Abstract
Background
The role of diet in the pathogenesis and treatment of chronic enteropathies (CE) in dogs is unresolved.
Objectives
To compare the ability of diets composed of hydrolyzed fish, rice starch, and fish oil without (HF) or with prebiotics, turmeric, and high cobalamin (HF+) against a limited ingredient diet containing mixed nonhydrolyzed antigens and oils (control) to resolve clinical signs and maintain serum cobalamin and folate concentrations in dogs with nonprotein losing CE (non‐PLE). To determine the ability of hydrolyzed fish diets to support recovery and remission in dogs with PLE.
Animals
Thirty‐one client‐owned dogs with CE: 23 non‐PLE, 8 PLE.
Methods
Randomized, blinded, controlled trial. Diets were fed for 2 weeks; responders continued for 12 weeks. Nonresponders were crossed over to another diet for 12 weeks. Response was determined by standardized clinical evaluation with long‐term follow‐up at 26 weeks. Concurrent medications were allowed in PLE.
Results
Nineteen of 23 (83%; 95% confidence interval [CI], 60%‐94%) non‐PLE CE responded clinically to their initial diet, with no difference between diets (P > .05). Four nonresponders responded to another diet, with sustained remission of 18/18 (100%; 95%CI, 78%‐100%) at 26 weeks. Serum cobalamin concentration was increased (P < .05) and maintained by diet. Serum folate concentration decreased posttreatment (P < .05) but was restored by dietary supplementation. Hydrolyzed fish diets supported weight gain, serum albumin concentration, and recovery (P < .05) in dogs with PLE.
Conclusions and Clinical Importance
Changing diet, independent of antigen restriction or supplemental ingredients, induced long‐term remission in dogs with non‐PLE CE. Serum cobalamin and folate concentrations were maintained by diet. Hydrolyzed fish diets supported clinical recovery and remission in PLE.
Keywords: cobalamin, curcumin, folate, inflammatory bowel disease, lymphangiectasia, prebiotics, protein‐losing enteropathy
Abbreviations
- CCECAI
canine chronic enteropathy clinical activity index
- CE
chronic enteropathy
- DM
dry matter
- GSD
German Shepherd dog
- HF
hydrolyzed fish
- HF+
hydrolyzed fish plus prebiotics, curcumin, and cobalamin
- MCT
medium chain triglycerides
- PLE
protein‐losing enteropathy
1. INTRODUCTION
The ability of diet to ameliorate signs of gastrointestinal disease in dogs that lack extra‐intestinal causes for their clinical signs (chronic enteropathy, CE) is well established. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 As monotherapy, diets restricted to antigens considered novel to the patient, or containing hydrolyzed proteins, induce remission in 60% to 88% of dogs with CE and normal serum albumin concentration. 1 , 3 , 4 , 5 Because many dogs do not relapse when challenged with dietary antigens suspected of inducing immune hypersensitivity, it is possible the response is related to restricting ingredients rather than immunogenic antigens. 1 , 3 , 4 , 5 The role of diet in dogs with protein‐losing enteropathies (PLE) is less clear because the prognosis is poor and they often receive concurrent medications or refuse to eat therapeutic diets. 3 , 4 , 5 , 7 , 10 , 12 , 16 , 18 As monotherapy, an antigen‐restricted diet that induced remission in non‐PLE was ineffective in PLE. 1 Diets that are limited in fat (<2.6 g fat/100 kcal), notably ultra‐low fat (0.31 g/100 kcal) antigen‐restricted diets, can induce remission in dogs with PLE attributed to lymphangiectasia and a subset refractory to immunosuppression. 3 , 4 , 7 , 12 , 13 , 19 , 20 However, dogs that respond to ultra‐low fat diets must transition to a nutritionally balanced diet with higher fat content, and may require concurrent medications to maintain remission. 12 , 19 The ability of diets containing hydrolyzed proteins and moderate amounts of fat to induce remission and support clinical recovery of dogs with PLE is unclear. The nutritional impact of CE is compounded by malassimilation of vitamins B12 (cobalamin), B9 (folate), and D. 21 , 22 , 23 , 24 , 25 Hypocobalaminemia is common, 1 , 6 , 23 , 24 , 25 , 26 may increase risk of death and induce metabolic derangements that overlap with folate metabolism. 1 , 6 , 24 , 27 The correlation of serum cobalamin and folate concentrations with dietary concentrations, 28 along with the ability of PO supplements to increase serum concentrations in dogs, 25 , 26 , 29 suggest dietary incorporation as a therapeutic approach. Advances in our understanding of the interplay among diet, the microbiome, and the host have identified additional micronutrients, such as “prebiotics” (eg, inulin, fructooligosaccharides [FOS], mannan oligosaccharides [MOS]) that promote microbial short chain fatty acid synthesis 30 , 31 and plant‐derived phytochemicals (eg, curcumin), that can ameliorate intestinal inflammation and promote barrier function. 30 , 32 The ability of these micronutrients to enhance dietary responses in CE has not been established.
Considering this background, we undertook a prospective, randomized, blinded, controlled clinical trial to (a) compare the ability of isocaloric therapeutic diets composed of hydrolyzed fish, rice starch, and fish oil (HF), or HF plus prebiotics, turmeric, and high vitamin B12 (HF+), and a limited ingredient maintenance diet containing a mixture of intact chicken and fish, corn, rice, chicken fat and fish oil (control) to resolve clinical signs and maintain serum cobalamin and folate concentrations in dogs with nonprotein losing CE, and (b) to determine if hydrolyzed fish diets support clinical recovery and remission in dogs with PLE.
2. MATERIALS AND METHODS
2.1. Patients
Client‐owned dogs diagnosed with chronic enteropathy (CE) at the Cornell University Hospital for Animals and referral base were eligible for this trial. The trial was approved by IACAUC: # 2017‐0129. Chronic enteropathy was defined by the presence of clinical signs of gastrointestinal disease (eg, vomiting, diarrhea, weight loss) for >3 weeks that were localized to the gastrointestinal tract by exclusion of extra‐intestinal, parasitic, infectious, or neoplastic causes. Diagnostic evaluation consisted of history, physical examination, CBC, biochemistry profile, baseline serum cortisol concentration, urinalysis, fecal examination, and serum cobalamin, and folate concentrations. Abdominal ultrasonography, endoscopy, intestinal biopsy, and measurement of trypsin‐like immunoreactivity were performed at the discretion of the attending clinician. Dogs with concurrent diseases in other organ systems were excluded. Chronic enteropathy was subcategorized by serum albumin concentrations as protein‐losing enteropathy (PLE) and nonprotein‐losing enteropathy (non‐PLE). In the non‐PLE group, dogs treated with antimicrobials, anti‐inflammatory immunomodulatory drugs, or both 3 weeks before presentation were excluded. Concurrent medications were allowed in the PLE group.
2.2. Diets
We formulated 2 therapeutic diets (HF, HF+) and a limited ingredient maintenance diet (control) that were isocaloric (calories, calculated: metabolizable energy [ME] 3778 kcal/kg, 15.82 megajoules [MJ]/kg; 397 kcal/cup, 1 cup = 105 g) and balanced (g/100 kcal) in protein (4.7), fat (3.97), crude fiber (0.32), ash (1.4), and nitrogen free extract (14.2). Therapeutic diets were composed of hydrolyzed fish, rice starch, and fish oil (HF), or HF plus prebiotics (inulin, 0.6%; FOS, 0.4%; MOS, 0.4%), turmeric (>3% curcumin), 33 mg/kg, and high vitamin B12 (10 mg/kg cyanocobalamin: HF+). The control diet contained a mixture of intact chicken and fish, corn, rice, chicken fat, and fish oil (Table 1). Cyanocobalamin was included in the diets at 0.04 mg/kg diet (control), 0.06 mg/kg diet (HF), and 10 mg/kg (HF+). Folic Acid was included in the diets at 0.3 mg/kg (control) and 0.45 mg/kg (HF, HF+; Table 1).
TABLE 1.
Composition of test diets calories (calculated): ME 3778 kcal/kg‐15.82 MJ/kg; 397 kcal/cup, 1 cup = 105 g; AS FED (g/100 kcal): protein 4.76, fat 3.97, fiber 0.32, ash 1.4, and NFE 14.16.
| Diet | Composition | Vitamins, prebiotics, and other | Protein | Fat | Carbohydrate |
|---|---|---|---|---|---|
| HF | Rice starch, hydrolyzed white fish, herring oil, powdered cellulose, potassium chloride, calcium carbonate, monocalcium phosphate, salt, vitamin A supplement, vitamin D3 supplement, vitamin E supplement, ascorbic acid, niacin, calcium pantothenate, riboflavin, pyridoxine hydrochloride, thiamine hydrochloride, biotin, folic acid, vitamin B12 supplement, choline chloride, beta‐carotene, zinc methionine hydroxy analogue chelate, manganese methionine hydroxy analogue chelate, ferrous glycine, copper methionine hydroxy analogue chelate, selenium yeast, calcium iodate, DL‐Methionine, taurine, mixed tocopherols (a preservative). | Cyanocobalamin 0.06 mg/kg; Folic acid 0.45 mg/kg | Hydrolyzed fish | Fish oil | Rice starch |
| HF+ | Rice starch, hydrolyzed white fish, herring oil, powdered cellulose, potassium chloride, inulin, fructooligosaccharide, yeast extract, calcium carbonate, monocalcium phosphate, salt, turmeric, vitamin A supplement, vitamin D3 supplement, vitamin E supplement, ascorbic acid, niacin, calcium pantothenate, riboflavin, pyridoxine hydrochloride, thiamine hydrochloride, biotin, folic acid, vitamin B12 supplement, choline chloride, beta‐carotene, zinc methionine hydroxy analogue chelate, manganese methionine hydroxy analogue chelate, ferrous glycine, copper methionine hydroxy analogue chelate, selenium yeast, calcium iodate, DL‐Methionine, taurine, mixed tocopherols (a preservative). | Cyanocobalamin 10 mg/kg; Folic Acid 0.45 mg/kg; Prebiotics (inulin 0.6%, FOS 0.4%, MOS 0.4%); Turmeric 33 mg/kg | Hydrolyzed fish | Fish oil | Rice starch |
| Control | Whole corn, rice, dehydrated chicken, chicken fat, dehydrated fish, fish oil, dried beet pulp, salt, brewers dried yeast, vitamin A supplement, vitamin D3 supplement, vitamin E supplement, ascorbic acid, niacin, calcium pantothenate, riboflavin, pyridoxine hydrochloride, thiamine hydrochloride, biotin, folic acid, vitamin B12 supplement, choline chloride, beta‐carotene, zinc methionine hydroxy analogue chelate, manganese methionine hydroxy analogue chelate, ferrous glycine, copper methionine hydroxy analogue chelate, selenium yeast, calcium iodate, DL‐Methionine, taurine, mixed tocopherols (a preservative). | Cyanocobalamin 0.04 mg/kg; Folic acid 0.3 mg /kg; brewers dried yeast (0.3%) | Dehydrated chicken; dehydrated fish | Chicken fat; fish oil | Corn, rice, dried beet pulp |
Note: Linoleic acid was provided by herring oil, fish oil, and white fish.
Abbreviations: FOS, fructooligosaccharides; MOS, mannanoligosaccharides.
2.3. Dietary trial
A clinical trials coordinator (CF) randomly assigned dogs to a diet group. Veterinarians, staff, and owners were blinded to treatment. Dogs without PLE were randomized to receive HF, HF+, or control without additional medications or supplemental vitamin B12 (cyanocobalamin). Based on the results of previous studies that demonstrated the inability of (a) an antigen‐restricted diet to induce remission in dogs with PLE 1 and (b) an ingredient‐restricted diet to maintain remission in non‐PLE CE, 9 the nontherapeutic Control diet was not considered standard‐of‐care for dogs with PLE. Dogs with PLE were randomized to receive either HF or HF+ with medications for PLE at the clinician's discretion. The addition of psyllium was permitted in dogs with incomplete resolution of large bowel diarrhea on the test diet. 8
Each diet was initially fed for 2 weeks, with responders, determined by improvement in clinical variable, continuing for 12 weeks. Nonresponders, 2 weeks or later, were randomly crossed over to receive another diet with the trial extended an additional 12 weeks at each cross‐over. Long‐term response was evaluated at 26 weeks.
A positive response to diet as monotherapy in CE non‐PLE was defined as decreased clinical disease activity and improved fecal consistency. A positive response to diet in PLE, where concurrent medications were permitted, was defined as weight gain and increase in serum albumin concentration. Standardized evaluations of clinical disease activity and fecal consistency were performed by the canine chronic enteropathy clinical activity index (CCECAI, 0‐27: insignificant disease, 0‐3; mild disease, 4‐5; moderate disease, 6‐8; severe disease, 9‐11; very severe disease, >12) 1 and a fecal chart (https://www.waltham.com/resources/waltham-booklets). Dogs with decreased clinical disease activity and a CCECAI score ≤3 at 12 or 26 weeks posttreatment were considered to be in clinical remission.
Owners provided feedback to a clinical trials coordinator (CF) every week for 12 weeks, with long‐term follow‐up at 26 weeks or later. Dogs were examined physically with blood sampled for serum cobalamin and folate concentrations at enrollment, and 6 and 12 weeks after starting the dietary trial, with additional samples obtained at the same time intervals after cross‐over.
2.4. Statistical analyses
Informed by a previous study that reported sustained remission in 13/15 dogs with non‐PLE CE fed a hydrolyzed diet compared to 2/7 consuming a therapeutic mixed antigen diet (P < .05), 9 we aimed to recruit 24 dogs with non‐PLE CE. Because our control diet lacked modifications thought to support intestinal health, we estimated that 1 in 10 dogs would respond, with treatment failures crossing to HF and HF+, yielding >80% power to detect differences between control and hydrolyzed diets. With an expected response rate of 60% to 88% to hydrolyzed diets we did not anticipate a significant difference in remission between HF and HF+, but sought to detect differences in disease activity and fecal quality. Although we randomized dogs to each diet, we had a relatively small sample size and compared baseline characteristics between groups for differences in disease severity. Specifically, we compared baseline CCECAI, fecal scores, serum cobalamin and folate concentrations, and body weight using Kruskal‐Wallis tests.
Because no nonparametric 2‐way analysis of variance (ANOVA) exists, we compared CCECAI and fecal scores at 2 weeks and 12 weeks among the diet groups using a Kruskal‐Wallis test with post hoc comparisons where necessary. We then separately compared the effect of time for all dogs without PLE, regardless of diet group, using a Friedman test with post hoc Durbin‐Conover pairwise comparisons where necessary.
We examined changes in serum cobalamin concentrations at baseline and 12 weeks and the magnitude of changes in concentrations (delta cobalamin: 12 weeks ‐ baseline) in non‐PLE dogs using a Kruskal‐Wallis test for initial comparison and a post hoc Dunn's test for pairwise comparison of Kruskal‐Wallis P < .05. Dogs with baseline serum cobalamin concentrations above the lower limit of the reference range that had previously received cobalamin parenterally were excluded from this comparison. We visually examined the relationships among serum cobalamin concentration, CCECAI, and fecal consistency scores at baseline.
We compared baseline and lowest available serum folate concentrations after starting the diets (either 6 or 12 weeks) in dogs with and without PLE using a Wilcoxon signed ranks test. After observing a possible decrease in serum folate concentrations, we identified 6 dogs that underwent folate supplementation and subsequent diet change to a diet supplemented with folate. We compared the changes in serum folate concentration in these dogs over time using a Friedman test. Finally, we compared baseline and lowest available serum folate concentrations in dogs that were fed the folate‐supplemented diet exclusively using a Wilcoxon signed ranks test.
To determine the ability of hydrolyzed fish diets to support clinical recovery and remission in dogs with PLE we examined changes in body weight, serum albumin concentration, fecal quality, and CCECAI at baseline and 12 weeks using a Wilcoxon signed rank test with Monte Carlo simulation.
3. RESULTS
3.1. Patient characteristics
3.1.1. Chronic enteropathy
Twenty‐three dogs with CE and normal serum albumin concentration were randomized to receive control, HF or HF+ diets (Table 2).
TABLE 2.
Patient demographics at baseline.
| Group | Diet | N | Age (years) | Sex | CCECAI (0–27) | Fecal score (0‐5) | Cobalamin (175‐800 pg/mL) | Folate (5‐20 ng/mL) | Prior diets | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Number of trials | Diet type | Antigens | |||||||||
| Non‐PLE | Control | 7 | 2 (1‐13) | 5FS,M,MC | 6 (3‐9) | 5 (3‐5) | 619 (149‐1176) | 14.9 (8.7‐22) | 5/7 | 2 (1‐5) | 3 FR, 3 HY, 2 H | 6 Ch, 3 Co, 3 Fi |
| HF | 7 | 4 (2‐7) | 5FS,M,MC | 4 (3‐8) | 3 (2‐4)* | 308 (183‐1001) | 12.8 (4.9‐19.2) | 5/7 | 1 (1‐4) | 2 FR, 2 HY, 2 H | 5 Ch, 1 Co, 1 Fi | |
| HF+ | 9 | 5 (2‐15) | 4FS,5MC | 5 (3‐6) | 4 (3.5‐5) | 623 (237‐969) | 11 (6.6‐23.2) | 6/9 | 2 (1‐8) | 1 FR, 4 HY, 3 H, 2AR | 7 Ch, 4 Co, 7 Fi | |
| PLE | HF | 5 | 6 (2‐13) | 2FS,2MC,M | 9 (4‐15) | 5 (2.5‐5) | 543 (149‐1008) | 9.1 (4.5‐12.5) | 4/5 | 1 (1‐3) | 2 FR, 1 HY, 3 H, 1 AR | 5 Ch, 2 Co, 3 Fi |
| HF + | 3 | 10 (1‐11) | 2FS,MC | 8 (5‐10) | 4 (3‐5) | 208 (149‐1513) | 19.8 (19‐24) | 2/3 | 1 (1‐3) | 1 FR, 2 HY, 1 H | 3 Ch, Co, 1 Fi | |
Note: Data shown as median (range).
Abbreviations: CCECAI, canine chronic enteropathy clinical activity index; Diet type: FR, fat restricted; HY, hydrolyzed protein; H, home‐prepared; AR, antigen restricted; Antigens: Ch, chicken; Co, corn; Fi, fish.
Fecal score of HF < Control (P < .05).
The distribution of breeds was: control: mixed breed (n = 3), and 1 each of Dogue de Bordeaux, Labrador Retriever, Great Dane, and Spinone, HF: German Shepherd (n = 2), and 1 each of whippet, Australian cattle dog, Dachshund, Irish Wolfhound, and mixed breed and HF+: Catahoula Leopard (n = 3), mixed breed (n = 3), and 1 each of German Shepherd, Leonberger, and corgi. We observed no differences in the age, sex, CCECAI, or serum concentrations of cobalamin and folate among groups at the time of enrollment (Table 2). One dog (control group) with a subnormal serum concentration of cobalamin (149 pg/mL) had received intermittent parenteral administration of cobalamin. One dog (HF group) had a subnormal serum folate concentration (4.85 ng/mL).
Baseline fecal scores were lower in dogs randomized to HF than in control (P < .05). Of the 23 dogs, 16 had previously failed dietary trials with a hydrolyzed protein (n = 9), home‐prepared (n = 7), fat‐restricted (n = 6), or antigen‐restricted (n = 2) diet, with a similar distribution across groups (Table 2, Table S1). All of the non‐PLE dogs randomized to the control diet previously consumed diets containing chicken, fish, and corn, and 8 of 16 dogs randomized to HF or HF+ previously had consumed fish (Table 2, Table S1).
Eleven dogs underwent abdominal ultrasonography, with no abnormalities detected in 6 dogs. Five dogs had abnormalities described as mild mesenteric lymphadenopathy (n = 3), reactive or hyperechoic mesentery (n = 2), mild mural thickening of the ileum or colon (n = 2), and mild hyperechoic stippling (n = 1).
Four dogs underwent intestinal biopsy (3 endoscopic, 1 surgical; 3 duodenum, 3 colon, 2 ileum) and histopathology. Inflammatory infiltrates consisting of lymphocytes, plasma cells, and eosinophils were described in each dog. The severity and distribution of inflammation were reported as mild‐to‐moderate enteritis and colitis in 3 dogs (control, HF, HF+) and marked, diffuse, chronic, enteritis, and colitis in 1 (control).
3.1.2. Protein‐losing enteropathy
Eight dogs with PLE (Table 2) were randomized to receive HF (n = 5) or HF+ (n = 3). The distribution of breeds by group was HF: mixed breed (n = 2), and 1 each of German Shepherd, Shi Tzu, French Bulldog, and HF+: 1 each of German Shepherd, Boston terrier, and mixed breed. Five dogs weighed <15 kg.
We observed no difference in the age, sex, CCECAI, fecal score, or serum concentrations of folate, cobalamin (Table 2), albumin (median, range: HF: 2.4, 1.5‐2.6; HF+: 2.5, 1.7‐2.9 g/L), or cholesterol (median, range: HF: 118, 91‐197; HF+: 104, 101‐140 mg/dL) between groups. Two dogs (1 on each diet) had subnormal serum cobalamin concentrations at baseline. Six dogs had received parenteral cobalamin before referral (4 HF, 2 HF+). One dog (randomized to HF) had a subnormal serum concentration of folate. Six of 8 dogs (4HF, 2HF+) previously had failed dietary trials with a home‐prepared (n = 4), hydrolyzed protein (n = 3), fat‐restricted (n = 3), or antigen‐restricted (n = 1) diet (Table 2) and 4 of 8 previously had consumed diets containing fish (Tables 2 and S2).
All dogs underwent abdominal ultrasonography. Abnormalities detected in 7 dogs included hyperechoic mucosa and diffuse enteropathy (n = 5), peritoneal effusion (n = 4), mural thickening (n = 3), linear hyperechoic striations (n = 2), hyperechoic mesentery (n = 2), and lymphadenopathy (n = 1).
Four dogs (3HF, 1 HF+) underwent intestinal biopsy (3 endoscopic, 1 surgical; 3 duodenum, 2 ileum, 1 colon) and histopathology. The dog that underwent surgical biopsy was a French bulldog (HF) diagnosed with focal granulomatous lymphangitis 33 with an incomplete response to surgical resection of the distal ileum, and treatment with metronidazole, dexamethasone, and parenteral cobalamin. The predominant histopathologic abnormalities in the other dogs were crypt cysts (n = 3), lymphangiectasia (n = 2), lymphoplasmacytic infiltrates (n = 2), and lymphoplasmacytic histiocytic infiltrates (n = 1) with severity reported as moderate to severe (2 HF diet, 1 HF+ diet).
Concurrent medications in PLE dogs at baseline included parenteral cobalamin (n = 6), glucocorticoids (n = 5), psyllium (n = 2), tylosin (n = 2), doxycycline (n = 1), clopidogrel (n = 1), and zonisamide (n = 1); Table S2.
3.2. Clinical response to diet
3.2.1. Chronic enteropathy
In the non‐PLE group (23 dogs), all dogs ate the test diets (7 control, 7 HF, 9 HF+), and diet as monotherapy was associated with marked decreases in CCECAI and fecal scores at 2 weeks (P < .05; Figure 1A,B) without an observable difference between diets. A positive clinical response was sustained in 19/23 (83%; 95% CI, 60%‐94%) dogs at 12 weeks, with further decreases in CCECAI and fecal scores (P < .05, 12 weeks vs 2 weeks, Figure 1A,B). Again, clinical responses appeared similar between diets (P < .05. Figure 1A‐C). Self‐resolving flares in clinical signs were observed in 2/19 (10%; 95% CI, 2% to 34%) dogs (1 HF+, vomiting 4 weeks; 1 control, vomiting and diarrhea, 10 weeks). Four dogs (1 control, 3 HF+) relapsed between 4 and 9 weeks and crossed over to another diet, and all achieved clinical remission at 12 weeks (HF, 3; HF+, 1; Figure 1C). Four of 23 dogs received supplemental psyllium (2 HF+, 1 HF, 1 control; Table S1). There was no difference (P > .05) in weight gain (12 weeks) between diets (% change from baseline and 12 weeks; median, range: control: 2.3, −0.25 to 6.6; HF: 0, 0‐15.2; HF+: 5, −0.5 to 9.9). Eighteen of 23 dogs were available for long‐term follow‐up, with sustained clinical remission in all dogs, including the 2 dogs with self‐resolving flares, at 26 weeks. Five dogs were lost to follow‐up: 2 euthanized for nongastrointestinal disease, 1 re‐homed, 1 with lost owner contact, and 1 with behavior issues. The 4 dogs with pretreatment histopathology responded clinically to their diets.
FIGURE 1.
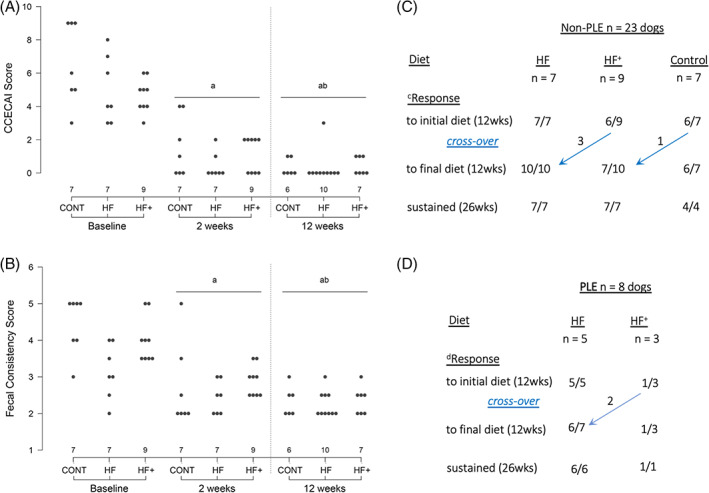
Clinical response to dietary modification. (A) Disease activity (CCECAI score) before and after diet non‐PLE Baseline and 2 weeks = initial diet, 12 weeks = final diet. (B) Fecal scores before and after diet non‐PLE Baseline and 2 weeks = initial diet, 12 weeks = final diet. (C) Clinical outcome in dogs with chronic enteropathy and normal serum albumin concentration. cResponse was defined as reduced disease activity and improved fecal consistency. (D) Clinical outcome in dogs with chronic enteropathy and protein‐losing enteropathy (PLE). dResponse was defined by increased body weight and serum albumin concentration. Concurrent medications were allowed a P < .001 vs baseline; b P < .01 vs 2 weeks.
3.2.2. Protein‐losing enteropathy
In the PLE group (5HF, 3HF+), all dogs found the diets palatable during the initial 2‐week period, and CCECAI scores decreased in 7 dogs (median, range: baseline 8.5, 4‐15; 2 weeks 4, 1‐11), and fecal consistency scores decreased in 4 (median, range: baseline 4.5, 2‐5; 2 weeks 3.25, 2‐5). Six dogs (5/5 HF, 1/3 HF+) completed the 12‐week trial on their initial diet (Figure 1D). Two dogs that relapsed on the HF+ diet at 6 weeks crossed over to the HF diet, with 1 achieving complete clinical remission at 12 weeks, and the other being withdrawn for inappetence in week 3 of the second trial (Figure 1D). Four dogs (3 HF, 1 HF+) received supplemental psyllium (Table S2). The 7 dogs with PLE that consumed hydrolyzed fish diets gained body weight (baseline vs 12 weeks; median, range: kg, + 4, −0.5‐7; %, + 17, −2.5‐64%; P = .02) and had increased serum albumin concentration (g/dL; median, range: baseline 2.4, 1.5‐2.9; 12 weeks 3, 2.3‐3.7; P < .05). Clinical disease activity also was significantly improved (CCECAI total score; median, range: baseline 9, 4‐15; 12 weeks 0, 0‐3; P = .02; CCECAI weight loss score; median, range: baseline 2, 1‐3; 12 weeks 0, 0‐0; P = .02), but fecal quality was more variable (median, range: baseline 5, 2‐5; 12 weeks 2.5, 2‐5; P > .05). Body condition score, recorded in 4/7 PLE dogs, was increased in each dog (9‐point score, median, range: baseline 2, 2‐4; 12 weeks 3.5, 3‐4). Clinical remission was sustained in all 7 PLE dogs (6 HF, 1 HF+) at 26 weeks, with 5 still receiving concurrent medications (corticosteroids, 4; tylosin, 1), and 2 large breed dogs, a 13‐year‐old mixed breed (HF) and 1‐year‐old German Shepherd (HF+), maintained on diet alone (Table S2, Figure S1B,C). During the trial, we observed marked changes in physical appearance and body condition, exemplified by hair re‐growth, improved haircoat condition, and weight gain (Figure S1A,C).
3.2.3. Serum cobalamin
To determine the ability of dietary cobalamin to maintain and normalize serum cobalamin concentration in dogs with CE we compared serum cobalamin concentrations at baseline and 12 weeks in 22 non‐PLE dogs consuming diets that contained cobalamin at 0.04 mg/kg (control), 0.06 mg/kg (HF), and 10 mg/kg (HF+) dry matter. One dog had received parenteral supplementation before enrollment (baseline serum cobalamin concentration, 184 pg/mL). There was no significant difference in baseline serum cobalamin concentrations among dogs randomized to receive HF, HF+, or control or visual correlation among baseline serum cobalamin and CCECAI and fecal scores. Serum cobalamin concentrations at 12 weeks were higher than baseline (P <.05), with higher concentrations in dogs fed HF+ than HF (P < .05), and no significant differences among control, HF+, and HF (Table 3). Serum cobalamin concentrations at 12 weeks were > 175 pg/mL (the lower limit of the reference interval) in all dogs, exceeding baseline concentrations in 18, and the upper limit of the reference interval (>800 pg/mL) in 11 dogs, including a dog with a baseline serum cobalamin concentration of 149 pg/mL. Serum cobalamin concentrations in 2 non‐PLE dogs that previously had received parenteral cobalamin were maintained by diet without the need for additional supplementation. In 2 dogs with PLE that did not receive parenteral cobalamin, serum cobalamin concentrations increased from 149 and 272 pg/mL at baseline to 1512 (HF+) and 580 (HF) pg/mL at 12 weeks, respectively. Collectively, in 3 dogs that initially received parenteral cyanocobalamin for hypocobalaminemia, serum cobalamin concentration (12 weeks, median, range) was maintained by diet (1001, 473‐1512 pg/mL) without exogenous supplementation.
TABLE 3.
Serum cobalamin before and after dietary modification.
| Group | Diet | N | Serum cobalamin (pg/ml) | ||
|---|---|---|---|---|---|
| Baseline | 12 weeks* | Delta | |||
| Non‐PLE | Control | 6 | 565 (149‐1176) | 688 (554‐1001) | 177 (−611 to 852) |
| HF | 7 | 275 (183‐1001) | 473 (251‐1373) | 74 (−386 to 789) | |
| HF+ | 9 | 619 (237‐969) | 853 (669‐1908)** | 335 (−83 to 1640) | |
Note: Data shown as median (range). Delta = 12 weeks‐baseline.
P < .05 12 weeks vs baseline.
P < .05 vs HF at 12 weeks.
3.2.4. Serum folate
Serum folate concentrations at baseline were within the normal range in all but 2 dogs (1 non‐PLE fed HF, 4.9 ng/mL; 1 PLE fed HF, 4.5 ng/mL). Unexpectedly, serum folate concentrations at 6 weeks were significantly (P < .05) lower than baseline in dogs with and without PLE regardless of the diet being fed (Table 4, Diet A). This finding was surprising because the folate content of the test diets (0.3‐0.45 mg/kg) exceeded the National Research Council (NRC) recommendations of 0.216 mg/kg for maintenance and growth, 34 and this result was confirmed by dietary analysis. To address this issue, we administered folic acid at >150 μcg/10 kg/day PO for 6 weeks to 6 dogs with normal serum folate concentrations at baseline (median, range; 10.15, 5.1‐17 ng/mL) and subnormal serum folate concentrations at 6 weeks (median, range; 2.84, 2.22‐3.94 ng/mL). Oral supplementation normalized serum folate concentrations (median, range; 9.15, 7.8‐19.8 ng/mL) in every dog, and re‐compounding the test diet with folic acid at 5 mg/kg maintained serum folate concentrations within the normal range in those dogs (n = 6; median, range; 7.21, 6.39‐15.4) and in dogs enrolled subsequently (n = 10, Table 4, Diet B).
TABLE 4.
Serum folate before and after dietary modification.
| Group | Diet batch A | N | Folate (ng/ml) | |
|---|---|---|---|---|
| Baseline | Post‐diet A* | |||
| Non‐PLE | Control | 5 | 14.9 (8.67‐21.9) | 4.29 (2.98‐5.14) |
| HF | 5 | 7.53 (4.85‐19.2) | 3.17 (2.22‐6.47) | |
| HF+ | 4 | 13.15 (6.9‐17.9) | 6.5 (2.7‐11.9) | |
| PLE | HF | 4 | 10.35 (8.47‐12.5) | 4.67 (2.96‐8.69) |
| HF+ | 0 | NA | NA | |
| Group | Diet batch B | N | Folate (ng/ml) | |
| Baseline | 12 weeks diet B | |||
| Non‐PLE | Control | 1 | 11.2 (11.2‐11.2) | 13.8 (13.8‐13.8) |
| HF | 2 | 10.62 (6.33‐14.9) | 19 (13‐25) | |
| HF+ | 6 | 13.45 (6.55‐23.2) | 15.25 (11.9‐20.7) | |
| PLE | HF | 0 | NA | NA |
| HF+ | 1 | 24 (24‐24) | 6.85 (6.85‐6.85) | |
Note: Data shown as median (range). Diet batch A: Folic acid 0.3 mg/kg (control) and 0.45 mg/kg (HF, HF+); Diet batch B: Folic acid 5 mg/kg (control, HF, HF+).
P < .05 vs baseline for all diets.
4. DISCUSSION
Our primary objective was to compare the ability of isocaloric diets composed of hydrolyzed fish, rice starch, and fish oil, with and without prebiotics, turmeric, and high cobalamin and a nontherapeutic, limited ingredient control diet containing nonhydrolyzed mixed protein sources to resolve clinical signs in dogs with nonprotein‐losing CE. We found that changing diet, independent of antigen restriction or supplementation, induced rapid and sustained remission. The ability of a diet containing nonnovel, mixed intact antigens to induce clinical remission supports the notion that restricting ingredients rather than antigens suspected of inducing immune hypersensitivity may be the basis of the clinical response, and calls into question the practice of dietary modification based on prior consumption of suspected immunogens. We extend previous studies linking PO intake of cobalamin and folate to serum concentrations in dogs and show that incorporating these vitamins into the diet can increase and normalize their serum concentrations in dogs with CE, and may preclude the need for exogenous supplementation. As a secondary objective we found that hydrolyzed fish diets with moderate fat support increase in body weight and serum albumin concentration, and sustained remission of dogs with PLE.
Our finding that dietary modification ameliorated clinical signs of gastrointestinal disease in 83% of non‐PLE dogs is consistent with responses to antigen‐restricted and hydrolyzed diets in 60% to 88% of dogs with CE and biopsy‐confirmed or suspected lymphocytic‐plasmacytic enteritis. 1 , 3 , 4 , 5 By incorporating an isocaloric limited ingredient control diet containing a mixture of nonhydrolyzed fish, chicken, and corn, we determined that beneficial responses were independent of antigen restriction, hydrolysis, or supplementation. It is difficult to reconcile our results with the widely accepted hypothesis of food‐responsive CE being driven by allergic reactions or adverse reactions to common dietary antigens. 1 , 3 , 4 , 5 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 Our results, in concert with the high response rates of dogs to diets that differ markedly in their composition but are formulated from relatively few ingredients, favor an effect of ingredients versus antigen restriction. In‐depth investigations of the etiopathogenesis of food‐responsive CE in dogs with lymphocytic‐plasmacytic enteritis have identified diet‐responsive changes in the ultrastructure of the microvillus membrane of enterocytes lining the small bowel, 15 reminiscent of those associated with gluten/gliadin in people, 32 , 35 and our findings suggest that yet‐to‐be‐defined environmental triggers (eg, emulsifiers, carrageenan, glyphosate) may be involved in the etiopathogenesis of CE in dogs. 5 , 7 , 36 , 37
It is unclear why the test diets induced remission in 17 dogs (16 non‐PLE, 1 PLE) that previously had failed from 1 to 8 therapeutic trials with hydrolyzed, antigen‐restricted, and home‐prepared antigen and fat‐restricted diets considered effective in dogs with CE. 3 , 4 , 5 We suspect the structure of our trial and owner compliance 16 and discrepancies between ingredients and labeling in commercial pet foods 38 , 39 may have played a role. From a practical perspective, our findings support the strategy of conducting multiple dietary trials in dogs with CE, with or without PLE, before escalating to use of antimicrobial or immunosuppressive agents. 5 , 7 , 16 We speculate that the high proportions of dogs with immunosuppression‐responsive versus food‐responsive enteropathy reported in some studies may reflect therapeutic escalation based on a single dietary trial. 40 , 41
In dogs with PLE, the role of diet as monotherapy is less clear because the prognosis is poor and such dogs often receive concurrent medications or refuse to eat therapeutic diets. 3 , 4 , 5 , 7 , 10 , 12 , 16 , 18 We showed that diets containing hydrolyzed proteins and moderate amounts of fat (3.97 g fat /100 kcal) support an increase in body weight, serum albumin concentration, and sustained remission. The long‐term remission in 2 dogs maintained on diet alone, including a German Shepherd dog with extensive mucosal hyperechoic striations attributed to lymphangiectasia, 42 concurs with other studies that included breeds predisposed to primary lymphangiectasia, 5 , 7 , 13 , 17 and underscores the need to further explore a broad spectrum of diets as monotherapy in dogs with PLE. Notably, the diets we employed all contained fish and fish oil (rich in n‐3 polyunsaturated fatty acids) which are associated with a decreased risk of inflammatory bowel disease in people. 43 Our current understanding of the role of dietary fat in dogs with and without CE and PLE is constrained by a focus on caloric density that equates beef tallow with olive oil and fish oil. Future studies should address its dietary origin, chemical composition, and functionality for the microbiome and host.
A secondary aim of our study was to examine the ability of dietary supplementation with prebiotics, turmeric, and high‐dose cobalamin to augment clinical responses relative to hydrolyzed fish and rice. Although we found no significant effect of these supplemental ingredients on clinical remission, disease activity (CCECAI), and fecal scores, our study was underpowered to detect differences between HF and HF+, and additional study is required to determine the impact on the enteric microenvironment. Eight dogs (4 non‐PLE, 4 PLE) received supplemental psyllium (4 HF+, 3 HF, 1 control), predominantly for residual signs of large bowel diarrhea from week 2 on, highlighting the need to optimize dietary fiber requirements in dogs with CE.
Our finding that diet‐supported serum cobalamin concentration in dogs previously maintained on parenteral cobalamin, and above the upper limit of the reference interval (>800 pg/mL) in 50% of dogs without PLE suggests that dietary incorporation may preclude the need for exogenous supplementation. We were surprised that serum concentrations of cobalamin in dogs consuming diets that ranged from 10 mg/kg (HF+) to 0.04 mg/kg DM (control) cobalamin, equating to PO intake of 1.57 mg at 10 mg/kg and 0.006 mg at 0.04 mg/kg for a 10 kg dog, were not more different. However, a study in dogs with subnormal serum cobalamin concentrations receiving PO cyanocobalamin at doses between 0.025 and 0.08 mg/kg body weight q24h also found substantial interindividual variation and a weak correlation (R 2 = 0.23) between PO intake and serum concentrations. 26 Because serum concentrations of cobalamin increased markedly in some of our patients fed cobalamin at 0.04 mg/kg dry matter, we speculate that resolution of disease and dysbiosis may have improved cobalamin availability, absorption, and homeostasis (eg, binding to transcobalamin 2 and cellular uptake) in dogs with CE.
Our finding that 2/31 CE dogs had subnormal serum concentrations of folate is consistent with previous studies. 22 , 23 , 24 , 26 Because we do not usually perform serial measurements of serum folate concentrations in dogs with CE with normal serum folate concentrations at the time of diagnosis, we did not anticipate serum folate concentrations would decrease in dogs with positive clinical responses to diets that exceed the NRC recommendations of 0.216 mg/kg dry matter. 34 The requirement for additional folic acid (5 mg/kg dry matter diet) to normalize serum folate concentrations during recovery from CE suggests an increased need for folate that may reflect changes in folate consumption and production by the microbiome as well as absorption, metabolism, and catabolism by the host. It remains to be determined if this phenomenon extends to other cohorts of dogs with CE and other diets.
Limitations of our study include the lack of endoscopic or histopathologic findings in many dogs with CE. However, the success of dietary trials in dogs that have undergone a comprehensive evaluation to exclude nongastrointestinal causes for their signs has meant that endoscopy and histopathology typically are reserved for dogs that fail dietary treatment or have PLE. Our patient population did not include breeds that are consistently predisposed to lymphangiectasia. 4 , 5 , 12 , 13 , 19 This bias may reflect the referral population, reluctance to enroll dogs with PLE on diets that are not restricted in fat, or successful treatment without a referral. Further study is required to elucidate the ability of dietary cobalamin and different amounts of cobalamin to normalize serum cobalamin concentrations in dogs with and without CE and cobalamin deficiency. Although we documented a substantial decrease in serum folate concentrations posttreatment, we did not determine its relationship to metabolic deficiency (eg, serum homocysteine concentrations).
In conclusion, we found that changing to a limited ingredient diet, independent of antigen restriction, protein hydrolysis, or supplementation with prebiotics, turmeric, and high‐dose cobalamin, was associated with long‐term clinical remission in dogs with CE and a subset of those with PLE. We found that dietary incorporation can increase and normalize serum concentrations of cobalamin and folate in dogs with CE and may preclude the need for exogenous supplementation. We determined that hydrolyzed diets with moderate fat content can support clinical recovery and remission in dogs with PLE.
Although a mechanistic understanding of the clinical responses to diet is undetermined, our findings support a clinical strategy of ingredient restriction and multiple dietary trials in dogs with CE and PLE before escalating to antimicrobial or immunosuppressive medications.
CONFLICT OF INTEREST DECLARATION
Dr. Simpson is a member of the Scientific Advisory Boards for Farmina Pet Foods and Nestle Purina Petcare. This conflict of interest was managed by the Office of Research Integrity and Assurance (ORIA) at Cornell University. No other authors declare a conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approved by the Cornell University IACUC: # 2017‐0129.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Figure S1: Clinical responses to dietary modification.
Table S1: Pretrial diets in dogs with nonprotein losing enteropathy.
Table S2: Pretrial diets, concurrent medications, and clinical outcome of dogs with protein‐losing enteropathy.
ACKNOWLEDGMENT
This study was funded by a grant from Farmina Pet Foods. We are grateful to the owners, veterinarians, and technical staff who supported this clinical trial.
Simpson KW, Miller ML, Loftus JP, Rishniw M, Frederick CE, Wakshlag JJ. Randomized controlled trial of hydrolyzed fish diets in dogs with chronic enteropathy. J Vet Intern Med. 2023;37(6):2334‐2343. doi: 10.1111/jvim.16844
REFERENCES
- 1. Allenspach K, Wieland B, Grone A, et al. Chronic enteropathies in dogs: evaluation of risk factors for negative outcome. J Vet Intern Med. 2007;21:700‐708. [DOI] [PubMed] [Google Scholar]
- 2. Allenspach K, Culverwell C, Chan D. Long‐term outcome in dogs with chronic enteropathies: 203 cases. Vet Rec. 2016;178(15):368.2‐368.368. [DOI] [PubMed] [Google Scholar]
- 3. Dandrieux JRS, Mansfield CS. Chronic enteropathy in canines: prevalence, impact and management strategies. Vet Med. 2019;10:203‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rudinsky AJ, Rowe JC, Parker VJ. Nutritional management of chronic enteropathies in dogs and cats. J Am Vet Med Assoc. 2018;253(5):570‐578. [DOI] [PubMed] [Google Scholar]
- 5. Simpson KW, Jergens AE. Pitfalls and progress in the diagnosis and management of canine inflammatory bowel disease. Vet Clin North Am Small Anim Pract. 2011;41(2):381‐398. [DOI] [PubMed] [Google Scholar]
- 6. Volkmann M, Steiner JM, Fosgate GT, Zentek J, Hartmann S, Kohn B. Chronic diarrhea in dogs—retrospective study in 136 cases. J Vet Intern Med. 2017;31(4):1043‐1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jablonski‐Wennogle SA, Stockman J, Webb CB. Prospective evaluation of a change in dietary therapy in dogs with steroid‐resistant protein‐losing enteropathy. J Small Anim Pract. 2021;62:756‐764. [DOI] [PubMed] [Google Scholar]
- 8. Leib MS. Treatment of chronic idiopathic large‐bowel diarrhea in dogs with a highly digestible diet and soluble fiber: pathology WNL. A retrospective review of 37 cases. J Vet Intern Med. 2000;14(1):27‐32. [DOI] [PubMed] [Google Scholar]
- 9. Mandigers PJ, Biourge V, van Den Ingh TS, et al. A randomized, open‐label, positively‐controlled field trial of a hydrolyzed protein diet in dogs with chronic small bowel enteropathy. J Vet Intern Med. 2010;24:1350‐1357. [DOI] [PubMed] [Google Scholar]
- 10. Marchesi MC, Timpano CC, Busechian S, et al. The role of diet in managing inflammatory bowel disease affected dogs: a retrospective cohort study on 76 cases. Vet Ital. 2017;6:297‐302. [DOI] [PubMed] [Google Scholar]
- 11. Nelson RW, Stookey LJ, Kazacos E. Nutritional management of idiopathic chronic colitis in the dog. J Vet Intern Med. 1988;2(3):133‐137. [DOI] [PubMed] [Google Scholar]
- 12. Okanishi H, Yoshioka R, Kagawa Y, Watari T. The clinical efficacy of dietary fat restriction in treatment of dogs with intestinal lymphangiectasia. J Vet Intern Med. 2014;28:809‐817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rudinsky AJ, Howard JP, Bishop MA, Sherding RG, Parker VJ, Gilor C. Dietary management of presumptive protein‐losing enteropathy in Yorkshire terriers. J Small Anim Pract. 2017;58:103‐108. [DOI] [PubMed] [Google Scholar]
- 14. Tams TR, Twedt DC. Canine protein‐losing gastroenteropathy syndrome. Compend Contin Educ Pract Vet. 1981;3:105‐114. [Google Scholar]
- 15. Walker D, Knuchel‐Takano A, McCutchan A, et al. A comprehensive pathological survey of duodenal biopsies from dogs with diet‐responsive chronic enteropathy. J Vet Intern Med. 2013;27:862‐874. [DOI] [PubMed] [Google Scholar]
- 16. Dandrieux JR, Martinez Lopez LM, Prakash N, Mansfield CS. Treatment response and long term follow up in nineteen dogs diagnosed with chronic enteropathy in Australia. Aust Vet J. 2019;97:301‐307. [DOI] [PubMed] [Google Scholar]
- 17. Kawano K, Shimakura H, Nagata N, et al. Prevalence of food‐responsive enteropathy among dogs with chronic enteropathy in Japan. J Vet Med Sci. 2016;78:1377‐1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Craven M, Simpson JW, Ridyard AE, Chandler ML. Canine inflammatory bowel disease: retrospective analysis of diagnosis and outcome in 80 cases (1995–2002). J Small Anim Pract. 2004;45:336‐342. [DOI] [PubMed] [Google Scholar]
- 19. Nagata N, Ohta H, Yokoyama N, et al. Clinical characteristics of dogs with food‐responsive protein‐losing enteropathy. J Vet Intern Med. 2020;34(2):659‐668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Peterson PB, Willard MD. Protein‐losing enteropathies. The veterinary clinics of North America. Small Animal Practice. 2003;33:1061‐1082. [DOI] [PubMed] [Google Scholar]
- 21. Allenspach K, Rizzo J, Jergens AE, Chang YM. Hypovitaminosis D is associated with negative outcome in dogs with protein losing enteropathy: a retrospective study of 43 cases. BMC Vet Res. 2017;13:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Batt RM, Morgan JO. Role of serum folate and vitamin B12 concentrations in the differentiation of small intestinal abnormalities in the dog. Res Vet Sci. 1981;32(1):17‐22. [PubMed] [Google Scholar]
- 23. German AJ, Day MJ, Ruaux CG, Steiner JM, Williams DA, Hall EJ. Comparison of direct and indirect tests for small intestinal bacterial overgrowth and antibiotic‐responsive diarrhea in dogs. J Vet Intern Med. 2003;17(1):33‐43. [DOI] [PubMed] [Google Scholar]
- 24. Schmitz SS, Gow A, Bommer N, et al. Diagnostic features, treatment, and outcome of dogs with inflammatory protein‐losing enteropathy. J Vet Intern Med. 2019;33:2005‐2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Toresson L, Steiner JM, Suchodolski J, et al. Oral cobalamin supplementation in dogs with chronic enteropathies and Hypocobalaminemia. J Vet Intern Med. 2016;30:101‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Toresson L, Steiner JM, Razdan P, et al. Comparison of efficacy of oral and parenteral cobalamin supplementation in normalizing low cobalamin concentrations in dogs: a randomized controlled study. Vet J. 2018;232:27‐32. [DOI] [PubMed] [Google Scholar]
- 27. Berghoff N, Suchodolski JS, Steiner JM. Association between serum cobalamin and methylmalonic acid concentrations in dogs. Vet J. 2012;191:306‐311. [DOI] [PubMed] [Google Scholar]
- 28. Davenport DJ, Ching RJ, Hunt JH, et al. The effect of dietary levels of folate and cobalamin on the serum concentration of folate and cobalamin in the dog. J Nutr. 1994;124(12 Suppl):2559S‐2562S. [DOI] [PubMed] [Google Scholar]
- 29. Domosławska A, Jurczak T, Janowski T. Oral folic acid supplementation decreases palate and/or lip cleft occurrence in Pug and Chihuahua puppies and elevates folic acid blood levels in pregnant bitches. Pol J Vet Sci. 2013;16(1):33‐37. [DOI] [PubMed] [Google Scholar]
- 30. Basson AR, Chen C, Sagl F, et al. Regulation of intestinal inflammation by dietary fats. Front Immunol. 2020;11:604989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Holscher HD. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes. 2017;8(2):172‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Burge K, Gunasekaran A, Eckert J, Chaaban H. Curcumin and intestinal inflammatory diseases: molecular mechanisms of protection. Int J Mol Sci. 2019;20(8):1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lecoindre A, Lecoindre P, Cadoré JL, et al. Focal intestinal lipogranulomatous lymphangitis in 10 dogs. J Small Anim Pract. 2016;57(9):465‐471. [DOI] [PubMed] [Google Scholar]
- 34. National Research Council (NRC) . Nutrient Requirements of Dogs and Cats. Washington, DC: National Academic Press; 2006. [Google Scholar]
- 35. Dunne MR, Byrne G, Chirdo FG, Feighery C. Coeliac disease pathogenesis: the uncertainties of a well‐known immune mediated disorder. Front Immunol. 2020;11:1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Raoul P, Cintoni M, Palombaro M, et al. Food additives, a key environmental factor in the development of IBD through gut dysbiosis. Microorganisms. 2022;10(1):167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhao J, Pacenka S, Wu J, et al. Detection of glyphosate residues in companion animal feeds. Environ Pollut. 2018;243:1113‐1118. [DOI] [PubMed] [Google Scholar]
- 38. Olivry T, Mueller RS. Critically appraised topic on adverse food reactions of companion animals (5): discrepancies between ingredients and labeling in commercial pet foods. BMC Vet Res. 2018;14:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ricci R, Granato A, Vascellari M, et al. Identification of undeclared sources of animal origin in canine dry foods used in dietary elimination trials. J Anim Physiol Anim Nutr. 2013;97(Suppl 1):32‐38. [DOI] [PubMed] [Google Scholar]
- 40. Benvenuti E, Pierini A, Bottero E, et al. Immunosuppressant‐responsive enteropathy and non‐responsive enteropathy in dogs: prognostic factors, short‐ and long‐term follow up. Animals. 2021;11:2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Holmberg J, Pelander L, Ljungvall I, Harlos C, Spillmann T, Häggström J. Chronic enteropathy in dogs‐epidemiologic aspects and clinical characteristics of dogs presenting at two Swedish animal hospitals. Animals. 2022;12(12):1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sutherland‐Smith J, Penninck DG, Keating JH, et al. Ultrasonographic intestinal hyperechoic mucosal striations in dogs are associated with lacteal dilation. Vet Radiol Ultrasound. 2007;48(1):51‐57. [DOI] [PubMed] [Google Scholar]
- 43. Mozaffari H, Daneshzad E, Larijani B, Bellissimo N, Azadbakht L. Dietary intake of fish, n‐3 polyunsaturated fatty acids, and risk of inflammatory bowel disease: a systematic review and meta‐analysis of observational studies. Eur J Nutr. 2020;59(1):1‐17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Clinical responses to dietary modification.
Table S1: Pretrial diets in dogs with nonprotein losing enteropathy.
Table S2: Pretrial diets, concurrent medications, and clinical outcome of dogs with protein‐losing enteropathy.


