Abstract
Background
Decreasing hyperinsulinemia is crucial in preventing laminitis in insulin dysregulated (ID) horses. Complementary pharmacological treatments that efficiently decrease postprandial hyperinsulinemia in ID horses are needed.
Objectives
Compare short‐term effects of canagliflozin vs placebo on glucose and insulin responses to an oral sugar test (OST) as well as the effects on body weight and triglyceride concentrations in horses with ID.
Animals
Sixteen privately‐owned ID horses.
Methods
A single‐center, randomized, double‐blind, placebo‐controlled, parallel design study. The horses were randomized (ratio 1:1) to either once daily PO treatment with 0.6 mg/kg canagliflozin or placebo. The study consisted of an initial 3‐day period for obtaining baseline data, a 3‐week double‐blind treatment period at home, and a 3‐day follow‐up period similar to the initial baseline period but with continued double‐blind treatment. Horses were subjected to an 8‐sample OST in the morning of the third day on both visits.
Results
Maximal geometric least square (LS) mean insulin concentration (95% confidence interval [CI]) during the OST decreased after 3 weeks of canagliflozin treatment compared with placebo (83.2; 55.4‐125.0 vs 215.2; 143.2‐323.2 μIU/mL). The geometric LS mean insulin response (insulin AUC0‐180) for canagliflozin‐treated horses was >66% lower compared with placebo. Least square mean body weight decreased by 11.1 (4‐18.1) kg and LS mean triglyceride concentrations increased by 0.99 (0.47‐1.5) mmol/L with canagliflozin treatment.
Conclusions and Clinical Importance
Canagliflozin is a promising drug for treatment of ID horses that requires future studies.
Keywords: equine, equine metabolic syndrome, horse, insulin dysregulation, SGLT2 inhibitor, sodium‐glucose co‐transporter 2 inhibitor
Abbreviations
- ∆AUCinsulin/∆AUCglucose
ratio of the incremental AUC for insulin and glucose during the OST
- ANCOVA
analysis of covariance
- BCS
body condition score
- CI
confidence interval
- CNS
cresty neck score
- CV
coefficient of variation
- glucose AUC0‐180
area under the plasma glucose vs time curve during the OST
- glucose C max
maximal glucose concentration during OST
- ID
insulin dysregulation
- insulin AUC0‐180
area under the plasma insulin vs time curve during the OST
- insulin C max
maximal insulin concentration during OST
- LS means
least squares means
- NSC
non‐structural carbohydrates
- OST
oral sugar test
- SGLT2 inhibitor
sodium‐glucose co‐transporter 2 inhibitor
- T2DM
type 2 diabetes mellitus
1. INTRODUCTION
Horses with insulin dysregulation (ID) respond to intake of non‐structural carbohydrates (NSC) with excessive postprandial hyperinsulinemia, 1 , 2 which is associated with laminitis. 3 Therefore, decreasing excessive hyperinsulinemia is crucial in preventing laminitis in these horses. The current strategy for decreasing postprandial hyperinsulinemia is to keep the intake of NSC low and, when appropriate, improve tissue insulin sensitivity by promoting weight loss and increasing exercise. 4 However, pharmacological treatments are needed, that will efficiently decrease postprandial hyperinsulinemia, especially in cases that are refractory to dietary management. Pioglitazone recently has been evaluated as a potential drug for decreasing postprandial hyperinsulinemia in ID horses, but efficacy is low. 5 Metformin hydrochloride has low PO bioavailability in horses, 6 and the efficacy of this drug in decreasing excessive postprandial insulin responses in naturally‐occurring ID recently has been questioned. 7
Sodium‐glucose co‐transporter 2 (SGLT2) inhibitors are a new treatment option for human adults with type 2 diabetes mellitus (T2DM). These drugs decrease hyperglycemia in T2DM patients by inhibition of glucose reabsorption in the proximal tubules in the kidneys, resulting in glucosuria. 8 The increase in urinary glucose excretion is partly balanced by increased hepatic glucose production, resulting in improved glycemic control with low risk of hypoglycemia. 9 Decreasing postprandial plasma glucose concentrations and thereby decreasing the postprandial insulin response may offer a novel therapeutic option for treating excessive hyperinsulinemia in ID horses.
Recently, the SGLT2 inhibitor velagliflozin was shown to effectively decrease the postprandial insulin response in ID ponies fed a challenge diet high in NSC. The treated ponies did not develop laminitis, whereas 36% of the untreated ponies did develop laminitis while receiving the same diet. 10 Over a 16‐week administration period, velagliflozin was shown to be well tolerated without any serious adverse effects, such as urinary tract infections, hypoglycemia, or negative energy balance. 11 In comparison with humans, where treatment with SGLT2 inhibitors results in weight loss, 8 16 weeks of treatment with velagliflozin had no effect on the ponies' body weight. 11 The SGLT2 inhibitor velagliflozin is, however, not currently a registered drug on the market. Canagliflozin, a commonly prescribed SGLT2 inhibitor for humans with T2DM, might be an appropriate off‐label alternative to velagliflozin for use in equine patients.
Therefore, our first objective was to compare the short‐term effects of canagliflozin vs placebo on glucose and insulin responses after an oral sugar test (OST) in horses with ID. The second objective was to determine how canagliflozin affected body weight and triglyceride concentrations after short‐term administration.
2. MATERIALS AND METHODS
2.1. Study design
Ours was a single‐center, randomized, double‐blind, placebo‐controlled, parallel‐design study in horses and ponies (hereafter collectively referred to as horses) with ID. Privately‐owned horses with ID were randomized using a 1:1 allocation ratio and an online web‐based randomization service (www.randomizer.org) to receive either once daily PO canagliflozin (0.6 mg/kg) or placebo. The staff conducting the trial and performing the analyses, as well as the horse owners, were blinded to the randomization throughout the trial.
The study consisted of an initial 3‐day period for obtaining baseline data, a 3‐week double‐blind treatment period at home, and a 3‐day follow‐up period similar to the initial baseline period but with continued double‐blind treatment. The baseline and follow‐up periods were conducted at the Equine Clinic at the University Animal Hospital, Swedish University of Agricultural Sciences (SLU).
Horse owners were instructed to keep the feeding constant 1 month before and over the entire study period by daily weighing of the feed on a scale. They also were instructed to maintain the horse on the same batch of roughage at least 2 weeks before the study and over the entire study period. The horses' exercise as well as turnout time in a dirt or sand paddock also were kept constant. All horse owners provided written informed consent.
2.2. Enrollment criteria
Client‐owned horses and ponies previously diagnosed with ID, referred to the University Animal Hospital for further evaluation, were enrolled. The diagnosis of ID was based on blood samples obtained by referring veterinarians during an OST using Dan Sukker glucose syrup at a dosage of 0.2 mL/kg body weight. 12 Horses with insulin concentrations >100 μIU/mL at blood sampling 60 to 90 minutes after PO sugar administration were eligible to participate in the study. Insulin concentrations >45 μIU/mL at 60 to 90 minutes during the OST are considered diagnostic for ID (Clinical Pathology Laboratory, University Animal Hospital, SLU; Lindåse S et al. Evaluation of an Oral Sugar Test to Diagnose Insulin Dysregulation. ACVIM Forum Research Abstract Program 2018;2183). Insulin concentrations were analyzed at the University Animal Hospital, SLU, using an insulin ELISA 13 (Equine Insulin ELISA; Mercodia AB, Uppsala, Sweden). Horses were excluded from the study if they were <4 years of age, had an ongoing acute episode of laminitis based on clinical examination, or had clinical evidence of pituitary pars intermedia dysfunction including assessment of EDTA plasma adrenocorticotropic hormone (ACTH) concentrations adjusted for season. Additional exclusion criteria included treatment with drugs, access to grass pasture for at least 1 month before enrollment, and systemic disease other than ID. Mares were excluded if they were pregnant or lactating. A flow diagram of patient enrollment is shown in Figure 1.
FIGURE 1.
Enrollment and randomization flow diagram for the 3‐week study. ID, insulin dysregulation.
2.3. Blinding and medication
The original blister package of canagliflozin was removed, and tablets were transferred to a primary package consisting of a plastic container with a study label containing information for identification of horse and owner. Placebo tablets, identical to the active drug, were not available. Instead, placebo consisting of lactose was dispensed in a similar plastic container as the active drug. Lactose was chosen because it is a compound of the canagliflozin tablets. Because the active drug and placebo were different in appearance, the primary containers were packed and dispensed in a secondary box, identical for both treatments. The difference in appearance and which drug was tested in the study were not revealed to clients. One of the authors, who did not act as an investigator, responded to questions regarding the treatment, dispensed the drug and placebo, and provided medication to the hospital staff who administered the treatment when the horses were admitted the second time for follow‐up. Owners, investigators, and hospital staff were unaware of treatment assignments.
Canagliflozin (300 or 100 mg tablets; Invokana, Mundipharma AB, Gothenburg, Sweden) at a target dosage of 0.6 mg/kg q24h or placebo was administered PO at 9 pm. Tablet size was adjusted by cutting a piece from tablets using a scalpel blade and discarding the unneeded piece. The weight of 1 adjusted tablet was made as exact as possible using a scale, and this tablet was used as a model for the horse owners so they could adjust their tablets exactly as possible without using a scale. At the time of medication, the tablet was placed in a small piece of apple and given to the horse by hand. One dose of placebo per horse contained between 600 and 800 mg of lactose depending on the size of the horse. Placebo was given mixed with a small amount of Luzern pellets (≤1 dL) mixed with water to a porridge and fed in a large bowl, ensuring that everything was consumed each time. Feeding of the horse was not allowed until 1 hour later for both canagliflozin and placebo treatment. The daily amount of the study medication was adjusted for the 3‐day follow‐up period for horses that had gained or lost weight during the treatment period so as to keep the medication dose as accurate as possible.
2.4. Experimental protocol
The horses arrived at the University Animal Hospital, SLU, initially for a 3‐day visit to obtain baseline data and a second time for a 3‐day follow‐up with continued double‐blind treatment after 3 weeks of treatment at home. On both clinical visits, the horses were allowed to acclimatize for 2 days to the environment where the experiment was to take place. Horses were maintained on their regular diet and fed the daily amount divided into 4 meals. They had daily access to a sand paddock but were kept in a stall at night and during the experimental procedures. Double‐blind treatment was given during the second visit at 9 pm every day, 1 hour before the last feeding of the day.
The second day on both clinical visits, clinical examination was performed and body condition score (BCS) and cresty neck score (CNS) were obtained. In addition, an IV (Intranule, 2.0 × 105 mm, Vygon France or Milacath—extended use 1.7 × 75 mm, Mila, USA, depending on the size of the horse) was inserted into a jugular vein using local anesthesia (EMLA, AstraZeneca AB, Södertälje, Sweden). On both clinical visits, feed was withheld on the morning of the third day, the horses' body weights were measured using a calibrated scale, and the OST started at 7 am.
Baseline sampling for the OST was performed via the jugular catheter within 10 minutes before PO administration of 0.5 mL Dan Sukker glucose syrup/kg body weight (Dan Sukker Glykossirap, Nordic Sugar A/S, Copenhagen, Denmark). Blood samples were obtained via the jugular catheter at 15, 30, 60, 90, 120, 150, and 180 minutes after PO sugar administration. After conclusion of the OST, the jugular catheter was removed and the feeding protocol resumed. For baseline samples, blood was transferred to vacutainer tubes containing lithium heparin and to vacutainer tubes without additive. All other blood samples were transferred only to vacutainer tubes with lithium heparin. The lithium heparin samples were centrifuged within 5 minutes of collection, whereas the samples without additive were allowed to clot at room temperature before being centrifuged. Plasma or serum was harvested immediately after centrifuging and frozen at −80°C. To minimize intrasubject variability, all samples from each horse were analyzed together in a single batch. Plasma insulin concentrations were analyzed in duplicate using an equine‐optimized ELISA 13 (Equine Insulin ELISA; Mercodia AB, Uppsala, Sweden), and concentrations were verified using a control. Plasma glucose and serum triglyceride concentrations were analyzed using automated clinical chemistry analyzers (YSI 2500 glucose/lactate analyzer, 14 Yellow Springs Instruments, Yellow Springs, Ohio and Architect c4000, Abbott Diagnostics, Abbott Park, Illinois, respectively).
2.5. Outcomes
Primary endpoints were determined by an OST and included change from baseline to the end of treatment for the area under the plasma insulin vs time curve (insulin AUC0‐180) and the maximal insulin concentration (insulin C max). The secondary endpoints were the corresponding values for area under the plasma glucose vs time curve (glucose AUC0‐180) and the maximal glucose concentration (glucose C max). Additional secondary endpoints were the difference from baseline to the end of treatment for fasting plasma glucose and insulin concentrations, serum triglycerides, and body weight. Exploratory endpoints included change in β‐cell responsiveness to glucose from baseline to the end of treatment measured as the ratio of the incremental area under the curve for insulin and glucose (∆AUCinsulin/∆AUCglucose).
2.6. Statistical methods
A sample size of 8 horses per treatment group was estimated to provide >80% power to detect mean decreases of 55% for the primary outcomes insulin AUC0‐180 and insulin C max in the canagliflozin‐treated horses using a 2‐sided t‐test with alpha 0.05. Data for calculations of placebo horses were based on a previous publication. 5
The insulin AUC0‐180 and glucose AUC0‐180 were calculated using the trapezoidal rule. The ∆AUCinsulin and ∆AUCglucose for each OST were obtained by subtracting the AUC for the total response by the corresponding fasting baseline AUC, which was obtained by taking the fasting values for plasma insulin or glucose × 180 min. 15 The ratio of ∆AUCinsulin/∆AUCglucose was used as an index to reflect insulin response to the concomitant plasma glucose concentration over the entire OST (ie, an index of β‐cell responsiveness to glucose). 2 , 16 , 17 , 18
Continuous data for baseline characteristics were evaluated for normality using the Shapiro‐Wilk test. Data for baseline clinical characteristics were summarized and displayed as mean ± SD, median (interquartile range), or count (n). Comparisons between groups were made using 2‐sample t‐test (2‐tailed), Wilcoxon rank sum, or chi‐squared tests as appropriate. Simple linear regression was used to estimate the linear relationship between change in serum triglyceride concentrations and decrease (%) in body weight.
The primary, secondary, and exploratory endpoints were analyzed using analysis of covariance (ANCOVA), with treatment (placebo vs canagliflozin) as a fixed factor and the corresponding baseline as a continuous covariate to adjust for baseline values. 19 The full model included an interaction term between the covariate and the treatment. The interaction term was removed from the model if not significant, and data were analyzed using an ANCOVA model with equal slopes. If the interaction between the covariate and the treatment was significant, the interaction was included in the model, with the conclusion that nonparallel lines are necessary to adequately describe the data. Comparisons of the treatment effect between canaglifozin and placebo then were performed at 3 levels of the covariate (baseline data): at the mean − 1 SD, mean, and mean + 1 SD.
Residuals from the ANCOVA were evaluated for normal distribution and equal variance using residual quantile plots and residuals by predicted plots, respectively. Non‐normally distributed data or data with unequal variance were log‐transformed before analysis. Adjusted means for normally‐distributed data are reported as least squares (LS) means ± SE of the mean, whereas non‐normally distributed data are reported as geometric LS means and 95% confidence interval (CI) after back‐transformation to the original scale. The LS means differences between treatments are reported with 2‐sided 95% CIs, but non‐normally distributed data are reported as geometric LS means ratio (expressed as percentage) with 2‐sided 95% CIs after back transformation of the LS means difference. This ratio is based on the following relationship for log‐transformed data: where and are LS means for the treatments.
The response of plasma insulin or glucose over time after treatment was analyzed using a mixed model for repeated measures with treatment (placebo vs canagliflozin), sampling point (0‐180 minutes) and treatment by sampling point interaction as categorical fixed factors. Corresponding baseline values for the pre‐treatment insulin AUC0‐180 or glucose AUC0‐180 were used as continuous covariates, and horse was a random factor in the model. The initial model included several interaction terms with the covariate but because they were not significant, they were excluded from the model in a stepwise manner. Because time points were not equidistant, a spatial power covariate structure was used to model the within‐horse covariance over time. Data were log‐transformed before analysis to obtain equal variance and normal distribution of residuals. Results are presented as geometric LS means and 95% CI after back‐transformation to its original scale.
Comparison between time points or between treatments at different levels of the covariate were performed using the Tukey‐Kramer post hoc test. Values where P < .05 were considered significant. All statistical analyses were performed using JMP Pro version 16.0.0 (SAS Institute Inc, Cary, North Carolina).
3. RESULTS
3.1. Study subjects
Of the 16 horses randomized to treatment, all animals completed the study (canagliflozin [n = 8] and placebo [n = 8]; Figure 1). The study included British native pony breeds (n = 6), crossbred ponies (n = 3), Icelandic horses (n = 3), Swedish native pony breeds (n = 2), and warmblood breeds (n = 2).
Baseline clinical characteristics of both groups are described in Table 1. Age, sex, weight, BCS, CNS, fasting plasma insulin, fasting plasma glucose, and frequency of previous episodes of laminitis were well‐matched between the 2 groups (Table 1).
TABLE 1.
Baseline clinical characteristics of participant insulin dysregulated horses.
| Variable | Placebo | Canagliflozin | P‐value |
|---|---|---|---|
| (n = 8) | (n = 8) | ||
| Age (years) | 16.0 ± 4.8 | 11.1 ± 5.6 | .08 |
| Sex (mare/gelding) | 4/4 | 5/3 | .6 |
| Weight (kg) | 282 ± 95 | 353 ± 146 | .3 |
| BCS | 6.75 (1.875) | 7.5 (1.375) | .5 |
| CNS | 3.5 (1.375) | 4 (1.25) | .8 |
| Fasting plasma insulin (μIU/mL) | 18.8 ± 12.3 | 32.1 ± 14.6 | .07 |
| Fasting plasma glucose (mmol/L) | 5.1 ± 0.4 | 5.2 ± 0.4 | .7 |
| Previous laminitis (yes/no) | 7/1 | 8/0 | .3 |
Note: Values are mean ± SD, median (interquartile range), or n/n (count number).
Abbreviations: BCS, body condition score; CNS, cresty neck score.
3.2. Fasting insulin and postprandial insulin responses
The ANCOVA for fasting insulin concentrations demonstrated covariate by treatment interaction (P < .001), and comparisons of adjusted treatment means therefore were made along the regression lines at 3 different values of the covariate (Table 2). No difference was observed in post‐treatment fasting insulin concentrations between placebo and canagliflozin‐treated horses in the lower range of insulin concentrations (P = .2). With increasing pre‐treatment fasting insulin concentrations, the difference in fasting insulin concentrations between placebo and canagliflozin‐treated horses increased (P ≤ .02; Table 2).
TABLE 2.
Comparison of primary, secondary, and exploratory endpoints between placebo and canagliflozin treated insulin dysregulated horses.
| Parameter | Placebo (n = 8) | Canagliflozin (n = 8) | Least square mean difference canagliflozin vs placebo | Geometric least square mean ratio canagliflozin/placebo (%) | P‐value |
|---|---|---|---|---|---|
| Primary endpoint | |||||
| Insulin AUC0‐180 (μIU/mL × min) | 24 832 (17 817‐34 610) | 8308 (5961‐11 579) | n/a | 33.5 (20.9‐53.6) | <.001 |
| Insulin C max (μIU/mL) | 215.2 (143.2‐323.2) | 83.2 (55.4‐125.0) | n/a | 38.7 (21.7‐69.0) | .004 |
| Secondary endpoint | |||||
| Fasting insulin (μIU/mL) | n/a | ||||
| Covariate at 10.7 (μIU/mL) | 9.0 ± 1.8 | 13.1 ± 2.7 | 4.1 (−3.1 to 11.2) | .8 | |
| Covariate at 25.4 (μIU/mL) | 22.1 ± 1.7 | 13.1 ± 1.6 | −9.0 (−14.2 to −3.9) | .02 | |
| Covariate at 40.1 (μIU/mL) | 35.2 ± 3.1 | 13.1 ± 1.7 | −22.1 (−29.8 to −14.3) | <.001 | |
| Fasting plasma glucose (mmol/L) | 4.9 ± 0.1 | 4.7 ± 0.1 | −0.2 (−0.5 to 0.04) | n/a | .09 |
| Glucose C max (mmol/L) | 8.4 ± 0.3 | 7.0 ± 0.3 | −1.4 (−2.5 to −0.4) | n/a | .01 |
| Glucose AUC0‐180 (mmol/L × min) | 1281 (1190‐1380) | 1094 (1016‐1178) | n/a | 85.4 (76.6‐95.2) | .01 |
| Body weight (kg) | 319 ± 2.2 | 308 ± 2.2 | −11.1 (−18.1 to −4.0) | .01 | |
| Triglycerides (mmol/L) | 0.48 ± 0.17 | 1.47 ± 0.17 | 0.99 (0.47 to 1.50) | .001 | |
| Exploratory endpoint | |||||
| ∆AUCinsulin/∆AUCglucose (mIU/mmol) | 58.1 (43.2‐78.1) | 25.1 (18.7‐33.8) | n/a | 43.2 (28.3‐66.1) | <.001 |
Note: Adjusted means for baseline values are presented as least square mean ± SE of the mean or geometric least square means with 95% confidence interval. Least square mean difference or geometric least square mean ratio for canagliflozin vs placebo are reported with 95% confidence intervals.
Abbreviations: ∆AUCinsulin/∆AUCglucose, ratio of incremental area under the curve for insulin and incremental area under the curve for glucose; glucose AUC0‐180, area under the glucose vs time curve at 0‐180 min; Glucose C max, maximal glucose concentration; insulin AUC0‐180, area under the plasma insulin vs time curve at 0 to 180 minutes; insulin C max, maximal insulin concentration; n/a, not applicable.
After PO administration of glucose syrup, the postprandial insulin response over time was decreased with canagliflozin treatment compared to placebo (main effect of treatment, P < .008; Figure 2A). Maximal insulin concentration (insulin C max) and insulin AUC0‐180 significantly decreased at 3 weeks with canagliflozin treatment (Table 2). The geometric LS mean insulin response (AUC0‐180) for canagliflozin‐treated horses was 33.5% (95% CI, 20.9‐53.6) of the geometric LS mean insulin response (AUC0‐180) for the placebo‐treated horses (ie, 66.5% decrease in insulin response with canagliflozin treatment). Four of 8 horses treated with canagliflozin had Insulin C max <65 μIU/mL, the diagnostic cut‐off for ID for the test (higher dose of Dan Sukker glucose syrup [0.5 mL/kg]; Clinical Pathology Laboratory, University Animal Hospital, SLU), whereas all placebo horses had Insulin C max >65 μIU/mL.
FIGURE 2.
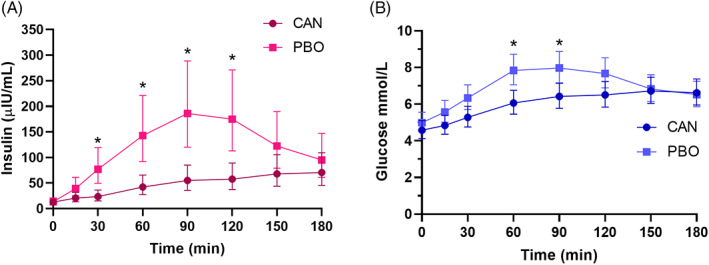
Geometric least square mean and 95% confidence interval concentration‐time profiles from before to 180 minutes after a 0.5 mL/kg dose of oral glucose syrup (Dan Sukker) for plasma insulin (A) and plasma glucose (B). CAN, canagliflozin; PBO, placebo. *Significantly different within a time point (P < .03).
3.3. Fasting glucose and postprandial glucose responses
No significant difference (P = .09) was observed in LS means fasting plasma glucose concentrations between canagliflozin (4.7 ± 0.1 mmol/L) and placebo‐treated horses (4.9 ± 0.1 mmol/L; Table 2). Horses that received canagliflozin achieved significantly (P < .02) lower glucose concentrations at 60 and 90 minutes after PO administration of glucose syrup compared with placebo (Figure 2B). Treatment with canagliflozin resulted in average 1.4 (95% CI, 0.4‐2.5) mmol/L lower glucose C max compared with placebo (P = .01). Glucose AUC0‐180 significantly decreased at 3 weeks with canagliflozin treatment (Table 2). The geometric LS mean glucose response (AUC0‐180) for canagliflozin‐treated horses was 85.4% (95% CI, 76.6‐95.2) of the geometric LS mean glucose response (AUC0‐180) for the placebo‐treated horses (P = .008; ie, 14.6% decrease in glucose response with canagliflozin treatment).
3.4. Body weight and triglyceride concentrations
Treatment with canagliflozin resulted in significantly (P = .01) higher adjusted mean decreases (mean, 11.1 kg; 95% CI, 4.0‐18.1 kg) in body weight from baseline to 3 weeks compared with placebo (Table 2). Canagliflozin increased serum triglyceride concentrations compared with placebo (Table 2) with adjusted mean difference of 0.99 (95% CI, 0.47‐1.50) mmol/L (P = .001). Post‐treatment, 2 of 8 placebo‐treated horses and all canagliflozin‐treated horses had serum triglyceride concentrations >0.51 mmol/L, the laboratory cutoff for increased triglyceride concentrations (Clinical Pathology Laboratory, University Animal Hospital, SLU). The increase in triglyceride concentrations was not accompanied by any clinical signs, such as altered attitude or decreased appetite. In fact, all horses continued to have normal clinical examinations. No significant associations were found between percentage decrease in body weight and change in serum triglyceride concentrations after 3 weeks of treatment assessed by simple linear regression in the group of canagliflozin (R 2 = .48; P = .06) and placebo (R 2 = .01; P = .8) treated horses.
3.5. β‐cell glucose sensitivity
Treatment with canagliflozin decreased the ∆AUCinsulin/∆AUCglucose ratio compared with placebo (P < .001; Table 2). The geometric LS mean ∆AUCinsulin/∆AUCglucose ratio for canagliflozin‐treated horses was 43.2% (95% CI, 28.3‐66.1) of the geometric LS mean ∆AUCinsulin/∆AUCglucose ratio for the placebo‐treated horses (P < .001; ie, 56.8% decrease in β‐cell responsiveness to glucose [∆AUCinsulin/∆AUCglucose ratio] with canagliflozin treatment).
4. DISCUSSION
Our randomized, placebo‐controlled, double‐blind study together with a previous prospective study (Frank N. Safety and Efficacy of Canagliflozin and Octreotide for Managing Insulin Dysregulation in Horses. In 2018 ACVIM Research Report Program 2018;2123‐2143) represents the first evidence for the efficacy of canagliflozin in decreasing the hyperinsulinemic response to PO glucose in ID horses. Treatment was well tolerated, and no clinical adverse effects were identified over the 3‐week treatment period. Our results showed that treatment with canagliflozin decreased the postprandial insulin response by 2 major mechanisms: decreased glycemic response and decreased β‐cell responsiveness to PO glucose. This resulted in a very efficient treatment effect, where canagliflozin on average decreased the hyperinsulinemic response by >66% compared with placebo in response to an OST. The use of SGLT2 inhibitors in clinical trials with ID horses has been reported previously, but those studies used velagliflozin, which is not yet a registered drug on the market. 10 , 11 In addition, some case series reported efficacy using canagliflozin or ertugliflozin in decreasing hyperinsulinemia in ID horses, but these reports were not prospective controlled clinical trials. 20 , 21 Currently, the SGLT2 inhibitors appear to be the most efficient pharmacological treatment in decreasing the insulin response to PO sugars in ID horses.
In agreement with previous studies using SGLT2 inhibitors in horses with ID, 10 , 11 we observed a significant decrease in the postprandial insulin response after treatment with canagliflozin. Despite decreases in insulin responses, only 50% of the treated horses had normal insulin concentrations after the OST. Our study included horses with moderate and marked ID, and it was therefore not expected that all horses treated with canagliflozin would have normal results on an OST. The horses in our study were evaluated between 10 and 13 hours after drug administration, which is approximately halfway between 2 treatments. Because the pharmacology and pharmacodynamics of canagliflozin in horses are unknown, information about the optimal dosage and treatment interval for this drug in this species is lacking. The dose and treatment interval in our study was based on a pilot study as well as on clinical experience of the research group using canagliflozin as treatment for ID.
Human patients with T2DM have high fasting plasma glucose concentrations. The glucose load filtered by the glomeruli during fasting therefore is increased compared with non‐diabetic individuals. 22 , 23 At the same time, diabetic patients have an increased tubular glucose transport maximum 22 as a result of upregulation of SGLT2 in the kidney. 24 Treatment with SGLT2 inhibitors in humans with T2DM will cause a non‐insulin dependent decrease in fasting plasma glucose concentration by a dose‐dependent increase in urinary glucose excretion. 25 In contrast, there is lack of significant treatment effect of SGLT2 inhibitors on fasting plasma glucose concentration in normal animals of different species. 22 , 26 This finding has been attributed to a counter‐regulatory mechanism that increases endogenous glucose production to exactly compensate for increased urinary loss. In addition, the urinary loss of glucose is lower during euglycemia in humans treated with SGLT2 inhibitors because lower filtered glucose load. 27 Horses with ID have been shown to have euglycemia with equivalent 12 or somewhat higher 28 fasting plasma glucose concentrations compared with horses with normal insulin regulation. The risk of hypoglycemia in ID horses treated with SGLT2 inhibitors thus likely is low. In ponies treated with velagliflozin, no evidence of hypoglycemia was observed after overnight fasting. 10 , 11 This observation is in agreement with our results, where no evidence of hypoglycemia was found in samples obtained after withholding feed overnight. In our study, fasting plasma glucose concentrations were similar between placebo and canagliflozin‐treated horses.
Compared with fasting situations, the postprandial state results in more glucose filtered through the kidney, which increases urinary glucose excretion and thereby decreases postprandial hyperglycemia in SGLT2‐treated human patients with T2DM. 25 This outcome is in agreement with our study where canagliflozin caused a decrease in glucose C max as well as in glucose AUC0‐180 compared with placebo. In comparison, treatment with velagliflozin in ID ponies has shown inconsistent results for decreasing the postprandial glucose response. 10 , 11
Canagliflozin decreased the insulin response to a greater extent compared with the glucose response. Thus, the high treatment effect of decreasing the insulin response may not be attributed solely to decrease in the glycemic response. This conclusion was supported by a mean decrease of 56.8% of the ∆AUCinsulin/∆AUCglucose ratio in canagliflozin compared with placebo‐treated horses. The index reflects the insulin response to the concomitant plasma glucose concentrations during the entire OST. 16 , 17 , 18 The ∆AUCinsulin/∆AUCglucose ratio index is increased in ID horses compared with horses with normal insulin regulation, suggesting that horses with ID have increased β‐cell responsiveness to PO glucose. 2 Treatment with canagliflozin in ID horses in our study decreased β‐cell responsiveness to PO glucose. In contrast, humans with T2DM have lower ∆AUCinsulin/∆AUCglucose ratios after PO glucose tolerance tests compared with healthy humans as a result of decreased β‐cell function. 17 Treatment with SGLT2 inhibitors in humans with T2DM leads to improved β‐cell function. 29 This effect has been attributed to a secondary effect of decreased plasma glucose concentration and amelioration of glucotoxicity, and not a direct effect on the β‐cell. 30 Interestingly, treatment with SGLT2 inhibitors in ID horses and humans with T2DM affects β‐cell responsiveness to glucose in opposite directions. However, it must be recognized that the ∆AUCinsulin/∆AUCglucose ratio is an estimate of β‐cell responsiveness to PO glucose and not a true measurement of the slope of a dose‐response relationship between insulin secretion and glucose concentrations. We did not measure C‐peptide in our study, and therefore we are not able to assess if the decrease in ∆AUCinsulin/∆AUCglucose ratio after canagliflozin treatment to some extent is explained by an increased clearance rate of insulin. Other explanations for the lower ∆AUCinsulin/∆AUCglucose ratios after canagliflozin treatment might be improved insulin sensitivity and thereby decreased β‐cell responsiveness because of the feedback loop that exists between insulin sensitivity and β‐cell function. 2 It is possible that the decreased glycemic response itself in canagliflozin‐treated horses had a positive impact on the β‐cell responsiveness to glucose, which is in alignment with the hypothesis that hyperglycemia (and secondary hyperinsulinemia) is a stimulus for progression of ID in horses. 31 Future studies should investigate in more detail the mechanism by which canagliflozin changes β‐cell responsiveness to glucose, because it may be a very important mechanism for decreasing the insulin response in canagliflozin‐treated ID horses.
It is well established that human patients receiving SGLT2 inhibitors experience gradual weight loss over time. The weight loss is likely secondary to increased glucose excretion, resulting in caloric loss. 23 , 32 In agreement with these studies, the canagliflozin‐treated horses in our study experienced weight loss over the treatment period compared to those treated with placebo. Unlike our study, weight loss was not evident in 2 other studies evaluating the treatment effect of velagliflozin in ID ponies. 10 , 11 The different findings between these studies likely are caused by different feeding regimens. In our study, the horses were fed roughage (hay or haylage) with low NSC content as part of the owners feeding strategy to keep the postprandial glycemic and insulin responses low, whereas the 2 studies with velagliflozin used a diet with more NSC and energy than recommended for ID horses. 10 , 11 Taken together, these studies suggest that adjusting the diet in ID horses treated with SGLT2 inhibitors might be necessary to control the rate of weight loss or maintain the body weight as appropriate. Although our study did not assess caloric intake over the study period, the horse owners were instructed to cautiously maintain feeding and training as constant as possible for 1 month before and during the treatment period.
In agreement with a previous study, where the weight of the ponies remained constant during treatment with SGLT2 inhibitors, 11 treatment with canagliflozin in our study caused an increase in serum triglyceride concentrations despite normal findings on physical examinations of the horses. Despite the weight loss in the canagliflozin‐treated horses in our study, no association was found between the increase in triglycerides and the magnitude of weight loss. The SGLT2 inhibitors thus appear to facilitate an increase in serum triglyceride concentrations in ID horses both in stages of normal and negative energy balance. This possibility requires additional studies, because triglycerides already may be increased in horses with ID. 4 In addition, the safety of long‐term use of SGLT2 inhibitors in equids is unknown at this time.
Our study had some limitations. It was a small, single‐center study with a rather short treatment period in light of potential life‐long treatment for some ID horses. In addition, it would have been valuable to measure urinary glucose output as well as urinalyses for identification of possible urinary tract infections. Because we did not assess the postprandial insulin response after a meal of the horses' regular roughage diet, we are unable to evaluate the efficacy of canagliflozin in decreasing the postprandial insulin response to normal low‐NSC feeding. Currently, no studies have evaluated the effect of SGLT‐2 inhibitors on the postprandial insulin response after a meal of roughage with <10% NSC content.
Taken together, these data indicate that treatment with canagliflozin efficiently decreases the postprandial insulin response after an OST in ID horses. The amelioration in insulin response was achieved by a combination of decreased postprandial glucose response and, perhaps most importantly, as a result of decreased β‐cell responsiveness to glucose. Canagliflozin thus is a promising drug for treatment of ID in horses that requires future study.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approved by the Ethical Committee for Animal Experiments, Uppsala, Sweden (Dnr: 5.8.18‐09082‐2020).
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENT
Funding provided by the Swedish‐Norwegian Foundation for Equine Research.
Lindåse S, Nostell K, Forslund A, Bergsten P, Bröjer J. Short‐term effects of canagliflozin on glucose and insulin responses in insulin dysregulated horses: A randomized, placebo‐controlled, double‐blind, study. J Vet Intern Med. 2023;37(6):2520‐2528. doi: 10.1111/jvim.16906
REFERENCES
- 1. Carslake H, Argo CM, Pinchbeck G, et al. Insulinaemic and glycaemic responses to three forages in ponies. Vet J. 2018;235:83‐89. [DOI] [PubMed] [Google Scholar]
- 2. Lindåse S, Nostell K, Söder J, Bröjer J. Relationship between β‐cell response and insulin sensitivity in horses based on the oral sugar test and the euglycemic hyperinsulinemic clamp. J Vet Intern Med. 2017;31:1541‐1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Patterson‐Kane JC, Karikoski NP, McGowan CM. Paradigm shifts in understanding equine laminitis. Vet J. 2018;231:33‐40. [DOI] [PubMed] [Google Scholar]
- 4. Durham AE, Frank N, McGowan CM, et al. ECEIM consensus statement on equine metabolic syndrome. J Vet Intern Med. 2019;33:335‐349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Legere RM, Taylor DR, Davis JL, et al. Pharmacodynamic effects of pioglitazone on high molecular weight adiponectin concentrations and insulin response after oral sugar in equids. J Equine Vet. 2019;82:102797. [DOI] [PubMed] [Google Scholar]
- 6. Tinworth KD, Boston RC, Harris PA, Sillence MN, Raidal SL, Noble GK. The effect of oral metformin on insulin sensitivity in insulin‐resistant ponies. Vet J. 2012;191:79‐84. [DOI] [PubMed] [Google Scholar]
- 7. Colmer SF, Adams AA, Adam E, et al. The effect of pre‐dosing with metformin on the insulin response to oral sugar in insulin‐dysregulated horses. Equine Vet J. 2023; doi: 10.1111/evj.13979. [DOI] [PubMed] [Google Scholar]
- 8. Deeks ED, Scheen AJ. Canagliflozin: a review in type 2 diabetes. Drugs. 2017;77:1577‐1592. [DOI] [PubMed] [Google Scholar]
- 9. Martinez R, Al‐Jobori H, Ali AM, et al. Endogenous glucose production and hormonal changes in response to Canagliflozin and Liraglutide combination therapy. Diabetes. 2018;67:1182‐1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Meier A, Reiche D, de Laat M, Pollitt C, Walsh D, Sillence M. The sodium‐glucose co‐transporter 2 inhibitor velagliflozin reduces hyperinsulinemia and prevents laminitis in insulin‐dysregulated ponies. PloS One. 2018;13:e0203655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Meier A, de Laat M, Reiche D, Fitzgerald D, Sillence M. The efficacy and safety of velagliflozin over 16 weeks as a treatment for insulin dysregulation in ponies. BMC Vet Res. 2019;15:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lindåse S, Nostell K, Bröjer J. A modified oral sugar test for evaluation of insulin and glucose dynamics in horses. Acta Vet Scand. 2016;58(Suppl 1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Öberg J, Bröjer J, Wattle O, Lilliehöök I. Evaluation of an equine‐optimized enzyme‐linked immunosorbent assay for serum insulin measurement and stability study of equine serum insulin. Comp Clin Path. 2012;21:1291‐1300. [Google Scholar]
- 14. Slough T, Gunkel C, Murray L, et al. A comparison of methodologies for measuring glucose concentrations in the horse. Prof Anim Sci. 2011;27:204‐214. [Google Scholar]
- 15. Allison DB, Paultre F, Maggio C, Mezzitis N, Pi‐Sunyer FX. The use of areas under curves in diabetes research. Diabetes Care. 1995;18:245‐250. [DOI] [PubMed] [Google Scholar]
- 16. Hannon TS, Kahn SE, Utzschneider KM, et al. Review of methods for measuring β‐cell function: design considerations from the restoring insulin secretion (RISE) consortium. Diabetes Obes Metab. 2018;20:14‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Utzschneider KM, Prigeon RL, Faulenbach MV, et al. Oral disposition index predicts the development of future diabetes above and beyond fasting and 2‐h glucose levels. Diabetes Care. 2009;32:335‐341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Utzschneider KM, Younes N, Rasouli N, et al. Shape of the OGTT glucose response curve: relationship with β‐cell function and differences by sex, race, and BMI in adults with early type 2 diabetes treated with metformin. BMJ Open Diabetes Res Care. 2021;9:e002264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vickers AJ, Altman DG. Analysing controlled trials with baseline and follow up measurements. BMJ. 2001;323:1123‐1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kellon EM, Gustafson KM. Use of the SGLT2 inhibitor canagliflozin for control of refractory equine hyperinsulinemia and laminitis. Open Vet J. 2022;12:511‐518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sundra T, Kelty E, Rendle D. Preliminary observations on the use of ertugliflozin in the management of hyperinsulinaemia and laminitis in 51 horses: a case series. Equine Vet Educ. 2022;35:311‐320. [Google Scholar]
- 22. Abdul‐Ghani MA, Norton L, Defronzo RA. Role of sodium‐glucose cotransporter 2 (SGLT 2) inhibitors in the treatment of type 2 diabetes. Endocr Rev. 2011;32:515‐531. [DOI] [PubMed] [Google Scholar]
- 23. Nauck MA. Update on developments with SGLT2 inhibitors in the management of type 2 diabetes. Drug des Devel Ther. 2014;8:1335‐1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rahmoune H, Thompson PW, Ward JM, Smith CD, Hong G, Brown J. Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non‐insulin‐dependent diabetes. Diabetes. 2005;54:3427‐3434. [DOI] [PubMed] [Google Scholar]
- 25. Komoroski B, Vachharajani N, Feng Y, Li L, Kornhauser D, Pfister M. Dapagliflozin, a novel, selective SGLT2 inhibitor, improved glycemic control over 2 weeks in patients with type 2 diabetes mellitus. Clin Pharmacol Ther. 2009;85:513‐519. [DOI] [PubMed] [Google Scholar]
- 26. Hoenig M, Clark M, Schaeffer DJ, Reiche D. Effects of the sodium‐glucose cotransporter 2 (SGLT2) inhibitor velagliflozin, a new drug with therapeutic potential to treat diabetes in cats. J Vet Pharmacol Ther. 2018;41:266‐273. [DOI] [PubMed] [Google Scholar]
- 27. List JF, Whaley JM. Glucose dynamics and mechanistic implications of SGLT2 inhibitors in animals and humans. Kidney Int Suppl. 2011;2011:S20‐S27. [DOI] [PubMed] [Google Scholar]
- 28. Nostell K, Lindåse S, Edberg H, Bröjer J. The effect of insulin infusion on heart rate and systemic blood pressure in horses with equine metabolic syndrome. Equine Vet J. 2019;51:733‐737. [DOI] [PubMed] [Google Scholar]
- 29. Al Jobori H, Daniele G, Adams J, et al. Empagliflozin treatment is associated with improved β‐cell function in type 2 diabetes mellitus. J Clin Endocrinol Metab. 2018;103:1402‐1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shyr ZA, Yan Z, Ustione A, Egan EM, Remedi MS. SGLT2 inhibitors therapy protects glucotoxicity‐induced β‐cell failure in a mouse model of human KATP‐induced diabetes through mitigation of oxidative and ER stress. PloS One. 2022;17:e0258054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. de Laat MA, Fitzgerald DM. Equine metabolic syndrome: role of the enteroinsular axis in the insulin response to oral carbohydrate. Vet J. 2023;294:105967. [DOI] [PubMed] [Google Scholar]
- 32. Pereira MJ, Lundkvist P, Kamble PG, et al. A randomized controlled trial of dapagliflozin plus once‐weekly exenatide versus placebo in individuals with obesity and without diabetes: metabolic effects and markers associated with bodyweight loss. Diabetes Ther. 2018;9:1511‐1532. [DOI] [PMC free article] [PubMed] [Google Scholar]


