Abstract
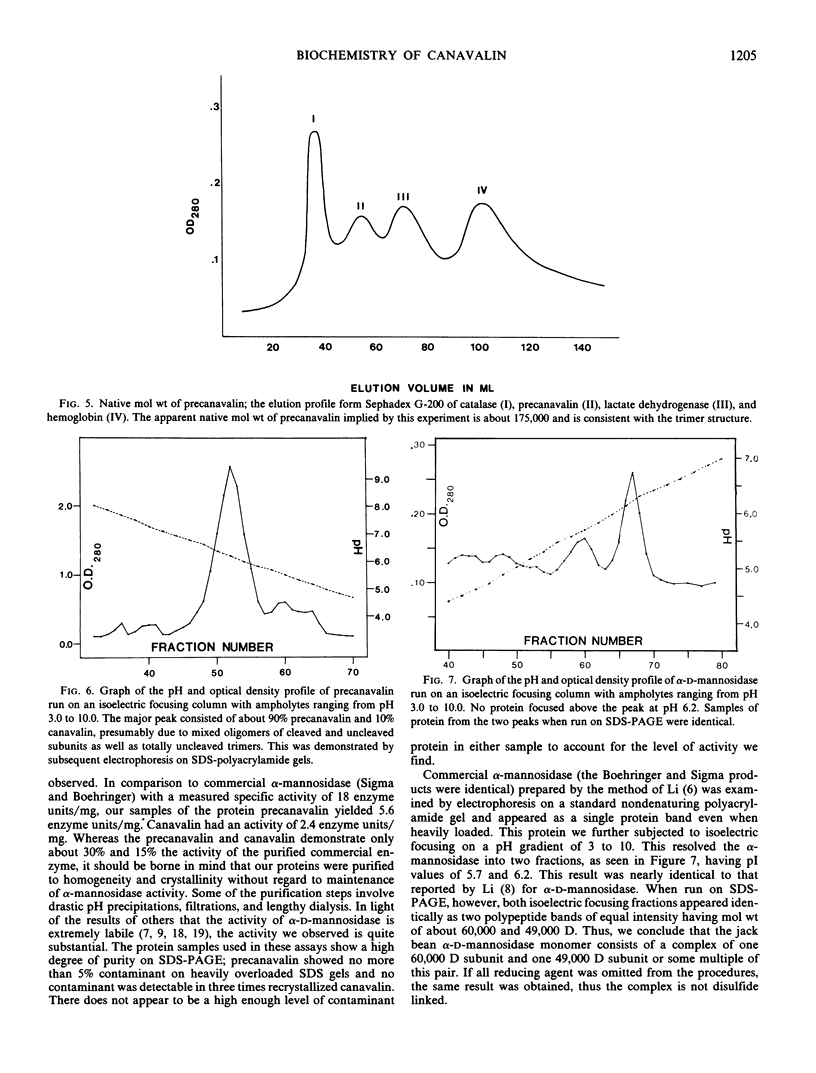
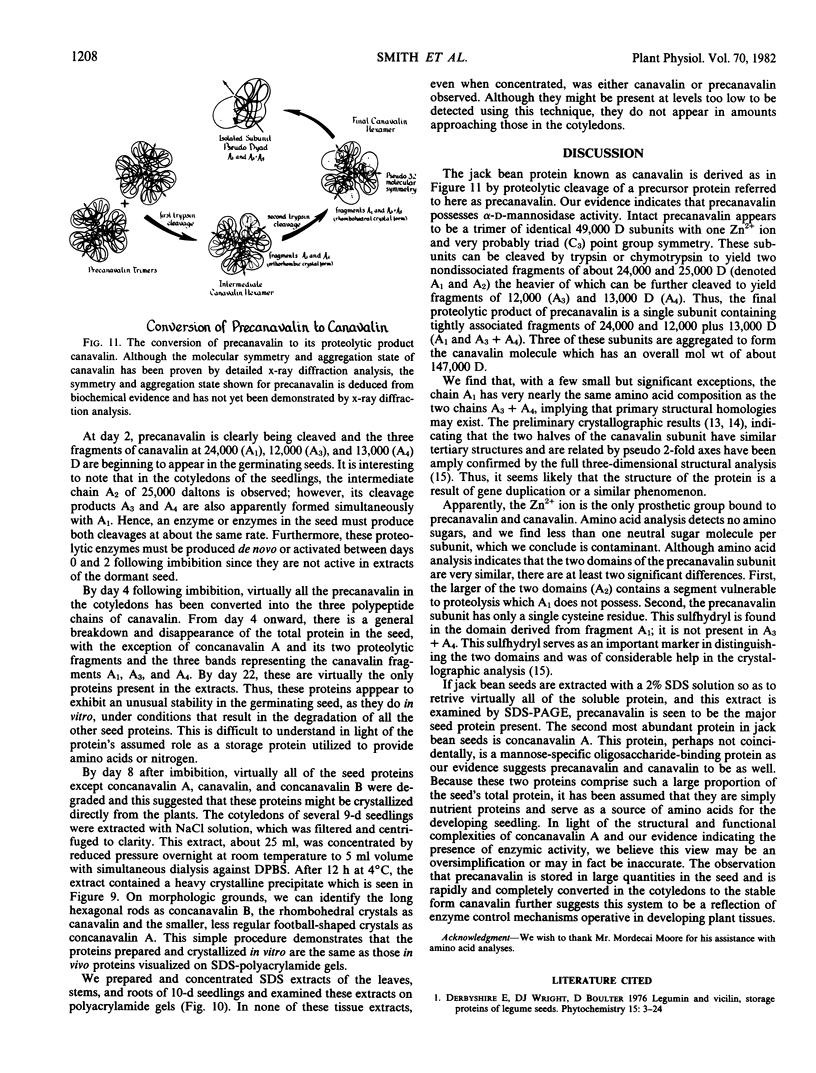
The structure of canavalin, a jack bean (Canavalis ensiformis) protein homologous to phaseolin, the major seed storage protein of Phaseolus vulgaris, has been investigated by x-ray crystallography and found to be a hexamer composed of three identical pairs of similar but nonidentical subunits related by a perfect 3-fold axis and pseudo dyad axes (strict C3 and pseudo D3). One member of each pair of subunits is derived from the amino terminal half of a precursor polypeptide of molecular weight 49,000 and the other from its carboxy terminal half. Thus, the crystallographic evidence indicates that the precursor polypeptide is a tandem duplicate and is structurally redundant (McPherson A. 1982 J Biol Chem 255: 10472). A number of physical and chemical properties of the protein in both the uncleaved and the cleaved form were investigated. These included the native molecular weights, amino acid analyses, number of exposed sulfhydryl groups, carbohydrate content, metal ion analysis, crystallization behavior, and the fate of the protein in developing seeds. It was also found that the purified precursor protein possesses a substantial level of α-d-mannosidase activity and seems to share a number of other physical and chemical properties with that enzyme.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liu T. Y., Chang Y. H. Hydrolysis of proteins with p-toluenesulfonic acid. Determination of tryptophan. J Biol Chem. 1971 May 10;246(9):2842–2848. [PubMed] [Google Scholar]
- Matsubara H., Sasaki R. M. High recovery of tryptophan from acid hydrolysates of proteins. Biochem Biophys Res Commun. 1969 Apr 29;35(2):175–181. doi: 10.1016/0006-291x(69)90263-0. [DOI] [PubMed] [Google Scholar]
- McPherson A., Jr, Rich A. X-ray crystallographic study of the quaternary structure of canavalin. J Biochem. 1973 Jul;74(1):155–160. doi: 10.1093/oxfordjournals.jbchem.a130218. [DOI] [PubMed] [Google Scholar]
- McPherson A., Jr, Spencer R. Preliminary structure analysis of canavalin from jack bean. Arch Biochem Biophys. 1975 Aug;169(2):650–661. doi: 10.1016/0003-9861(75)90209-x. [DOI] [PubMed] [Google Scholar]
- McPherson A. The three-dimensional structure of canavalin at 3.0 A resolution by X-ray diffraction analysis. J Biol Chem. 1980 Nov 10;255(21):10472–10480. [PubMed] [Google Scholar]
- Snaith S. M. Characterization of jack-bean alpha-D-mannosidase as a zinc metalloenzyme. Biochem J. 1975 Apr;147(1):83–90. doi: 10.1042/bj1470083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snaith S. M., Levvy G. A. Purification and properties of alpha-D-mannosidase from jack-bean meal. Biochem J. 1968 Dec;110(4):663–670. doi: 10.1042/bj1100663. [DOI] [PMC free article] [PubMed] [Google Scholar]