Abstract
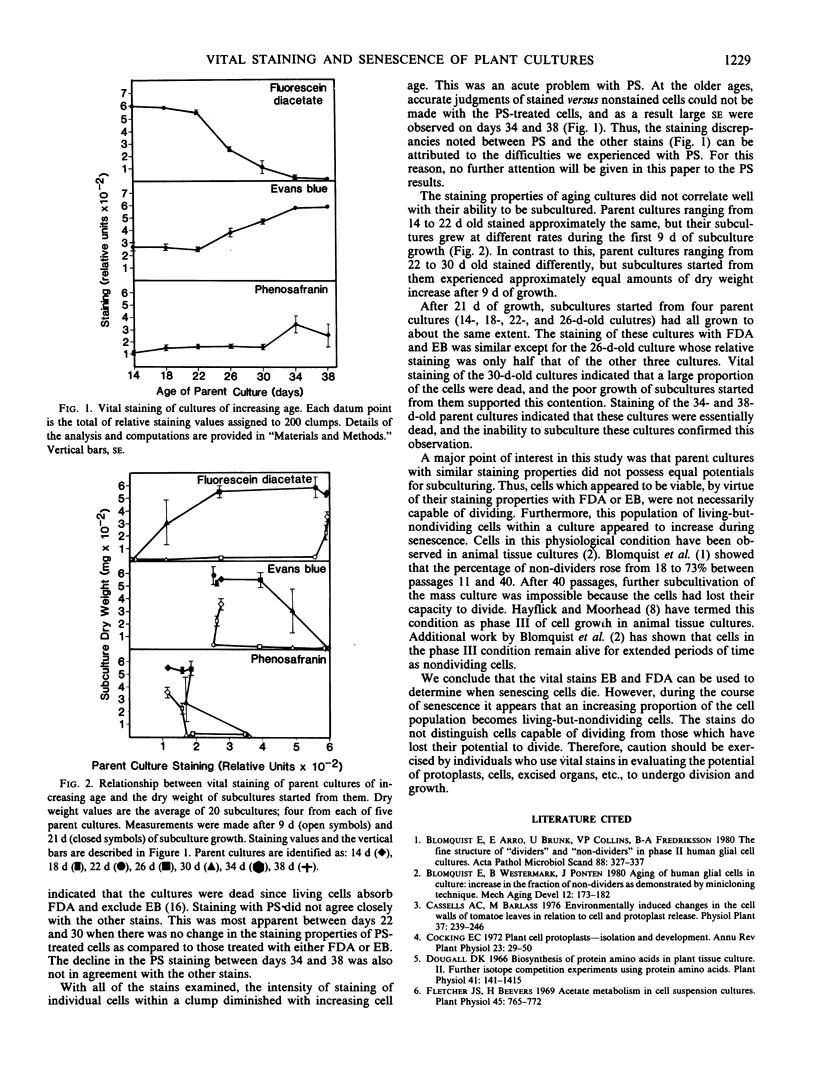
The vital staining properties of rose cultures (Rosa cv Paul's Scarlet) of increasing age were compared with their ability to be subcultured. At 4-day intervals beginning on day 14, after cell division and expansion had stopped, cells were stained separately with Evans blue, fluorescein diacetate, and phenosafranine. The degree to which parent cultures stained with each of these dyes was compared to the dry weight of their subcultures harvested after 9 and 21 days of growth.
Staining with either Evans blue or fluorescein diacetate was demonstrated to be a good means of establishing when senescing cells died. However, the staining properties of aging cultures did not correlate well with their ability to be subcultured, because an increasing proportion of the living cells appeared to lose their ability to divide as senescence progressed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blomquist E., Arro E., Brunk U., Collins V. P., Fredriksson B. A. The fine structure of "dividers" and "non-dividers" in phase II human glial cell cultures. Acta Pathol Microbiol Scand A. 1980 Sep;88(5):327–337. doi: 10.1111/j.1699-0463.1980.tb02503.x. [DOI] [PubMed] [Google Scholar]
- Blomquist E., Westermark B., Pontén J. Ageing of human glial cells in culture: increase in the fraction of non-dividers as demonstrated by a minicloning technique. Mech Ageing Dev. 1980 Feb;12(2):173–182. doi: 10.1016/0047-6374(80)90093-7. [DOI] [PubMed] [Google Scholar]
- Dougall D. K. Biosynthesis of Protein Amino Acids in Plant Tissue Culture II Further Isotope Competition Experiments Using Protein Amino Acids. Plant Physiol. 1966 Nov;41(9):1411–1415. doi: 10.1104/pp.41.9.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher J. S., Beevers H. Acetate metabolism in cell suspension cultures. Plant Physiol. 1970 Jun;45(6):765–772. doi: 10.1104/pp.45.6.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palta J. P., Levitt J., Stadelmann E. Q. Plant viability assay. Cryobiology. 1978 Apr;15(2):249–255. doi: 10.1016/0011-2240(78)90035-4. [DOI] [PubMed] [Google Scholar]
- Pech J. C., Romani R. J. Senescence of Pear Fruit Cells Cultured in a Continuously Renewed, Auxin-deprived Medium. Plant Physiol. 1979 Nov;64(5):814–817. doi: 10.1104/pp.64.5.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavik N. S., Widholm J. M. Inhibition of deoxyribonuclease activity in the medium surrounding plant protoplasts. Plant Physiol. 1978 Aug;62(2):272–275. doi: 10.1104/pp.62.2.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widholm J. M. The use of fluorescein diacetate and phenosafranine for determining viability of cultured plant cells. Stain Technol. 1972 Jul;47(4):189–194. doi: 10.3109/10520297209116483. [DOI] [PubMed] [Google Scholar]


