Abstract
Background:
Infusion set function remains the limiting factor of insulin pump therapy due to nonmetabolic complications. Here, we tested an investigational extended-wear infusion set prototype with a soft, angled, wire-reinforced cannula with three additional side holes, and compared failure mechanisms and tissue response with a commercial Teflon control.
Methods:
A total of 48 Teflon and 48 prototype infusion sets were inserted subcutaneously every other day for 14 days in 12 swine and infused with dilute insulin. After two weeks, tissue around cannulas was excised, and occlusions, leaks, and kinks were determined. Tissue was processed and stained to assess the total area of inflammation (TAI) and the inflammatory layer thickness (ILT) around the cannulas. Data were analyzed using Fisher’s exact, analysis of variance-general linear model, Kruskal-Wallis, and post hoc tests.
Results:
On average, the TAI surrounding the investigational cannula was 52.6% smaller than around the commercial control. The ILT was 66.3% smaller around investigational cannulas. Kinks occurred in 2.1% (investigational) vs 32.4% (commercial) cannulas (P < .001). There was no difference in occlusion alarms and leaks onto skin.
Conclusions:
The data suggest that the infusion set prototype elicits less inflammation over an extended wear time and is resistant to kinking, compared with a commercial Teflon device. This is consistent with previously published data on the impact of cannula material/angle on the inflammatory tissue response. We highlight the following important aspects of infusion set design: (1) secure skin adhesion, (2) reliable cannula insertion, (3) automatic removal of the stylet, (4) cannula material/design that resists kinking, and (5) minimization of local tissue inflammation.
Keywords: insulin infusion set, insulin pump therapy, CSII therapy, swine model, extended-wear infusion set, inflammatory tissue response
Continuous subcutaneous (SC) insulin infusion using an insulin infusion set (IIS) and open-loop or hybrid closed-loop system has shown the best outcome for people living with type 1 diabetes as tight glycemic control reduces long-term complications.1-3 Nonetheless, the maintenance of the insulin infusion site remains the limiting factor of insulin pump therapy due to a series of nonmetabolic complications, such as local SC tissue inflammation, insulin infusion cannula dislodgement, cannula bending/kinking, and fibrous scar formation.4,5 Localized tissue damage and inflammation have a variable effect on the delivery of insulin into the adjacent vascular tissue and the absorption of insulin from the SC space into the circulation, and have been shown to increase with IIS wear time.6-9 Previous animal studies from our group have shown that insulin may leak into the infusion set hub, leak onto the skin surface due to superficial cannula insertion, or reflux from the SC tissue onto the skin surface due to noncompliant inflammatory tissue that surrounds the inserted cannula.6,8 In addition, insulin flow into the adjacent vascular tissue may be partially or completely obstructed by cannula kinking/bending.5,10,11
Variable insulin absorption makes it difficult for the insulin pump user to maintain good glycemic control and avoid hypoglycemic and hyperglycemic events. Although changing out an IIS every two to three days remains the general recommendation, many users choose to prolong wear time, when possible.12-14 With the advent of automated insulin delivery systems, the combination of both glucose measurement and insulin delivery in a single device seems the ultimate goal to decrease the number of cannula insertions and associated complications. As continuous glucose monitoring (CGM) devices can be worn for seven days or longer, there is an urgent need to extend the wear time of IISs, to realize a single-device approach. In this iterative, preclinical study in nondiabetic swine, we evaluated a novel extended-wear IIS prototype with a soft and flexible, cannula material, kink-resistant coil reinforcement, and three additional side holes for enhanced insulin delivery compared with a commercial Teflon control IIS. We hypothesized that this new cannula design would elicit less SC tissue trauma and inflammation and may decrease the incidence of IIS failure modes, such as kinking, occlusion, and insulin leakage.
Methods
Investigational Extended-Wear Insulin Infusion Set Prototype
The investigational extended-wear insulin infusion set (investigational IIS) prototype was developed through iterative in vitro and in vivo studies (data not shown) by Capillary Biomedical Inc (Irvine, California) and Thomas Jefferson University (TJU, Philadelphia, Pennsylvania). The investigational IIS contained a soft polymer (Nylon-derivative), 13.5-mm-long cannula with a steel coil–reinforced wall, one distal hole, and three proximal holes spaced 2 mm apart in a helical pattern (Figure 1a). The wire-reinforced cannula wall eliminates the risk of flow obstruction due to kinking or bending (Figure 1b). Multiple holes along the cannula shaft provide redundancy: If one hole becomes obstructed, insulin can still leave the cannula and spread into the surrounding SC tissue along the path of least resistance. Flow through multiple holes delivers insulin into adjacent vascular SC tissue with a greater surface area (Figure 1a) compared with a single distal hole. The investigational IIS prototypes were manufactured by Capillary Biomedical in accordance with relevant quality system regulations (21 CFR 820; Code of Federal Regulations). The investigational IIS base, tubing, and insulin pump connector were packaged and sterilized for animal use. As this was an iterative study, different methods were utilized to insert the investigational IIS cannula into the SC tissue of the swine’s abdomen:
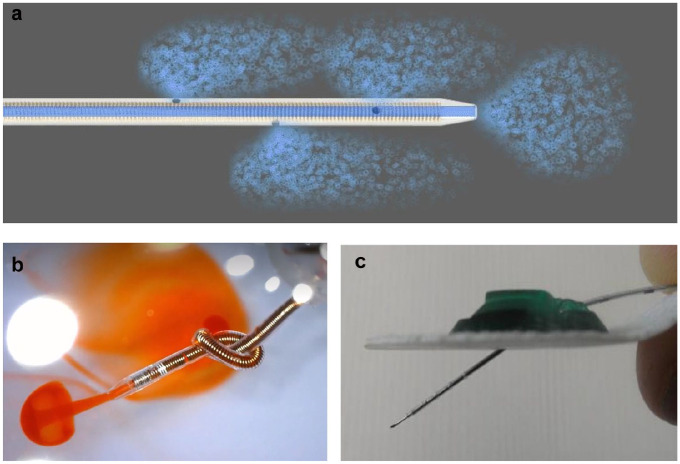
Figure 1.
(a) Schematic representation of insulin flow (blue) through the investigational prototype extended-wear cannula and out through the four holes. (b) The coil-reinforced wall guarantees flexibility and kink resistance of the cannula. This demonstration shows orange dye flowing through the four holes, even when the 24-gauge cannula is tied in a knot. (c) The investigational IIS prototype with a soft Nylon-derivative cannula and stiff stainless-steel stylet with a sharp needle distal tip. Markings facilitated manual insertion of the 13.5-mm-long cannula into the subcutaneous tissue at an angle of approximately 35°. The stylet was removed after insertion.
Abbreviation: IIS, insulin infusion set.
Method A (24/48 devices): The investigational IIS cannula was inserted manually at an angle of approximately 35° after making a pilot hole using a 22- or 24-gauge sharp needle (Beckton Dickinson, Franklin Lakes, New Jersey) through the epidermis and dermis. The cannula was manually advanced using a guiding needle (stylet) to the appropriate depth (marked on the cannula in black) before attaching the green base (Figure 1c) over the cannula. The stylet was manually removed after insertion into the SC tissue, taking care not to move the inserted cannula.
Method B (24/48 devices): The investigational IIS cannula was inserted at an angle of approximately 35° using a first-generation spring-loaded automated inserter (Capillary Biomedical, Irvine, California). After adhering the IIS base to the skin surface, the inserter’s spring mechanism advanced the needle (stylet) and cannula through the epidermis and dermis to the appropriate depth and location within the SC tissue. The stylet was manually retracted without moving the inserted cannula. Commercial Insulin Infusion Set (Control IIS). The commercial IISs were manufactured by ConvaTec (Deeside) and distributed by Animas Corporation (West Chester, Pennsylvania) as the Inset Infusion System®; and by Medtronic MiniMed (Northridge, California) as the MiniMed Quick-set®. The commercial IIS had a 6-mm nonflexible Teflon cannula with one distal hole that was inserted using an automated spring-loaded inserter at a 90° angle. After adhering the IIS base to the skin surface, the inserter’s spring mechanism advanced the needle (stylet) and cannula through the epidermis and dermis to the appropriate depth and location within the SC tissue. The device automatically removed the stylet from the tissue without moving the inserted cannula.
In Vivo Study and Statistics
The in vivo study methods have been described in detail elsewhere. 8 Twelve healthy nondiabetic Yorkshire female swine, aged three to six months and weighing 60 to 70 kg, were used as a model of human SC tissue.15,16 The study and all procedures were approved by the TJU Institutional Animal Care and Use Committee. As this was an iterative research program to drive development of a novel extended-wear IIS, there was no formal power calculation conducted. Animals were given one week of acclimation before study start. One investigational IIS and one commercial IIS were inserted into the SC tissue of the swine’s abdomen every other day for two weeks using aseptic technique. Both types of IISs were protected by a layer of Tegaderm bandage, kinesiology athletic tape, several layers of elastic stockinet, and a custom swine vest with 12 pockets. Each IIS was connected to an insulin pump (OneTouch® Ping®, Animas Corporation, West Chester, Pennsylvania or Paradigm® REAL-Time RevelTM, Medtronic MiniMed, Northridge, California). Dilute insulin lispro/Humalog (Eli Lilly, Indianapolis, Indiana) was continuously infused at a concentration of 5 units/mL (U-5) at a rate of 0.05 units/h. Insulin was diluted with commercial sterile diluent containing phenol and m-Cresol (Eli Lilly, Indianapolis, Indiana). A 70-μL bolus of dilute insulin was given over 45 seconds twice daily (before each meal) to mimic a pump user’s routine. In summary, dilute insulin was infused through six investigational IISs and six commercial IISs using the same basal/bolus pattern of delivery at the end of each 14-day study. Our goal was to deliver insulin into the SC tissue using a fluid volume and pattern of delivery similar to an adult human with type 1 diabetes. Dilute insulin was required to minimize the risk of symptomatic hypoglycemia in the nondiabetic swine. Interstitial glucose concentration was monitored using two commercial CGMs (Dexcom G4 Platinum, Dexcom, Inc, San Diego, California) with alarms for hypoglycemia and telemetry to staff cell phones. Blood glucose (BG) levels were monitored with intermittent capillary BG measurements using a commercial glucose meter and test strips (Bayer Contour Next, Parsippany, New Jersey).
After 14 days, swine were placed under general anesthesia and a final 70-μL bolus of dilute insulin and X-ray contrast agent (70% U-100 lispro, 30% IsoVue 300) was infused through each IIS using an insulin pump (OneTouch Ping). Occlusion alarms were recorded if they occurred. For subsequent micro-CT imaging and histopathological analysis, the insulin infusion cannula and surrounding skin and SC tissue were excised ten minutes after the bolus infusion, and each specimen was gently frozen in isopentane cooled with dry ice. Thereafter, the swine was euthanized by intravenous injection of Beuthanasia (1 mL/4.5 kg body weight) solution while under general anesthesia. Insulin infusion set-tissue specimens were imaged using a micro-CT scanner (Inveon, Siemens Medical Solutions USA, Knoxville, Tennessee) to determine cannula kinks/bends and cannula leakage onto the skin surface (RadiAnt DICOM Viewer, Medixant). A kink was defined as a bend in the cannula >90°. Following micro-CT imaging, IIS-tissue specimens were processed, and stained with Masson’s Trichrome to measure the total area of inflammation (TAI) and inflammatory layer thickness (ILT) surrounding the cannula. For the histology measurements, specimens with at least 25% cannula channel view were considered for data analysis. The histology slides were analyzed by TJU research personnel, averaging multiple measurements (see Eisler et al 2019 Supplementary Material I for detailed description of histopathological analyses). 8
Data were analyzed using analysis of variance-general linear model and the Kruskal-Wallis Test (Systat Version 13 and SPSS Version 25). Post hoc analyses were performed using the Tukey honestly significant difference test and pairwise comparisons for nonparametric data were performed using Dwass-Steel-Chritchlow-Fligner test. Comparison of failure mechanisms was done by means of Fisher’s exact testing. A P < .05 was set for statistical significance.
Results
No swine developed an infection or inflammation requiring IIS removal or analgesic medication. No animal developed symptomatic hypoglycemia. Micro-CT data from one animal could not be used for analysis of failure mechanisms (kink/leak/occlusion) due to a problem with the X-ray contrast solution. Data from 11 animals were included in the failure analysis, summarized in Table 1 and Figure 2.
Table 1.
Cannula Kinks, Leaks Onto the Skin Surface, and Occlusions (Per Animas Insulin Pump Alarm) by Infusion Set Type.
| Kink | Ctrl. IIS | 13/40 | 32.5% | P = .0002 |
| Inv. IIS | 1/48 | 2.1% | ||
| Leak | Ctrl. IIS | 3/40 | 7.5% | P = .326 |
| Inv. IIS | 7/48 | 14.6% | ||
| Occlusion (pump 3alarm) | Ctrl. IIS | 1/40 | 2.5% | P = 1 |
| Inv. IIS | 1/48 | 2.1% |
Total percentage is calculated based on number of infusion sets included in the analysis (40 commercial IISs with a Teflon cannula inserted at a 90° angle, 48 investigational IIS prototypes with a Nylon cannula inserted at a 35° angle).
Abbreviations: IIS, insulin infusion set; Inv. investigational; Ctrl., control.
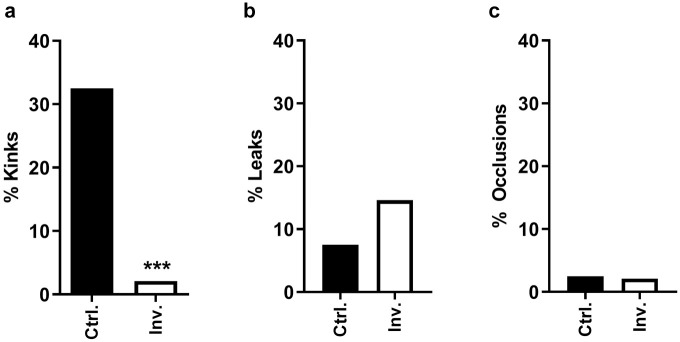
Figure 2.
Insulin infusion set failure mechanisms. (a) Percentage of cannula kinks/bends. (b) Percentage of leaks onto the skin surface (all types included). (c) Percentage of cannula occlusions as evidenced by insulin pump alarm.
Abbreviations: Inv., investigational; Ctrl., control.
The 32.5% (13/40) of the commercial Teflon IIS cannulas kinked, while only 2.1% (1/48) of the investigational IIS cannulas revealed a bend >90° in micro-CT imaging (P < .001). The one investigational IIS cannula with a significant bend was inserted using the first-generation spring-loaded inserter which did not deploy sufficient force to advance the cannula through the dermis to the appropriate depth in the SC tissue. The cannula was manually advanced using forceps which resulted in the bend. Only one of the kinked commercial Teflon cannulas (four days of wear time) caused an insulin pump occlusion alarm.
Leakages of insulin/X-ray contrast agent onto the skin surface (between the skin and the adhesive) were more common for investigational IIS specimens than for Teflon controls (7/48 versus 3/40).
However, upon more thorough investigation (analysis of insertion videos, re-revision of micro-CT 3D images, and histology), we identified three types of leaks: (1) leakages through the most proximal hole (ie, closest to plastic base) due to superficial insertion of the investigational IIS cannula (2/48), (2) leakages into the external investigational IIS hub due to malalignment of the insulin tubing pin and septum after insertion with the prototype spring-loaded inserter (1/48), and (3) contrast agent refluxing up the cannula shaft due to the layer of inflammatory tissue causing a physical barrier in the SC tissue (4/48 investigational IISs and 3/40 control IISs). Insertion method A caused leakage type 1, while insertion method B caused leakage type 2, both of which caused insulin/contrast agent to leak into the IIS hub and onto the skin surface, as evidenced by micro-CT imaging (Figure 3a and 3b). Identifying the mechanical causes of the type 1 and type 2 leaks was key to redesigning the investigational IIS hub connection and automated inserter to ensure reliable device performance.
Figure 3.
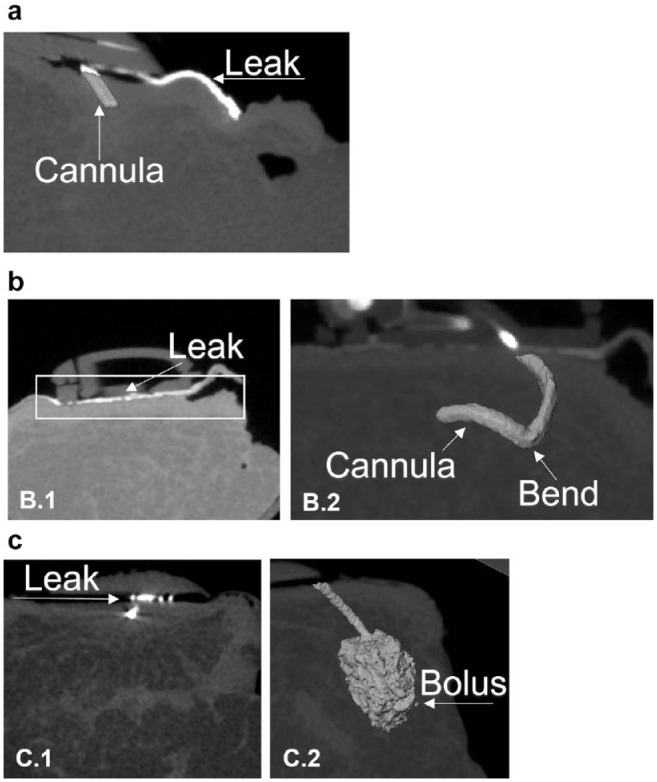
Types of leaks using micro-CT imaging. (a) “Type 1 leak”: Incomplete and superficial cannula insertion caused the most proximal hole to remain above the skin surface, and insulin/X-ray contrast (white) leaked on the IIS adhesive and skin surface. No insulin/contrast was delivered into the SC tissue. (b) “Type 2 leak”: The first-generation spring-loaded inserter caused incomplete and superficial cannula insertion. Subsequent manual advancement of the cannula into the SC tissue using forceps led to both a bend in the cannula (b2) and a misalignment between the IIS tubing pin and the septum within the IIS hub, causing insulin/contrast to leak into the hub. No insulin/contrast was delivered into the SC tissue (b1). (c) “Type 3 leak”: High resistance to flow around a Teflon control IIS cannula caused insulin/contrast to reflux from the SC tissue onto the skin surface, along the path of least resistance (c1). In general, the volume of insulin/contrast that refluxed from the SC tissue onto the skin surface was minimal, as evidenced by the large SC bolus (c2). Note, that because of the angled cannula, it is only possible to view the leak and the bolus in two separate 2D images—c1 and c2 show the same cannula from different angles/depths in the SC tissue.
Abbreviations: CT, computerized tomography; IIS, insulin infusion set; SC, subcutaneous.
Of clinical significance, reflux upward from the SC tissue onto the skin surface caused negligible loss of insulin/contrast agent (approximately 5 µL of a 70 µL bolus; Figure 3c) in both the investigational IIS and commercial control IIS tested. The percentage of type 3 leakages from the SC tissue upward onto the skin surface was comparable between the groups (3/40 [7.5%] control IISs and 4/48 [8.3%] investigational IISs). There was no pattern between wear time and type 3 leakage for the investigational IIS; one IIS leaked immediately after insertion, others leaked on days 2, 12, and 14 of wear time. In the Teflon control group, type 3 leakage occurred with two IISs worn for eight days and one IIS worn for two days. The control IIS that had a type 3 leak on day 8 of wear time had a kink in the Teflon cannula that did not cause a pump occlusion alarm.
There was no difference in the occurrence of pump occlusion alarms between IIS types (Table 1). One pump occlusion alarm occurred in the commercial control group with an IIS indwelling for four days and one alarm occurred in the investigational IIS group with an IIS indwelling for 14 days.
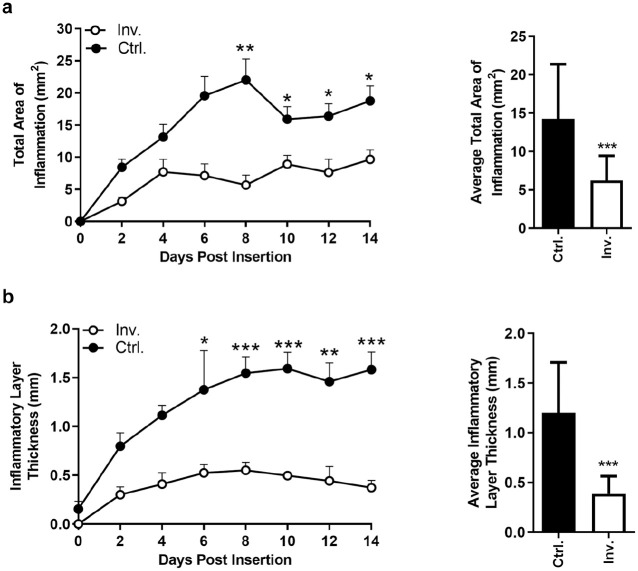
All IIS-tissue specimens were excised from the 12 animals without difficulty. In total, 33 investigational IIS and 46 commercial control IIS specimens with >25% channel view were included in the final histology analysis after review and approval by the TJU investigators. Of note, it was more challenging to locate the insertion channel from a tissue specimen with an angled investigational IIS cannula compared with the straight commercial control IIS cannula. Over 14 days of IIS wear time, TAI and ILT were consistently larger around the commercial Teflon cannula, with a statistically significant difference after six (ILT) and eight days (TAI) of IIS wear time, respectively (Figure 4). On average, the TAI was 52.6% smaller (6.23 vs 14.27 mm2, P < .001) and the ILT was 66.3% smaller (0.39 vs 1.20 mm, P < .001) around the angled investigational IIS cannula, and at least 56% (ILT) smaller or 41% (TAI) smaller on individual days compared with commercial Teflon cannula (Table 2). Figure 5 shows examples of stained SC tissue histology around commercial Teflon IIS cannula and investigational IIS cannula and the progression of inflammation over 14 days of wear time. Of interest, there was no statistically significant difference in TAI and ILT between insertion methods A and B (data not shown).
Figure 4.
Subcutaneous inflammatory tissue response to the insulin infusion cannula. (a) TAI (tissue disruption, immune cells, bleeding, etc) around the cannula insertion channel. The TAI around the investigational IIS cannula (Inv.) stabilizes after four days of wear time at a low level, while the TAI around the control Teflon cannula (Ctrl.) continues to increase, and peaks on day 8 of wear time. (b) ILT (cellular debris, immune cells, collagen deposition, etc) around the inserted IIS cannula. The ILT around the investigational IIS cannula stabilizes at a low level at four to six days of wear time, while the ILT around the control Teflon cannula continues to increase in thickness and peaks around day 10 of wear time.
Abbreviations: TAI, total area of inflammation; IIS, insulin infusion set; Inv., investigational; Ctrl., control; ILT, inflammatory layer thickness.
*P < .05. **P < .005. ***P < .001.
Table 2.
ILT and TAI by Day of Wear Time and Infusion Set Type.
| Day | IIS type | Mean ± SD | % difference |
|---|---|---|---|
| ILT (mm) | |||
| 0 | Inv. | 0.00 ± 0.00 | 00.00 |
| Ctrl. | 0.00 ± 0.00 | ||
| 2 | Inv. | 0.18 ± 0.05 | −77.82 |
| Ctrl. | 0.83 ± 0.28 | ||
| 4 | Inv. | 0.48 ± 0.25 | −60.21 |
| Ctrl. | 1.20 ± 0.29 | ||
| 6 | Inv. | 0.45 ± 0.05 | −70.58 |
| Ctrl. | 1.52 ± 0.77 | ||
| 8 | Inv. | 0.51 ± 0.15 | −68.33 |
| Ctrl. | 1.63 ± 0.38 | ||
| 10 | Inv. | 0.56 ± 0.26 | −64.08 |
| Ctrl. | 1.57 ± 0.34 | ||
| 12 | Inv. | 0.62 ± 0.21 | −56.44 |
| Ctrl. | 1.43 ± 0.39 | ||
| 14 | Inv. | 0.51 ± 0.25 | −66.47 |
| Ctrl. | 1.52 ± 0.34 | ||
| TAI (mm2) | |||
| 0 | Inv. | 0.00 ± 0.00 | 00.00 |
| Ctrl. | 0.00 ± 0.00 | ||
| 2 | Inv. | 3.13 ± 1.28 | −62.90 |
| Ctrl. | 8.43 ± 3.11 | ||
| 4 | Inv. | 7.71 ± 3.92 | −41.27 |
| Ctrl. | 13.13 ± 4.83 | ||
| 6 | Inv. | 8.93 ± 0.87 | −54.33 |
| Ctrl. | 19.55 ± 6.71 | ||
| 8 | Inv. | 5.67 ± 3.09 | −74.22 |
| Ctrl. | 22.01 ± 8.05 | ||
| 10 | Inv. | 8.92 ± 3.34 | −43.94 |
| Ctrl. | 15.91 ± 4.77 | ||
| 12 | Inv. | 9.34 ± 2.99 | −43.00 |
| Ctrl. | 16.38 ± 4.75 | ||
| 14 | Inv. | 9.65 ± 3.33 | −48.56 |
| Ctrl. | 18.77 ± 5.18 | ||
The difference between values in % is shown in the last column. IISs were freshly inserted on day 0.
Abbreviations: ILT, inflammatory layer thickness; TAI, total area of inflammation; IIS, insulin infusion set; Inv., investigational; Ctrl., control; SD, standard deviation.
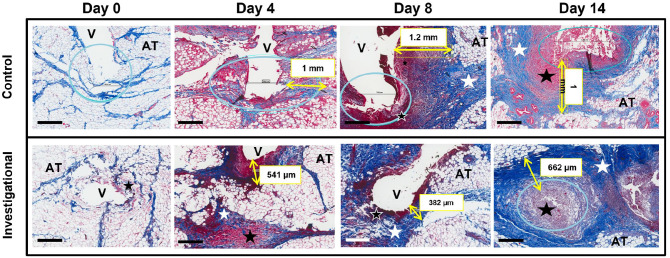
Figure 5.
Progression of the acute inflammatory response in the subcutaneous tissue around the insulin infusion set cannula over 14 days of wear time. The black bar in the left lower corner is equal to 500 µm. After two hours of cannula insertion (day 0), the SC tissue is merely disrupted by stylet/cannula insertion with no cellular infiltration. On day 4 of wear time, there is infiltration of inflammatory cells and fibrin (dense red areas) and collagen deposition (light blue). The area around the cannula contains red blood cells, platelets, fibrin clot, immune cells, damaged adipose cells, and connective tissue. By day 8 and eventually day 14, there is a dense layer of inflammatory tissue and collagen deposited around the cannula.
Abbreviations: blue circle, cannula tip; yellow arrow and box, inflammatory layer thickness; white star, collagen deposition; black star, inflamed abnormal tissue; AT, healthy adipose tissue; V, void caused by cannula insertion channel; SC, subcutaneous.
Discussion
In this study, we systematically compared the inflammatory tissue response elicited by the insertion and maintenance of a novel extended-wear insulin infusion set prototype with a soft flexible angled cannula to that of a commercial control IIS with a straight, more rigid Teflon cannula.
Our data suggest that the novel cannula design of the investigational IIS causes less tissue damage and inflammation over an extended wear time (14 days) in an ambulatory nondiabetic swine model and is highly resistant to kinking. The soft, coil-reinforced, Nylon-derivative material was chosen for the investigational IIS cannula because it is already used in Food and Drug Administration (FDA)-approved catheter products designed for delivering local anesthetic medication around peripheral and spinal nerves over a several-day period without causing significant inflammation. 17
Limitations to this study include the use of a nondiabetic swine model, and the absence of glucose clamp data for a direct correlation between the inflammatory tissue response and the insulin absorption/action (insulin pharmacokinetics/pharmacodynamics). Although swine skin and SC tissue are similar to that of an adult human, multiple factors in the research setting may affect the animal’s immune response to IIS insertion, maintenance, and the infusion of insulin (nutrition, stress, body movement). 15 In addition, use of a nondiabetic model eliminated the potential effects of chronic hyperglycemia on the immune/foreign body response. 18
Since this was an iterative process to develop an extended-wear infusion set, two different methods of cannula insertion were tested. We initially utilized a manual method for inserting the stylet/cannula through the tough dermis, and then utilized an investigational device that automated stylet/cannula insertion. This difference in insertion technique may have impacted the inflammatory tissue response as well as the failure mechanisms; however, the extent of this impact cannot be determined given the small sample size in each group.
A substantial limitation in every histological study is the fact that tissue staining can merely provide a snapshot of the actual tissue response. We attempted to overcome this limitation by examining tissue histology around cannula that was freshly inserted, and cannula that infused dilute insulin into the SC tissue for 2, 4, 6, 8, 10, 12, and 14 days of wear time. Since an individual slide of stained tissue may not show the region with the most severe inflammatory response, we examined slides that showed a significant length of the cannula insertion channel, and we averaged the thickness of the surrounding layer of inflammatory tissue at multiple proximal and distal locations.
It is currently recommended by clinicians and insulin pump manufacturers to insert a new IIS every two to three days to ensure safe and effective BG control.12,19 Users find this frequent site change and rotation inconvenient and often maintain their infusion site longer than recommended.12-14 Based on clinical experience, there is evidence that cannula wear time might be extended in a subgroup of insulin pump users without worsening glucose control.14,20,21 Of note, a recently modified commercial IIS from Medtronic plc (Dublin) with a Teflon cannula recently received CE Mark and FDA clearance for a seven-day wear time. 22
The insertion and maintenance of an IIS can lead to nonmetabolic complications, such as infection, skin inflammation due to the adhesive, and acute inflammation/fibrous tissue formation within the SC tissue.5,23,24 The inflammatory process changes dynamically over wear time due to ongoing tissue trauma caused by the cannula and proinflammatory insulin and insulin preservatives (eg, m-Cresol).6,7,25,26 The evidence of the impact of preservatives on the inflammatory tissue response is conflicting, as it has been shown that a decrease of preservatives is associated with the formation of insulin aggregates (fibrils) which are known to increase inflammation.27-29 In contrast, a recent well-designed IIS study in diabetic swine concluded that the insulin molecule itself is the major pro-inflammatory agent when infused into the SC tissue, rather than the excipients. 30 The current study infused insulin lispro diluted with commercial Lilly diluent (commonly used for managing diabetes in infants and small children) using typical adult human basal/bolus volumes to expose the SC tissue to both insulin molecules and the lispro excipients, while minimizing the risk of symptomatic hypoglycemia in a nondiabetic animal.
Bending/kinking of the insulin infusion cannula below the skin can lead to partial or complete obstruction of insulin flow and reflux of insulin onto the skin surface, leading to unexplained hyperglycemia and/or diabetic ketoacidosis.4,5 In a survey by Pickup and colleagues, 64% of Teflon IIS users reported cannula kinking, 54% cannula occlusion, and 16% leakage at the insertion site. 5 Others reported a kinking rate of 15% on the first day of IIS wear and an occlusion rate of 61% on the third day.13,31
Inflammation and IIS failure modes are strongly associated with increased variability of the absorption of insulin from the SC space into the circulation, especially after using the infusion set for more than three days.6-9 Variable and unreliable insulin absorption can lead to dangerous complications such as hyperglycemia, hypoglycemia, and diabetic ketoacidosis with an estimated lifetime economic cost of approximately $85 000 per patient.32-35 Furthermore, the repeated trauma of IIS insertion and maintenance contributes to the development of avascular scar tissue and may result in eventual loss of infusion sites.36,37 It is thus of substantial clinical importance to develop an extended-wear IIS that minimizes local tissue inflammation and fibrous scar formation. Furthermore, changing an IIS less often may eliminate more than half of the environmental waste associated with insulin pump therapy.30,31
As part of the iterative development of the investigational IIS prototype, we tested two different methods of cannula insertion: (1) manual advancement of the cannula through a pilot hole in the epidermis, dermis, and SC tissue, and (2) advancement of the cannula through the epidermis and dermis into the SC tissue using a first-generation spring-loaded automated inserter. For some prototypes, it was difficult to ensure proper manual insertion through a premade hole in the skin, while the automated inserter spring often did not employ a strong enough force to advance the cannula to the appropriate depth within the SC tissue. Several of the cannulas needed to be manually advanced to a deeper insertion depth using a forceps. We tested final insertion depth and proper placement by infusing a 70-µL bolus of insulin mixed with X-ray contrast agent and subsequent micro-CT imaging. One of the investigational IIS cannulas was not inserted to the appropriate depth, and leaked insulin/contrast agent though the most proximal hole onto the skin surface. Of clinical significance, superficial cannula insertion was the most common cause of substantial leakage onto the skin surface (type 1 leak). Also of clinical significance, a minimal amount of insulin/contrast refluxed from the SC tissue upward onto the skin surface over the 14 days of wear time (type 3 leak). The dermis-cannula interface acted as a robust mechanical barrier.
Our results confirm the importance of an automated spring-loaded introducer design, where the stylet and cannula are reliably inserted through the epidermis, dermis, and SC tissue at the optimal angle and depth. The data highlight the following important aspects of IIS design: (1) secure adhesion of the IIS base to the skin surface, (2) reliable cannula insertion to the appropriate depth within the SC tissue using a sharp stylet and a strong spring mechanism, (3) reliable removal of the needle/stylet without moving the inserted cannula, (4) cannula material and design that resist kinking, and (5) cannula material and design that minimize local tissue inflammation.
Results of this study confirm previous findings of a rapid increase in the TAI and the ILT surrounding the inserted cannula. 8 This increase in tissue trauma and inflammation was significantly less pronounced around investigational IIS with a soft and flexible Nylon-derivative cannula inserted at a 35° angle, compared with commercial IIS with a more rigid Teflon cannula inserted at a 90° angle. Of potential clinical significance, the area and thickness of the surrounding layer of inflammatory tissue stabilized after four days of wear time using the investigational IIS, while the inflammation around the commercial IIS cannula continued to increase in thickness and area for at least six days post cannula insertion, and often continued to increase over the 14 days of wear time. These results suggest that the soft and flexible investigational IIS cannula causes less ongoing inflammation and tissue trauma. Overall, the TAI and the ILT surrounding the cannula were reduced by more than 50% directly comparing the investigational IIS with the commercial IIS when inserted side-by-side, and infused with the same dilute insulin lispro formulation, the same volume of delivery, and the same basal/bolus pattern of delivery for 14 days of wear time. We have previously shown that both the type of material and the angle of cannula insertion have a significant effect on tissue disruption and insulin spread into the adjacent SC tissue, with softer materials and slanted angles eliciting less inflammation.6-8
Conclusions
In conclusion, our results suggest that the investigational IIS prototype design may cause significantly less overall tissue inflammation over an extended wear time and is resistant to kinking compared with a commercial IIS with a Teflon cannula inserted at a 90° angle. However, a larger animal study using diabetic swine will be required to strengthen these findings. Insulin infusion set testing in swine confirmed that superficial cannula insertion is a common cause of insulin leakage onto the skin surface in both types of IIS. This preclinical swine data highlighted the importance of reliably inserting the IIS cannula into the SC tissue at the appropriate depth, angle, and hole location. This information was used to develop an optimized automated IIS inserter that reliably inserts the soft flexible cannula into the SC tissue at the appropriate angle and depth. Human clinical trials are currently underway to evaluate the feasibility, safety, and efficacy of the novel investigational IIS prototype in a real-world setting in adults with type 1 diabetes. The results of this study highlight the importance of considering cannula material, design, and method of insertion in the development of extended-wear IISs.
Acknowledgments
We thank the technicians and veterinarians from the TJU Office of Animal Resources for their support, as well as the student volunteers assisting with animal work.
Footnotes
Abbreviations: ANOVA GLM, analysis of variance-general linear model; BG, blood glucose; CFR, Code of Federal Regulations; CGM, continuous glucose monitoring; CT, computed tomography; FDA, Food and Drug Administration; HSD, honestly significant difference; IACUC, Institutional Animal Care and Use Committee; IIS, insulin infusion set; ILT, inflammatory layer thickness; SC, subcutaneous; SD, standard deviation; TAI, total area of inflammation; TJU, Thomas Jefferson University.
Author Contributions: JRK contributed to data analysis and interpretation and drafted the manuscript. GE, MCT, AK, DD, ARD, CL, and JIJ contributed to in vivo studies and data acquisition. PS and JIJ contributed to the study concept and design, data interpretation, drafting and critical revision of the manuscript, and were responsible for study supervision. MCT was responsible for the statistical analysis and interpretation of the data, and critical revision of the manuscript for important intellectual content. MLT is responsible for developing part of the protocol and for data analysis and interpretation. JIJ is the guarantor of this work and as such has full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Data Availability: Data are available upon reasonable request.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JIJ is a founder, equity owner, and consultant to Capillary Biomedical and has received research supports. PJS is a founder, equity owner, and employee of Capillary Biomedical. MCT is an equity owner and member of Capillary Biomedical’s Scientific Advisory Board. JRK is a consultant to and equity owner of Capillary Biomedical.
Ethics Approval: The study and animal procedures were approved by the Thomas Jefferson University (TJU) Institutional Animal Care and Use Committee.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was sponsored by Capillary Biomedical and funded by the Juvenile Diabetes Research Foundation (award 2-IND-2016-232 M-X).
ORCID iDs: Jasmin R. Kastner  https://orcid.org/0000-0002-0821-3778
https://orcid.org/0000-0002-0821-3778
Jeffrey I Joseph  https://orcid.org/0000-0002-4945-2070
https://orcid.org/0000-0002-4945-2070
References
- 1. Summers JC, Briganti EM, Fitzgerald ZA, Lambers LNJ, Cohen ND. Long-term effectiveness of continuous subcutaneous insulin infusion in the prevention of hypoglycemia in adults with type 1 diabetes. Diabetes Technol Ther. 2019;21:423-429. [DOI] [PubMed] [Google Scholar]
- 2. Senn JD, Fischli S, Slahor L, Schelbert S, Henzen C. Long-term effects of initiating Continuous Subcutaneous Insulin Infusion (CSII) and Continuous Glucose Monitoring (CGM) in people with type 1 diabetes and unsatisfactory diabetes control. J Clin Med. 2019;8:E394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tubili C, Folco UD, Nardone MR, Clementi A. A single-center long-term Continuous Subcutaneous Insulin Infusion (CSII) experience: higher fractional use is associated with less diabetes complications. J Diabetes Sci Technol. 2017;11:1057-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Heinemann L. Insulin infusion sets: a critical reappraisal. Diabetes Technol Ther. 2016;18:327-333. [DOI] [PubMed] [Google Scholar]
- 5. Pickup JC, Yemane N, Brackenridge A, Pender S. Nonmetabolic complications of continuous subcutaneous insulin infusion: a patient survey. Diabetes Technol Ther. 2014;16:145-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hauzenberger JR, Hipszer BR, Loeum C, et al. Detailed analysis of insulin absorption variability and the tissue response to continuous subcutaneous insulin infusion catheter implantation in swine. Diabetes Tech Therapeut. 2017;19:641-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hauzenberger JR, Münzker J, Kotzbeck P, et al. Systematic in vivo evaluation of the time-dependent inflammatory response to steel and Teflon insulin infusion catheters. Sci Rep. 2018;8:1132-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eisler G, Kastner JR, Torjman MC, et al. In vivo investigation of the tissue response to commercial Teflon insulin infusion sets in large swine for 14 days: the effect of angle of insertion on tissue histology and insulin spread within the subcutaneous tissue. BMJ Open Diabetes Res Care. 2019;7:e000881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clausen TS, Kaastrup P, Stallknecht B. Effect of insulin catheter wear-time on subcutaneous adipose tissue blood flow and insulin absorption in humans. Diabetes Technol. Ther. 2019;11:575-580. [DOI] [PubMed] [Google Scholar]
- 10. Kuroda K, Takeshita Y, Kaneko S, Takamura T. Bending of a vertical cannula without alarm during insulin pump therapy as a cause of unexpected hyperglycemia: a Japanese issue? J Diabetes Investig. 2015;6:739-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Heinemann L, Krinelke L. Insulin infusion set: the Achilles heel of continuous subcutaneous insulin infusion. J Diabetes Sci Technol. 2012;6:954-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schmid V, Hohberg C, Borchert M, Forst T, Pfützner A. Pilot study for assessment of optimal frequency for changing catheters in insulin pump therapy-trouble starts on day 3. J Diabetes Sci Technol. 2010;4:976-982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Patel PJ, Benasi K, Ferrari G, et al. Randomized trial of infusion set function: steel versus teflon. Diabetes Technol Ther. 2013;16:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Waldenmaier D, Zschornack E, Buhr A, Pleus S, Haug C, Freckmann G. A prospective study of insulin infusion set use for up to 7 days: early replacement reasons and impact on glycemic control. Diabetes Tech Therapeut. 2020. doi: 10.1089/dia.2019.0445. [DOI] [PubMed] [Google Scholar]
- 15. Sullivan TPP, Eaglstein WHH, Davis SCC, Mertz P. The pig as a model for human wound healing. Wound Repair Regen. 2001;9:66-76. [DOI] [PubMed] [Google Scholar]
- 16. Wang JF, Olson ME, Reno CR, Wright JB, Hart DA. The pig as a model for excisional skin wound healing: characterization of the molecular and cellular biology, and bacteriology of the healing process. Comp Med. 2001;51:341-348. [PubMed] [Google Scholar]
- 17. Toledano RD, Tsen LC. Epidural catheter design: history, innovations, and clinical implications. Anesthesiology. 2014;121:9-17. [DOI] [PubMed] [Google Scholar]
- 18. Soto RJ, Merricks EP, Bellinger DA, Nichols TC, Schoenfisch MH. Influence of diabetes on the foreign body response to nitric oxide-releasing implants. Biomaterials. 2018;157:76-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thethi TK, Rao A, Kawji H, et al. Consequences of delayed pump infusion line change in patients with type 1 diabetes mellitus treated with continuous subcutaneous insulin infusion. J Diabetes Complications. 2010;24:73-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Simic A, Schøndorff PK, Stumpe T, et al. Survival assessment of the extended-wear insulin infusion set featuring lantern technology in adults with type 1 diabetes by the glucose clamp technique. Diabetes Obes Metab. 2021:231402-231408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lal RA, Hsu L, Zhang J, Schøndorff PK, Heschel M, Buckingham B. Longevity of the novel ConvaTec infusion set with Lantern technology. Diabetes Obes Metab. 2021. doi: 10.1111/dom.14395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. US Food & Drug Administration. 510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY K210544. 2105;44. https://www.accessdata.fda.gov/cdrh_docs/reviews/K210544.pdf. Accessed April 7, 2022
- 23. Zhang E, Cao Z. Tissue response to subcutaneous infusion catheter. J Diabetes Sci Technol. 2019;14:226-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ross PL, Milburn J, Reith DM, Wiltshire E, Wheeler BJ. Clinical review: insulin pump-associated adverse events in adults and children. Acta Diabetol. 52:1017–1024. [DOI] [PubMed] [Google Scholar]
- 25. Conwell LS, Pope E, Artiles AM, Mohanta A, Daneman A, Daneman D. Dermatological complications of continuous subcutaneous insulin infusion in children and adolescents. The Journal of Pediatrics. 2008;152:622–628 [DOI] [PubMed] [Google Scholar]
- 26. Mulka A, Lewis BE, Mao L, et al. Phenolic preservative removal from commercial insulin formulations reduces tissue inflammation while maintaining euglycemia. ACS Pharmacol Transl Sci. 2021;4:1161–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Teska BM, Alarcõn J, Pettis RJ, Randolph TW, Carpenter JF. Effects of phenol and meta-cresol depletion on insulin analog stability at physiological temperature. J Pharm Sci. 2014;103:2255–2267. [DOI] [PubMed] [Google Scholar]
- 28. Kerr D, Wizemann E, Senstius J, Zacho M, Ampudia-Blasco FJ. Stability and performance of rapid-acting insulin analogs used for continuous subcutaneous insulin infusion: a systematic review. J Diabetes Sci Technol. 2013;7:1595–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lewis BE, Mulka A, Mao L, et al. Insulin derived fibrils induce cytotoxicity in vitro and trigger inflammation in murine models. J Diabetes Sci Technol. 1932. 2968211033868. doi: 10.1177/19322968211033868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Swinney MR, Cox AL., Hawkins ED, et al. Insulin, not the preservative m-cresol, instigates loss of infusion site patency over extended durations of CSII in diabetic swine. J Pharm Sci. 2021;110:1418–1426. [DOI] [PubMed] [Google Scholar]
- 31. van Bon AC, Bode BW, Sert-Langeron C, DeVries JH, Charpentier G. Insulin glulisine compared to insulin aspart and to insulin lispro administered by continuous subcutaneous insulin infusion in patients with type 1 diabetes: a randomized controlled trial. Diabetes Technol Ther. 2011;13:607–614. [DOI] [PubMed] [Google Scholar]
- 32. Deiss D, Cobelli C, Charpentier G, et al. Insulin infusion set use: European perspectives and recommendations. Diabetes Technol Ther. 2016;18:517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhuo X, Zhang P, Hoerger TJ. Lifetime direct medical costs of treating type 2 diabetes and diabetic complications. Am J Prev Med. 2013;45:253–261. [DOI] [PubMed] [Google Scholar]
- 34. Nwadiugwu MC, Bastola DR, Haas C, Russell D. Identifying glycemic variability in diabetes patient cohorts and evaluating disease outcomes. J Clin Med. 2021;10:1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lindvig A, Tran MP, Kidd R, Tikkanen CK, Gæde P. The economic burden of poor glycemic control associated with therapeutic inertia in patients with type 2 diabetes in Denmark. Curr Med Res Opin. 2021;37:949–956. [DOI] [PubMed] [Google Scholar]
- 36. Richardson T, Kerr D. Skin-related complications of insulin therapy. Am J Clin Dermatol. 2003;4:661–667. [DOI] [PubMed] [Google Scholar]
- 37. Heinemann L. Insulin absorption from lipodystrophic areas: a (neglected) source of trouble for insulin therapy? J Diabetes Sci Technol. 2010;4:750–753. [DOI] [PMC free article] [PubMed] [Google Scholar]