Key Clinical Message
Acute thrombus formation on the delivery sheath is rare condition during percutaneous left atrial appendage occlusion. We presented two cases that transesophageal echocardiography (TEE) showed a floating thrombus attached to the tip of delivery sheath during the procedure. Cerebral embolic protection devices were used to prevent neurological events after thrombus was detected. The neurological function was not impaired post‐procedure.
Keywords: cerebral embolic protection, left atrial appendage occlusion, thrombolysis, thrombus
3D transesophageal echocardiography view showed acute thrombus formation on the delivery sheath during left atrial appendge occlusion.

1. INTRODUCTION
Left atrial appendage occlusion (LAAO) is an alternative to oral anticoagulants for prevention of stroke in patients with atrial fibrillation who are not optimal candidates for long‐term anticoagulation. Acute thrombus formation on the delivery sheath during left atrial appendage occlusion is rare condition. Periprocedural stroke during LAAO is extremely unacceptable. We described the use of placement of cerebral embolic protection devices to prevent neurological events when acute thrombus formation during the implantation of LACbes (Shanghai PushMed, Shanghai, China).
2. CASE PRESENTATION
2.1. Case 1
A 59‐year‐old male with hypertension, diabetes, atrial fibrillation, prior twice stroke history, and prior ICD implantation due to cardiac arrest presented for selective percutaneous left atrial appendage occlusion (LAAO). The patient received catheter direct atrial flutter ablation therapy 6 months ago, but electrocardiogram and 24‐h Holter showed atrial fibrillation at present (average heart rate 86 bpm). The patient's CHA2DS2‐VASc score was 4, and his HASBLED score was 3. He was unwilling to take long‐term oral anticoagulants because of high risk for bleeding. During the hospitalization, soft tissue hemorrhage occurred. Before the procedure, the patient's coagulation function test was normal.
An 6F sheath was placed in the right femoral vein; intravenous heparin 3000 U was administered. After transesophageal echocardiography (TEE)‐guided trans‐septal puncture, heparin 4500 U was given. Then, a 12F delivery sheath and pigtail catheter were positioned in the LAA. Activated clotting time (ACT) measured 254 s. An angiogram and TEE were performed to assess the appendage morphology. TEE revealed a cactus shaped LAA free of thrombus. LAA emptying velocity was 40 cm/s. Mild spontaneous echocardiographic contrast was in the LAA. The patient then underwent successfully implantation of a 22‐mm LACbes device with no para‐device leak. After the occlusion device release, TEE showed a 20 mm length floating thrombus attached to the delivery sheath tip (Figure 1A,B). ACT was measured 112 s, and heparin 3000 U was added immediately. We tried to suck the thrombus through long sheath but failed. ACT measured 124 s after 5 min. Heparin was added in divided doses without any effect on the thrombus resolution. Cerebral embolic protection devices (ev3 SpiderFX) were implanted in the bilateral internal carotid arteries and urokinase 500,000 U was administered to achieve ACT >250 s until TEE showed thrombus dissolved. After thrombolytic therapy, cerebral and renal artery angiogram were conducted and showed no embolism sign. Thrombus debris was detected in the filter after removal (Figure 2). Rivaroxaban and aspirin were initiated, and the patient was closely monitored post‐operation. The neurological function was not impaired, and cerebral CT showed old infarcts 1 day after procedure.
FIGURE 1.

(A, B) 2D and 3D TEE showed a floating thrombus attached to the delivery sheath; 3D TEE view: yellow arrow indicate sheath and red arrow indicate thrombus.
FIGURE 2.

Thrombus debris was detected in a filter (right).
3. FOLLOW‐UP
No neurological events occurred during follow‐up. 6 and 12 months after the procedure, TEE revealed a well‐seated 22‐mm LACbes device with no residual flow around the device and no device related thrombus (DRT).
3.1. Case 2
A 59‐year‐old male with atrial fibrillation and heart failure presented to hospital. The patient's CHA2DS2‐VASc score was 2, and his HASBLED score was 1. He chose LAAO because of poor compliance with long‐term oral anticoagulants.
At the beginning of the procedure, TEE revealed a chicken wing shaped LAA free of thrombus. LAA emptying velocity was 28.9 cm/s and LAA EF 34%, no spontaneous echocardiographic contrast in the LAA. Intravenous heparin 3000 U was given after right femoral vein puncture. After TEE‐guided trans‐septal puncture, a 12F delivery sheath and pigtail catheter were delivered to the left atrial, intravenous heparin 4100 U were administered. At this time, TEE suddenly showed a mobile thrombus whose proximal part was connected to the 12F delivery sheath (Figure 3A,B). Immediately, the ACT measured 161 s. Heparin 2000 U was added and ACT measured 168 s after 5 min. Heparin was added again, and we tried to suck the thrombus through the long sheath, thrombus still attached to the outside of the sheath. Cerebral embolic protection devices (ev3 SpiderFX) were implanted in the bilateral internal carotid arteries; TEE showed the amount of thrombus gradually decreased until disappeared. When TEE showed thrombus dissolution, we rechecked ACT which was 260 s. Then, a 24‐mm LACbes device was implanted with no para‐device leak and no thrombus on the device surface. The total heparin dosage used was 18,000 U. None was showed in the filters after withdraw cerebral protection devices. Rivaroxaban and aspirin were initiated, and the patient was closely monitored post‐operation. The neurological function was not impaired after the procedure.
FIGURE 3.
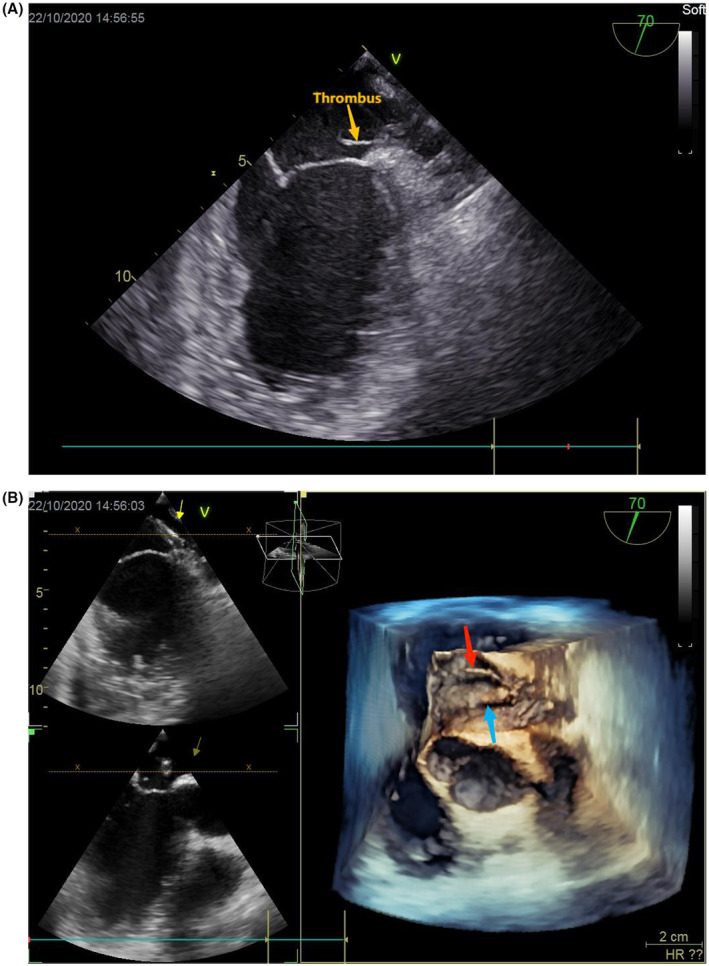
(A, B) 2D and 3D TEE showed a floating thrombus attached to the delivery sheath; 3D TEE view: blue arrow indicates sheath, and red arrow indicates thrombus.
4. FOLLOW‐UP
No neurological events occurred during follow‐up. TEE revealed a well‐seated LACbes device with no residual flow around the device and no DRT at 1‐ and 3‐month follow‐up.
5. DISCUSSION
Left atrial appendage occlusion is an alternative to oral anticoagulants for prevention of stroke in patients with atrial fibrillation who are not optimal candidates for long‐term anticoagulation. 1 Device related thrombus (DRT) is considered an important issue and associated with increased risk of ischemic events after LAAO. 2 , 3 , 4 Current published report suggests that DRT occurs about 3.7% of patients between 3 and 6 months post‐procedure of LAAO. 3 The mechanism underlying DRT is incompletely understood. Some known factors such as hypercoagulability disorder, pericardial effusion, renal insufficiency, implantation depth >10 mm, and non‐paroxysmal atrial fibrillation are risk predictors of DRT following LAAO. 5
Intraprocedural thrombosis during LAAO has been rarely reported. A case report firstly described the acute thrombus formation on the surface of the occlusion device immediately after release. 6 The patient's recent COVID‐19 infection may contribute to acute thrombus formation. Thrombus formation on the delivery sheath during LAAO has been reported. 7 , 8 To prevent thrombus migration, thrombus were retrieved and sucked by sheath without cerebral embolic protection device. However, suction of thrombus within the left atrial by sheath may have high risk of embolism. Prevention of periprocedural complications especially stroke is a major issue during LAAO.
In our case‐series, we followed the standard LAAO procedure and heparin was used at a dosage 100 U/kg. We found that: (1) floating thrombus formed on the tip of the delivery sheath during LAAO; (2) ACT value was <200 s when acute clotting formed and which only achieved acceptable low limit value after repeatedly giving heparin even thrombolytic therapy; (3) we try to suck the thrombus but failed for both cases; (4) it is feasible and safe to place cerebral embolic protection devices to prevent neurological events; and (5) in the follow‐up, there are no DRT and new‐onset stroke.
Periprocedural stroke during LAAO which is a preventive therapy for stroke in patients with atrial fibrillation is extremely unacceptable. Although heparin was generally administered to prevent thrombosis in the procedure, heparin‐induced anticoagulation has a high interindividual variability either in terms of dosage or time duration requiring a frequent ACT monitoring. However, optimal ACT cutoff value is currently unknown during LAAO. Previously mentioned reports showed ACT >250 s when thrombus formed on the deliver sheath during LAAO procedure. Preoperative coagulation tests were within normal reference values although our two patients take rivaroxaban. In addition, persistent low ACT value in the procedure indicated that heparin resistance and stasis of left atrial dilation predispose to coagulopathy and consequently to thrombus formation. Unfortunately, antithrombin III in blood samples were not tested. A study demonstrate that lower ACT level was significantly associated with the development of procedure‐related silent cerebral embolism. 9 Underlying genetic susceptibility of heparin resistance induced lower ACT level and impaired left atrial endothelial function both would be more likely to activate coagulopathy. Further studies should be conducted to determine the optimal ACT level for LAAO procedure. The use of cerebral embolic protection devices during percutaneous LAAO was a feasible and safe therapeutic option for patients with LAA thrombus. 10 It is hard to suck when acute floating thrombus formed on the sheath after occlusion device released. Placement of cerebral embolic protection devices could be a rational option for neurological protection. Indeed, our two cases showed no neurological function impairment after the procedure. In addition, continuous drip of heparin saline to the delivery and guide system may be ensure the local heparin concentration around the instruments in the left atrium.
6. CONCLUSION
Acute thrombus formation on the delivery sheath during LAAO is rare. The need of anticoagulation and the frequency of ACT monitoring should be highlighted. Cerebral protection device may be a feasible management for neurological function protection.
AUTHOR CONTRIBUTIONS
Yangyang Yu: Writing – review and editing. Fang Du: Writing – original draft. Feng Zhao: Validation. Hao Hu: Supervision.
CONFLICT OF INTEREST STATEMENT
The authors report no financial relationships or conflicts of interest regarding the content herein.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
Yu Y, Du F, Zhao F, Hu H. Acute thrombus formation on the delivery sheath during left atrial appendage occlusion: Case reports with placement of cerebral protection devices. Clin Case Rep. 2023;11:e8113. doi: 10.1002/ccr3.8113
Yangyang Yu and Fang Du contributed equally to the manuscript.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in PubMed at https://pubmed.ncbi.nlm.nih.gov/.
REFERENCES
- 1. Reddy VY, Doshi SK, Kar S, et al. 5‐year outcomes after left atrial appendage closure: from the PREVAIL and PROTECT AF trials. J Am Coll Cardiol. 2017;70:2964‐2975. [DOI] [PubMed] [Google Scholar]
- 2. Dukkipati SR, Kar S, Holmes DR, et al. Device‐related thrombus after left atrial appendage closure: incidence, predictors, and outcomes. Circulation. 2018;138:874‐885. [DOI] [PubMed] [Google Scholar]
- 3. Alkhouli M, Busu T, Shah K, Osman M, Alqahtani F, Raybuck B. Incidence and clinical impact of device‐related thrombus following percutaneous left atrial appendage occlusion: a meta‐analysis. JACC Clin Electrophysiol. 2018;4:1629‐1637. [DOI] [PubMed] [Google Scholar]
- 4. Fauchier L, Cinaud A, Brigadeau F, et al. Device‐related thrombosis after percutaneous left atrial appendage occlusion for atrial fibrillation. J Am Coll Cardiol. 2018;71:1528‐1536. [DOI] [PubMed] [Google Scholar]
- 5. Simard T, Jung RG, Lehenbauer K, et al. Predictors of device‐related thrombus following percutaneous left atrial appendage occlusion. J Am Coll Cardiol. 2021;78:297‐313. [DOI] [PubMed] [Google Scholar]
- 6. Manongi N, Volodarskiy A, Goldbarg S. Acute formation of thrombus on a left atrial appendage occlusion device immediately after detachment. JACC Case Rep. 2023;19:101933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Walia R, Lo LW, Lam YY, Yu WC, Chen SA. Long sheath filling defect during left atrial appendage occlusion device placement. Int J Cardiol. 2015;199:193‐194. [DOI] [PubMed] [Google Scholar]
- 8. Talanas G, Sanna GD, Portoghese M, Parodi G. Sudden floating thrombus formation on the delivery cable before Amplatzer‐Amulet device deployment during left atrial appendage occlusion: the need for a standardized anticoagulation. Catheter Cardiovasc Interv. 2020;95:408‐410. [DOI] [PubMed] [Google Scholar]
- 9. Wang Z, Wang K, Lu S, et al. Surgical and percutaneous left atrial appendage intervention: silent cerebral embolism considerations. Eur J Cardiothorac Surg. 2023;63:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sharma SP, Cheng J, Turagam MK, et al. Feasibility of left atrial appendage occlusion in left atrial appendage thrombus: a systematic review. JACC Clin Electrophysiol. 2020;6:414‐424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are openly available in PubMed at https://pubmed.ncbi.nlm.nih.gov/.


