates the opposite situation. Black dots correspond to droplets without fluorescence. The used color scheme does not represent the real fluorochrome emission light color.
Discussion
In species with large and complex genomes, a correlation between the CO hotspot and the DSB hotspot distribution is observed [6]. However, most studies in higher plants have been done with a limited resolution of about 10 kb on average or more, and the conclusions were based on associations between the relatively large mapping intervals with COs and other chromatin features [25]. The low resolution does not allow for reliable searching of epigenetic marks or common DNA sequence motifs such as the 13 bp degenerate motif overrepresented in COs in mammals [50]. In wheat, the only feature known to be prerequisite for strong control of the recombination frequency is presence of the functional Ph1 locus [20], [30], [51]. In our previous study [34] we acquired a wheat mapping population with unprecedently high mapping resolution of recombining 4A chromatin of T. aestivum cv. CS and T. militinae 7G chromatin and identified recombination hotspot. This allowed us to address the following questions: If the COs in wheat are directed repeatedly to the same regions? Do the hotspot and regular recombination site share a common sequence motif or a chromatin mark for CO occurrence and are both under the Ph1 locus control? The presumption that COs are directed to preferred loci is supported by the observation of similar CO patterns in wheat mapping populations derived from more related parental lines [30].
Characterization of CO sites sequences and recombination frequency
Two loci with significantly different CO frequencies were identified: the H1 locus (a recombination hotspot) has a sixfold higher recombination frequency compared to the chromosome average, on the other hand, the Rec7 locus (defined as a regular CO site) has a recombination frequency the same as the chromosome average [34]. The loci were high-density mapped, share one flanking marker, and are comparable in size: 1.586 kb and 2.538 kb for H1 and Rec7, respectively. To facilitate and increase reliability of the CO frequency and distribution analysis, we have developed a new method that combines pollen nuclei sorting and droplet digital PCR. This allowed testing of almost 66,000 gametes to confirm a 5.94-fold higher frequency of COs at the H1 region in comparison to Rec7 (Fig. 3). Such recombination hotspots seem to be common in the Eukaryotes [52]. Drouaud et al. [53] found regions in the A. thaliana genome having five times more COs than the chromosome average and that CO rates negatively correlate with the GC content and do not correlate either with genes, pseudogenes, transposable elements, or dispersed repeats. In our case, sequencing of both regions from the parental lines (CS vs 8.1) revealed a higher sequence identity of H1 (only two additional polymorphisms) in comparison to the Rec7 locus where 26 additional SNPs were identified (Fig. 1B, Supplementary file 2). In the Ph1 mapping population, the location of CO in the Rec7 was delimited to only 19 bp (Fig. 1B) suggesting that the proximity of SNPs does not prohibit CO formation under the control of the Ph1 locus. However, this contradicts findings in yeasts that a single mismatch is sufficient to inhibit recombination between otherwise identical sequences [54]. Similarly in wheat, Fan et al. (24) concluded that the Ph1 locus readily rejects polymorphic DNAs as candidates for CO, leading to high CO stringency. Nevertheless, Dooner [55], [56] demonstrated that crossovers are sensitive to the level and type of polymorphism within the maize bz gene, with transposon insertions suppressing crossovers to a greater degree than single nucleotide changes. Other studies show that CO rates are higher in regions with higher sequence similarity - lower genetic diversity facilities homologous pairing and recombination [57], [58], [59]. To address these contradictive findings, we examined SNP density along the terminal part of wheat chromosome arm 4AL in the parental lines. SNP density within six selected COs including the Rec7 and H1 from the study of Janáková et al. [34] showed that the COs are all located in regions with average SNP density (Fig. 2B).
This observation suggested that presence of SNPs in wheat does not have a completely restrictive effect on recombination frequency and that loci with extreme SNP density are not favorable for CO machinery. Nevertheless, larger chromosomal rearrangements may have a strong effect on recombination suppression [56]. Such a situation was observed in the mapping population derived from a cross of wheat cv. Tähti with line 8.1 where no recombination in the QPm-tut.4A region was observed. PCR with primers for the Rec7 and H1 region in cv. Tähti did not yield PCR products suggesting the presence of indels or other larger structural changes. This conclusion was supported by the significant difference and smaller size of the cv. Tähti 4A chromosome compared to the cv. CS 4A chromosome [60], [61].
Another aspect of chromatin structure associated with the CO frequency is a lower nucleosome density due to low GC content. Such a situation is often associated with Transcription Starting Sites (TSS), promoter regions and TATA box-like structures, and DBS hotspots. On the other hand, the Transcription Terminator Sites (TTS) are also associated with AT-rich stretches (e.g., PolyA signal) but not that often with the DBS hotspots [5], [8], [32]. In the H1 recombining regions, the GC content is slightly below (44.5 %) the genome average (48 %) and differs from the findings above (Fig. 1C). Moreover, the Rec7 recombining subregions have significantly lower GC content (31 %, 30 bp sliding window) with areas going down to only 6 % (Fig. 1C). The AT-rich regions are known for low nucleosome occupancy which was before associated with the CO formation [8]. However, for reliable association establishment, more CO sites must be detailly assessed. There is also a local difference in the Rec7 subregions, the 19 bp CO subregion from the Ph1 population has about 6 % higher GC content than the subregion from the ph1 mapping population. If the Ph1 protein/protein complex can be affected by such structural changes would require more work.
Epigenetic marks at identified CO sites
Besides the primary DNA structure, the state, function, and reconstruction of chromatin are significantly affected by epigenetic marks such as histone acetylation, phosphorylation, methylation, ubiquitylation, and others (reviewed in [62]). In plants, DNA methylation occurs in three contexts: CG, CHG, and CHH (H = any base except G; [63], [64], [65]). As mentioned above, CO hotspots in plants are observed in regions with decreased nucleosome occupancy which often overlaps with DNA hypomethylated gene TSSs or TTSs [5]. Although the Rec7 and H1 do not overlap with the TSS or TTS sites, they show a highly hypomethylated pattern (Fig. 1D-F) with the exception for T. militinae chromatin where the Rec7 Ph1 recombination site overlap with higher CHH methylation (Fig. 1E, F) suggesting low sensitivity of recombination to the CHH methylation. In contrast, CHG methylation is the most suppressed in the regions with COs (Fig. 1D-F). In the Rec7 site, the low methylation level could be the result of low GC content, but the H1 is also hypomethylated with average GC content confirming the high frequency of CO formation in association with low DNA methylation as described before [5], [66], [67]. Nevertheless, the presence of CHH methylation in the CO loci conflicts with the conclusions of Zhao et al. [68] that crossover redistribution is driven by a loss of CHH methylation in gene regions of maize. This raises a question whether CO loci in transcriptionally active genes are marked differently from COs in intergenic regions?
Besides DNA methylation, also methylation and acetylation of histone proteins play a significant role in chromatin behavior. H3K4me3, H3K9ac, and H3K27ac are markers of euchromatin, whereas H3K27me3 is a marker of facultative heterochromatin. Among them, trimethylation of histone H3 at lysine 4 (H3K4me3) is one of the most studied epigenetic marks since it is associated with transcriptionally active and nucleosome depleted chromatin (especially TSS) [69], [70], [71], DSB hotspots and initiation of meiotic recombination [72] and initiation of V(D)J recombinations [73]. Nevertheless, Borde et al. [72] showed that the amount of H3K4me3 relative to the total amount of histone H3 does not change significantly during meiosis, suggesting that this mark does not interfere with meiotic processes even though it was found highly dynamic. It seems that the H3K4me3 may be a preferential marker of active open chromatin and association with the COs is a coincidence due to the accessibility of chromatin by proteins. Another marker for open chromatin is its sensitivity to DNase I digestion (DHS) [74]. The DHS and H3K4me3 were found to be present in all regions of recombination of the Rec7 and H1 loci in the CS genome (Fig. 1G). On the other hand, the H3K9me2 was found to be associated with heterochromatin and its compactness, especially with the presence of the CG and non-CG DNA methylations [75], and this mark is mostly absent in the Rec7 and H1 loci (Fig. 1G), what agrees with the association of open chromatin and CO formation. It is worth adding that the histone modification H3K27me3 (overrepresented at the H1), was determined to be one of the strongest predictors of crossover activity at such loci in wheat [76]. Even the positions of the epigenetic determinants are derived from the leaf tissue and not from meiocytes, where the meiosis is taking place, they are suggesting that both regions are part of euchromatin which is considered biologically active. If there is any biological function of the regions in somatic cells would require additional study.
Role of the Ph1 locus in the occurrence of recombination within the H1 and Rec7 sites
According to Martín et al. [77], the main role of the Ph1 locus during meiosis is to stabilize wheat polyploidy by controlling the accuracy of homologous synapsis during early meiosis and by regulating CO formation. The first analysis of wheat genetic map in the presence and absence of Ph1 revealed a similar distribution of COs along chromosomes [78], suggesting its role in the interference of CO frequency, but not their distribution. Later it was confirmed that the change in the Ph1 status does not affect the CO pattern in a chromosome arm but its frequency [24], [79]. However, this does not agree with our analysis where the CO pattern in the Ph1 and ph1 mapping populations (8425 and 1255 individuals with 15 and 23 COs, respectively, from the study of Janáková et al., [34]) were compared. It revealed that 30.4 % of the CO loci were shared between both mapping populations. This shows that the Ph1 presence or absence does change CO distribution along wheat chromosomes but increases their number by suppressing the wheat Ph1 locus. This also raises the question if all CO loci are affected by the Ph1 locus? Thus, the CO frequency was compared in the Rec7 and H1 loci between plants with the Ph1 and ph1 genome composition. While the Rec7 locus showed a 40 % increase in CO frequency at the ph1 genome composition, the H1 locus showed no difference, suggesting that the H1 locus is not under the control of the Ph1 locus. This implies that the recombination in wheat is controlled by additional independent pathways. Moreover, it seems that preferences in chromosome composition after recombination and gamete formation are not under the Ph1 influence. A twofold preference of pollen generative nucleus formation with the 4A chromosome composed of T. militinae at the terminal part of the long arm, with no difference for Ph1 or ph1 allele presence was observed. The segregation distortion is a widespread phenomenon in plants and animals affecting the evolution of species and can have different mechanisms [80]. In this case, no obvious selection advantage for the distortion was recognized and a possible mechanism is also unclear.
Conclusions
In this work, for the first time, we characterized wheat “regular” CO site and the recombination hotspot to as little as 19 and 1164 bp, respectively and developed a new reliable method (ddPing) for large-scale recombination frequency measurement in the particular CO sites. We have shown that not all CO sites are under Ph1 locus control the same as gamete segregation distortion indicating additional independent pathway in wheat recombination control. Moreover, the CHH methylation and SNP proximity were not confirmed as recombination inhibitors in contrast to larger chromatin reconstructions. On the other hand, the open chromatin and lack of DNA methylation were found associated with the recombination events, and the COs were found repeatedly directed to specific regions. Understanding recombination mechanisms may become a base for the development of efficient tools for wheat gene pool enrichment from related species and fast and effective breeding.
CRediT authorship contribution statement
Maciej Majka: Data curation, Funding acquisition, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. Eva Janáková: Investigation, Writing – review & editing. Irena Jakobson: Investigation, Writing – review & editing. Kadri Järve: Conceptualization, Funding acquisition, Resources, Writing – review & editing. Petr Cápal: Methodology. Zuzana Korchanová: Investigation, Writing – review & editing. Adam Lampar: Investigation, Writing – review & editing. Jakub Juračka: Software, Visualization, Writing – review & editing. Miroslav Valárik: Conceptualization, Funding acquisition, Project administration, Supervision, Visualization, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The technical assistance of Marie Seifertová, Romana Šperková, and Jitka Weiserová is highly appreciated. This work was supported by the Czech Science Foundation (award 18-11688S), the Czech Republic Ministry of Agriculture (award QK1710302 and QK22010293), ERDF project “Plants as a tool for sustainable global development” (No. CZ.02.1.01/0.0/0.0/16_019/0000827), Estonian Ministry of Rural Affairs, State Programme on Plant Breeding, and the National Science Center (ETIUDA6-2018/28/T/NZ9/00073).
Footnotes
Peer review under responsibility of Cairo University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2023.01.002.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Primers and sequences of recombination sites
Data availability
The raw sequence data of bisulfite converted regions Rec7 and H1 of lines CS, TM and 8.1 were deposited at NCBI GEO data sets: GSE220598.
References
- 1.Zickler D., Kleckner N. Meiotic chromosomes: integrating structure and function. Annu Rev Genet. 1999;33:603–754. doi: 10.1146/annurev.genet.33.1.603. [DOI] [PubMed] [Google Scholar]
- 2.Hunter N. Meiotic recombination: the essence of heredity. Cold Spring Harb Perspect Biol. 2015;7:1–35. doi: 10.1101/cshperspect.a016618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernandes J.B., Duhamel M., Seguela-Arnaud M., Froger N., Girard C., Choinard S., et al. FIGL1 and its novel partner FLIP form a conserved complex that regulates homologous recombination. PLoS Genet. 2018;14:e1007317. doi: 10.1371/journal.pgen.1007317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lukaszewski A.J., Curtis C.A. Physical distribution of recombination in B-genome chromosomes of tetraploid wheat. Theor Appl Genet. 1993;86:121–127. doi: 10.1007/BF00223816. [DOI] [PubMed] [Google Scholar]
- 5.Tock A.J., Henderson I.R. Hotspots for Initiation of Meiotic Recombination. Front Genet. 2018;9:521. doi: 10.3389/fgene.2018.00521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zelkowski M., Olson M.A., Wang M., Pawlowski W. Diversity and determinants of meiotic recombination landscapes. Trends Genet. 2019;35:359–370. doi: 10.1016/j.tig.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Baker K., Bayer M., Cook N., Dreissig S., Dhillon T., Russell J., et al. The low-recombining pericentromeric region of barley restricts gene diversity and evolution but not gene expression. Plant J. 2014;79:981–992. doi: 10.1111/tpj.12600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi K., Zhao X., Kelly K.A., Venn O., Higgins J.D., Yelina N.E., et al. Arabidopsis meiotic crossover hot spots overlap with H2A.Z nucleosomes at gene promoters. Nat Genet. 2013;45:1327–1338. doi: 10.1038/ng.2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drouaud J., Khademian H., Giraut L., Zanni V., Bellalou S., Henderson I.R., et al. Contrasted patterns of crossover and non-crossover at Arabidopsis thaliana meiotic recombination hotspots. PLoS Genet. 2013;9:e1003922. doi: 10.1371/journal.pgen.1003922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu H., Zheng Z., Dooner H.K. Recombination rates between adjacent genic and retrotransposon regions in maize vary by 2 orders of magnitude. Proc Natl Acad Sci USA. 2002;99:1082–1087. doi: 10.1073/pnas.022635499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He Y., Wang M., Dukowic-Schulze S., Zhou A., Tiang C.-L., Shilo S., et al. Genomic features shaping the landscape of meiotic double-strand-break hotspots in maize. Proc Natl Acad Sci. 2017;114:12231–12236. doi: 10.1073/pnas.1713225114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Massy B. Distribution of meiotic recombination sites. Trends Genet. 2003;19:514–522. doi: 10.1016/S0168-9525(03)00201-4. [DOI] [PubMed] [Google Scholar]
- 13.Jeffreys A.J., Neumann R. The rise and fall of a human recombination hotspot. Nat Genet. 2009;41:625–629. doi: 10.1038/ng.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Novák P., Guignard M.S., Neumann P., Kelly L.J., Mlinarec J., Koblížková A., et al. Repeat-sequence turnover shifts fundamentally in species with large genomes. Nat Plants. 2020;6:1325–1329. doi: 10.1038/s41477-020-00785-x. [DOI] [PubMed] [Google Scholar]
- 15.Choi K., Henderson I.R. Meiotic recombination hotspots – a comparative view. Plant J. 2015;83:52–61. doi: 10.1111/tpj.12870. [DOI] [PubMed] [Google Scholar]
- 16.Tiemann-Boege I., Schwarz T., Striedner Y., Heissl A. The consequences of sequence erosion in the evolution of recombination hotspots. Philos Trans R Soc B. 2017;372:20160462. doi: 10.1098/rstb.2016.0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.International Wheat Genome Sequencing Consortium (IWGSC). Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science 2018;361:7191. [DOI] [PubMed]
- 18.Riley R., Chapman V. Genetic control of the cytologically diploid behaviour of hexaploid wheat. Nature. 1958;182:713–715. [Google Scholar]
- 19.Sears E.R., Okamoto M. Intergenomic chromosome relationships in hexaploid wheat. Proc 10th Int Congr Genet Montreal. 1958;2:258–259. [Google Scholar]
- 20.Griffiths S., Sharp R., Foote T.N., Bertin I., Wanous M., Reader S., et al. Molecular characterization of Ph1 as a major chromosome pairing locus in polyploid wheat. Nature. 2006;439:749–752. doi: 10.1038/nature04434. [DOI] [PubMed] [Google Scholar]
- 21.Lukaszewski A.J., Kopecký D. The Ph1 locus from wheat controls meiotic chromosome pairing in autotetraploid rye (Secale cereale L.) Cytogenet Genome Res. 2010;129:117–123. doi: 10.1159/000314279. [DOI] [PubMed] [Google Scholar]
- 22.Sears E.R. Induced mutant with homoeologous pairing in common wheat. Can J Genet Cytol. 1977;19:585–593. [Google Scholar]
- 23.Al-Kaff N., Knight E., Bertin I., Foote T., Hart N., Griffiths S., et al. Detailed dissection of the chromosomal region containing the Ph1 locus in wheat Triticum aestivum: with deletion mutants and expression profiling. Ann Bot. 2008;101:863–872. doi: 10.1093/aob/mcm252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fan C., Hao M., Jia Z., Neri C., Chen X., Chen W., et al. Some characteristics of crossing over in induced recombination between chromosomes of wheat and rye. Plant J. 2021;105:1665–1676. doi: 10.1111/tpj.15140. [DOI] [PubMed] [Google Scholar]
- 25.Darrier B., Rimbert H., Balfourier L., Pingault L., Josselin A.-A., Servin B., et al. High-resolution mapping of crossover events in the hexaploid wheat genome suggests a universal recombination mechanism. Genetics. 2017;206:1373–1388. doi: 10.1534/genetics.116.196014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.International Wheat Genome Sequencing Consortium (IWGSC). A chromosome-based draft sequence of the hexaploid bread wheat (Triticum aestivum) genome. Science 2014;345:1251788. [DOI] [PubMed]
- 27.Sherman J.D., Stack S.M. Two-dimensional spreads of synaptonemal complexes from solanaceous plants. VI. High-resolution recombination nodule map for tomato (Lycopersicon esculentum) Genetics. 1995;141:683–708. doi: 10.1093/genetics/141.2.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tenaillon M.I., Sawkins M.C., Anderson L.K., Stack S.M., Doebley J., Gaut B.S. Patterns of diversity and recombination along chromosome 1 of maize (Zea mays ssp. mays L.) Genetics. 2002;162:1401–1413. doi: 10.1093/genetics/162.3.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saintenac C., Falque M., Martin O.C., Paux E., Feuillet C., Sourdille P. Detailed recombination studies along chromosome 3B provide new insights on crossover distribution in wheat (Triticum aestivum L.) Genetics. 2009;181:393–403. doi: 10.1534/genetics.108.097469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gardiner L.J., Wingen L.U., Bailey P., Joynson R., Brabbs T., Wright J., et al. Analysis of the recombination landscape of hexaploid bread wheat reveals genes controlling recombination and gene conversion frequency. Genome Biol. 2019;20:69. doi: 10.1186/s13059-019-1675-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Falque M., Anderson L.K., Stack S.M., Gauthier F., Martin O.C. Two types of meiotic crossovers coexist in maize. Plant Cell. 2009;21:3915–3925. doi: 10.1105/tpc.109.071514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pan J., Sasaki M., Kniewel R., Murakami H., Blitzblau H.G., Tischfield S.E., et al. A hierarchical combination of factors shapes the genome-wide topography of yeast meiotic recombination initiation. Cell. 2011;144:719–731. doi: 10.1016/j.cell.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fayos I., Mieulet D., Petit J., Meunier A.C., Perin C., Nicolas A., et al. Engineering meiotic recombination pathways in rice. Plant Biotechnol J. 2019;17:2062–2077. doi: 10.1111/pbi.13189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Janáková E., Jakobson I., Peusha H., Abrouk M., Škopová M., Šimková H., et al. Divergence between bread wheat and Triticum militinae in the powdery mildew resistance QPm.tut-4A locus and its implications for cloning of the resistance gene. Theor Appl Genet. 2019;132:1061–1072. doi: 10.1007/s00122-018-3259-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jakobson I., Peusha H., Timofejeva L., Järve K. Adult plant and seedling resistance to powdery mildew in a Triticum aestivum × Triticum militinae hybrid line. Theor Appl Genet. 2006;112:760–769. doi: 10.1007/s00122-005-0181-2. [DOI] [PubMed] [Google Scholar]
- 36.Jakobson I., Reis D., Tiidema A., Peusha H., Timofejeva L., Valárik M., et al. Fine mapping, phenotypic characterization and validation of non-race-specific resistance to powdery mildew in a wheat-Triticum militinae introgression line. Theor Appl Genet. 2012;125:609–623. doi: 10.1007/s00122-012-1856-0. [DOI] [PubMed] [Google Scholar]
- 37.Abrouk M., Balcárková B., Šimková H., Komínková E., Martis M., Jakobson I., et al. The in silico identification and characterization of a bread wheat/Triticum militinae introgression line. Plant Biotechnol J. 2017;15:249–256. doi: 10.1111/pbi.12610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hernandez P., Martis M., Dorado G., Pfeifer M., Gálvez S., Schaaf S., et al. Next-generation sequencing and syntenic integration of flow sorted arms of wheat chromosome 4A exposes the chromosome structure and gene content. Plant J. 2012;69:377–386. doi: 10.1111/j.1365-313X.2011.04808.x. [DOI] [PubMed] [Google Scholar]
- 39.Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 40.Michaels S.D., Amasino R.M. A robust method for detecting single-nucleotide changes as polymorphic markers by PCR. Plant J. 1998;14:381–385. doi: 10.1046/j.1365-313x.1998.00123.x. [DOI] [PubMed] [Google Scholar]
- 41.Untergasser A., Cutcutache I., Koressaar T., Ye J., Faircloth B.C., Remm M., et al. Primer3: new capabilities and interfaces. Nucleic Acids Res. 2012;40:e115. doi: 10.1093/nar/gks596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Šimková H., Svensson J.T., Condamine P., Hřibová E., Suchánková P., Bhat P.R., et al. Coupling amplified DNA from flow-sorted chromosomes to high-density SNP mapping in barley. BMC Genomics. 2008;9:294. doi: 10.1186/1471-2164-9-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ivaničová Z., Jakobson I., Reis D., Šafář J., Milec Z., Abrouk M., et al. Characterization of new allele influencing flowering time in bread wheat introgressed from Triticum militinae. New Biotechnol. 2016;33:718–727. doi: 10.1016/j.nbt.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 44.Altschul S.F., Madden T.L., Schäffer A.A., Zhang J., Zhang Z., Webb W., et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Solovyev V., Kosarev P., Seledsov I., Vorobyev D. Automatic annotation of eukaryotic genes, pseudogenes and promoters. Genome Biol. 2006;7:10.1-10.12. doi: 10.1186/gb-2006-7-s1-s10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Danecek P., Bonfield J.K., Liddle J., Marshall J., Ohan V., Pollard M.O., et al. Twelve years of SAMtools and BCFtools. GigaScience. 2021;10(2) doi: 10.1093/gigascience/giab008. giab008 [33590861] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gruntman E., Qi Y., Slotkin R.K., Roeder T., Martienssen R.A., Sachidanandam R. Kismeth: Analyzer of plant methylation states through bisulfite sequencing. BMC Bioinf. 2008;9:371. doi: 10.1186/1471-2105-9-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Doležel J., Binarová P., Lucretti S. Analysis of nuclear DNA content in plant cells by flow cytometry. Biol Plant. 1989;31:113–120. [Google Scholar]
- 49.Röder M.S., Korzun V., Wendehake K., Plaschke J., Tixier M.H., Leroy P., et al. A microsatellite map of wheat. Genetics. 1998;149:2007–2023. doi: 10.1093/genetics/149.4.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Myers S., Freeman C., Auton A., Donnelly P., McVean G. A common sequence motif associated with recombination hot spots and genome instability in humans. Nat Genet. 2008;40:1124–1129. doi: 10.1038/ng.213. [DOI] [PubMed] [Google Scholar]
- 51.Greer E., Martín A.C., Pendle A., Colas I., Jones A.M.E., Moore G., et al. The Ph1 locus suppresses Cdk2-type activity during premeiosis and meiosis in wheat. Plant Cell. 2012;24:152–162. doi: 10.1105/tpc.111.094771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stapley J., Feulner P.G.D., Johnston S.E., Santure A.W., Smadja C.M. Variation in recombination frequency and distribution across eukaryotes: patterns and processes. Philos Trans R Soc B. 2017;372:20160455. doi: 10.1098/rstb.2016.0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Drouaud J., Camilleri C., Bourguignon P.-Y., Canaguier A., Berard A., Vezon D., et al. Variation in crossing-over rates across chromosome 4 of Arabidopsis thaliana reveals the presence of meiotic recombination “hot spots”. Genome Res. 2006;16:106–114. doi: 10.1101/gr.4319006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Datta A., Hendrix M., Lipstich M., Jinks-Robertson S. Dual roles for DNA sequence identity and the mismatch repair system in the regulation of mitotic crossing-over in yeast. Proc Natl Acad Sci USA. 1997;94:9757–9762. doi: 10.1073/pnas.94.18.9757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dooner H.K. Genetic fine structure of the BRONZE locus in maize. Genetics. 1986;113:1021–1036. doi: 10.1093/genetics/113.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dooner H.K., He L. Maize genome structure variation: interplay between retrotransposon polymorphisms and genic recombination. Plant Cell. 2008;20:249–258. doi: 10.1105/tpc.107.057596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rodgers-Melnick E., Bradbury P.J., Elshire R.J., Glaubitz J.C., Acharya C.B., Mitchell S.E., et al. Recombination in diverse maize is stable, predictable, and associated with genetic load. Proc Natl Acad Sci USA. 2015;112:3823–3828. doi: 10.1073/pnas.1413864112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bouchet S., Olatoye M.O., Marla S.R., Perumal R., Tesso T., Yu J., et al. Increased power to dissect adaptive traits in global Sorghum diversity using a nested association mapping population. Genetics. 2017;206:573–585. doi: 10.1534/genetics.116.198499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Serra H., Choi K., Zhao X., Blackwell A.R., Kim J., Henderson I.R. Interhomolog polymorphism shapes meiotic crossover within the Arabidopsis RAC1 and RPP13 disease resistance genes. PLOS Genet. 2018;14(12):e1007843. doi: 10.1371/journal.pgen.1007843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vrána J., Kubaláková M., Číhalíková J., Valárik M., Doležel J. Preparation of sub-genomic fractions enriched for particular chromosomes in polyploid wheat. Biol Plant. 2015;59:445–455. [Google Scholar]
- 61.Tsõmbalova J., Karafiátová M., Vrána J., Kubaláková M., Peuša H., Jakobson I., et al. A haplotype specific to North European wheat (Triticum aestivum L.) Genet Resour Crop Evol. 2017;64:653–664. [Google Scholar]
- 62.Bannister A., Kouzarides T. Regulation of chromatin by histone modifications. Cell Res. 2011;21:381–395. doi: 10.1038/cr.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Law J.A., Jacobsen S.E. Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat Rev Genet. 2010;11:204–220. doi: 10.1038/nrg2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lindroth A.M., Cao X., Jackson J.P., Zilberman D., McCallum C.M., Henikoff S., et al. Requirement of CHROMOMETHYLASE3 for maintenance of CpXpG methylation. Science. 2001;292:2077–2080. doi: 10.1126/science.1059745. [DOI] [PubMed] [Google Scholar]
- 65.Harris K.D., Zemach A. Contiguous and stochastic CHH methylation patterns of plant DRM2 and CMT2 revealed by single-read methylome analysis. Genome Biol. 2020;194:1–19. doi: 10.1186/s13059-020-02099-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mirouze M., Lieberman-Lazarovich M., Aversano R., Bucher E., Nicolet J., Reinders J., et al. Loss of DNA methylation affects the recombination landscape in Arabidopsis. Proc Natl Acad Sci. 2012;109:5880–5885. doi: 10.1073/pnas.1120841109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yelina N.E., Lambing C., Hardcastle T.J., Zhao X., Santos B., Henderson I.R. DNA methylation epigenetically silences crossover hot spots and controls chromosomal domains of meiotic recombination in Arabidopsis. Genes Dev. 2015;29:2183–2202. doi: 10.1101/gad.270876.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao M, Ku JC, Liu B, Yang D, Yin L, Ferrell TJ, et al. The mop1 mutation affects the recombination landscape in maize. Proc Natl Acad Sci 2021;118:e2009475118. [DOI] [PMC free article] [PubMed]
- 69.Barski A., Cuddapah S., Cui K., Roh T.Y., Schones D.E., Wang Z., et al. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129:823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 70.Pokholok D.K., Harbison C.T., Levine S., Cole M., Hannett N.M., Lee T.I., et al. Genome-wide map of nucleosome acetylation and methylation in yeast. Cell. 2005;122:517–527. doi: 10.1016/j.cell.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 71.Zhang A., Wei Y., Shi Y., Deng X., Gao J., Feng Y., et al. Profiling of H3K4me3 and H3K27me3 and their roles in gene subfunctionalization in allotetraploid cotton. Front Plant Sci. 2021;12 doi: 10.3389/fpls.2021.761059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Borde V., Robine N., Lin W., Bonfils S., Géli V., Nicolas A. Histone H3 lysine 4 trimethylation marks meiotic recombination initiation sites. EMBO J. 2009;28:99–111. doi: 10.1038/emboj.2008.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bettridge J., Na C.H., Pandey A., Desiderio S. H3K4me3 induces allosteric conformational changes in the DNA-binding and catalytic regions of the V(D)J recombinase. PNAS. 2017;114:1904–1909. doi: 10.1073/pnas.1615727114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bajic M., Maher K.A., Deal R.B. Identification of open chromatin regions in plant genomes using ATAC-seq. Methods Mol Biol. 2018;1675:183–201. doi: 10.1007/978-1-4939-7318-7_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Champagne K.S., Kutateladze T.G. Structural insight into histone recognition by the ING PHD fingers. Curr Drug Targets. 2009;10:432–441. doi: 10.2174/138945009788185040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tock A.J., Holland D.M., Jiang W., Osman K., Sanchez-Moran E., Higgins J.D., et al. Crossover-active regions of the wheat genome are distinguished by DMC1, the chromosome axis, H3K27me3, and signatures of adaptation. Genome Res. 2021;31:1614–1628. doi: 10.1101/gr.273672.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Martín A.C., Shaw P., Phillips D., Reader S., Moore G. Licensing MLH1 sites for crossover during meiosis. Nat Commun. 2014;5:1–5. doi: 10.1038/ncomms5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dubcovsky J., Luo M.C., Dvorak J. Differentiation between homoeologous chromosomes 1A of wheat and 1Am of Triticum monococcum and its recognition by the wheat Ph1 locus. Proc Natl Acad Sci USA. 1995;92:6645–6649. doi: 10.1073/pnas.92.14.6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang W., Zhu X., Zhang M., Chao S., Xu S., Cai X. Meiotic homoeologous recombination-based mapping of wheat chromosome 2B and its homoeologues in Aegilops speltoides and Thinopyrum elongatum. Theor Appl Genet. 2018;131:2381–2395. doi: 10.1007/s00122-018-3160-0. [DOI] [PubMed] [Google Scholar]
- 80.Lyttle T.W. Segregation distorters. Annu Rev Genet. 1991;25:511–557. doi: 10.1146/annurev.ge.25.120191.002455. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primers and sequences of recombination sites
Data Availability Statement
The raw sequence data of bisulfite converted regions Rec7 and H1 of lines CS, TM and 8.1 were deposited at NCBI GEO data sets: GSE220598.