Abstract
Background
Growing evidence from dogs and humans supports the abundance of mutation‐based biomarkers in tumors of dogs. Increasing the use of clinical genomic diagnostic testing now provides another powerful data source for biomarker discovery.
Hypothesis
Analyzed clinical outcomes in dogs with cancer profiled using SearchLight DNA, a cancer gene panel for dogs, to identify mutations with prognostic value.
Animals
A total of 127 cases of cancer in dogs were analyzed using SearchLight DNA and for which clinical outcome information was available.
Methods
Clinical data points were collected by medical record review. Variables including mutated genes, mutations, signalment, and treatment were fitted using Cox proportional hazard models to identify factors associated with progression‐free survival (PFS). The log‐rank test was used to compare PFS between patients receiving and not receiving targeted treatment before first progression.
Results
Combined genomic and outcomes analysis identified 336 unique mutations in 89 genes across 26 cancer types. Mutations in 6 genes (CCND1, CCND3, SMARCB1, FANCG, CDKN2A/B, and MSH6) were significantly associated with shorter PFS. Dogs that received targeted treatment before first progression (n = 45) experienced significantly longer PFS compared with those that did not (n = 82, P = .01). This significance held true for 29 dogs that received genomically informed targeted treatment compared with those that did not (P = .05).
Conclusion and Clinical Importance
We identified novel mutations with prognostic value and demonstrate the benefit of targeted treatment across multiple cancer types. These results provide clinical evidence of the potential for genomics and precision medicine in dogs with cancer.
Keywords: dog, genes, mutations, outcomes, precision medicine, progression‐free survival, real‐world, targeted therapy
Abbreviations
- CNV
copy number variant
- INDEL
insertion/deletion
- ITD
internal tandem duplication
- MCT
mast cell tumor
- NGS
next‐generation sequencing
- PFS
progression‐free survival
- SNV
single nucleotide variant
- SOC
standard of care
- VUS
variants of uncertain significance
1. INTRODUCTION
Next‐generation sequencing (NGS) and better understanding of cancer's genomic basis have fundamentally altered the treatment paradigm for human oncology patients, shifting the therapeutic strategy away from a histology‐centered and “one‐treatment‐fits‐all” basis to a biomarker‐guided, personalized, and often tumor type‐agnostic approach. 1 , 2 As a result, more Food and Drug Administration (FDA)‐approved molecularly‐guided treatment options are available, accompanied by companion diagnostic tests to facilitate selection of patients for these targeted approaches. 3 , 4 , 5
In parallel, veterinary oncology is experiencing similar shifts toward a genomics‐centered approach. Growing knowledge of individual mutations in dogs with cancer supports the utility of genomics for diagnosis, prognostication, and treatment. For example, single gene‐based tests already are available for diagnostic support, such as BRAF mutations for urothelial carcinomas and KIT mutations for mast cell tumors (MCTs). 6 , 7
Genomic mutations also have shown prognostic value in dogs with cancer, such as the TP53 mutation association with shorter survival in dogs with osteosarcoma. 8 Additionally, mutations are being leveraged for their sensitivity to targeted treatments, such as KIT mutations to toceranib phosphate. 9 , 10 Additional mutations have the potential to be targeted in the correct genomic context, as evidenced by early clinical data on BRAF mutations with vemurafenib and HER2 mutations with lapatinib. 11 , 12 However, more robust studies are warranted to prove the clinical benefit of mutation biomarker‐driven targeted treatment.
Although genomics continues to demonstrate its utility for cancers of dogs in the single‐ or multigene setting, a need exists to systematically evaluate the clinical relevance of a holistic mutation profile of individuals or cohorts. Such comprehensive mutation landscape data has become increasingly available as more tumors have been profiled using genome‐wide approaches. 12 , 13 , 14 , 15 , 16 , 17 Furthermore, a recent real‐world clinical genomics study provides an early compelling view of the untapped potential in genomics and precision medicine for dogs with cancer. 18 This study uncovered gene‐level prognostic indications for several cancer genes and the potential association of mutant genes with response to targeted treatments, based on point mutation detection in 48 genes. However, more data is needed to expand the breadth and depth of genomic data utilization for the management of cancer in dogs, including assessment of a broad spectrum of cancer genes and key mutation types, mutation‐level clinical relevance, and, particularly, for assessment of treatment responses in dogs with cancer that are treated based on genomic biomarker information.
Here, we leverage high‐quality clinical and genomic data from 134 cancer‐bearing dogs profiled by Searchlight DNA to find mutation‐level prognostic associations, and, to critically assess the impact of treatment decisions based on patient‐specific mutations. Our objective was to identify genomic and clinical variables that could be associated with progression‐free survival (PFS). In addition, we evaluated the benefit of genomic‐guided targeted treatment in PFS. We hypothesized that at least 1 clinical or genomic variable would be associated with PFS.
2. MATERIALS AND METHODS
2.1. Patient records
In our retrospective observational analysis of data from medical records and genomic reports, Vidium Animal Health's patient database was searched for patients with SearchLight DNA reports delivered between September 2020 and May 2022. Inclusion criteria consisted of the following standards: (1) a definitive cytologic or histologic diagnosis of cancer, (2) at least 3 months since SearchLight DNA analysis was performed, and (3) the identification of at least 1 mutation biomarker in the SearchLight DNA report. Veterinarians whose patients met the inclusion criteria provided outcome data. Veterinarians were given the option of either directly completing an online questionnaire (Table 1) or sending patient records to be reviewed and utilized by Vidium's medical oncologist (EC) to complete the questionnaire on the veterinarian's behalf. The questionnaire provided a systematic and focused collection of key data points, including the initial cancer diagnosis, initial treatments (defined as any treatments given until first progression), first progression, subsequent progression(s), death (or date of last visit, if applicable), and demographic data (including breed, age, and sex). The primary outcome endpoint was progression‐free survival (PFS), which was chosen instead of overall survival to filter out noise associated with variability in uncontrolled clinical cohorts such as that of the current study.
TABLE 1.
Medical records data gathered in the form of an online questionnaire completed for each dog included for clinical outcome analysis.
| Initial cancer diagnosis | Diagnosis and stage (lymph node and distant disease status) |
| Date of diagnosis | |
| Diagnostic tests performed | |
| Initial treatment (s) | Treatments performed (including surgery, radiation therapy, conventional chemotherapy, targeted therapy, immunotherapy, other therapies) |
| Date(s) of treatment(s) | |
| Initial best response (minimal residual disease, complete response, partial response, stable disease, not yet evaluated, no response) | |
| Whether initial therapies were broadly considered standard of care a | |
| First progression | Date of progression |
| Site(s) of progression | |
| Targeted therapy administered at first or any subsequent progression, best response to targeted therapy | |
| Subsequent progression | Date(s) of progression |
| Site(s) of progression | |
| Current status of patient (alive, deceased, lost to follow‐up) | Date of death |
| Type of death (euthanasia or natural; cancer‐related or not) | |
| Date of last follow‐up |
Ad hoc question.
2.2. Genomic data
SearchLight DNA is a cancer NGS gene panel for dogs that utilizes hybrid capture‐based enrichment of canine genes that play a role in cancer to detect single nucleotide variants (SNVs) and small insertions or deletions (INDELs), copy number variants (CNVs), and internal tandem duplications (ITDs) based on tumor‐only sequencing. SearchLight DNA consists of SureSelect probes (Agilent Technologies) targeting 1358 exonic and 429 exon‐proximal regions of 120 cancer genes. SearchLight DNA analysis was performed for each patient as previously described. 19 Briefly, fine‐needle aspirate (FNA) samples or biopsy specimens (fresh frozen, formalin‐fixed, or formalin‐fixed paraffin‐embedded [FFPE] blocks, scrolls, or slides) of suspected tumors were submitted by veterinarians directly or by the veterinarians' reference laboratories to Vidium Animal Health. Once received, samples were processed and embedded if not already performed (for formalin‐fixed or fresh frozen tissues), tumor content was determined, and DNA was extracted as previously described 19 within 2 to 3 days of receipt. Libraries were constructed using Agilent SureSelect XT HS Low Input Target Enrichment with Pre‐Capture Pooling (Agilent Technologies), and were sequenced using Illumina sequencing instruments. DNA sequence data were analyzed using a custom tumor‐only genomics pipeline for SNV, CNV, and ITD identification. All samples were sequenced to at least ×200 coverage. Somatic (tumor‐specific) and putative pathogenic germline mutations were retained for further annotation after filtering of population germline mutations. Each sample passed multiple quality control validation steps, both pre‐ and postsequencing. If a sample failed ≥1 quality control metric, in‐depth manual data review or repeated sampling and sequencing were performed to ensure data quality. All mutations then were used to query a comprehensive proprietary genomic biomarker database (Vidium Insight; Vidium Animal Health) for annotation as biomarkers of diagnosis, prognosis, treatment response or variants of uncertain significance (VUSs) using an automated process, along with supporting evidence levels for each biomarker association based on consensus guidelines used for humans. 20 A diagnostic biomarker was defined as a mutation found to be enriched in a specific tumor type and suspected to contribute to cancer development (ie, pattern of mutation, frequency, functional data, or some combination of these supports their role in cancer). We defined “diagnostic biomarkers” more broadly as those that include any mutations that have been identified in certain cancers (both solid tumors and liquid). A prognostic biomarker was defined as a mutation associated with clinical outcome, and a therapeutic biomarker as a mutation associated with response to a specific treatment.
Each SearchLight DNA report was reviewed by a Vidium genomic scientist (GW). Mutation data and annotations were extracted and harmonized to include gene identification (ID), transcript identification (ID), genomic coordinates, protein coordinates, mutation types (SNVs, CNVs, and ITDs), and variant allele frequency for SNVs and Log2 ratio for CNVs. Validated or predicted pathogenic mutations based on literature and in silico algorithms (FATHMM, SIFT), were selected in subsequent statistical analyses. 21 , 22
2.3. Statistical analysis
Progression‐free survival (PFS) was defined as the time from the date of initial diagnosis to the date of first progression or death, whichever was earlier. Patients were excluded from PFS analysis if PFS could not be determined (eg, dates of diagnosis, first progression, death, or last visit were missing). Patients lost to follow‐up were censored at the time of their last visit. To determine potential association of PFS with patient demographics, genomics, and treatments, the following variables were fitted using univariable and multivariable Cox proportional hazard models: mutated genes, gene: mutation (eg, TP53:copy number loss), cancer diagnosis, age, breed (individual breeds; pure or mixed), sex (male or female), whether targeted treatment was administered (yes or no), and whether the patient received standard of care treatment (SOC; yes, no or unknown). Standard of care treatment was defined as broad standard practice to treat a specific cancer (eg, surgery, systemic treatment) to the best knowledge of a medical oncologist (EC), recognizing that variations of SOC exist among individual clinicians. For gene and mutation analyses, a threshold of genes and mutations present in a minimum of 5 cases was selected. Variables with P value < .2 from univariable analyses were fitted for multivariable analysis. The P values from the log‐rank test and Kaplan‐Meier curves were generated using the R software program and an online tool (https://www.statskingdom.com/kaplan-meier.html), comparing patients with and without significant genes or mutations, patients receiving versus not receiving targeted treatment before first progression or death, and patients receiving genomic‐informed targeted treatment versus genomic‐agnostic treatment (targeted treatment or not). A P value of ≤.05 was considered significant. The follow‐up period was calculated as the time elapsed from the date of diagnosis to the date of death or the date of last visit, whichever came first. For the comparison of cancer type (both solid tumors and liquid) and age distribution among different groups, and evaluating significance between gene co‐occurrences, the Chi‐squared and t‐tests were performed, respectively.
3. RESULTS
3.1. Study population
Questionnaires or medical record requests were sent to 121 veterinarians for 259 patients. Completed questionnaires and received records from 50 veterinarians for 134 patients were reviewed. Three were excluded because SearchLight DNA did not identify any somatic mutations.
3.2. Progression‐free survival
Four patients were excluded from PFS analyses because PFS could not be derived as a consequence of missing data (eg, dates of initial diagnosis, first progression, death, or last visit). The remaining 127 patients were included in the PFS analysis, of which 20 patients were censored because of loss of follow‐up or nonoccurrence of progression before the data collection date, and therefore only the date of last visit was available for these patients. Median and mean PFS for the 127 patients were 113 and 167 days, respectively (range, 5‐959 days). Six patients who were alive at the time of questionnaire completion did not have a date of last visit. The mean and median follow‐up period for the remaining 121 patients were 261 and 173 days, respectively (range, 5‐1596 days). Demographic data and distribution of cancer diagnoses are tabulated in Tables 2 and 3, respectively.
TABLE 2.
Demographic data of 127 dogs included for clinical outcome analysis.
| Variable | n (%) or median (range) |
|---|---|
| Age (years) | 9 (2–18) |
| Sex | |
| Female intact | 3 (2.4) |
| Female spayed | 55 (43.3) |
| Male intact | 4 (3.1) |
| Male castrated | 63 (49.6) |
| Unknown | 2 (1.5) |
| Breed a | n (%) |
|---|---|
| Mixed b | 46 (36.2) |
| Golden Retriever | 11 (8.7) |
| Beagle | 5 (3.9) |
| Labrador Retriever | 5 (3.9) |
| Boxer | 4 (3.1) |
| German Shepherd Dog | 4 (3.1) |
| Boston Terrier | 3 (2.4) |
| Chihuahua | 3 (2.4) |
| Rottweiler | 3 (2.4) |
Breeds represented by ≥3 cases are tabulated.
Included unidentified/unlisted breeds and breeds listed as “other” or “unknown.”
TABLE 3.
Distribution of cancer diagnoses in 127 dogs included for clinical outcome analysis.
| Diagnosis | n (%) |
|---|---|
| Lymphoma a | 18 (14.2) |
| Melanoma b | 13 (10.2) |
| Sarcoma c | 11 (8.7) |
| Hemangiosarcoma d | 10 (7.9) |
| Osteosarcoma e | 9 (7.1) |
| Mast cell tumor f | 8 (6.3) |
| Solid tumor g | 6 (4.7) |
| Mammary carcinoma h | 6 (4.7) |
| Histiocytic sarcoma i | 5 (3.9) |
| Lung carcinoma j | 5 (3.9) |
| Neoplasia k | 5 (3.9) |
| Neuroendocrine carcinoma l | 4 (3.1) |
| Renal carcinoma | 4 (3.1) |
| Hepatocellular carcinoma | 4 (3.1) |
| Anal sac apocrine gland adenocarcinoma | 3 (2.4) |
| Squamous cell carcinoma n | 3 (2.4) |
| Gastrointestinal stromal tumor (cecal) | 2 (1.6) |
| Soft tissue sarcoma m | 2 (1.6) |
| Urothelial carcinoma o | 2 (1.6) |
| Bile duct carcinoma | 1 (0.8) |
| Liposarcoma (subcutis) | 1 (0.8) |
| Mesothelioma | 1 (0.8) |
| Nasal adenocarcinoma | 1 (0.8) |
| Nephroblastoma | 1 (0.8) |
| Ovarian papillary adenocarcinoma | 1 (0.8) |
| Thymic carcinoma | 1 (0.8) |
Types of lymphoma included multicentric B‐cell (n = 8), multicentric T‐cell (n = 3), multicentric non‐immunophenotyped (n = 2), hepatosplenic (not immunophenotyped, n = 1), cutaneous T‐cell (n = 2), cutaneous (not immunophenotyped, n = 1), and mediastinal T‐cell (n = 1).
Melanoma sites included the digit (n = 4), oral mucosa (n = 6), lung (metastatic from malignant oral melanoma [n = 2] or malignant cutaneous melanoma [n = 1]), and lymph node (metastatic from malignant oral melanoma [n = 1]).
Sarcomas arose from multiple sites, including the oral mucosa (n = 1), spleen (n = 3), liver (n = 1), skin (1), kidney (n = 1), lymph node (n = 3), and within the abdomen (originating organ not identified, n = 1).
Hemangiosarcoma sites included the spleen (n = 5), peripheral lymph node (n = 1), subcutis (n = 1), liver (n = 1), and tonsil (n = 1), and atrium (n = 1).
Osteosarcoma sites were rib (n = 1), lungs (metastatic from appendicular osteosarcoma, n = 1), lymph node (metastatic from appendicular osteosarcoma, n = 1), and appendicular (n = 6).
Mast cell tumor sites included the skin (grade 2/high, n = 3; grade 2/low, n = 1; grade 3/high, n = 1), lymph node (metastatic from previous high‐grade cutaneous mast cell tumor [n = 1], and subcutis [n = 2]).
Solid tumors included tumors that did not appear hematopoietic and included those with no specific diagnosis from the kidney (n = 1), skin (n = 1), muscle (metastatic from thyroid carcinoma, n = 1), and lymph node (carcinoma with unknown primary, n = 3).
Mammary carcinomas included simple carcinoma (n = 2), complex carcinoma (n = 1), anaplastic carcinoma (n = 1), and unspecified carcinoma (n = 2).
Histiocytic sarcoma sites included the, oral gingiva (n = 1), synovium (n = 1), liver (n = 1), and spleen (n = 2).
Lung carcinoma included carcinoma (n = 2), poorly differentiated carcinoma (n = 2), adenocarcinoma (n = 1), and bronchoalveolar carcinoma (n = 1).
Neoplasias were defined as such if pathology reports did not provide other definitive descriptors.
Neuroendocrine carcinoma sites included the liver (n = 4) and perispinal mass (n = 1).
Soft tissue sarcomas were grade 2 (n = 1) and grade 3 (n = 1).
Squamous cell carcinomas were from the lymph node (metastatic from previous tonsillar squamous cell carcinoma, n = 1), oral gingiva (n = 1), and frontal sinus (n = 1).
Both urothelial carcinomas were transitional cell carcinomas arising from the trigone of the urinary bladder.
3.3. Specific genes and mutations were significantly associated with PFS
Genomic analysis by Searchlight DNA identified 336 unique mutations in 89 genes, among 127 patients with diagnoses across 26 cancer types (both solid tumors and liquid; Table S1). The frequency of each gene and each mutation were tabulated, and genes and mutations present in >5 cases were included in the PFS univariable analyses. Mutations in 6 genes (CCND1, SMARCB1, FANCG, CDKN2A/B, MSH6, and CCND3) were significantly associated with shorter PFS in univariable analysis (Figure 1).
FIGURE 1.
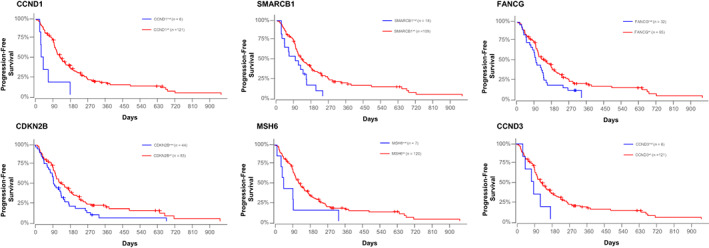
Kaplan‐Meier plots depicting progression‐free survival (PFS) for 127 dogs included for clinical outcome analysis. Each plot represents 1 of the 6 genes (CCND1, SMARCB1, FANCG, CDKN2B, MSH6, and CCND3) significantly associated with shorter PFS in univariate analysis. Blue curves represent dogs with a mutation, and red curves represent dogs without a mutation in the respective gene. Log‐rank P‐values, hazard ratios, and 95% confidence intervals are CCND1: P = .002 (HR, 3.746 [95% CI: 1.621‐8.656]); SMARCB1: P = .01 (HR, 1.984 [95% CI: 1.153‐3.413]); FANCG: P = .02 (HR, 1.652 [95% CI: 1.066‐2.561]); CDKN2B: P = .03 (HR, 1.545 [95% CI: 1.037‐2.303]); MSH6: P = .04 (HR, 2.253 [95% CI: 1.041‐4.877]); CCND3: P = .06 (HR, 2.251 [95% CI: 0.977‐5.184]). Censored patients are identified by a vertical tick mark.
Of the 127 cases that had PFS data, 66 cases harbored mutations in ≥1 of these 6 genes (6‐gene‐positive group), with the remaining 61 cases lacking mutations in these 6 genes (6‐gene‐negative group). Distribution of the genes, mutation types, cancer diagnoses, and age in these 127 patients are plotted in Figure 2. CDKN2A/B were primarily affected by copy number losses, followed by truncating mutations. SMARCB1, FANCG, and MSH6 were primarily affected by copy number losses, whereas CCND1 and CCND3 were altered by a mix of SNVs and copy number gains. Among the 6‐gene‐positive group, significant co‐occurrence of FANCG/CDKN2A/B (P < .001) and MSH6/CDKN2B (P = .002) was observed. The genes FANCG and CDKN2A/B are 10 megabases apart on chromosome 11, thus it is likely that FANCG and CDKN2A/B mutations co‐occurred because of genomic proximity. On the other hand, MSH6 is located on chromosome 10. No significant differences were observed for age (P = .62) and cancer type (P = .38) between the 6‐gene‐positive group and 6‐gene‐negative group.
FIGURE 2.
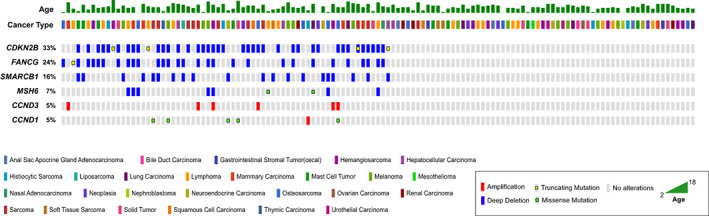
Oncoprint depicting the distribution of genes, mutation types, cancer diagnoses, and age in 127 dogs included for clinical outcome analysis. Each column of bars represents 1 patient.
3.4. Targeted treatment was significantly associated with prolonged PFS
Forty‐five patients received targeted treatment before first disease progression or death, and 82 patients did not receive targeted treatment before progression or death. Significantly improved PFS (P = .01; Figure 3) was found for the 45 patients that received targeted treatment (median PFS, 136 days; mean PFS, 213 days; range, 24‐959) compared to the 82 patients that did not receive targeted treatment (median PFS, 103.5 days; mean PFS, 142 days; range, 5‐647 days). The Kaplan‐Meier tail was visibly prolonged for both groups, although a distinct vertical separation existed in the right two‐thirds of the curve.
FIGURE 3.
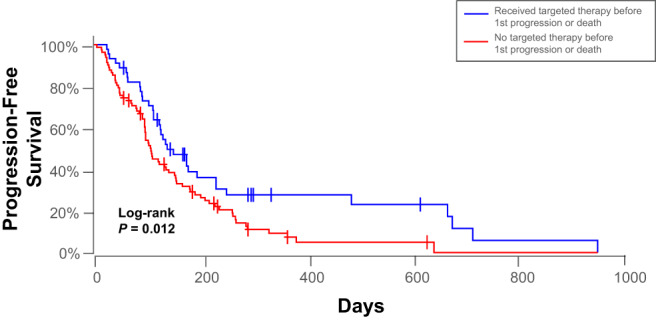
Kaplan‐Meier plots depicting significant differences in progression‐free survival between 45 dogs that received targeted therapy before 1st progression or death (blue) and 82 dogs that did not receive targeted therapy before 1st progression or death. Censored patients are identified by a vertical tick mark.
Of the 45 dogs that received targeted treatment before first progression, the majority of dogs (29/45, 64%) received treatment that was directly informed by genomic results. The other 16 dogs (16/45, 36%) received targeted treatment that was not informed by genomic results. Among these 16 dogs, 2 had started targeted treatment before tumor sample collection for SearchLight DNA analysis, 7 did not have any therapeutic biomarkers identified in their report, 3 had started targeted treatment before tumor sample collection for genomic analysis and did not have any therapeutic biomarkers identified in their genomic report, and 4 received toceranib regardless of therapeutic biomarkers indicating other (non‐toceranib) available targeted therapeutics in the genomic report. The most commonly used targeted treatment in these 16 dogs was toceranib, prescribed in 14/16 (87.50%) dogs. The remaining 2 dogs either received dasatinib or verdinexor.
In the 29 dogs that received genomically informed targeted treatment (median PFS, 139 days; mean PFS, 221 days; range, 24‐721 days), significantly improved survival was found (P = .05; Figure 4) compared to the other 98 dogs (median PFS, 107.5 days; mean PFS, 151 days; range, 5‐959 days). The difference was most marked between 200 and 700 days survival time, showing >30% of the genomically informed cohorts having no progression during this time period, compared with approximately 10% from the other group. In addition, nearly 30% of dogs that received genomically informed targeted treatment survived an additional year or more during the 200 to 700 day period. In these dogs, the most common drug used was olaparib (12/29, 41.40%), followed by sirolimus (8/29, 27.60%), trametinib (5/29, 17.20%), and 1 each of palbociclib (1/29, 3.45%), toceranib (1/29, 3.45%), lapatinib (1/29, 3.45%), or a trametinib/sirolimus combination (1/29, 3.45%; Table 4). For all dogs, other conventional treatments, such as surgery, systemic chemotherapy, radiation therapy, immunotherapy (most commonly Oncept canine melanoma DNA vaccine), or any combination were commonly given with or without targeted treatment (Table S2).
FIGURE 4.
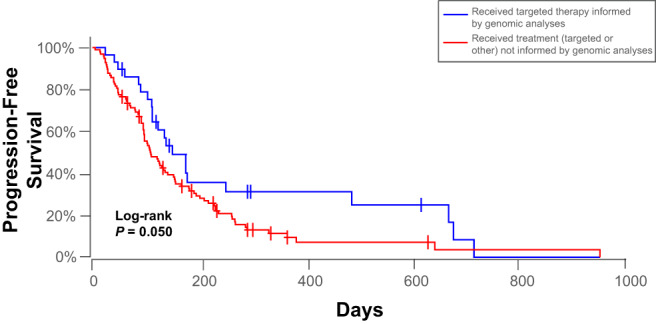
Kaplan‐Meier plots demonstrating significant differences in progression‐free survival between 29 dogs that received genomically informed targeted therapy (blue) and 98 dogs that received treatment that was not informed by genomic analysis (red). Censored patients are identified by a vertical tick mark.
TABLE 4.
Outcomes according to cancer type and dosing of targeted therapies given to 29 dogs that received these in the genomically informed setting.
| Cancer diagnosis | n | Mutation‐informing targeted therapy | Variant Allele frequency or CNV Log2FC | Drug given | Drug dosage | Schedule | Initial best response | Progression‐free survival |
|---|---|---|---|---|---|---|---|---|
| Hemangiosarcoma (cardiac) | 1 | PIK3CA p.His1047Arg | 0.05 | Sirolimus | 0.1 mg/kg | Once daily | Partial response | 124 |
| Hemangiosarcoma (spleen) | 1 | PTEN p.Tyr177fs | 0.08 | Sirolimus | 0.09 mg/kg | Once daily | Minimal residual disease | 113 |
| Hepatic sarcoma | 1 | ATM CNL, CHEK2 CNL | −0.56, −0.47 | Olaparib | 1.4 mg/kg | Twice daily | Minimal residual disease | 136 |
| Lymphoma (cutaneous, not immunophenotyped) | 1 | NRAS CNG | 0.68 | Trametinib | Not reported | Not reported | Stable disease | NA (DEATH) |
| Lymphoma (mediastinal, T cell) | 1 | PTEN p.Arg233 a , PTEN CNL | 0.06, −0.97 | Sirolimus | 0.08 mg/kg | Once daily | Stable disease | 48 |
| Lymphoma (multicentric) | 1 | PTEN CNL | −0.92 | Sirolimus | 0.09 mg/kg | Once daily | Complete response | 151 |
| Mast cell tumor | 1 | KIT ITD | N/A | Toceranib | 2.8 mg/kg | Monday/Wednesday/Friday | Minimal residual disease | 721 |
| Melanoma (digit) | 3 | ATM CNL, BRCA2 CNL | −0.38, 0.34 | Olaparib | 2.5 mg/kg | Once daily | Minimal residual disease | NA (CENSORED) |
| CDK4 CNG | 1.36 | Palbociclib | 0.2 mg/kg | Once daily | Minimal residual disease | NA (CENSORED) | ||
| NRAS p.Gln61Arg | 0.4 | Trametinib | 0.02 mg/kg | Once daily | Minimal residual disease | NA (CENSORED) | ||
| Melanoma (metastatic lung) | 1 | NF1 p.Gln857 a | 0.38 | Trametinib | 0.4 mg/m2 | Once daily | Not evaluated (arrived deceased) | NA (DEATH) |
| Melanoma (oral) | 4 | ATM CNL | −0.76 | Olaparib | 3.16 mg/kg | Once daily | Minimal residual disease | 91 |
| FANCL p.Val13fs | 0.52 | Olaparib | 2.9 mg/kg | Once daily | Complete response | 490 | ||
| ATM p.Glu2815fs, ATM CNL, CHEK2 CNL | 0.69, −0.59, −0.33 | Olaparib | 2.5 mg/kg | Once daily | Partial response | 112 | ||
| NRAS p.Gln61Lys, PTEN CNL | 0.30, −0.44 | Trametinib + Sirolimus | Not reported | Once daily for both | Stable disease | 673 | ||
| Neoplasia (oral mass) | 1 | BRAF p.Val588Glu | 0.19 | Trametinib | 0.5 mg/m2 | Once daily | Partial response | 176 |
| Osteosarcoma (appendicular) | 3 | PALB2 CNL | −0.35 | Olaparib a | 2.8 mg/kg | Once daily | Minimal residual disease | NA (CENSORED) |
| PTEN CNL | −1.29 | Sirolimus | 0.1 mg/kg | Once daily | Minimal residual disease | 179 | ||
| PTEN CNL | −3.24 | Sirolimus | 0.1 mg/kg | Every other day | Minimal residual disease | 41 | ||
| Pulmonary carcinoma | 2 | ERBB2 p.Val662Glu | 0.11 | Lapatinib b | 2 mg/kg | Once daily | Stable disease | 87 |
| BRAF p.Val588Glu | 0.09 | Trametinib | 0.5 mg/m2 | Once daily | Partial response | 113 | ||
| Renal carcinoma | 2 | ATM CNL | −0.79 | Olaparib | 2.33 mg/kg | Once daily | Stable disease | NA (DEATH) |
| ATM CNL | −0.42 | Sirolimus | 0.1 mg/kg | Monday/Wednesday/Friday | Minimal residual disease | NA (CENSORED) | ||
| Renal sarcoma | 1 | ATM CNL, CHEK2 CNL | −1.00, −0.97 | Olaparib | 2.33 mg/kg | Once daily | Minimal residual disease | 176 |
| Sarcoma (splenic stromal) | 1 | CHEK2 CNL | −0.70 | Olaparib | 2.67 mg/kg | Once daily | Lost to Follow‐Up (Censored) | NA (CENSORED) |
| Soft tissue sarcoma | 2 | ATM CNL | −0.57 | Olaparib | 2 mg/kg | Twice daily | Minimal residual disease | NA (CENSORED) |
| TSC2 CNL | −0.72 | Sirolimus | 0.1 mg/kg | Every other day | Stable disease | 682 | ||
| Squamous cell carcinoma (gingiva) | 1 | CHEK2 CNL | −0.83 | Olaparib | 2.6 mg/kg | Once daily | Minimal residual disease | 61 |
| Thymic carcinoma | 1 | ATM CNL, BRCA2 CNL | −0.79, −0.71 | Olaparib | 2.6 mg/kg | Once daily | Minimal residual disease | 139 |
One patient received olaparib 2.8 mg/kg once daily for 7 days starting day of carboplatin chemotherapy; after completion of carboplatin course, this patient received continuous olaparib at the same dose once daily.
This patient received lapatinib 2 mg/kg once daily while receiving concurrent carboplatin chemotherapy; after completion of carboplatin course, this patient received continuous lapatinib 11 mg/kg once daily.
3.5. Variables not associated with PFS
Other variables included in PFS analyses, including cancer diagnosis, patient age, patient breed, patient sex, and whether the patient received SOC, were not significantly associated with PFS.
4. DISCUSSION
Our study represents the first investigation into the association between genomic mutations identified by a comprehensive cancer gene panel and outcomes in dogs with cancer, finding outcomes differences based on whether or not treatment was informed by genomic mutation biomarkers. We found that both genomic and clinical factors were significantly associated with PFS in our cohort of 127 dogs. At the genomic level, mutations in 6 genes were associated with shorter PFS. At the clinical level, dogs that received targeted treatment before first progression experienced longer PFS compared with those that did not receive targeted treatment, and this observation held true for those patients that received targeted treatment that was genomically informed. Moreover, the cases spanned 26 different tumor types, multiple breeds, and a wide range of ages, supporting broad application of these prognostic factors. These findings provide crucial insight into the effectiveness of targeted treatment and the impact of genomic profiling on treatment decision‐making.
Pivotal to our study is the rigor of SearchLight DNA, a comprehensive genomic profiling assay for dogs. The panel's broad coverage of 120 well‐established cancer genes, coupled with the evaluation of CNVs and ITDs in addition to SNVs, allowed for robust detection of genomic mutations linked with cancer. The CNVs are especially important because they account for a large source of mutations in cancers (both solid tumors and liquid) of humans and dogs and comprise many clinically useful biomarker associations. 23 , 24 In addition, multiple sample types, including FFPE, FNA, and fresh frozen samples were included for Searchlight DNA analysis allowing flexibility for the veterinarian with no impact on sample quality and assay performance. Importantly, the Vidium Insight knowledgebase, which drives the biomarker associations in SearchLight DNA, consists of an expanding dataset of genes and biomarkers across diverse cancer types, covering 419 genes, 2842 biomarkers, and over 40 cancer types (both solid tumors and liquid) as of March 2023. To date, SearchLight DNA has sequenced >1300 tumors, with an overall number of mutations of approximately 12 000.
Our study identified novel associations between an unfavorable outcome and mutations in 6 genes: CDKN2A/B, SMARCB1, FANCG, MSH6, CCND1, and CCND3. These genes have established roles in cancer development, and our findings add to the growing body of evidence emphasizing their prognostic relevance. Of note, the majority of mutations identified in these genes are well‐characterized or predicted pathogenic mutations (ie, variants that can cause cancer development or progression, or both) such as copy number loss mutations in the tumor suppressor genes CDKN2A/B, SMARCB1, FANCG, MSH6, and copy number gain mutations in the oncogenes CCND1 and CCND3. 25 , 26 , 27
Each of these genes has an established connection to a wide variety of cancers (both solid tumors and liquid) in humans and dogs. The genes CDKN2A and CDKN2B often are deleted together as a single oncogenic event because of their close proximity on the chromosome, and their inactivation is associated with a poor prognosis in human sarcoma patients and shorter survival in non‐Hodgkin's lymphoma in dogs. 28 , 29 Although little prognostic evidence currently exists for the remaining 3 tumor suppressor genes (SMARCB1, FANCG, and MSH6), they are known cancer genes enriched in human cancer patients or associated with cancer predisposition. 30 , 31 , 32 The oncogenes CCND1 and CCND3 frequently are activated as a result of oncogenic mutations or copy number gains in cancer and have been identified in tumors of dogs, such as osteosarcoma. 33 Despite these known associations, a gap remains in knowledge regarding the impact of these mutations on prognosis. Further research is required to determine their specific roles in cancer prognosis.
Targeted treatment, both in genomically informed and uninformed contexts, significantly improved PFS. The most common targeted treatments prescribed were toceranib, olaparib, sirolimus, and trametinib, each with variable success in improving PFS. Among these, olaparib, sirolimus, and trametinib were informed by prior genomic analysis, suggesting that genomically guided treatment might lead to better outcomes. Conversely, toceranib often was prescribed in a genomically uninformed context, because most of these patients either had genomic analysis performed on a sample collected after toceranib was already instituted empirically, did not have therapeutic biomarkers identified in the genomic analysis or both. Toceranib's use in these settings is understandable, given its open‐label availability, tolerability, and wide use since its FDA approval for treatment of mast cell tumors in dogs. Its efficacy may be related to its broad tyrosine kinase inhibition activity against multiple gene targets, such as KIT, VEGFRA, PDGFRA/B, and FLT3, or potentially unidentified off‐target effects. 10 , 34 , 35 Interestingly, non‐ITD mutations such as copy number gains that likely resulted in KIT activation were identified in 2 of these patients, and their PFS times were 232 and 303 days, contributing to the high median PFS of the cohort receiving targeted treatment.
Genomically informed targeted treatment led to a significant improvement in PFS, lending credence to the importance of genomic profiling in guiding treatment decisions. Approximately 30% of dogs that received genomically informed treatment survived an additional year or more without further progression. Olaparib was the most commonly prescribed drug, likely because of its numerous biomarker associations in our cohort. As a PARP inhibitor that is FDA‐approved in humans for the treatment of BRCA1 or BRCA2 mutant ovarian, breast, and pancreatic cancers, as well as prostate cancers with deleterious mutations in many homologous recombination repair genes (eg, ATM, CDK12, CHEK1, CHEK2, FANCL, and PALB2), olaparib targets many genes in this pathway that are frequently mutated in SearchLight DNA‐sequenced cases. Similarly, sirolimus, as a mammalian target of rapamycin (mTOR) inhibitor, is associated with inactivating mutations in PTEN and TSC2, which are also commonly seen in SearchLight DNA cases. Trametinib, a mitogen‐activated protein kinase kinase (MEK) inhibitor, is frequently reported for its association with mutations in BRAF, NF1, and KRAS/NRAS/HRAS, PTPN11, and PIK3CA. Each of these drugs' contributions to the improvement of PFS may not only have been a result of the relatively high number of patients receiving these drugs, but also could have been a result of effective dosing. The most common olaparib dosage was 2 to 3 mg/kg/day, a dosage below which no dogs showed hematologic toxicity in the new drug application to the FDA. 36 Similarly, the dosages of sirolimus (0.08‐0.1 mg/kg/day) and trametinib (0.4‐0.5 mg/m2/day) have been used safely in small clinical studies of dogs. 37 , 38 , 39 , 40 , 41 Although evaluating adverse effects of targeted treatments was outside the scope of our study, these drugs appeared well tolerated. Whether individual drug doses were efficacious cannot be determined given the relatively small sample size for each drug and the variation of doses and dosing schedules. However, the aggregate of dogs administered genomically informed targeted treatments had significantly improved PFS compared with the remainder of the cohort, suggesting the benefit of targeted treatments when guided by genomic analysis, as corroborated by larger studies in humans. 42 , 43 Moreover, these dosages can be used to inform future biomarker‐driven prospective trials.
Our study had some limitations, including its retrospective nature and small sample size. Sampling and selection biases that could have been selected for patients responding favorably to targeted or other treatments may have been introduced by having veterinarians voluntarily choose to submit cases for the study. Type 1 statistical error also is possible based on the small sample size. Moreover, the selection of drugs, variability in dosing among clinicians, decisions to combine with other treatments, and timing of targeted treatment relative to other therapeutic modalities were clinician‐dependent and therefore nonstandardized across treatments.
In conclusion, we evaluated the use of genomic profiling to improve canine cancer patient survival. We used a robust test that evaluates 120 known cancer‐related genes and captures multiple vital mutation events. We also utilized a structured biomarker annotation system built on a comprehensive and rigorous database with ongoing literature curation, which is critical for standardization of this precision medicine approach. Our findings support the clinical relevance of targeted treatments in veterinary oncology. Despite some potential limitations, our results illustrate the potential for improved PFS in dogs with cancer. As our understanding of genomic biomarkers continues to expand and our repertoire of targeted treatments grows, even more pronounced improvement in patient outcomes hopefully will be possible.
CONFLICT OF INTEREST DECLARATION
Authors are employees of Vidium Animal Health, a subsidiary of the Translational Genomics Research Institute. SearchLight DNA is a product developed and provided by Vidium Animal Health. Dr Min Tang was contracted by Vidium Animal Health to perform statistical analyses.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Table S1. Mutation data arranged in alphabetical order by gene for 127 dogs included in clinical outcome analysis.
Table S2. Other therapies received by 127 dogs included in clinical outcome analysis.
ACKNOWLEDGMENT
No funding was received for this study. We are grateful to Drs Carrie Hume, Cecilia Lopez, Erin Roof, Heidi Ward, Jesse Dawson, Kimberly Shaffer, Krystal Harris, Olivia Stephenson, Roberta Portela, Samantha Bajorek, Zoe Williams, and all veterinary staff for providing cases, clinical follow‐up, and support for this study.
Chon E, Sakthikumar S, Tang M, et al. Novel genomic prognostic biomarkers for dogs with cancer. J Vet Intern Med. 2023;37(6):2410‐2421. doi: 10.1111/jvim.16893
REFERENCES
- 1. Brown NA, Elenitoba‐Johnson KSJ. Enabling precision oncology through precision diagnostics. Annu Rev Pathol. 2020;15:97‐121. [DOI] [PubMed] [Google Scholar]
- 2. Tsimberidou AM, Fountzilas E, Nikanjam M, Kurzrock R. Review of precision cancer medicine: evolution of the treatment paradigm. Cancer Treat Rev. 2020;86:102019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rosenbaum JN, Weisman P. The evolving role of companion diagnostics for breast cancer in an era of next‐generation omics. Am J Pathol. 2017;187:2185‐2198. [DOI] [PubMed] [Google Scholar]
- 4. Ocana A, Ethier JL, Diez‐Gonzalez L, et al. Influence of companion diagnostics on efficacy and safety of targeted anti‐cancer drugs: systematic review and meta‐analyses. Oncotarget. 2015;6:39538‐39549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yoo C, Park YS. Companion diagnostics for the targeted therapy of gastric cancer. World J Gastroenterol. 2015;21:10948‐10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thamm DH, Avery AC, Berlato D, et al. Prognostic and predictive significance of KIT protein expression and c‐KIT gene mutation in canine cutaneous mast cell tumours: a consensus of the Oncology‐Pathology Working Group. Vet Comp Oncol. 2019;17:451‐455. [DOI] [PubMed] [Google Scholar]
- 7. Mochizuki H, Shapiro SG, Breen M. Detection of BRAF mutation in urine DNA as a molecular diagnostic for canine urothelial and prostatic carcinoma. PloS One. 2015;10:e0144170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kirpensteijn J, Kik M, Teske E, et al. TP53 gene mutations in canine osteosarcoma. Vet Surg. 2008;37:454‐460. [DOI] [PubMed] [Google Scholar]
- 9. Gil da Costa RM. C‐kit as a prognostic and therapeutic marker in canine cutaneous mast cell tumours: from laboratory to clinic. Vet J. 2015;205:5‐10. [DOI] [PubMed] [Google Scholar]
- 10. London CA, Malpas PB, Wood‐Follis SL, et al. Multi‐center, placebo‐controlled, double‐blind, randomized study of oral toceranib phosphate (SU11654), a receptor tyrosine kinase inhibitor, for the treatment of dogs with recurrent (either local or distant) mast cell tumor following surgical excision. Clin Cancer Res. 2009;15:3856‐3865. [DOI] [PubMed] [Google Scholar]
- 11. Rossman P, Zabka TS, Ruple A, et al. Phase I/II trial of Vemurafenib in dogs with naturally occurring, BRAF‐mutated urothelial carcinoma. Mol Cancer Ther. 2021;20:2177‐2188. [DOI] [PubMed] [Google Scholar]
- 12. Lorch G, Sivaprakasam K, Zismann V, et al. Identification of recurrent activating HER2 mutations in primary canine pulmonary adenocarcinoma. Clin Cancer Res. 2019;25:5866‐5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thomas R, Wiley CA, Droste EL, Robertson J, Inman BA, Breen M. Whole exome sequencing analysis of canine urothelial carcinomas without BRAF V595E mutation: short in‐frame deletions in BRAF and MAP2K1 suggest alternative mechanisms for MAPK pathway disruption. PLoS Genet. 2023;19:e1010575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sakthikumar S, Elvers I, Kim J, et al. SETD2 is recurrently mutated in whole‐exome sequenced canine osteosarcoma. Cancer Res. 2018;78:3421‐3431. [DOI] [PubMed] [Google Scholar]
- 15. Wang G, Wu M, Maloneyhuss MA, et al. Actionable mutations in canine hemangiosarcoma. PLoS One. 2017;12:e0188667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shapiro SG, Raghunath S, Williams C, et al. Canine urothelial carcinoma: genomically aberrant and comparatively relevant. Chromosome Res. 2015;23:311‐331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Elvers I, Turner‐Maier J, Swofford R, et al. Exome sequencing of lymphomas from three dog breeds reveals somatic mutation patterns reflecting genetic background. Genome Res. 2015;25:1634‐1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wu K, Rodrigues L, Post G, et al. Analyses of canine cancer mutations and treatment outcomes using real‐world clinico‐genomics data of 2119 dogs. npj Precis Oncol. 2023;7:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chon E, Wang G, Whitley D, et al. Genomic tumor analysis provides clinical guidance for the management of diagnostically challenging cancers in dogs. J Am Vet Med Assoc. 2023;261:668‐677. [DOI] [PubMed] [Google Scholar]
- 20. Li MM, Datto M, Duncavage EJ, et al. Standards and guidelines for the interpretation and reporting of sequence variants in cancer: a joint consensus recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J Mol Diagn. 2017;19:4‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shihab HA, Rogers MF, Gough J, et al. An integrative approach to predicting the functional effects of non‐coding and coding sequence variation. Bioinformatics. 2015;31:1536‐1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ng PC, Henikoff S. SIFT: predicting amino acid changes that affect protein function. Nucleic Acids Res. 2003;31:3812‐3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Harbers L, Agostini F, Nicos M, Poddighe D, Bienko M, Crosetto N. Somatic copy number alterations in human cancers: an analysis of publicly available data from the cancer genome atlas. Front Oncol. 2021;11:700568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shlien A, Malkin D. Copy number variations and cancer. Genome Med. 2009;1:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McNeal AS, Liu K, Nakhate V, et al. CDKN2B loss promotes progression from benign melanocytic nevus to melanoma. Cancer Discov. 2015;5:1072‐1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Klochendler‐Yeivin A, Fiette L, Barra J, Muchardt C, Babinet C, Yaniv M. The murine SNF5/INI1 chromatin remodeling factor is essential for embryonic development and tumor suppression. EMBO Rep. 2000;1:500‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mueller A, Odze R, Jenkins TD, et al. A transgenic mouse model with cyclin D1 overexpression results in cell cycle, epidermal growth factor receptor, and p53 abnormalities. Cancer Res. 1997;57:5542‐5549. [PubMed] [Google Scholar]
- 28. Bui NQ, Przybyl J, Trabucco SE, et al. A clinico‐genomic analysis of soft tissue sarcoma patients reveals CDKN2A deletion as a biomarker for poor prognosis. Clin Sarcoma Res. 2019;9:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Modiano JF, Breen M, Valli VE, et al. Predictive value of p16 or Rb inactivation in a model of naturally occurring canine non‐Hodgkin's lymphoma. Leukemia. 2007;21:184‐187. [DOI] [PubMed] [Google Scholar]
- 30. Rogers CD, van der Heijden MS, Brune K, et al. The genetics of FANCC and FANCG in familial pancreatic cancer. Cancer Biol Ther. 2004;3:167‐169. [DOI] [PubMed] [Google Scholar]
- 31. Kariola R, Raevaara TE, Lonnqvist KE, et al. Functional analysis of MSH6 mutations linked to kindreds with putative hereditary non‐polyposis colorectal cancer syndrome. Hum Mol Genet. 2002;11:1303‐1310. [DOI] [PubMed] [Google Scholar]
- 32. Sevenet N, Sheridan E, Amram D, et al. Constitutional mutations of the hSNF5/INI1 gene predispose to a variety of cancers. Am J Hum Genet. 1999;65:1342‐1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Das S, Idate R, Regan DP, et al. Immune pathways and TP53 missense mutations are associated with longer survival in canine osteosarcoma. Commun Biol. 2021;4:1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. London CA, Hannah AL, Zadovoskaya R, et al. Phase I dose‐escalating study of SU11654, a small molecule receptor tyrosine kinase inhibitor, in dogs with spontaneous malignancies. Clin Cancer Res. 2003;9:2755‐2768. [PubMed] [Google Scholar]
- 35. Liao AT, Chien MB, Shenoy N, et al. Inhibition of constitutively active forms of mutant kit by multitargeted indolinone tyrosine kinase inhibitors. Blood. 2002;100:585‐593. [DOI] [PubMed] [Google Scholar]
- 36. Olaparib New Drug Application Food & Drug Administration; 2014. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/206162Orig1s000PharmR.pdf
- 37. Takada MMS, Jones A, Onsager A, Borgen J, Yuzbasiyan‐Gurkan V, Vail D. Phase I clinical trial to evaluate the tolerability of trametinib in dogs with cancer. In: Veterinary Cancer Society Annual Meeting, Norfolk, Virginia; 2022:44.
- 38. LeBlanc AK, Mazcko CN, Cherukuri A, et al. Adjuvant Sirolimus does not improve outcome in pet dogs receiving standard‐of‐care therapy for appendicular osteosarcoma: a prospective, randomized trial of 324 dogs. Clin Cancer Res. 2021;27:3005‐3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Urfer SR, Kaeberlein TL, Mailheau S, et al. A randomized controlled trial to establish effects of short‐term rapamycin treatment in 24 middle‐aged companion dogs. Geroscience. 2017;39:117‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yi H, Brooks ED, Thurberg BL, Fyfe JC, Kishnani PS, Sun B. Correction of glycogen storage disease type III with rapamycin in a canine model. J Mol Med (Berl). 2014;92:641‐650. [DOI] [PubMed] [Google Scholar]
- 41. Paoloni MC, Mazcko C, Fox E, et al. Rapamycin pharmacokinetic and pharmacodynamic relationships in osteosarcoma: a comparative oncology study in dogs. PLoS One. 2010;5:e11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Andre F, Filleron T, Kamal M, et al. Genomics to select treatment for patients with metastatic breast cancer. Nature. 2022;610:343‐348. [DOI] [PubMed] [Google Scholar]
- 43. Le Tourneau C, Delord JP, Goncalves A, et al. Molecularly targeted therapy based on tumour molecular profiling versus conventional therapy for advanced cancer (SHIVA): a multicentre, open‐label, proof‐of‐concept, randomised, controlled phase 2 trial. Lancet Oncol. 2015;16:1324‐1334. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Mutation data arranged in alphabetical order by gene for 127 dogs included in clinical outcome analysis.
Table S2. Other therapies received by 127 dogs included in clinical outcome analysis.


