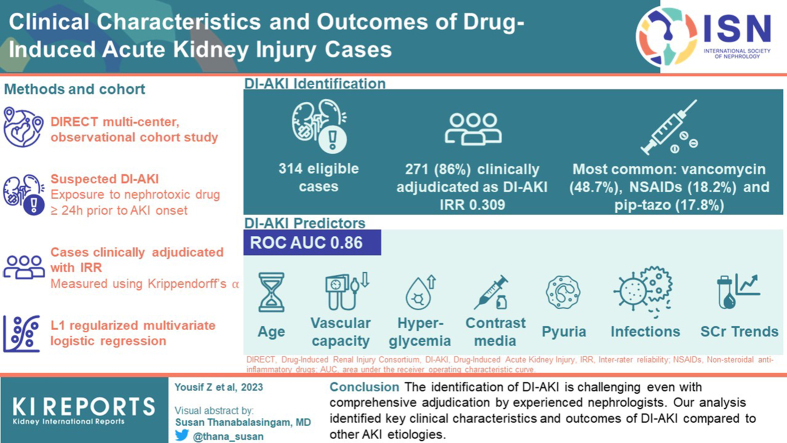
Abstract
Introduction
Drug-induced acute kidney injury (DI-AKI) is a frequent adverse event. The identification of DI-AKI is challenged by competing etiologies, clinical heterogeneity among patients, and a lack of accurate diagnostic tools. Our research aims to describe the clinical characteristics and predictive variables of DI-AKI.
Methods
We analyzed data from the Drug-Induced Renal Injury Consortium (DIRECT) study (NCT02159209), an international, multicenter, observational cohort study of enriched clinically adjudicated DI-AKI cases. Cases met the primary inclusion criteria if the patient was exposed to at least 1 nephrotoxic drug for a minimum of 24 hours prior to AKI onset. Cases were clinically adjudicated, and inter-rater reliability (IRR) was measured using Krippendorff’s alpha. Variables associated with DI-AKI were identified using L1 regularized multivariable logistic regression. Model performance was assessed using the area under the receiver operating characteristic curve (ROC AUC).
Results
A total of 314 AKI cases met the eligibility criteria for this analysis, and 271 (86%) cases were adjudicated as DI-AKI. The majority of the AKI cases were recruited from the United States (68%). The most frequent causal nephrotoxic drugs were vancomycin (48.7%), nonsteroidal antiinflammatory drugs (18.2%), and piperacillin/tazobactam (17.8%). The IRR for DI-AKI adjudication was 0.309. The multivariable model identified age, vascular capacity, hyperglycemia, infections, pyuria, serum creatinine (SCr) trends, and contrast media as significant predictors of DI-AKI with good performance (ROC AUC 0.86).
Conclusion
The identification of DI-AKI is challenging even with comprehensive adjudication by experienced nephrologists. Our analysis identified key clinical characteristics and outcomes of DI-AKI compared to other AKI etiologies.
Keywords: drug-induced acute kidney injury, nephrotoxicity
Graphical abstract
DI-AKI is a common adverse drug event affecting approximately 14% to 26% of the hospitalized adult population.1, 2, 3, 4 Hospitalized patients, particularly critically ill patients, are often exposed to numerous nephrotoxic drugs, and the risk of AKI has been shown to increase by 53% for each nephrotoxic drug exposure.5 When a patient experiences AKI, clinicians must evaluate concomitant risk factors and the nephrotoxic risk profile for each drug exposure based on the published literature to determine causality for each exposure and discontinue causal drugs where feasible.2 This is challenging given current gaps in the literature, which include variable definitions of DI-AKI employed in studies, lack of diagnostic markers of drug-specific injury, and few published studies on AKI subphenotypes.6,7 Clinical manifestations and the onset of DI-AKI vary by drug and can be overlooked with short-term drug exposures. There is significant clinical heterogeneity among patients, and consequently, it is very challenging to determine the role of drugs amid other etiologies of AKI, such as sepsis or hypotension. Causality assessment tools for adverse drug events have been shown to perform well for general adverse drug events, with some limitations.8, 9, 10 These tools have not been validated for DI-AKI, which requires a careful evaluation of competing clinical risk factors and concurrent nephrotoxin exposures by an experienced clinician.11
The study aims were as follows: (i) to describe the injury, risk factors, and outcomes of DI-AKI and (ii) to identify the best predictive variables that differentiate high probability DI-AKI cases from other etiologies of AKI in inpatient settings in a well-characterized cohort of adult patients with clinically adjudicated hospital-acquired DI-AKI.
Methods
Study Populations, Eligibility Criteria, and Data Collection
In this study, we retrospectively analyzed data from hospitalized adult patients, aged 18 years or older, enrolled in the DIRECT study (NCT02159209), which was conducted during the period from February 2013 to December 2015.12 We refer the reader to the DIRECT study methodology which has been published previously.12 In summary, DIRECT is an international, multicenter, and observational cohort study of enriched DI-AKI cases that underwent clinical adjudication. The study was approved by the institutional research board for human subjects at the coordinating center, and subjects provided informed consent. Each of the 42 participating centers was required to follow institutional requirements for human subject research approval and informed consent.
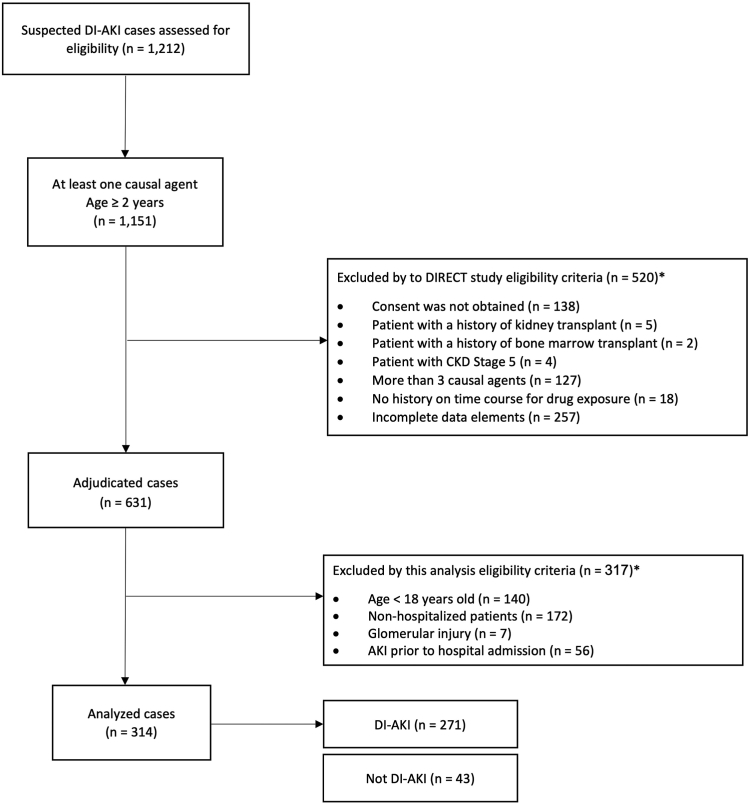
Suspected DI-AKI cases identified by the principal investigator at each participating site were electronically screened for eligibility, enrolled by the site, and underwent clinical adjudication. Eligible cases included subjects who experienced AKI stage 2 or higher during their hospitalization, defined as a doubling in SCr from baseline after exposure to candidate nephrotoxic drug(s) for a minimum of 24 hours (Supplementary Table S1). AKI was staged according to the 2012 Kidney Disease Improving Global Outcomes criteria using the peak to baseline SCr ratio.13 Baseline SCr was defined as the lowest SCr within 90 days before drug exposure. In patients who presented without baseline SCr, nadir SCr during hospitalization was used to back-calculate baseline SCr. Cases were excluded if the patients had undergone kidney or bone marrow transplants, had chronic kidney disease stage 5, had exposure to more than 3 possible causal drugs, or had incomplete patient information on the time course of drug exposure. For this analysis, we excluded patients who presented to the hospital with AKI or those with biopsy proven glomerular injury (Figure 1).11
Figure 1.
Flow diagram of subject disposition through DIRECT study and cohort selection. AKI, acute kidney injury; CKD, chronic kidney disease; DI-AKI, drug-induced acute kidney injury; DIRECT, Rationale and Design of the Genetic Contribution to Drug Induced Renal Injury Study. ∗Cases may meet one or many of the exclusion criteria.
Data was collected at predefined time points based on causal drug exposure and course of injury, including hospital admission, predrug exposure, start of drug exposure, onset of AKI, peak SCr, drug discontinuation or dosage adjustment, nadir SCr, hospital discharge, 28 days and 90 days postinjury. Data elements collected included vital signs, laboratory results, urine studies, physical examination, AKI risk factors, kidney replacement therapy, and survival status (Table 1). Definitions of AKI risk factors were provided to site investigators. For example, increased vascular capacity was defined as clinical events leading to reduced blood perfusion to the kidney (e.g., mean arterial pressure <65 mm Hg, sepsis). Hyperglycemia was defined as blood sugar >110 mg/dl or patient was on insulin. In this analysis, AKI risk factors were present if they were recorded in the 72 hours preceding AKI onset. Urinalysis findings were defined as: minimal (<1+), moderate (1+ to 2+), and heavy (>2+) for protein and glucose in the urine. Positive urinary sediment findings were defined as minimal (<6), moderate (6–20), and heavy (>20) for white and red blood cells, presence of either hyaline, granular, cellular hemoglobin, fatty, waxy, tubular, or broad casts.
Table 1.
Assessment schedule of hospitalized patients in DIRECT study
| Clinical data | Hospital admission | Pre-drug exposure | Drug exposure | AKI onset | Peak SCr or peak severity of injury | Drug discontin uation | Nadir SCr or resolution of injury | Hospital discharge | Status at days 28 and 90 |
|---|---|---|---|---|---|---|---|---|---|
| Physical exam | X | X | X | X | X | X | X | X | |
| AKI risk factors | X | X | X | X | X | X | X | X | |
| Vital signs | X | X | X | X | X | X | X | X | |
| Laboratory tests | X | X | X | X | X | X | X | X | |
| SCr | X | X | X | X | X | X | X | X | X |
| Urine study | X | ||||||||
| KRT | X | X | X | X | X | X | |||
| Survival status | X | X |
AKI, acute kidney injury; KRT, kidney replacement therapy; SCr, serum creatinine.
Peak SCr or peak severity of injury was defined as the highest SCr recorded throughout hospitalization.
This table was adapted from Awdishu et al.12 “Rationale and design of the genetic contribution to Drug Induced Renal Injury (DIRECT) study.” Kidney Int Rep. 2016;1:288-298.
Structured data elements were extracted electronically from the electronic health record where feasible, whereas unstructured data was collected manually. All data was deidentified and populated in electronic case report forms stored in a web-based database. A web platform was developed, allowing the adjudicators to evaluate the clinical data elements across study timepoint and perform clinical adjudication (Supplementary Figure S1).
Clinical Adjudication
All cases enrolled in the DIRECT study underwent clinical adjudication by a panel of 9 nephrologists and 1 pharmacist. All cases were reviewed by the pharmacist (LA) to ensure complete and accurate data prior to simultaneous adjudication by 2 independent nephrologists (EM, DC, JC, RC, AL, SG, MZ, or DS) as DI-AKI versus Not DI-AKI. If a unanimous agreement between the 2 adjudicators was not achieved, a third adjudicator (RM) was introduced to resolve the tie and achieve an agreement. None of the adjudicators were involved with the clinical care of the cases they adjudicated.
Each case was summarized by visually displaying SCr graphs with drug exposure dates overlayed and a summary of AKI risk factors and clinical findings at each timepoint including but not limited to vital signs, laboratory values, procedures, contrast exposure, drug doses, and drug concentrations (Supplementary Figure S1). To standardize the adjudication process and assure the reproducibility of findings, adjudicators were provided with a list of candidate nephrotoxic drugs (Supplementary Table S1) and were trained on the adjudication process, which included completing 2 causality assessment tools, the Naranjo Probability Scale (Supplementary Figure S2) and the Liverpool Probability Scale (Supplementary Figure S3) for each candidate nephrotoxic drug.9,10 Using the causality scores and reviewing the case summary, adjudicators completed the Adjudicator Probability Scale using a web-based rubric to determine the contribution of candidate nephrotoxic drugs and concomitant risk factors for AKI (Supplementary Figure S4). Naranjo Probability Scale is a questionnaire tool that involves 10 “Yes”, “No”, or “Unknown” questions. The adverse event rating is determined based on the total score as “Definite” (≥9), “Likely” (5–8), “Possible” (1–4), “Unlikely” (≤0) (Supplementary Figure S2). Liverpool Probability Scale is a decision tree flowchart to establish an adverse event probability rating of “Definite”, “Probable”, “Possible”, or “Unlikely” (Supplementary Figure S3). Guided by the Naranjo Probability Scale and Liverpool Probability Scale, adjudicators completed the Adjudicator Probability Scale for each case. Adjudicator Probability Scale is comprised of “Definite”, “Probable”, “Possible”, or “Unlikely” probability rating for nephrotoxic drugs and concomitant risk factors. For example, in the case of a patient who developed AKI after treatment with gentamicin and ibuprofen, the clinical adjudicator may determine that gentamicin was a “Definite” cause and ibuprofen and underlying and concomitant risk factors were “Unlikely” causes of AKI. Finally, each case was adjudicated as DI-AKI versus Not DI-AKI by adjudicators.
Descriptive Statistical Analysis and IRR
Cases were grouped into DI-AKI or Not DI-AKI, and we summarized the patient demographics, risk factors, and outcomes using descriptive statistics such as mean (SD), median (inter-quartile range), or counts (%), where appropriate. Continuous variables were analyzed by 2 independent samples t-test or the Wilcoxon rank-sum test, as appropriate. Categorical variables were analyzed by chi-square test or Fisher exact test, as appropriate. We measured the IRR of DI-AKI adjudication (DI-AKI vs. Not DI-AKI) between the 2 primary adjudicators across all cases using Krippendorff’s alpha statistic. Krippendorff’s alpha is a reliability coefficient developed to measure the agreement among adjudicators. Unlike other IRR measures, Krippendorff’s alpha can be applied to any number of adjudicators, not only 2 adjudicators. In addition, it can be used for small sample sizes and missing data.14
Multivariable Analysis of DI-AKI
We examined 166 clinical variables from the DIRECT study and grouped variables under the following domains: demographics (3), past medical history (25), preadmission medications (5), psychosocial (2), AKI risk factors (15), physical examination (17), vitals (9), trends-vitals (6), labs (25), trends - labs (18), urinalysis (20), contrast exposure (4), urine chemistry (10), kidney biopsy (4), and hemodialysis and critical illness (3).
Given the critical role of SCr trends and temporal relationship to drug exposure,2 we developed a set of variables to capture the relative change in SCr across several time points of the study (Supplementary Table S2) using the following formula:
To evaluate the relationship between contrast agent exposure and AKI, we developed 2 variables as folows: (i) contrast volume (ml) administered between hospital admission up to AKI onset, and (ii) number of days between contrast exposure date and AKI onset. For subjects with multiple exposures, we considered the exposure closest to AKI onset.15,16
When constructing the multivariable model, we aimed to identify a parsimonious set of predictors that best distinguish DI-AKI cases from other etiologies of AKI. To achieve that, we employed a staged approach to variable selection. First, we eliminated variables that met the following criteria: (i) past medical history variables with <2% prevalence, (ii) laboratory variables with >30% missing except for blood eosinophil count, (iii) urinalysis variables with >30% missing except for urinary sediments, and (iv) urine chemistry variables with >30% missing except for creatinine and protein. Continuous variables were imputed by median value substitution. Categorical variables were imputed by most frequent value substitution. Second, we performed univariable analyses between the remaining variables and the DI-AKI ascertainment outcome, removing predictors with a P > 0.1. Finally, we utilized penalized logistic regression using the L1 penalty (Least Absolute Shrinkage and Selection Operator—LASSO) with k-fold cross-validation (k = 10) to select a final subset of the predictor variables.17
The final subset of predictors identified by the variable selection procedure described above was used in an unpenalized logistic regression model with DI-AKI as the outcome. We evaluated model discrimination using the ROC AUC, and model calibration with an observed-to-expected calibration plot and the Hosmer–Lemeshow statistic.18 Confidence interval (CI) of ROC AUC were generated using bootstrapping (100 bootstrap samples).19 We assessed the performance of our model’s sensitivity and specificity at Youden’s index, a commonly used cutoff that attempts to maximize sensitivity and minimize false positives.20
Results
Patient Cohort and Clinical Adjudication
In the DIRECT study, 1212 AKI cases were screened, of which 631 cases underwent clinical adjudication. A total of 314 hospitalized adult cases met the eligibility criteria for this analysis (Figure 1). The majority of cases were from the United States (68%), followed by India (11%), and the United Kingdom (10%), respectively (Supplementary Table S3). Of the 314 cases, 271 (86%) were adjudicated as DI-AKI with unanimous agreement in 233 (74.2%) cases, corresponding to poor agreement (Krippendorff’s alpha statistic 0.309). The proportion of cases requiring a tiebreaker was comparable between the cases clinically adjudicated as DI-AKI and Not DI-AKI (26.1% vs. 21.0%, P = 0.55). Assessment of clinical notes from tiebreaker adjudication of Not DI-AKI cases indicated that drug causality could not be established due to concerns related to temporality in relationship to AKI onset, drug exposure (dosing or concentration), or competing AKI risk factors (Supplementary Table S4).
The cohort demographics are summarized in Table 2. Males represented 51% of the cohort with a median (inter-quartile range) age of 55 (31) years. Caucasian patients represented about half of the cohort (55%). The most frequent comorbidities were hypertension (44.6%), diabetes mellitus (30.6%), and chronic kidney disease (19.4%). Except for diabetes mellitus (33.2% vs. 16.3%, P = 0.03), the prevalence of comorbidities was similar between DI-AKI and Not DI-AKI groups. A total of 76 (24.2%) cases developed AKI during their intensive care unit stay, of which a larger proportion was clinically adjudicated as Not DI-AKI compared to DI-AKI (21.4% vs. 41.9%, P < 0.01) (Table 2).
Table 2.
Characteristics of the cohort
| Characteristics | Entire cohort (N = 314) | DI-AKI (n = 271) | Not DI-AKI (n = 43) | P-Value |
|---|---|---|---|---|
| Demographics | ||||
| Age, yrs, median (IQR) | 55 (31) | 55 (31) | 61 (32) | 0.09 |
| Race, Caucasian, n (%) | 174 (54) | 142 (52) | 33 (74) | 0.13 |
| Male, n (%) | 160 (51) | 139 (51) | 21 (49) | 0.87 |
| BSA, m2, median (IQR) | 1.91 (0.28) | 1.91 (0.30) | 1.91 (0.34) | 0.54 |
| ICU admission at AKI onset, n (%) | 76 (24.2) | 58 (21.4) | 18 (41.9) | <0.01 |
| Baseline eGFR, category, n (%)a | 0.40 | |||
| >90 ml/min | 176 (56.1) | 156 (57.6) | 20 (46.5) | |
| 60–89 ml/min | 67 (21.3) | 59 (21.8) | 8 (18.6) | |
| 45–59 ml/min | 40 (12.7) | 33 (12.2) | 7 (16.3) | |
| 30–44 ml/min | 19 (6.1) | 15 (5.5) | 4 (9.3) | |
| 15–29 ml/min | 12 (3.8) | 8 (3.0) | 4 (9.3) | |
| Past Medical History, n (%) | ||||
| Congestive heart failure | 36 (11.5) | 30 (11.1) | 6 (14) | 0.61 |
| CAD | 36 (11.5) | 30 (11.1) | 6 (14) | 0.61 |
| CKD | 61 (19.4) | 52 (19.2) | 9 (20.9) | 0.84 |
| COPD | 32 (10.2) | 30 (11.1) | 2 (5) | 0.28 |
| Diabetes mellitus | 96 (30.6) | 89 (32.8) | 7 (16) | 0.03 |
| Hypertension | 140 (44.6) | 120 (44.3) | 20 (47) | 0.87 |
| Leukemia or lymphoma | 19 (6.1) | 18 (6.6) | 1 (2) | 0.49 |
| Liver cirrhosis | 26 (8.3) | 21 (7.7) | 5 (12) | 0.38 |
| Malignancy–chemotherapy | 27 (8.6) | 22 (8.1) | 5 (12) | 0.40 |
| Nephrotoxic drugs exposure, n (%) | ||||
| Number of candidate nephrotoxic drugs, mean (SD) | 1.5 (0.67) | 1.5 (0.68) | 1.4 (0.63) | 0.24 |
| Vancomycin | 153 (48.7) | 143 (53.8) | 10 (23.3) | < 0.01 |
| NSAIDs | 57 (18.2) | 57 (21.0) | 0 (0) | < 0.01 |
| Piperacillin/tazobactam | 56 (17.8) | 49 (18.1) | 7 (16.3) | 0.99 |
| Cephalosporin antibiotics | 34 (10.8) | 23 (8.5) | 11 (25.6) | < 0.01 |
| Aminoglycoside antibiotics | 30 (9.6) | 29 (10.7) | 1 (2.33) | 0.10 |
| Proton pump inhibitors | 27 (8.6) | 19 (7.01) | 8 (18.6) | 0.20 |
| Fluoroquinolone antibiotics | 20 (6.4) | 15 (5.5) | 5 (11.6) | 0.17 |
| Other antibiotics | 15 (4.8) | 14 (5.2) | 1 (2.33) | 0.70 |
| Calcineurin inhibitor drugs | 13 (4.1) | 10 (3.7) | 3 (7.0) | 0.40 |
| Loop diuretics | 13 (4.1) | 6 (2.2) | 7 (16.3) | < 0.01 |
| Antiviral drugs | 10 (3.2) | 9 (3.3) | 1 (2.3) | 1.00 |
| Penicillin antibiotics | 10 (3.2) | 9 (3.3) | 1 (2.3) | 1.00 |
| Antineoplastic drugs | 10 (3.2) | 9 (3.3) | 1 (2.3) | 1.00 |
| Sulfonamide antibiotics | 8 (2.5) | 7 (2.6) | 1 (2.3) | 1.00 |
| Antifungal drugs | 7 (2.2) | 7 (2.6) | 0 (0) | 0.60 |
| β-lactam antibiotics | 7 (2.2) | 5 (1.9) | 2 (4.7) | 0.25 |
| Other drugs | 5 (1.6) | 4 (1.5) | 1 (2.3) | 0.52 |
| Antiepileptic drugs | 2 (0.6) | 2 (0.74) | 0 (0) | 1.00 |
| ARBs | 1 (0.3) | 0 (0) | 1 (2.3) | 0.14 |
| Carbapenem antibiotics | 1 (0.3) | 1 (0.4) | 0 (0) | 1.00 |
| Gout suppressant drugs | 1 (0.3) | 1 (0.4) | 0 (0) | 1.00 |
ARBs, angiotensin receptor blockers; BSA, body surface area; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease; DI-AKI, drug-induced acute kidney injury; eGFR, estimated glomerular filtration rate; ICU, intensive care unit; NSAIDs, nonsteroidal antiinflammatory drugs.
eGFR was calculated using serum creatinine between 90 days to 12 months prior to hospital admission.21
Patients clinically adjudicated as DI-AKI and Not DI-AKI groups were exposed to a comparable mean (SD) number of candidate drugs (1.5 [0.68] vs. 1.4 [0.63], P = 0.24). Vancomycin was the most frequent candidate nephrotoxic drug (48.7%), followed by NSAIDs (18.2%) and piperacillin/tazobactam (17.8%) (Table 2). The proportion of certain candidate causal drugs was significantly different between DI-AKI and Not DI-AKI groups. A greater proportion of clinically adjudicated DI-AKI cases had vancomycin (53.8% vs. 23.3%, P < 0.01) and NSAIDs (21.0% vs. 0.0%, P < 0.01) suspected as candidate causal drugs compared to Not DI-AKI cases. Whereas a greater proportion of Not DI-AKI cases had cephalosporins (25.6% vs. 8.5%, P < 0.01) and loop diuretics (16.3% vs. 2.2%, P < 0.01) suspected as candidate causal drugs compared to DI-AKI cases. The median vancomycin trough concentration was higher in the cases adjudicated as DI-AKI compared to the cases adjudicated as Not DI-AKI (31.9 vs. 27.6 mcg/dl, P = 0.43).
Our evaluation of AKI risk factors revealed that among patients adjudicated as DI-AKI, a lower proportion had undergone cardiac surgery (3.3% vs. 11.3%, P < 0.01) and had increased vascular capacity (8.1% vs. 11.6%, P = 0.03), and a higher proportion had hyperglycemia (48.3% vs. 30.2%, P = 0.03) (Table 3). The proportion of AKI risk factors was comparable between cases that required and those that did not require a tiebreaker adjudicator (Supplementary Table S5). A total of 33 patients underwent kidney biopsy, of whom 15 (45%) had histopathology consistent with acute interstitial nephritis, 8 (24%) with acute tubular necrosis (ATN), and 7 (21%) as mixed pattern of acute interstitial nephritis and ATN (Supplementary Table S6). Interestingly, all cases with kidney biopsy results were adjudicated as DI-AKI.
Table 3.
Proportion of AKI risk factors
| AKI risk factor, n (%) | Entire cohort (N = 314) | DI-AKI (n = 271) | Not DI-AKI (n = 43) | P-value |
|---|---|---|---|---|
| Hyperglycemia | 144 (45.86) | 131 (48.34) | 13 (30.23) | 0.03 |
| Severe infection or sepsis | 56 (17.83) | 50 (18.45) | 6 (13.95) | 0.67 |
| Extracellular fluid loss | 41 (13.06) | 38 (14.02) | 3 (6.98) | 0.33 |
| Red blood cells transfusion | 39 (12.42) | 30 (11.07) | 9 (20.93) | 0.08 |
| Intravascular fluid loss | 37 (11.78) | 34 (12.55) | 3 (6.98) | 0.44 |
| Other procedures | 31 (9.9) | 29 (10.7) | 2 (4.7) | 0.28 |
| Liver disease | 30 (9.55) | 24 (8.86) | 6 (13.95) | 0.27 |
| Anesthetic agent | 28 (8.92) | 22 (8.12) | 6 (13.95) | 0.24 |
| Cardiac failure | 27 (8.6) | 25 (9.23) | 2 (4.65) | 0.56 |
| Increased vascular capacity | 14 (4.46) | 9 (3.32) | 5 (11.63) | 0.03 |
| Cardiac surgery | 7 (2.23) | 3 (1.11) | 4 (9.3) | <0.01 |
| Vascular surgery | 4 (1.27) | 3 (1.11) | 1 (2.33) | 0.45 |
| Hepatorenal syndrome | 3 (0.96) | 3 (1.11) | 0 (0) | 1 |
| Hemorrhage | 2 (0.64) | 2 (0.74) | 0 (0) | 1 |
AKI, acute kidney injury; DI-AKI, drug-induced acute kidney injury; RBC, red blood cell.
AKI risk factors were considered to be present if they were recorded in the 72 hours preceding AKI onset.
Increased vascular capacity was defined as clinical events leading to reduced blood perfusion (e.g., mean arterial pressure <65 mm Hg, sepsis). Hyperglycemia was defined as blood sugar >110 mg/dl or the patient is on insulin.
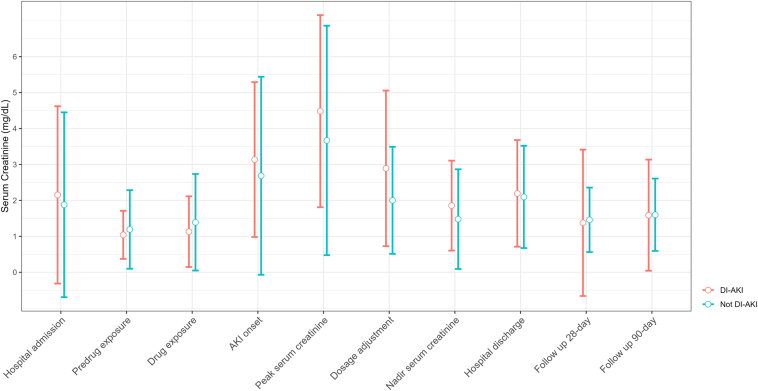
The mean (SD) baseline SCr was not statistically different between DI-AKI and Not DI-AKI groups (0.91 [0.44] vs. 0.98 [0.81] mg/dl, P = 0.60) and increased to a maximum of 4.5 (2.7) versus 3.7 (3.2) mg/dl (P = 0.12) (Table 4). At discharge, the SCr remained elevated at 2.2 (1.5) versus 2.1 (1.4) mg/dl (P = 0.72), reflecting the severity of the injury (Figure 2). A significantly greater proportion of patients clinically adjudicated as DI-AKI had AKI stage 3 (73.7% vs. 48.8%, P < 0.01). The rate of acute kidney disease was significantly higher in the DI-AKI group at discharge (61.3% vs. 32.6%, P < 0.01), but it was nonsignificant at 28-day (27.3% vs. 25.6%, P = 1) and 90-day (14.8% vs. 11.6%, P = 0.79) follow-up.22 No statistically significant differences between the groups were observed for the duration from drug exposure to AKI onset, length of hospital stay, acute kidney disease, need for renal replacement therapy, and inpatient mortality (Table 4).
Table 4.
AKI related characteristics and outcomes
| Variable | DI-AKI (n = 271) | Not DI-AKI (n = 43) | P-value |
|---|---|---|---|
| Previous DI-AKI, n (%) | 10 (3.7) | 2 (4.8) | 1 |
| Start of drug exposure to AKI onset, days, median (IQR) | 5 (8) | 3 (3.5) | 0.24 |
| Baseline SCr, mg/dl, mean (SD) | 0.91 (0.44) | 0.98 (0.81) | 0.60 |
| Peak SCr, mg/dl, mean (SD) | 4.5 (2.7) | 3.7 (3.2) | 0.12 |
| Discharge SCr, mg/dl, mean (SD) | 2.2 (1.5) | 2.1 (1.4) | 0.72 |
| 28-days SCr, mg/dl, mean (SD) | 1.6 (1.6) | 1.6 (1.0) | 0.96 |
| 90-days SCr, mg/dl, mean (SD) | 1.4 (2.0) | 1.5 (0.9) | 0.28 |
| AKI stage 3 during hospitalization, n (%)a | 194 (73.7) | 21 (48.8) | <0.01 |
| AKD at hospital discharge, n (%)b | 166 (61.3) | 14 (32.6) | <0.01 |
| AKD at 28 days, n (%)b | 74 (27.3) | 11 (25.6) | 1 |
| AKD at 90 days, n (%)b | 40 (14.8) | 5 (11.6) | 0.79 |
| Biopsy, n (%) | 33 (12.2) | 0 (0) | <0.01 |
| Need for KRT during hospitalization, n (%) | 70 (2.6) | 11 (2.6) | 1 |
| Duration of hospital stay, days, median (IQR) | 17 (23) | 17 (21) | 0.83 |
| Inpatient mortality, n (%) | 15 (5.5) | 4 (9.3) | 0.52 |
AKD, acute kidney disease; AKI, acute kidney injury; DI-AKI, drug-induced acute kidney injury; IQR, interquartile range; KRT, kidney replacement therapy; SCr, serum creatinine.
AKI Stage 3 was defined as 3 times baseline serum creatinine meeting the 2012 Kidney Disease Improving Global Outcomes criteria.
AKD was defined as 1.5 × baseline SCr.
Figure 2.
Mean serum creatinine trends across study time points grouped by DI-AKI ascertainment. AKI, acute kidney injury; DI-AKI, drug-induced acute kidney injury.
Clinical Predictors of DI-AKI Adjudication
A total of 166 clinical variables (Supplementary Table S7) were summarized, evaluated, and 28 predictions were chosen by the feature selection process (Supplementary Table S8). In Table 5, we list the odds ratios (ORs) from the multivariable logistic model for statistically significant predictors differentiating DI-AKI in hospitalized adults from other etiologies of AKI. The temporality and pattern of SCr rise prior to nephrotoxic drug exposure reduced the probability of DI-AKI adjudication. For every 10% increase in SCr from predrug exposure to the start of drug exposure, DI-AKI adjudication odds were reduced by 43% (OR = 0.57, 95% CI 0.41–0.77). AKI risk factors of volume status, such as increased vascular capacity, receipt of red blood cells transfusions, and remarkable ascites on physical examination, reduced the odds of DI-AKI as well (Table 5).
Table 5.
Significant DI-AKI predictors from multivariable logistic regression analysis
| Variable | Odds ratio (95% CI) | P-value |
|---|---|---|
| Demographics | ||
| Age, 10 yrs | 0.69 (0.51–0.92) | 0.01 |
| AKI risk factors | ||
| Increased vascular capacity | 0.07 (0.01–0.54) | 0.01 |
| Red blood cells transfusion | 0.18 (0.05–0.65) | < 0.01 |
| Hyperglycemia | 3.72 (1.26–12.1) | 0.02 |
| Contrast exposure | ||
| Days between AKI onset and contrast exposure, days | 1.08 (1.02–1.15) | 0.02 |
| Physical exam | ||
| Confirmed infection | 3.84 (1.35–11.3) | 0.01 |
| Ascites | 0.17 (0.04–0.74) | 0.02 |
| Trends–labs | ||
| Relative SCr diff. (Drug Exposure, Pre-Drug Exposure), 10% | 0.57 (0.41–0.77) | < 0.01 |
| Platelets diff. (AKI Onset, Drug Exposure), 100 × 109/l | 0.40 (0.21–0.73) | < 0.01 |
| WBC diff. (AKI onset, drug exposure), 5 × 109/l | 0.64 (0.41–0.98) | 0.04 |
| Urinalysis | ||
| WBC–heavy, AKI onset | 0.16 (0.03–0.84) | 0.03 |
AKI, acute kidney injury; CI, confidence internal; ICU, intensive care unit; SCr, serum creatinine; WBC, white blood cells
Increased vascular capacity was defined as clinical events leading to reduced blood perfusion to the kidney (e.g., mean arterial pressure <65 mm Hg, sepsis). Hyperglycemia was defined as blood sugar >110 mg/dl or 6.05 mmol/l, or patient is on insulin. Predrug Exposure time point was defined as the day prior to treatment with a candidate nephrotoxic drug. Drug Exposure time point was defined as the first day of treatment with the candidate nephrotoxic drug. AKI Onset time point was defined as the first day of AKI meeting the 2012 Kidney Disease Improving Global Outcomes AKI stage 2 criteria. Relative SCr Diff. (Drug Exposure, Predrug Exposure) was defined as the relative change in SCr between Drug Exposure and Pre-Drug Exposure time points. WBC in urinalysis was defined as minimal (< 6), moderate (6–20), and heavy (> 21).
Trends of platelets and white blood cell counts affected DI-AKI ascertainment. For every 100 × 109/l increase in platelets count from the start of drug exposure to AKI onset, there was a decrease in the odds of DI-AKI adjudication by 60% (OR = 0.40, 95% CI 0.21–0.73). Similarly, for every 5 × 109/l increase in white blood cell count from the start of drug exposure to AKI onset, there was a decrease in the odds of DI-AKI adjudication by 36% (OR = 0.64, 95% CI 0.41–0.98). Lastly, an increase in the time duration between exposure to contrast medium and AKI onset increased the odds of DI-AKI adjudication by 8% (OR = 1.08, 95% CI 1.01–1.15) for every additional day (Table 5).
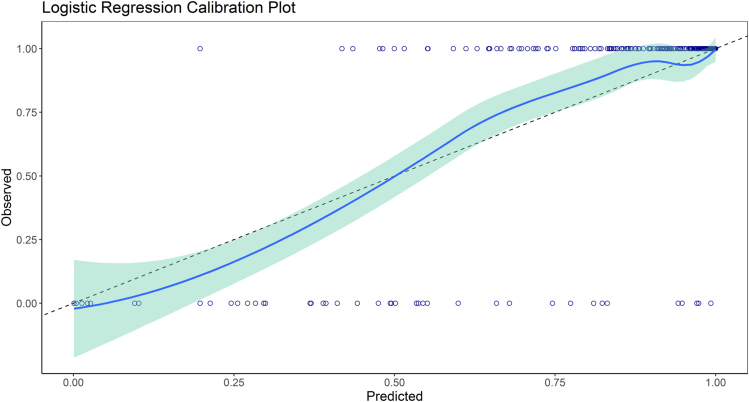
The ROC AUC of the final model was 0.86 (95% CI 0.78–0.90). The Hosmer– Lemeshow test was statistically significant (X-squared = 17.16, df = 8, P = 0.02); despite that, the model appeared to have acceptable overall calibration, judged by the smoothed calibration curve with agreement across the range of predicted probabilities (Figure 3).23 At the Youden’s index, the model sensitivity, specificity, positive predictive value, and negative predictive value were 0.88, 0.84, 0.97, and 0.53, respectively.
Figure 3.
Smoothed calibration curves for the observed-to-expected predicted probability plot for the logistic regression model.
Discussion
In the DIRECT study, a well characterized cohort of AKI cases underwent causality assessment and clinical adjudication to differentiate DI-AKI from other etiologies. In this analysis, we summarize the clinical characteristics and outcomes from comprehensive data (i.e., demographics, past medical history, laboratory results, vital signs, and AKI risk factors) of hospitalized adult patients with suspected DI-AKI and generated a statistical model of clinical predictors that differentiates clinically adjudicated DI-AKI from other AKI etiologies. Our multivariable model achieved a performance of 0.86 (95% CI 0.78–0.90) measured by ROC AUC, demonstrating a comprehensive approach that could improve the recognition of DI-AKI. This analysis was conducted in a multinational cohort of patients, making our findings widely applicable to a range of populations.24 Our work contributes to the understanding of AKI subphenotypes as it relates to drug-induced causes and can enhance future research focused on developing prospective AKI prediction models.25,26
Our analysis revealed that most clinical characteristics and risk factors were shared between the DI-AKI and Not DI-AKI groups, attesting to the complexity of establishing a causal relationship between drug exposure and AKI. Treatment with drugs such as vancomycin and NSAIDs was more frequent in the DI-AKI group, reflecting the adjudicator's knowledge of key factors in establishing a causal relationship such as pharmacological mechanisms, epidemiology of DI-AKI, dose-toxicity relationship, utilization, and availability of drug concentrations. In contrast, treatment with loop diuretics and cephalosporin antibiotics was more frequent in the Not DI-AKI group, reflecting adjudicator uncertainty on causality. Although all drugs included in this analysis have been established as nephrotoxic agents, their relevant contribution to AKI in clinically adjudicated cases is yet to be fully understood, especially in the context of their clinical indications, underlying patient diagnosis, and concomitant AKI risk factors.27
AKI adjudication by nephrologists compared to biomarker-based definitions has been evaluated in previous studies with good agreement. However, most studies have focused on establishing the occurrence of AKI rather than adjudication of AKI subphenotypes. The lack of standard definitions or consensus criteria for AKI- phenotypes complicates clinical adjudication. In the Tribe-AKI study, Koyner et al.28 reported poor IRR (Fleiss’ kappa 0.046) between clinical adjudicators in differentiating ATN versus prerenal azotemia in patients undergoing cardiac surgery.29 In the DIRECT study, investigators recruited suspected DI-AKI cases and employed standardized definitions with criteria for phenotypes resulting in a relative improvement in the IRR of clinical adjudication (Krippendorff’s alpha statistic = 0.309); despite this, the reliability remained below the significance threshold of 0.677.14 This demonstrates that AKI ascertainment is consistently a debatable decision, even among experienced clinicians. IRR can be improved by using standardized consensus definitions of DI-AKI, capturing the relevant clinical variables for these definitions in a consistent method across sites, and training clinical adjudicators to reduce variability in the adjudication process.12
We report certain risk factors associated with the adjudication of DI-AKI cases from other causes of AKI. Although certain traditional risk factors of AKI may not be selected by the final multivariable model, it is expected because this analysis aimed to identify distinctive clinical characteristics of DI-AKI using an enriched cohort, yielding results specific to this unique subset of AKI cases. The following variables made DI-AKI ascertainment less likely: age,11 increased vascular capacity,30 diabetes,31 heavy pyuria,32 and increase in SCr prior to treatment with nephrotoxic drugs.11 These findings align with our current understanding of AKI etiologies and risk factors. It is worth noting that most variables from the multivariable model were associated with lowering the odds of DI-AKI adjudication. This observation could be attributed to 2 factors. First, the DIRECT study eligibility criteria aimed to select DI-AKI cases by recruiting patients exposed to nephrotoxic drugs before AKI onset.12 This resulted in the absence of traditional variables associated with establishing the adverse drug event. Second, there is a scarcity of biomarkers and well-established clinical criteria specific to DI-AKI, making the diagnosis complex by requiring the evaluation of the contribution of competing etiologies.7 Therefore, the multivariable model results should not be interpreted as causative or protective of DI-AKI; however, these predictors inform the identification of probable DI-AKI cases.
Kidney biopsy is a valuable tool for determining the pathophysiology of AKI, which helps to inform the causality ascertainment when paired with clinical observations.33 The rate of biopsy-confirmed acute interstitial nephritis cases in this analysis was 66% compared to 5% to 27% reported in other AKI studies. This is not surprising given the number of antibiotics, NSAID, and proton pump inhibitor cases included in this analysis.34 Whereas nephrotoxic agents are responsible for 70% to 90% of acute interstitial nephritis cases, ATN is multifactorial and can be caused by ischemia, sepsis, or nephrotoxins. The rate of biopsy-confirmed ATN varies and can reach up to 50% in cases of severe sepsis.34,35 Although kidney biopsy may reveal histopathologic patterns associated with DI-AKI, it is challenging to include its findings in statistical modeling studies due to the limited number of AKI cases with biopsy results.
Our multivariable logistic regression model achieved good performance with a ROC AUC of 0.86 for the outcome of DI-AKI. This finding supports the hypothesis that a probabilistic model can be used to identify DI-AKI cases with good discrimination. The high specificity of 0.84 enables the ruling out of AKI cases that are not induced by exposure to nephrotoxic drugs. The positive predictive value of 0.97 ensures that cases deemed to be DI-AKI by the algorithm are highly probable cases. Probabilistic models allow the user to manipulate the thresholds for varying levels of sensitivity and specificity for defining cohorts in secondary data use analyses. The calibration plot appears relatively uniform, indicating that the agreement between the estimated and observed number of events is consistent across all cases. The variables selected in our model use clinical data often available as structured data in the electronic health record of patients in the United States and other countries.36 This significantly improves the utility of our model when used to analyze clinical data extracted from electronic health record or clinical data repositories.
We note limitations that will require refinement and future investigation. First, this is a retrospective analysis of enriched DI-AKI cases that were selected by DIRECT study principal investigators, explaining the 86% DI-AKI adjudication rate in this study.12 It is not uncommon for statistical models to be developed using cohorts with high disease incidence. This may systematically give overestimated risk estimates when applied to a new unenriched cohort. Recalibration methods (e.g., calibration-in-the-large) may be applied to correct for poor calibration when performing external validation.23 Second, this analysis focused on identifying predictors of DI-AKI adjudication in patients with hospital-acquired AKI, making the study findings not applicable to community-acquired DI-AKI or other subtypes of DI-AKI. Third, it is important to acknowledge the potential effect of confounding on our results. We hypothesize that the increased risk of DI-AKI associated with confirmed infection may be confounded by the nephrotoxic antimicrobials prescribed to treat infections in hospitalized patients.5,37 For example, a hospitalized patient with bacteremia may receive high doses of vancomycin and piperacillin/tazobactam until stable or culture results favors narrow-spectrum antibiotics. Fourth, it is well-established that drugs have varying degrees of nephrotoxicity. For example, aminoglycosides are associated with a higher risk of kidney injury compared to cephalosporins.38,39 Our analysis did not adjust for the varying risk of nephrotoxicity; however, adjudicators inherently associate varying risks according to their knowledge of the literature. The inclusion of drugs’ relative nephrotoxicity risk as a variable could potentially improve the model performance in identifying DI-AKI cases.40 Fifth, in this study, AKI was defined and staged according to the Kidney Disease Improving Global Outcomes criteria. However, the onset of nephrotoxicity for certain drugs (e.g., cisplatin, ifosfamide, and proton pump inhibitors) may not fit the Kidney Disease Improving Global Outcomes acute timeline. In addition, the clinical presentation of nephrotoxicity varies among individual drugs and drug classes depending on the mechanism of injury. For example, aminoglycosides cause ATN, whereas calcineurin inhibitors may cause different types of injury, including hemodynamic insults, ATN, or thrombotic microangiopathy. The DIRECT study evaluated a wide spectrum of nephrotoxic drugs that cause injury through various mechanisms. Due to the cohort size limitation, it was not feasible to analyze drug classes or mechanisms of injury separately. This limitation could affect the generalizability of this research selecting towards mechanisms of nephrotoxicity associated with short-term drug exposure.41 Since the DIRECT study was completed, new nephrotoxic drugs have been developed and highly utilized (e.g., checkpoint inhibitors) that were not captured in this study.42 Similarly, new evidence has emerged demonstrating the limitation of SCr as a biomarker for nephrotoxicity due to commonly used drugs.43,44 This highlights the need for biomarker research to improve the diagnosis of DI-AKI. Lastly, validation with a new cohort was not feasible within the scope of the analysis because the low DI-AKI prevalence would have required the screening and adjudication of a significant number of new AKI cases.45
In conclusion, this analysis demonstrates that DI-AKI cases are challenging to distinguish from other AKI subtypes. The IRR of DI-AKI ascertainment is poor, even among experienced nephrologists. Statistical modeling offers a promising comprehensive approach to identifying probable DI-AKI from other AKI subtypes with good performance characteristics. Future studies are needed to externally validate our model and to construct causality assessment tools specific to DI-AKI.
Disclosure
PN is an employee of Sema4 Mount Sinai venture, Stamford, Connecticut, USA. VJ has received grant funding from GSK, Baxter Healthcare, and Biocon; and honoraria from AstraZeneca, Boehringer Ingelheim, Baxter, NephroPlus and Zydus Cadilla, under the policy of all honoraria being paid to the organization. All the other authors have no competing interests.
Acknowledgment
This study was funded by the International Serious Adverse Events Consortium. ZKY acknowledges grant funding from the National Library of Medicine (award number T15LM011271) and the University of California, San Diego Skaggs School of Pharmacy and Pharmaceutical Sciences.
Footnotes
Figure S1. Patient summary as presented in the DIRECT study web platform.
Figure S2. Naranjo causality assessment scale.
Figure S3. Liverpool causality assessment scale.
Figure S4. Clinical adjudication form of DIRECT study.
Table S1. Candidate nephrotoxic drugs evaluated in DIRECT study.
Table S2. Timepoint-pairs referenced to calculate the relative change in serum creatinine.
Table S3. Distribution of cases by country.
Table S4. Rationale for Not DI-AKI adjudication by tiebreaker adjudicator.
Table S5. Incidence of AKI risk factors stratified by requiring a tiebreaker adjudicator.
Table S6. Kidney biopsy findings.
Table S7. List of candidate predictor variables with their domains, timepoints and summary statistics.
Table S8. DI-AKI predictors from multivariable logistic regression analysis.
Supplementary Material
Figure S1. Patient summary as presented in the DIRECT study web platform.
Figure S2. Naranjo causality assessment scale.
Figure S3. Liverpool causality assessment scale.
Figure S4. Clinical adjudication form of DIRECT study.
Table S1. Candidate nephrotoxic drugs evaluated in DIRECT study.
Table S2. Timepoint-pairs referenced to calculate the relative change in serum creatinine.
Table S3. Distribution of cases by country.
Table S4. Rationale for Not DI-AKI adjudication by tiebreaker adjudicator.
Table S5. Incidence of AKI risk factors stratified by requiring a tiebreaker adjudicator.
Table S6. Kidney biopsy findings.
Table S7. List of candidate predictor variables with their domains, timepoints and summary statistics.
Table S8. DI-AKI predictors from multivariable logistic regression analysis.
References
- 1.Bartoli E. Adverse effects of drugs on the kidney. Eur J Intern Med. 2016;28:1–8. doi: 10.1016/j.ejim.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Awdishu L., Mehta R.L. The 6R’s of drug induced nephrotoxicity. BMC Nephrol. 2017;18:124. doi: 10.1186/s12882-017-0536-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehta R.L., Kellum J.A., Shah S.V., et al. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schiffl H., Fischer R. Five-year outcomes of severe acute kidney injury requiring renal replacement therapy. Nephrol Dial Transpl. 2008;23:2235–2241. doi: 10.1093/ndt/gfn182. [DOI] [PubMed] [Google Scholar]
- 5.Cartin-Ceba R., Kashiouris M., Plataki M., Kor D.J., Gajic O., Casey E.T. Risk factors for development of acute kidney injury in critically ill patients: a systematic review and meta-analysis of observational studies. Crit Care Res Pract. 2012;2012 doi: 10.1155/2012/691013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ostermann M., Zarbock A., Goldstein S., et al. Recommendations on acute kidney injury biomarkers from the acute disease quality initiative consensus conference: a consensus statement. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.19209. [DOI] [PubMed] [Google Scholar]
- 7.Griffin B.R., Faubel S., Edelstein C.L. Biomarkers of drug-induced kidney toxicity. Ther Drug Monit. 2019;41:213–226. doi: 10.1097/FTD.0000000000000589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan L.M., Al-Harthi S.E., Osman A.M.M., Sattar M.A.A.A., Ali A.S. Dilemmas of the causality assessment tools in the diagnosis of adverse drug reactions. Saudi Pharm J. 2016;24:485–493. doi: 10.1016/j.jsps.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naranjo C.A., Busto U., Sellers E.M., et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 10.Gallagher R.M., Kirkham J.J., Mason J.R., et al. Development and inter-rater reliability of the Liverpool adverse drug reaction causality assessment tool. PLoS One. 2011;6 doi: 10.1371/journal.pone.0028096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta R.L., Awdishu L., Davenport A., et al. Phenotype standardization for drug induced kidney disease Europe PMC funders group. Kidney Int. 2015;88:226–234. doi: 10.1038/ki.2015.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Awdishu L., Nievergelt C.M., Davenport A., et al. Rationale and design of the genetic contribution to drug induced renal injury (DIRECT) study. Kidney Int Rep. 2016;1:288–298. doi: 10.1016/j.ekir.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kellum J.A., Lameire N., Aspelin P., et al. Kidney disease: Improving global outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. doi: 10.1038/kisup.2012.1. [DOI] [Google Scholar]
- 14.Shelley M., Krippendorff K. Content analysis: an introduction to its methodology. J Am Stat Assoc. 1984;79:240. doi: 10.2307/2288384. [DOI] [Google Scholar]
- 15.James M.T., Samuel S.M., Manning M.A., et al. Contrast-induced acute kidney injury and risk of adverse clinical outcomes after coronary angiography: a systematic review and meta-analysis. Circ Cardiovasc Interv. 2013;6:37–43. doi: 10.1161/CIRCINTERVENTIONS.112.974493. [DOI] [PubMed] [Google Scholar]
- 16.Vandenberghe W., Hoste E. Contrast-associated acute kidney injury: does it really exist, and if so, what to do about it? F1000Res. 2019;8:F1000. doi: 10.12688/f1000research.16347.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tibshirani R. Regression shrinkage and selection via the lasso. J R Stat Soc B. 1996;58:267–288. doi: 10.1111/j.2517-6161.1996.tb02080.x. [DOI] [Google Scholar]
- 18.Scott A.J., Hosmer D.W., Lemeshow S. Applied logistic regression. Biometrics. 1991;47 doi: 10.2307/2532419. [DOI] [Google Scholar]
- 19.Allen D.M. The relationship between variable selection and data Agumentation and a method for prediction. Technometrics. 1974;16:125–127. doi: 10.1080/00401706.1974.10489157. [DOI] [Google Scholar]
- 20.Youden W.J. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::AID-CNCR2820030106>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 21.Levey A.S., Stevens L.A., Schmid C.H., et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chawla L.S., Bellomo R., Bihorac A., et al. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol. 2017;13:241–257. doi: 10.1038/nrneph.2017.2. [DOI] [PubMed] [Google Scholar]
- 23.Van Calster B., McLernon D.J., Van Smeden M., et al. Calibration: the Achilles heel of predictive analytics. BMC Med. 2019;17:1–7. doi: 10.1186/S12916-019-1466-7/TABLES/1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lang T., Siribaddana S. Clinical trials have gone global: is this a good thing? PLoS Med. 2012;9:6. doi: 10.1371/journal.pmed.1001228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gameiro J., Branco T., Lopes J.A. Artificial intelligence in acute kidney injury risk prediction. J Clin Med. 2020;9:678. doi: 10.3390/JCM9030678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yousif Z., Awdishu L. Clinical practice: mini-review drug-induced acute kidney injury risk prediction models. Nephron. 2023;147:44–47. doi: 10.1159/000526267. [DOI] [PubMed] [Google Scholar]
- 27.Kane-Gill S.L., Goldstein S.L. Drug-induced acute kidney injury: a focus on risk assessment for prevention. Crit Care Clin. 2015;31:675–684. doi: 10.1016/j.ccc.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Koyner J.L., Garg A.X., Thiessen-Philbrook H., et al. Adjudication of etiology of acute kidney injury: experience from the TRIBE-AKI multi-center study. BMC Nephrol. 2014;15:105. doi: 10.1186/1471-2369-15-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu K.D., Vijayan A., Rosner M.H., Shi J., Chawla L.S., Kellum J.A. Clinical adjudication in acute kidney injury studies: findings from the pivotal TIMP-2∗IGFBP7 biomarker study. Nephrol Dial Transplant. 2016;31:1641–1646. doi: 10.1093/NDT/GFW238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blantz R.C. Pathophysiology of pre-renal azotemia. Kidney Int. 1998;53:512–523. doi: 10.1046/j.1523-1755.2003_t01-1-00784.x. [DOI] [PubMed] [Google Scholar]
- 31.Oliveira J.F.P., Silva C.A., Barbieri C.D., Oliveira G.M., Zanetta D.M.T., Burdmann E.A. Prevalence and risk factors for aminoglycoside nephrotoxicity in intensive care units. Antimicrob Agents Chemother. 2009;53:2887–2891. doi: 10.1128/AAC.01430-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsiao C.Y., Yang H.Y., Hsiao M.C., Hung P.H., Wang M.C. Risk factors for development of acute kidney injury in patients with urinary tract infection. PLoS One. 2015;10 doi: 10.1371/JOURNAL.PONE.0133835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brachemi S., Bollée G. Renal biopsy practice: what is the gold standard? World J Nephrol. 2014;3:287–294. doi: 10.5527/wjn.v3.i4.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nast C.C. Medication-Induced interstitial Nephritis in the 21st Century. Adv Chronic Kidney Dis. 2017;24:72–79. doi: 10.1053/J.ACKD.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 35.Diaz de León M., Moreno S.A., Gonzalez D.J.G.B. Severe sepsis as a cause of acute renal failure. Crit Care. 2008;12:1–7. doi: 10.1186/CC6823/TABLES/3. [DOI] [Google Scholar]
- 36.Horton S., Fleming K.A., Kuti M., et al. The top 25 laboratory tests by volume and revenue in five different countries. Am J Clin Pathol. 2019;151:446–451. doi: 10.1093/AJCP/AQY165. [DOI] [PubMed] [Google Scholar]
- 37.Paul M., Benuri-Silbiger I., Soares-Weiser K., Leibovici L. β lactam monotherapy versus β lactam-aminoglycoside combination therapy for sepsis in immunocompetent patients: systematic review and meta-analysis of randomised trials. BMJ. 2004;328:668. doi: 10.1136/BMJ.38028.520995.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pierson-Marchandise M., Gras V., Moragny J., et al. The drugs that mostly frequently induce acute kidney injury: a case−noncase study of a pharmacovigilance database. Br J Clin Pharmacol. 2017;83:1341–1349. doi: 10.1111/bcp.13216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hosohata K., Inada A., Oyama S., Furushima D., Yamada H., Iwanaga K. Surveillance of drugs that most frequently induce acute kidney injury: a pharmacovigilance approach. J Clin Pharm Ther. 2019;44:49–53. doi: 10.1111/jcpt.12748. [DOI] [PubMed] [Google Scholar]
- 40.Wang L., McGregor T.L., Jones D.P., et al. Electronic health record-based predictive models for acute kidney injury screening in pediatric inpatients. Pediatr Res. 2017;82:465–473. doi: 10.1038/pr.2017.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mody H., Ramakrishnan V., Chaar M., et al. A review on drug-induced nephrotoxicity: pathophysiological mechanisms, drug classes, clinical management, and recent advances in mathematical modeling and simulation approaches. Clin Pharmacol Drug Dev. 2020;9:896–909. doi: 10.1002/CPDD.879. [DOI] [PubMed] [Google Scholar]
- 42.Perazella M.A., Shirali A.C. Immune checkpoint inhibitor nephrotoxicity: what do we know and what should we do? Kidney Int. 2020;97:62–74. doi: 10.1016/J.KINT.2019.07.022. [DOI] [PubMed] [Google Scholar]
- 43.Miano T.A., Hennessy S., Yang W., et al. Association of vancomycin plus piperacillin-tazobactam with early changes in creatinine versus cystatin C in critically ill adults: a prospective cohort study. Intensive Care Med. 2022;48:1144–1155. doi: 10.1007/S00134-022-06811-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Côté J.M., Kane-Gill S.L., Murray P.T. A ray of hope in the discord: is adding piperacillin–tazobactam to vancomycin truly more nephrotoxic? Intensive Care Med. 2022;48:1208–1210. doi: 10.1007/S00134-022-06861-4/FIGURES/1. [DOI] [PubMed] [Google Scholar]
- 45.Ramspek C.L., Jager K.J., Dekker F.W., Zoccali C., van Diepen M. External validation of prognostic models: what, why, how, when and where? Clin Kidney J. 2021;14:49–58. doi: 10.1093/CKJ/SFAA188. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.