Abstract
Lipohypertrophy is a common skin complication associated with insulin-treated diabetes. The impact of lipohypertrophy as a contributing factor to suboptimal glycemic control, glucose variability, and hypoglycemia is often under-recognized by health care professionals. In a recent Webinar on April 26, 2023, Diabetes Technology Society asked international experts to provide updates on the latest knowledge related to lipohypertrophy for practicing clinicians and educators, researchers, and industries involved in insulin delivery. A recording of the Webinar is freely available on the Diabetes Technology Society Web site (https://www.diabetestechnology.org/).
Keywords: lipohypertrophy, insulin, diabetes, ultrasound, glucose management, diabetes education
Introduction
Lipohypertrophy (LH) is characterized by increased size and proliferation of adipose tissue in the subcutaneous (SC) space related to injections or infusions of insulin. Various techniques have been used to diagnose LH, including visual inspection, palpation, and ultrasound. Despite existing knowledge of the adverse consequences of LH on achieved glycemic outcomes, it remains a common complication of insulin therapy and poses a significant burden on people with diabetes (PWDs) and health care systems.
For several decades, lipoatrophy (LA) was the predominant problem associated with the use of older animal insulins that contained impurities and provoked antibodies. 1 However, after the introduction of purified human insulins in the 1980s, LH became the most common form of abnormality at injection sites. 2 Human insulin was even used to inject around areas of LA to try to restore the absent SC fat. Lipodystrophy in the form of SC lumpiness, often with associated discomfort at injection sites and which resembled neither LA nor LH, was also commonly observed before the 1980s. 3
Many patients deliberately use areas of LH for insulin administration because the injections are not as painful. However, this perpetuates the problem, and these fatty areas were occasionally misdiagnosed as lipomas, sometimes even resulting in their surgical removal. Although LA was uncommon after the introduction of human insulin, it still did occur with these highly purified insulins as has been described in a few case reports. 4
Skin Complications of Diabetes
Skin complications are common in PWDs but are often neglected.
Despite the high prevalence of LH in PWDs using insulin, there is a lack of awareness of this condition.
Lipohypertrophy areas are not advised for insulin pump placement because they interfere with ideal glucose management.
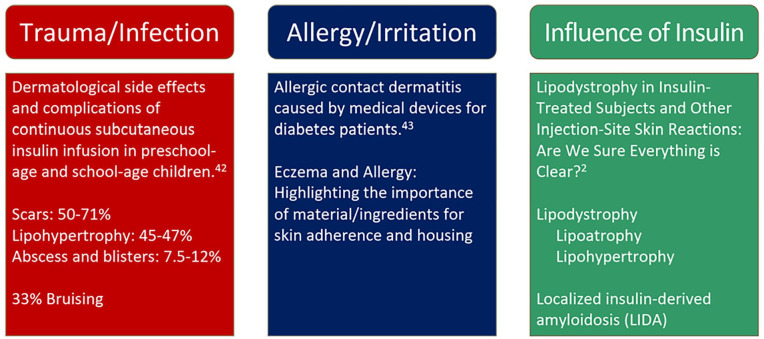
Three common types of localized skin complications associated with diabetes include (1) trauma or infection as a side effect of the use of continuous subcutaneous insulin infusion (CSII) sets, (2) allergy or irritation due to prolonged contact with skin adherence material for diabetes devices, and (3) lipodystrophy in insulin-treated individuals (Figure 1). In a 2017 survey examining self-reported skin complications associated with CSII and continuous glucose monitors (CGMs) in children and adults with diabetes, eczema and wounds were the most commonly reported complications.7,8 Several risk factors were determined for developing skin complications. Longer duration of use of insulin infusion sets and use of CGMs is related to a greater risk of developing skin complications, with most skin complications appearing after four to six months of use of these devices. 7 About 51% of individuals with existing skin complications at baseline continued to suffer from skin complications, while 12% developed new onset of skin complications at six months follow-up. 7 The survey also found that individuals with skin complications at baseline were 13 times more likely to continue exhibiting skin complications in the future. 7
Figure 1.
Common factors associated with localized skin complications in people with diabetes using insulin infusion sets or continuous glucose monitors.
Source: Major findings for each category are referenced from the works of Schober et al, 5 Herman et al, 6 and Gentile et al. 2
Lipohypertrophy is a vastly under-recognized condition in PWDs. In the self-reported survey conducted by Berg et al,7,8 less than 5% of pediatric individuals with type 1 diabetes (T1D) reported any level of LH. However, when a separate cohort of children and young adults with T1D were evaluated for LH during outpatient clinical visits, 48% were diagnosed with LH. 9 The disparity between the prevalence of perceived LH and clinically diagnosed LH demonstrates a lack of awareness and different diagnostic methods used by health care professionals (HCPs). Using visual inspection and palpation, the method most often used by the majority of HCPs, 37% to 64% of individuals screened appear to have LH10 -20 with some variation in how systematically the inspection is performed.2,21 Ultrasound is a useful tool in LH detection because of its ability to reveal structural changes in deeper levels of SC tissue before there are any visual changes on the surface.20,22 -24
Besides the duration of device use, several other potential risk factors for LH have emerged. The number of injections per day, reuse of needles, and lack of site rotation correlate with an increased prevalence of LH, and some reports have described more LH in pen users than pump users.9,17,25 However, the latter needs additional follow-up studies in individuals wearing pumps for a prolonged period since LH develops over time, and little is known about time to disappearance and factors necessary for the healing of the SC tissue. The types of infusion sets are also thought to influence the risk for LH. Novel seven-day infusion sets may lead to fewer cases of LH, but studies are underway to quantify this hypothesis.26,27 Additives in different types of insulin may also have some influence because of the introduction of foreign bodies that can activate the immune system. Insulin delivery via jet injectors has been speculated to decrease LH risk. However, the use of this type of instrument has not been widely adopted, and few studies examined its effect on LH risk. 28
Diagnosis and Pathology of Lipohypertrophy
Lipohypertrophy due to incorrect injection technique is commonplace and frequently not recognized by HCPs.
Histology shows that areas with insulin-induced LH contain a significantly larger number of macro-adipocytes and fibrosis.
Implementing affordable and structured clinical diagnostic paths is necessary to identify LH in routine diabetes care.
In 2022, there were approximately 537 million adults with diabetes, with around 200 million requiring insulin treatment, according to the International Diabetes Federation. Almost half of insulin users have been found to have LH,13,29 suggesting that around 90 million PWDs are affected by LH. As mentioned earlier, common risk factors for the development of LH are failure to rotate injection sites, concentrating injections in a small area, and needle reuse. Additional factors include injection of cold insulin, a high number of injections per day, large injection volumes, and using long and thick needles. A list of common risk factors for LH is presented in Figure 2. Clinical problems associated with LH include glycemic variability, unpredictable and severe hypoglycemia, high hemoglobin A1c levels, increased health care costs due to greater insulin requirements, and a negative effect on the quality of life. In addition, there appears to be an association between the presence of LH and the risk of micro- and macrovascular complications; the explanation for this association may be that both abnormalities are associated with increased glycemic variability.
Figure 2.
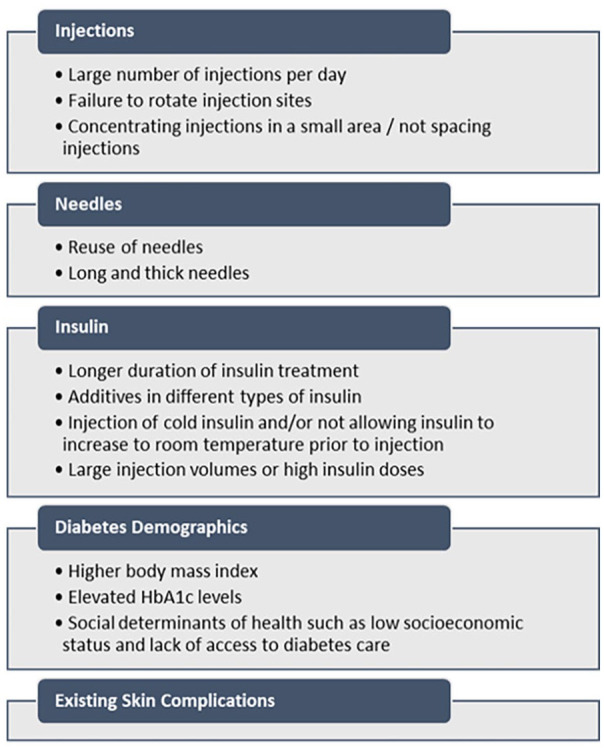
Common risk factors of lipohypertrophy. 30
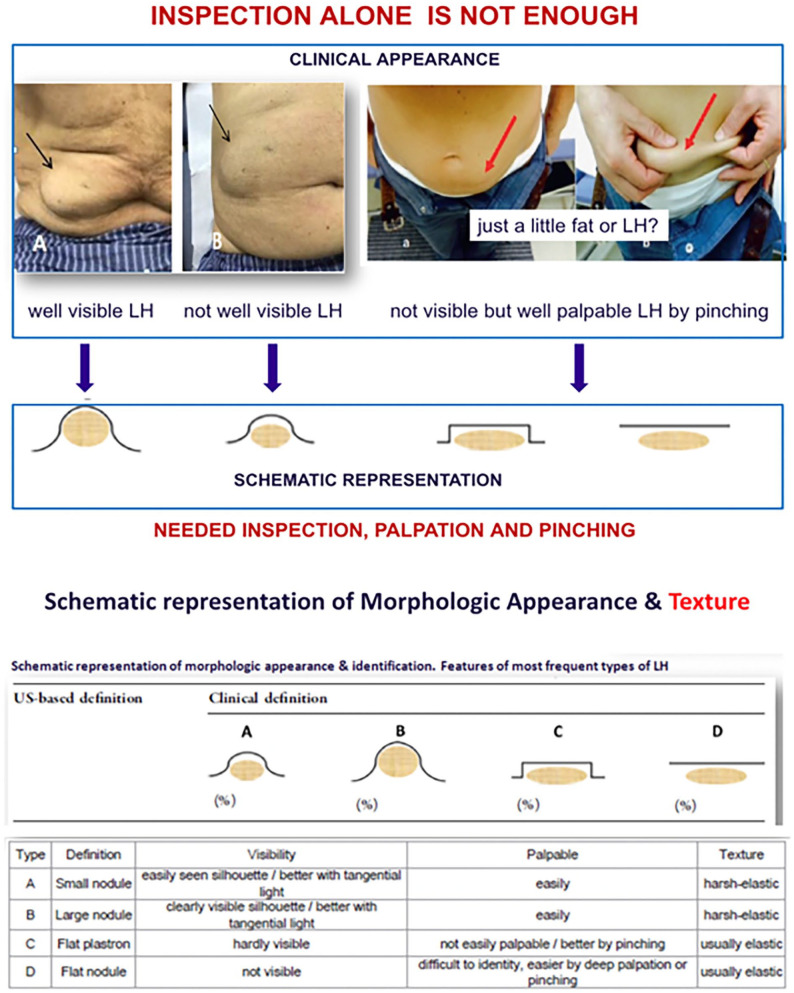
The presentation of LH can be variable. Schematic representations of four common types of LH are shown in Figure 3. Lipohypertrophy can be detected through visual inspection and palpation. However, a pinching maneuver is needed to diagnose less visible types of LH that contain flat nodules that are elastic. Deep palpation is also required to determine the texture of the suspected LH areas in these situations. The pinching maneuver also allows identification of the sides and position of a collection of LH even when it is not visible to the naked eye. In addition, changing the positioning of the individual can increase the detectability of less visible LH. Rotation of the body along the longitudinal axis also allows the profile of the skin to detect the presence of LH. In a study conducted by Gentile et al, 21 it was shown that the ability to diagnose LH varies greatly between trained and untrained HCPs. For areas of LH smaller than 4 cm in diameter, trained professionals are 45% more likely to correctly detect LH. 21 In another study by Gentile et al, 29 a survey was conducted to determine how PWDs learn about proper insulin injection techniques and site rotation. Only 48% of PWDs reported to have received education on site rotation by HCPs. 29 These findings indicate that HCPs need to be trained in LH detection techniques, and they also need to educate PWDs who inject insulin on LH prevention to improve outcomes. Health care professionals should also follow up with individuals to assess whether the amount of LH changes over time by comparing progressive lesion improvement with metabolic parameters (e.g., glucose time-in-range and variability).
Figure 3.
Schematic representations, examples, and descriptions of four common types of lipohypertrophy.
Source: Figure modified from Gentile et al 31 under the Creative Commons Attribution—Non-Commercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/).
Histology images of LH obtained through scanning electron microscopy have shown that 75% of the SC tissue is composed of macro-adipocytes with a significantly larger size than adjacent normal adipocytes. 32 This may be associated with an increase in fibrosis and apoptosis. 32 However, the underlying mechanism for developing these characteristics in LH areas remains to be determined.
Many PWDs report not having received education about proper insulin injection techniques. For example, in a study from Italy, 50% of insulin-injecting PWDs had not been referred for education on this topic. 29 This is especially true for individuals starting insulin during a hospital admission, where almost 70% report receiving no education on proper insulin injection techniques. 29 In another study of adults discharged on insulin from endocrinology units in hospitals, a similar proportion (71%) were taught how to inject and provided with insulin pens during the hospital stay. 33 Previous data on the durability of education related to injection techniques suggest that the impact wanes within six months.33 -35 As expected, the LH percentage is significantly higher in those not receiving specific training.
In conclusion, clinicians may not recognize the burden of LH and the value of education to prevent LH among insulin-treated individuals. People with diabetes would benefit from increasing HCP awareness of LH and establishing standardized educational practices for wwinsulin users during hospital admission. 32 The amount of time devoted to diabetes education to ensure that PWDs inject insulin correctly is a good investment. 34
Ultrasound Imaging of Lipohypertrophy
Ultrasound assessment of LH is more sensitive than palpation in detecting LH.
Ultrasound examination of LH shows that insulin-related tissue changes are more complex than LH alone (dermal thickening, inflammatory changes, and necrotic tissue).
Ultrasound may provide a clinically feasible method for detecting LH and may also be useful in mediating behavior changes in PWDs because it visualizes the tissue changes for them.
Multiple pathways can generate SC tissue changes following insulin exposure. These include inflammation and the anabolic effects of insulin on adipocytes, resulting in LH and hyperplasia of fat cells. These tissue changes can be detected using ultrasound. Continuous glucose monitor or flash glucose monitoring data can also reveal evidence of decreased insulin action, which can be a warning sign for individuals suffering from LH, LA, or other skin-mediated problems that interfere with insulin absorption. 17
Ultrasound is about 30% more sensitive in detecting LH than palpation. 36 However, its clinical utility for widespread use outside of a research setting remains unclear. In a study conducted by Hashem et al, 37 74 individuals with T1D underwent an ultrasound examination of LH regions with unaffected areas as reference regions. Ultrasound images revealed that healthy tissues had clearly defined demarcation lines of the muscle sheath, SC tissue, and dermis, whereas LH-affected areas were blurred with diffuse borders, which had generally increased reflectivity without a defined structure (Figure 4). These tissue changes are reflected in nearly all grades of LH and are consistent with inflammatory changes. Using ultrasound, some nodules have a flat profile underneath the skin surface and hence would not necessarily protrude visibly at the skin surface. The midpoint of the depth of these LH nodules correlated with the length of needles the individuals were using, and there is evidence of left- or right-handed distribution. A novel clinical solution suggested in the study was alternating different-length needles to distribute insulin exposure, although this hypothesis is untested. Hypoechogenic areas suggestive of necrotic tissue were also observed within the LH nodules in 30% of participants. 37 In addition, a recent cross-sectional study of adolescents and young adults (n = 95) with T1D found that participants with LH had significantly higher levels of TNF-α, which is an inflammatory marker. 4
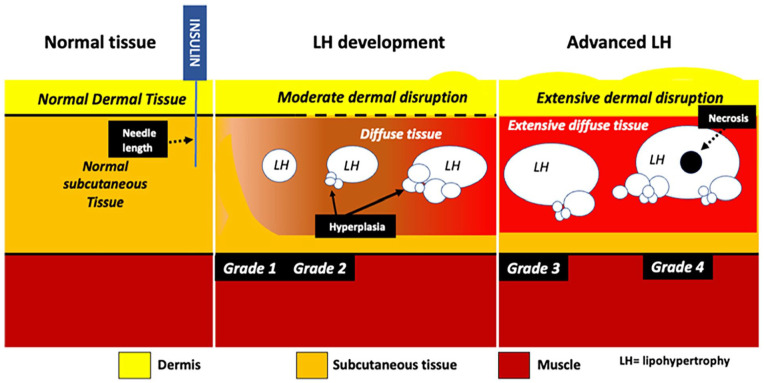
Figure 4.
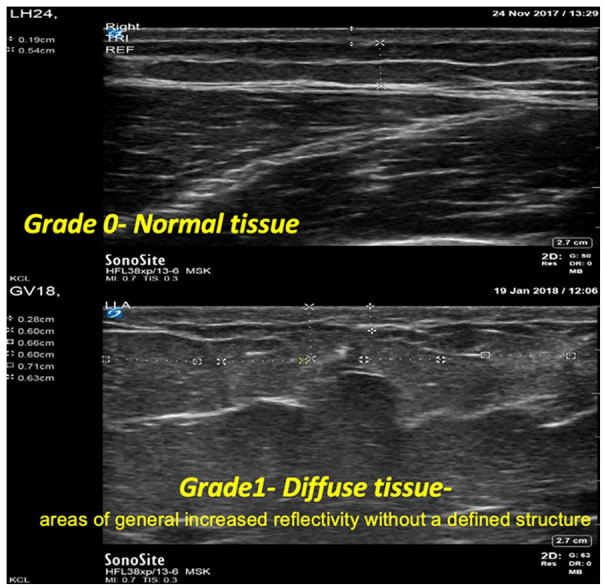
Ultrasound images of normal tissue and diffuse tissue affected by lipohypertrophy. Affected tissues demonstrate blurred lines between muscle sheath and subcutaneous tissue layers.
Studies suggest an average of 10 nodules per individual affected by LH, widely distributed but mainly located in the abdomen and thighs. 38 To determine the severity of each LH area, a severity indicator based on the size and the number of nodules to produce a parameter on potential severity, as shown in Figure 5, has been described. 37 Increasing severity, as indicated by this model, is associated with increasing size and number of LH nodules and increasing amounts of inflammation, thickening, and loss of differentiation between the dermis and SC tissue. The insulin antibody load was also examined in individuals with varying severity of LH, and the antibody titer was associated with the severity of LH as defined in the model. 4
Figure 5.
Conceptual model used to determine the severity of lipohypertrophy.
Source: Figure reproduced from Hashem et al 37 under the Creative Commons Attribution—Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work noncommercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is noncommercial. See http://creativecommons.org/licenses/by-nc/4.0/.
It is uncertain whether LH tissue will resolve on its own without interventions. However, LH certainly leaves an archeological footprint in the affected tissue. Emerging technologies are being developed to clinically integrate ultrasound into care. 39 The development of portable ultrasound scanners connected to a mobile app has increased the accessibility of this technology for individuals affected by LH. However, this technology for diagnosing LH is not available everywhere. Education and behavioral support combined with technological advancement are needed to help individuals change their behavior.
Pharmacokinetics and Pharmacodynamics of Insulin in the Presence of Lipohypertrophy
Injection or infusion of insulin in areas containing LH can adversely affect insulin pharmacokinetics (PK) and pharmacodynamics (PD).
Insulin absorption and action are reduced and more variable when injected into LH compared with normal SC tissue, which increases the amount of insulin needed and raises the societal costs of insulin.
Lipohypertrophy is associated with a higher risk of uncontrolled diabetes, which can lead to more emergency room visits, hospitalizations, and absenteeism. Each of these consequences of LH carries its own health and economic burden on both PWDs and health care systems.
Successful insulin therapy relies on the reproducible absorption of insulin from the SC tissue. Many factors are known to impact the PK and PD properties of SC insulin, and LH is one of them. Injection or infusion of insulin into LH areas can impact insulin PK, and insulin is absorbed more slowly than application in other areas unaffected by LH. It is, therefore, important to avoid infusion of insulin into areas with LH since the unstable absorption of insulin from these areas leads to high glucose variability and unpredictable hypoglycemic and hyperglycemic events. This advice is challenged by the decreased pain sensitivity in LH areas, making site rotation for some pump users less attractive. The unpredictable insulin absorption from LH areas is likely to also disturb the function of automated insulin delivery systems. Therefore, LH poses a significant barrier to PWDs’ desired glucose management. Glucose control in individuals with LH is improved when they inject insulin into sites without detectable LH. Few systematic and appropriate PK studies concerning the impact of LH on the PK of injected insulin have been published, and there are a limited number of studies about the PK of insulin infused into the SC tissue.
Defective absorption of insulin in palpably abnormal injection sites was first examined in a study conducted by Young et al 40 that demonstrated slower clearance of 125I-insulin from injection sites with LH. In another study, Thow et al 41 discovered a 22% difference between injecting insulin in unaffected sites compared with abnormal sites. They also found that the thickness of palpably abnormal sites is greater than control sites, indicating a difference in morphology in the affected tissues 41 . Johansson et al 42 also highlighted the clinical importance of the impact of LH on insulin absorption and further investigated the differences in absorption of a single SC dose of insulin administered directly into LH tissues in individuals with T1D. They confirmed impaired absorption of insulin aspart into LH tissue which yielded 25% lower Cmax of plasma insulin. 42
Impaired insulin absorption also translates into differences in the PD properties, which affect blood glucose management and the ability of PWDs to keep their blood glucose at target levels. A crossover study by Famulla et al 43 looked at the effect of insulin injection into LH tissue on postprandial blood glucose control. They performed a euglycemic clamp and a mixed meal tolerance test in 13 individuals with T1D with LH that was confirmed by physical examination and ultrasound. 43 In the six-hour clamp studies, they found that insulin area under the curve (AUC) and Cmax are lower in LH injections than in normal site injections. 43 The glucose infusion rate was also lower, and intrasubject variability was higher with LH injections. 43 In the five-hour mixed meal tolerance test, they found that insulin AUC was 46% lower, while blood glucose was nearly 40% higher for more than five hours. 43 Maximum blood glucose also increased by 25% with LH injections. 43 From these findings, it can be concluded that insulin absorption and action are reduced and more variable when this substance is injected into LH compared with normal SC tissue.
Several types of economic benefits have been associated with reducing the risk of LH, a treatable and preventable condition. In a 52-week prospective randomized controlled study of 713 individuals with insulin-treated type 2 diabetes (T2D) and LH, implementing an intensive educational program significantly reduced LH sizes and rates of symptomatic and severe hypoglycemia. 35 In that study, the cost of managing severe hypoglycemia in the intervention group (IG) decreased by 83% at the end of the study. 35 Likewise, the cost due to symptomatic hypoglycemia decreased by 93%. 35 Moreover, by the end of the study, the IG used approximately 14 fewer units of insulin per day compared with their baseline use which resulted in a cost saving of 123 € per participant. 35 Lipohypertrophy is associated with a higher risk of hypoglycemia, hyperglycemia, and glucose fluctuations, which can lead to more emergency room visits, hospitalization, and absenteeism. Each of these consequences of LH carries its own health and economic burden on both PWDs and health care systems. As LH impairs the SC absorption of insulin, this can lead to an increased dose of insulin being applied over time. Lipohypertrophy, therefore, has a massive impact on insulin PK/PD and represents a considerable economic burden. Given that many individuals treated with insulin experience LH and may have worse glycemic outcomes as a result, better educational programs for PWDs and HCPs are needed to raise awareness of this condition, increase prevention with regular assessment of the skin, and improve outcomes.
How to Prevent Lipohypertrophy
To prevent LH, education that includes proper insulin injection/infusion technique, site selection, and rotation is crucial for PWDs injecting insulin or wearing an insulin pump.
Education for HCPs that raises awareness of the serious consequences of LH, emphasizes the importance of ongoing assessment of injection/infusion set sites, and teaches prevention strategies is needed.
Health care professionals need multimedia resources and referrals readily available to reinforce education on injection technique, site selection, and an individualized site rotation plan.
When PWDs inject into the same site with LH, insulin is 25% to 30% less effective. 43 If HCPs fail to check sites and increase insulin doses and PWDs decide to move to a new site, then hypoglycemia could occur. In the previously described survey reporting significant gaps in the knowledge of the correct insulin injection technique among adults with insulin-treated T2D, it was noteworthy that 15% of the respondents felt that their peers taking insulin were more experienced and knew more about insulin injection technique than their HCPs. 29
Smith et al 44 conducted a prospective 18-center study in the United Kingdom with 75 PWDs to determine whether knowledge can make a difference in insulin injection technique. They received a comprehensive educational intervention and were switched to using 4-mm needles. In the end, there was a reported 50% reduction or complete disappearance of LH at all injection sites. 44 Several strategies shown in Table 1 can be applied to promote safe and effective insulin injections and optimal insulin absorption. Using 4-mm needles can ensure injection into the SC layer and prevent injection into the muscle layer. In addition, the needle should be injected at a straight 90° angle. The pinching maneuver is not recommended for injections except for children below six years and very thin adults. The four recommended injection sites are the abdomen, the upper arm, the thigh, and the buttock. Individuals should inspect and palpate the site before injection to look for abnormal tissue formation. A new needle must also be used every time since used needles are not as sharp and do not penetrate the skin as easily or can cause more tissue trauma in the SC space. Needle reuse should particularly be avoided for insulin pens, since clogging could occur, and the individual may not get a full dose of insulin.
Table 1.
Strategies to Promote Safe and Effective Insulin Injections and Absorption.
| Strategy | Recommendation | Rationale |
|---|---|---|
| Short needle length for children and adults | Pen needles: 4 mm Syringe needles: 6 mm |
Decreases risk of intramuscular injection, promotes comfort, and improves motivation to perform self-care |
| Inject 4-mm needle perpendicular (90°) to skin | Skinfold should be raised only for young children (<6 years) and very thin adults. | Lifting the skinfold increases the distance between the skin surface and the muscle. |
| Injection and infusion sites may include abdomen, upper arm, thigh, and buttock. | Inspect site for LH, edema, infection, inflammation, ulceration, and cleanliness. | Site rotation and inspection every time will facilitate avoiding injecting into areas of LH. |
| Develop a simple plan together with PWDs to rotate sites. | For injections in the community, disinfect the site if not clean. Avoid injecting through clothing since the site cannot be assessed. | If alcohol is used to disinfect, allow alcohol to dry before injection. |
Source: Table adapted from Frid et al 45 under the Creative Commons Attribution—Non Commercial—NoDerivs (CC BY-NC-ND 4.0) license.
Abbreviations: LH, lipohypertrophy; PWDs, people with diabetes.
A site rotation plan can be established to prevent LH development. As shown in Figure 6, rotating injections into four consecutive abdominal sites and four consecutive thigh sites can help lower the risk of LH and ease the burden for PWDs to keep track of their injection sites. It can also be beneficial to associate an injection area with the day of the week, and the injection sites for basal insulin and mealtime insulin can also be allocated to designated areas. People with diabetes can also utilize an available smartphone application to track and receive guidance on injection sites. 46 A clip-on electronic injection log device is under development to help track injection sites in the abdomen. 47 It attaches directly to the insulin pen and activates when the pen is picked up and positioned at an anchor point in front of the navel. 47 Light from a light-emitting diode and vibration from a motor on the device show where to inject according to the PWD’s injection plan. 47
Figure 6.
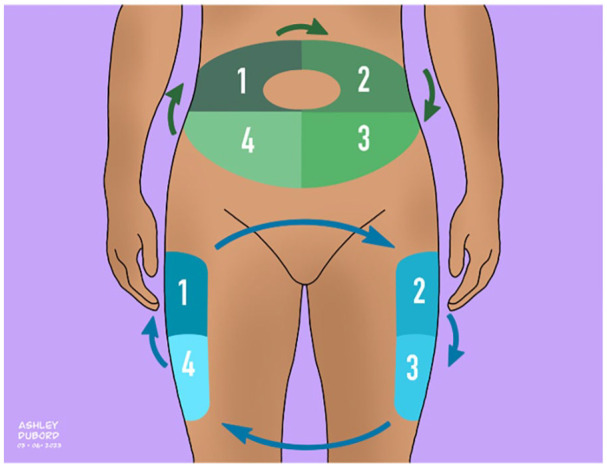
Insulin injection site rotation plan.
Source: Figure courtesy of Ashley Y. DuBord.
The psychological challenges of injections also need to be considered regarding LH prevention and management. Emotional concerns and anxiety need to be addressed first. Health care professionals should encourage PWDs to express any frustration, anger, fear, or concerns about taking insulin injections. They should let the PWDs know that they will do their best to teach them how to make each injection as comfortable as possible and will support them to master the skill. It is also important to point out that insulin is a natural hormone and that taking it does not mean they “failed.” Finally, HCPs should discuss with PWDs how insulin will help achieve glycemic goals, which may help them feel better and lower their risk of LH and diabetes complications. Therefore, it is essential for HCPs to take the time to address the psychological challenges of PWDs taking insulin, demonstrate good injection techniques, and create a site rotation plan with individuals to decrease their risk of LH and help them achieve their glycemic goals. Inspecting injection sites and educating PWDs that take insulin on best practices for rotating injection sites and properly injecting insulin should be a priority during diabetes visits. Research has shown that educating both HCPs and PWDs on ways to prevent LH is worth the effort.9,16
In summary, five practices to decrease the risk of developing LH need to be considered. Individuals injecting insulin should
Search for regions of existing LH to avoid injecting insulin into them.
Follow constant injection site rotation to ensure a distance of at least 1 cm between two successive injections.
Use the entire surface of the injection areas of the abdomen, external and rear sides of the arms, upper external side of the thighs, and buttocks.
Wherever possible, use 32-gauge, 4-mm needles and avoid injecting ice-cold insulin.
Avoid skin massage after injection.
Conclusion
Six conclusions about LH to consider include (1) LH affects almost half of PWDs using insulin and is often overlooked by HCPs; (2) LH can result in decreased, late, and unpredictable insulin absorption, leading to hypoglycemia and glycemic variability; (3) LH can be difficult to diagnose because some presentations have a flat profile underneath the skin; (4) visual inspection, palpation, and maneuvers incorporating pinching techniques and body positioning changes can increase LH’s detectability; (5) ultrasound increases the sensitivity of LH detection, and recently developed portable scanners can make this diagnostic method more accessible to PWDs; and (6) education on proper injection techniques and site rotations for HCPs and PWDs can help prevent LH development and reduce the economic burden on PWDs taking insulin. Given the adverse effects on clinical outcomes and costs of insulin therapy caused by LH, it is crucial to understand, diagnose, treat, and prevent this common complication of insulin therapy.
Acknowledgments
The authors thank Annamarie Sucher-Jones for her expert editorial assistance and Ashley Y. DuBord for her artistic contribution.
Footnotes
Abbreviations: AUC, area under the curve; CGM, continuous glucose monitor; CSII, continuous subcutaneous insulin infusion; HCP, health care professional; IG, intervention group; LA, lipoatrophy; LH, lipohypertrophy; PD, pharmacodynamics; PK, pharmacokinetics; PWDs, people with diabetes; SC, subcutaneous; T1D, type 1 diabetes; T2D, type 2 diabetes.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: L.H. is a consultant for a number of companies that are developing novel diagnostic and therapeutic options for diabetes treatment. He is a shareholder of the Profil Institut für Stoffwechselforschung GmbH, Neuss, Germany. D.K. has received remuneration for participation in Advisory Boards from Sanofi, Novo Nordisk, and Abbott Diabetes Care. He also has received research support from Novo Nordisk and Abbott Diabetes Care and has financial interests in Glooko, Proteomics, Better Therapeutics, Hi.Health, and SNAQ. D.C.K. is a consultant to Better, Eoflow, Integrity, Lifecare, Nevro, Novo, Sanofi, and Thirdwayv.
T.T., R.E.A., J.H., A.M.Y., J.S., S.G., A.F., and J.J.S. have no relevant discloses.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The webinar was supported by a grant from Embecta.
ORCID iDs: Tiffany Tian  https://orcid.org/0009-0003-1417-6445
https://orcid.org/0009-0003-1417-6445
Rachel E. Aaron  https://orcid.org/0009-0005-5120-2264
https://orcid.org/0009-0005-5120-2264
Jingtong Huang  https://orcid.org/0000-0002-3119-9361
https://orcid.org/0000-0002-3119-9361
Andrea M. Yeung  https://orcid.org/0000-0002-5592-453X
https://orcid.org/0000-0002-5592-453X
Jannet Svensson  https://orcid.org/0000-0002-9365-0728
https://orcid.org/0000-0002-9365-0728
Sandro Gentile  https://orcid.org/0000-0002-9059-6121
https://orcid.org/0000-0002-9059-6121
Angus Forbes  https://orcid.org/0000-0003-3331-755X
https://orcid.org/0000-0003-3331-755X
Lutz Heinemann  https://orcid.org/0000-0003-2493-1304
https://orcid.org/0000-0003-2493-1304
Jane Jeffrie Seley  https://orcid.org/0000-0003-1582-4320
https://orcid.org/0000-0003-1582-4320
David Kerr  https://orcid.org/0000-0003-1335-1857
https://orcid.org/0000-0003-1335-1857
David C. Klonoff  https://orcid.org/0000-0001-6394-6862
https://orcid.org/0000-0001-6394-6862
References
- 1. Reeves WG, Allen BR, Tattersall RB. Insulin-induced lipoatrophy: evidence for an immune pathogenesis. Br Med J. 1980;280(6230):1500-1503. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1601688/. Accessed June 20, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gentile S, Strollo F, Ceriello A; AMD-OSDI Injection Technique Study Group. Lipodystrophy in insulin-treated subjects and other injection-site skin reactions: are we sure everything is clear? Diabetes Ther. 2016;7(3):401-409. doi: 10.1007/s13300-016-0187-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mier A, Weerakoon J, Dandona P. Bilateral abdominal lipohypertrophy after continuous subcutaneous infusion of insulin. Br Med J (Clin Res Ed). 1982;285(6354):1539. doi: 10.1136/bmj.285.6354.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Singha A, Bhattacharjee R, Dalal BS, Biswas D, Choudhuri S, Chowdhury S. Associations of insulin-induced lipodystrophy in children, adolescents, and young adults with type 1 diabetes mellitus using recombinant human insulin: a cross-sectional study. J Pediatr Endocrinol Metab. 2021;34(4):503-508. doi: 10.1515/jpem-2020-0556. [DOI] [PubMed] [Google Scholar]
- 5. Schober E, Rami B. Dermatological side effects and complications of continuous subcutaneous insulin infusion in preschool-age and school-age children. Pediatr Diabetes. 2009;10(3):198-201. doi: 10.1111/j.1399-5448.2008.00477.x. [DOI] [PubMed] [Google Scholar]
- 6. Herman A, de Montjoye L, Tromme I, Goossens A, Baeck M. Allergic contact dermatitis caused by medical devices for diabetes patients: a review. Contact Dermatitis. 2018;79(6):331-335. doi: 10.1111/cod.13120. [DOI] [PubMed] [Google Scholar]
- 7. Berg AK, Nørgaard K, Thyssen JP, et al. Skin problems associated with insulin pumps and sensors in adults with type 1 diabetes: a cross-sectional study. Diabetes Technol Ther. 2018;20(7):475-482. doi: 10.1089/dia.2018.0088. [DOI] [PubMed] [Google Scholar]
- 8. Berg AK, Olsen BS, Thyssen JP, et al. High frequencies of dermatological complications in children using insulin pumps or sensors. Pediatr Diabetes. 2018;19(4):733-740. doi: 10.1111/pedi.12652. [DOI] [PubMed] [Google Scholar]
- 9. Kordonouri O, Lauterborn R, Deiss D. Lipohypertrophy in young patients with type 1 diabetes. Diabetes Care. 2002;25(3):634. doi: 10.2337/diacare.25.3.634. [DOI] [PubMed] [Google Scholar]
- 10. Pozzuoli GM, Laudato M, Barone M, Crisci F, Pozzuoli B. Errors in insulin treatment management and risk of lipohypertrophy. Acta Diabetol. 2018;55(1):67-73. doi: 10.1007/s00592-017-1066-y. [DOI] [PubMed] [Google Scholar]
- 11. Deng N, Zhang X, Zhao F, Wang Y, He H. Prevalence of lipohypertrophy in insulin-treated diabetes patients: a systematic review and meta-analysis. J Diabetes Investig. 2017;9(3):536-543. doi: 10.1111/jdi.12742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Peng S, Xu M, Zhao H, et al. Gender differences in prevalence and clinical correlates of lipohypertrophy in insulin-exposed patients with diabetes mellitus. Diabetes Metab Syndr Obes. 2022;15:3871-3887. doi: 10.2147/DMSO.S392324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thewjitcharoen Y, Prasartkaew H, Tongsumrit P, et al. Prevalence, risk factors, and clinical characteristics of lipodystrophy in insulin-treated patients with diabetes: an old problem in a new era of modern insulin. Diabetes Metab Syndr Obes. 2020;13:4609-4620. doi: 10.2147/DMSO.S282926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Al Hayek AA, Robert AA, Braham RB, Al Dawish MA. Frequency of lipohypertrophy and associated risk factors in young patients with type 1 diabetes: a cross-sectional study. Diabetes Ther. 2016;7(2):259-267. doi: 10.1007/s13300-016-0161-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Al Ajlouni M, Abujbara M, Batieha A, Ajlouni K. Prevalence of lipohypertrophy and associated risk factors in insulin-treated patients with type 2 diabetes mellitus. Int J Endocrinol Metab. 2015;13(2):e20776. doi: 10.5812/ijem.20776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ucieklak D, Mrozinska S, Wojnarska A, Malecki MT, Klupa T, Matejko B. Insulin-induced lipohypertrophy in patients with type 1 diabetes mellitus treated with an insulin pump. Int J Endocrinol. 2022;2022:9169296. doi: 10.1155/2022/9169296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lombardo F, Bombaci B, Alibrandi A, Visalli G, Salzano G, Passanisi S. The Impact of insulin-induced lipodystrophy on glycemic variability in pediatric patients with type 1 diabetes. Children (Basel). 2022;9(7):1087. doi: 10.3390/children9071087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Korkmaz FN, Gökçay Canpolat A, Güllü S. Determination of insulin-related lipohypertrophy frequency and risk factors in patients with diabetes. Endocrinol Diabetes Nutr (Engl Ed). 2022;69(5);354-361. doi: 10.1016/j.endinu.2021.07.002. [DOI] [PubMed] [Google Scholar]
- 19. Blanco M, Hernández MT, Strauss KW, Amaya M. Prevalence and risk factors of lipohypertrophy in insulin-injecting patients with diabetes. Diabetes Metab. 2013;39(5):445-453. doi: 10.1016/j.diabet.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 20. Ji L, Sun Z, Li Q, et al. Lipohypertrophy in China: prevalence, risk factors, insulin consumption, and clinical impact. Diabetes Technol Ther. 2017;19(1):61-67. doi: 10.1089/dia.2016.0334. [DOI] [PubMed] [Google Scholar]
- 21. Gentile S, Guarino G, Giancaterini A, Guida P, Strollo F; AMD-OSDI Italian Injection Technique Study Group. A suitable palpation technique allows to identify skin lipohypertrophic lesions in insulin-treated people with diabetes. Springerplus. 2016;5:563. doi: 10.1186/s40064-016-1978-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang W, Huang R, Chen Y, Tu M. Values of ultrasound for diagnosis and management of insulin-induced lipohypertrophy: a prospective cohort study in China. Medicine (Baltimore). 2021;100(29):e26743. doi: 10.1097/MD.0000000000026743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kasperska-Czyzyk T, Stefanski P, Elwertowski M. Ultrasonographic assessment of subcutaneous lipohypertrophy at insulin injection sites. Diabetes Res Clin Pract. 2000;50(suppl 1):78. [Google Scholar]
- 24. Luo D, Shi Y, Zhu M, et al. Subclinical lipohypertrophy—easily ignored complications of insulin therapy. J Diabetes Complications. 2021;35(3):107806. doi: 10.1016/j.jdiacomp.2020.107806. [DOI] [PubMed] [Google Scholar]
- 25. Vardar B, Kizilci S. Incidence of lipohypertrophy in diabetic patients and a study of influencing factors. Diabetes Res Clin Pract. 2007;77(2):231-236. doi: 10.1016/j.diabres.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 26. Zhang G, Romo-Anselmo E, Kwa T, Cohen O, Vigersky R, Chattaraj S. Advances in insulin infusion set in the new era of automated insulin delivery: a systematic review. J Diabetes Sci Technol. 2023;17(2):302-313. doi: 10.1177/19322968221145731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kastner JR, Eisler G, Torjman MC, et al. In Vivo study of the inflammatory tissue response surrounding a novel extended-wear kink-resistant insulin infusion set prototype compared with a commercial control over two weeks of wear time [published online ahead of print May 9, 2022]. J Diabetes Sci Technol. doi: 10.1177/19322968221093362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Han HS, Hong JY, Kwon TR, et al. Mechanism and clinical applications of needle-free injectors in dermatology: literature review. J Cosmet Dermatol. 2021;20(12):3793-3801. doi: 10.1111/jocd.14047. [DOI] [PubMed] [Google Scholar]
- 29. Gentile S, Guarino G, Della Corte T, Marino G, Satta E, Pasquarella M. Why do so many people with type 2 diabetes who take insulin have lipohypertrophy? fate or educational deficiencies? Diabetes Ther. 2022;6:1-13. doi: 10.1007/s13300-022-01341-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huang J, Yeung AM, Kerr D, et al. Lipohypertrophy and insulin: an old dog that needs new tricks [published online ahead of print April 23, 2023]. Endocr Pract. doi: 10.1016/j.eprac.2023.04.006. [DOI] [PubMed] [Google Scholar]
- 31. Gentile S, Guarino G, Corte TD, et al. Insulin-induced skin lipohypertrophy in type 2 diabetes: a multicenter regional survey in Southern Italy. Diabetes Ther. 2020;11(9):2001-2017. doi: 10.1007/s13300-020-00876-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sandro G, Felice S, Nicoletta D, et al. Gr upSM injection-related local side effects in the treatment of diabetes mellitus: a methodological approach and possible solutions. Consensus statement of AMD-OSDI study group on injection technique. https://www.semanticscholar.org/paper/Gr-upSM-Injection-Related-Local-Side-Effects-in-the-Sandro-Felice/d29d49e463d1dbe98bde0089177870f2080bc46c. Published 2016. Accessed May 8, 2023. [Google Scholar]
- 33. Gentile S, Guarino G, Della Corte T, et al. Role of structured education in reducing lypodistrophy and its metabolic complications in insulin-treated people with type 2 diabetes: a randomized multicenter case-control study. Diabetes Ther. 2021;12(5):1379-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gentile S, Guarino G, Della Corte T, et al. The durability of an intensive, structured education-based rehabilitation protocol for best insulin injection practice: the ISTERP-2 study. Diabetes Ther. 2021;12(9):2557-2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gentile S, Guarino G, Della Corte T. The economic burden of insulin injection-induced lipohypertophy. Role of education: the ISTERP-3 study [Published Correction Appears in Adv Ther. 2022]. Adv Ther. 2022;39(5):2192-2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vandemergel X. Point-of-care ultrasound (POCUS) in the field of diabetology. Int J Chronic Dis. 2021;2021:8857016. doi: 10.1155/2021/8857016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hashem R, Mulnier H, Abu Ghazaleh H, et al. Characteristics and morphology of lipohypertrophic lesions in adults with type 1 diabetes with ultrasound screening: an exploratory observational study. BMJ Open Diabetes Res Care. 2021;9(2):e002553. doi: 10.1136/bmjdrc-2021-002553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gentile S, Guarino G, Della Corte T, et al. Lipohypertrophy in elderly insulin-treated patients with type 2 diabetes. Diabetes Ther. 2021;12(1):107-119. doi: 10.1007/s13300-020-00954-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bandari E, Beuzen T, Habashy L, et al. Machine learning decision support for detecting lipohypertrophy with bedside ultrasound: proof-of-concept study. JMIR Form Res. 2022;6(5):e34830. doi: 10.2196/34830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Young RJ, Hannan WJ, Frier BM, Steel JM, Duncan LJ. Diabetic lipohypertrophy delays insulin absorption. Diabetes Care. 1984;7(5):479-480. doi: 10.2337/diacare.7.5.479. [DOI] [PubMed] [Google Scholar]
- 41. Thow JC, Johnson AB, Marsden S, Taylor R, Home PD. Morphology of palpably abnormal injection sites and effects on absorption of isophane(NPH) insulin. Diabet Med. 1990;7(9):795-799. doi: 10.1111/j.1464-5491.1990.tb01494.x. [DOI] [PubMed] [Google Scholar]
- 42. Johansson UB, Amsberg S, Hannerz L, et al. Impaired absorption of insulin aspart from lipohypertrophic injection sites. Diabetes Care. 2005;28(8):2025-2027. doi: 10.2337/diacare.28.8.2025. [DOI] [PubMed] [Google Scholar]
- 43. Famulla S, Hövelmann U, Fischer A, et al. Insulin injection into lipohypertrophic tissue: blunted and more variable insulin absorption and action and impaired postprandial glucose control. Diabetes Care. 2016;39(9):1486-1492. doi: 10.2337/dc16-0610. [DOI] [PubMed] [Google Scholar]
- 44. Smith M, Clapham L, Strauss K. UK lipohypertrophy interventional study. Diabetes Res Clin Pract. 2017;126:248-253. [DOI] [PubMed] [Google Scholar]
- 45. Frid AH, Kreugel G, Grassi G, et al. New insulin delivery recommendations. Mayo Clin Proc. 2016;91(9):1231-1255. [DOI] [PubMed] [Google Scholar]
- 46. BDTM diabetes care app. Date unknown. Accessed May 8, 2023. https://www.embecta.com/en-us/products-and-solutions/products/product-families/bd-diabetes-care-app/.
- 47. Klarskov CK, Hamid YH, Tjalk-Bøggild R, Tarnow L, Kristensen PL. A new medical device for improved rotation of insulin injections in type 1 diabetes mellitus: a proof-of-concept study. J Diabetes Sci Technol. 2021;15(5):1111-1120. doi: 10.1177/1932296820950688. [DOI] [PMC free article] [PubMed] [Google Scholar]