Abstract
Background:
The 2022 American Diabetes Association (ADA) Standards of Care recommends considering use of continuous glucose monitoring (CGM) for insulin-managed diabetes mellitus (DM), but equitable access remains challenging. This study evaluates socioeconomic and demographic metrics associated with CGM use.
Methods:
RStudio 2021.09.1+372 was utilized to perform uni- and bivariable analysis, as well as binomial logistic regression modeling for categorical CGM use (yes/no) on the most recent cross-section from the Type 1 Diabetes Exchange (T1DX) Registry 2016-2018 cohort (n = 22 418).
Results:
Compared with White Non-Hispanic participants, Black Non-Hispanic (OR = 0.45, CI = 0.36-0.57, P < 0.001) and American Indian/Alaskan Native individuals (OR = 0.33, CI = 0.14-0.70, P = 0.008) had lower odds of CGM use. Compared with private insurance, government insurance had reduced odds of CGM use (OR = 0.59, CI = 0.52-0.66, P < 0.001). Individuals earning $100,000 or more were twice as likely to use CGMs (OR = 2.06, CI = 1.75-2.45, P < 0.001) compared with those earning <$25,000 annually. Subgroup analysis based on income bracket demonstrated that government insured individuals earning <$25,000 annually were the least likely to use CGMs (OR = 0.44, CI = 0.32-0.61, P < 0.001), as compared with private insurance.
Conclusions:
T1DX Registry data demonstrate that CGM use follows the inverse care law, with health technology utilization inversely related to disease burden. Federal policies promoting CGM use in Medicare and Medicaid populations can facilitate the ADA’s recommendation for patients with insulin-managed diabetes mellitus.
Keywords: Continuous Glucose Monitoring, Data Science, Diabetes Mellitus, Health Equity, Type 1 Diabetes Exchange Registry
Introduction
A promising development in the field of diabetes care has been the continuous glucose monitor (CGM), which is seen as an advancement in self-monitoring blood glucose (SMBG) techniques.1-3 CGM technology has demonstrated significant improvement in glycemic management, higher glucose monitoring satisfaction, and reduced incidence of diabetes complications, such as severe hypoglycemia (SH) and diabetic ketoacidosis (DKA).4-11 The increasing body of literature, both in randomized controlled trials (RCTs) and real-world evidence (RWE), supports CGM use for pediatric, adolescent, and adult persons with type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM).8,12 The American Diabetes Association (ADA) Standards of Medical Care in Diabetes 13 recommends considering initiation of CGM technology for all patients with DM requiring insulin therapy, and to sustain CGM access across all third-payer insurance types.
The advancing standard of care for insulin-managed DM has been impacted by ongoing changes to insurance, especially the beginning of Medicare coverage for CGM technology in 2017.12,14 In 2021, Medicare eligibility criteria allowed more patients with DM to initiate CGM use by removing the requirement for frequent SMBG monitoring four times daily. 14 Despite this progress, tenacious disparities persist. Reduced CGM utilization has been shown to correlate with socioeconomic status (SES), racial-ethnic disparities, and insurance type.15-17 Progress on increasing CGM use is led by diabetes advocacy groups, who push for coverage expansion for Medicaid and private payers. To study the evolving influences on CGM use, this study aims to evaluate a large database of insulin-managed patients with DM, the Type 1 Diabetes Exchange (T1DX) Registry.
The T1DX Registry 18 is a longitudinal study of persons living with T1DM, collecting socioeconomic, demographic, and diabetes care metrics from 81 endocrinology centers across the US. 19 In this analysis, we utilized the most recent cross-section (2016-2018) of this publicly available, de-identified registry to evaluate socioeconomic and demographic metrics associated with CGM use.7,10,16,20
Methods
Study Population
Participants in the T1DX Registry 19 from January 1, 2016, to March 31, 2018 were included in this study, with descriptive statistics provided in Table 1. This cohort, 20 as well as the database’s inclusion/exclusion criteria, consent process, and baseline data collection process16,21 were previously described. Figure 1 outlines the study sample selection process. Unique patient ID’s (n = 22,884) within the T1DX Registry were evaluated. Patient ID’s which did not contain responses (n = 466) for the outcome of interest (categorical CGM use—yes/no) were excluded, providing the final analytic study population (n = 22,418). This study was deemed not human subjects research by the Case Western Reserve University Institutional Review Board.
Table 1.
Descriptive Statistics of the T1DX Registry 2016-2018 Cohort.
| Characteristic | CGM use | Total cohort, N = 22 418 | P-value a | |
|---|---|---|---|---|
| Overall, N = 22 418 | No, N = 15 379 | Yes, N = 7039 | ||
| Mean Hemoglobin A1c, Median (IQR) | 8.15 (7.48, 8.93) | 8.32 (7.64, 9.15) | 7.80 (7.25, 8.43) | <.001 |
| Gender, n (%) | .009 | |||
| F | 11 315 (51%) | 7658 (50%) | 3657 (52%) | |
| M | 11 064 (49%) | 7695 (50%) | 3369 (48%) | |
| T | 12 (<0.1%) | 8 (<0.1%) | 4 (<0.1%) | |
| Age group, n (%) | <.001 | |||
| <18 years old | 10 549 (47%) | 7320 (48%) | 3229 (46%) | |
| 18-64 years old | 10 660 (48%) | 7187 (47%) | 3473 (49%) | |
| ≥65 years old | 1209 (5.4%) | 872 (5.7%) | 337 (4.8%) | |
| Race/ethnicity, n (%) | <.001 | |||
| White Non-Hispanic | 18 147 (82%) | 11 931 (78%) | 6216 (89%) | |
| Black Non-Hispanic | 1265 (5.7%) | 1137 (7.4%) | 128 (1.8%) | |
| Hispanic or Latino | 1851 (8.3%) | 1457 (9.5%) | 394 (5.6%) | |
| Asian | 239 (1.1%) | 161 (1.1%) | 78 (1.1%) | |
| Native Hawaiian/other Pacific Islander | 32 (0.1%) | 27 (0.2%) | 5 (<0.1%) | |
| American Indian/Alaskan Native | 96 (0.4%) | 82 (0.5%) | 14 (0.2%) | |
| More than one race | 626 (2.8%) | 472 (3.1%) | 154 (2.2%) | |
| Patient annual income, n (%) | <.001 | |||
| Less than $25,000 | 1,791 (11%) | 1549 (14%) | 242 (4.5%) | |
| $25,000-$35,000 | 1329 (8.1%) | 1089 (9.9%) | 240 (4.4%) | |
| $35,000-less than $50,000 | 1927 (12%) | 1477 (13%) | 450 (8.3%) | |
| $50,000-less than $75,000 | 2774 (17%) | 1900 (17%) | 874 (16%) | |
| $75,000-less than $100,000 | 2911 (18%) | 1867 (17%) | 1044 (19%) | |
| $100,000 or more | 5686 (35%) | 3123 (28%) | 2563 (47%) | |
| Level of education, n (%) | <.001 | |||
| Less than ninth grade | 722 (3.4%) | 509 (3.5%) | 213 (3.2%) | |
| Some high school | 814 (3.9%) | 725 (5.0%) | 89 (1.3%) | |
| High school graduate or GED | 2625 (12%) | 2163 (15%) | 462 (6.9%) | |
| Some college | 4,018 (19%) | 3,121 (22%) | 897 (13%) | |
| College graduate | 8233 (39%) | 5278 (37%) | 2955 (44%) | |
| Postgraduate degree | 4651 (22%) | 2581 (18%) | 2070 (31%) | |
| Patient is a current smoker, n (%) | 633 (3.0%) | 541 (3.7%) | 92 (1.4%) | <.001 |
| BMI, median (IQR) | 23.9 (20.5, 27.8) | 24.0 (20.8, 27.9) | 23.7 (19.8, 27.6) | <.001 |
| Self-reported DKA episode in the past year, n (%) | 1001 (4.5%) | 839 (5.5%) | 162 (2.3%) | <.001 |
| Self-reported severe hypoglycemia Episode in the past year, n (%) | 310 (1.4%) | 239 (1.6%) | 71 (1.0%) | .001 |
| Type of insurance, n (%) | ||||
| Private insurance | 15,224 (68%) | 9526 (62%) | 5698 (81%) | |
| Government insurance | 5297 (24%) | 4441 (29%) | 856 (12%) | |
| Single service (vision, dental) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Multiple insurance | 612 (2.7%) | 440 (2.9%) | 172 (2.4%) | |
| Unknown | 1040 (4.6%) | 771 (5.0%) | 269 (3.8%) | |
| Uninsured | 245 (1.1%) | 201 (1.3%) | 44 (0.6%) | |
Abbreviations: CGM, continuous glucose monitoring; IQR, interquartile range; GED, general educational development; BMI, body mass index; DKA, diabetic ketoacidosis.
Wilcoxon rank-sum test; Fisher’s exact test; Pearson’s chi-squared test.
Figure 1.
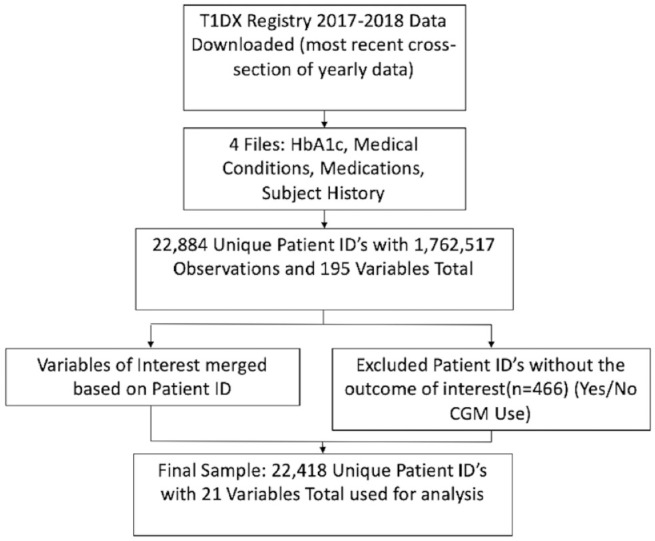
Sample selection for statistical analysis. Abbreviation: CGM, continuous glucose monitoring.
Data Processing
The variables of interest for this study were collected from the 2016 to 2018 Hemoglobin A1c and Subject information files. Unique patient IDs were used to merge variables for age, gender, race/ethnicity, current smoking status, body mass index (BMI), flags for DKA, flags for SH, insurance type, insulin delivery method, pump and CGM model and manufacturer, and the number of days in the past month a CGM was used.
For specific variables, data pre-processing required recoding to condense factors, collapse vectors, and create summary metrics. For HbA1c, self-reported values for each patient ID were averaged using the mean. In addition, the continuous variable for age was factored into three groups: <18 years old, 18-64 years old, and ≥65 years old. Level of education was also condensed from 15 factor levels to 6 (less than ninth grade, some high school, high school graduate or GED, some college, college graduate, postgraduate degree).
Five separate categorical vectors for insurance status were condensed to create two new variables. The first vector created was a categorical (yes/no) for whether the patient has any type of insurance. The second insurance vector was a leveled factor displaying the type of insurance for each patient. Nested if else logic was used, with values greater than 1 recoded as multiple insurance, and independent factor levels for government, private, single service, unknown, and uninsured insurance status.
Outcomes
CGM use
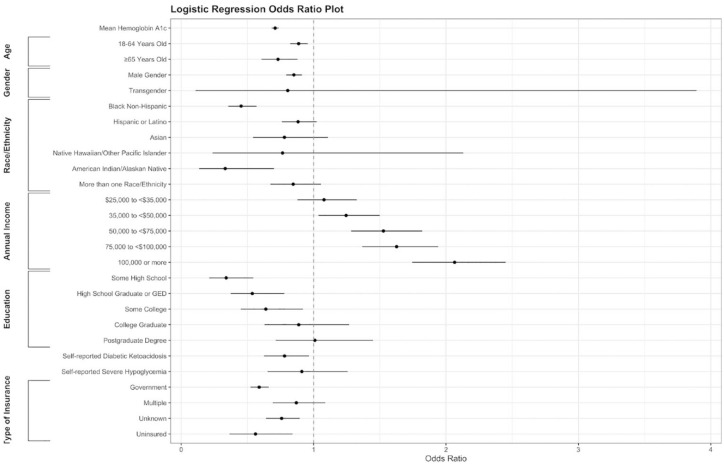
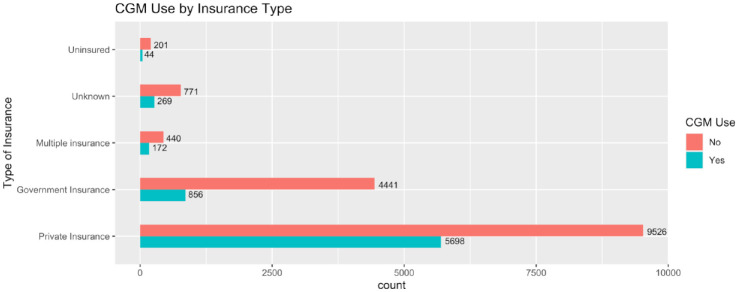
Categorical yes/no CGM use (the study’s primary outcome) is a variable obtained from the 2016-2018 T1DX Registry Subject File and merged to the Hemoglobin A1c dataframe by unique patient ID. Unique Patient ID’s that were missing responses for CGM use were excluded from analysis (n = 466). Logistic regression modeling for odds of CGM use is demonstrated in Table 2, with graphic visualization of the data provided as an odds plot in Figure 2. Patient self-reported insurance type based on CGM use is also reported in Figure 3.
Table 2.
Logistic Regression Model for Odds of CGM Use.
| Odds of CGM use | |||
|---|---|---|---|
| Characteristic | OR | 95% CI | P-value |
| Mean hemoglobin A1c | 0.71 | 0.68, 0.74 | <.001 |
| Age group | |||
| <18 years old | – | – | |
| 18-64 years old | 0.89 | 0.82, 0.96 | .002 |
| ≥65 years old | 0.73 | 0.61, 0.88 | <.001 |
| Gender | |||
| F | – | – | |
| M | 0.85 | 0.79, 0.91 | <.001 |
| T | 0.8 | 0.11, 3.89 | .8 |
| Race/ethnicity | |||
| White Non-Hispanic | – | – | |
| Black Non-Hispanic | 0.45 | 0.36, 0.57 | <.001 |
| Hispanic or Latino | 0.88 | 0.76, 1.02 | .1 |
| Asian | 0.78 | 0.54, 1.11 | .2 |
| Native Hawaiian/other Pacific Islander | 0.77 | 0.24, 2.13 | .6 |
| American Indian/Alaskan Native | 0.33 | 0.14, 0.70 | .008 |
| More than one race | 0.85 | 0.67, 1.06 | .14 |
| Patient annual income | |||
| Less than $25 000 | – | – | |
| $25 000-$35 000 | 1.08 | 0.88, 1.33 | .5 |
| $35 000-less than $50 000 | 1.25 | 1.04, 1.50 | .02 |
| $50 000-less than $75 000 | 1.53 | 1.28, 1.82 | <.001 |
| $75 000—less than $100 000 | 1.63 | 1.37, 1.94 | <.001 |
| $100 000 or more | 2.06 | 1.75, 2.45 | <.001 |
| Level of education | |||
| Less than ninth grade | – | – | |
| Some high school | 0.34 | 0.21, 0.54 | <.001 |
| High school graduate or GED | 0.54 | 0.37, 0.78 | <.001 |
| Some college | 0.64 | 0.45, 0.92 | .014 |
| College graduate | 0.89 | 0.63, 1.27 | .5 |
| Postgraduate degree | 1.01 | 0.71, 1.45 | >.9 |
| Self-reported DKA episode in the past year | |||
| No | – | – | |
| Yes | 0.78 | 0.63, 0.96 | .024 |
| Self-reported severe hypoglycemia episode in the past year | |||
| No | – | – | |
| Yes | 0.91 | 0.65-1.26 | .6 |
| Type of insurance | |||
| Private insurance | – | – | |
| Government insurance | 0.59 | 0.52, 0.66 | <.001 |
| Multiple insurance | 0.87 | 0.69, 1.09 | .2 |
| Unknown | 0.76 | 0.64, 0.89 | .001 |
| Uninsured | 0.56 | 0.36, 0.84 | .007 |
Abbreviations: CGM, continuous glucose monitoring; OR, odds ratio; CI, confidence interval; GED, general educational development; DKA, diabetic ketoacidosis.
Figure 2.
Odds plot (aka forest plot) for continuous glucose monitoring use. Abbreviation: GED, general educational development.
Figure 3.
Patient self-reported insurance type based on CGM use. Abbreviation: CGM, continuous glucose monitoring.
Median sample HbA1c
Intra-participant mean HbA1c is a value that was calculated by the study team, which was determined from the raw data within the T1DX Registry of self-reported HbA1c values. Median sample HbA1c histogram and univariable analysis were done to evaluate measures of central tendency and dispersion, and bivariable analysis comparing median sample HbA1c between CGM users and non-CGM users. A frequency plot demonstrating this comparison is presented in Figure 4.
Figure 4.
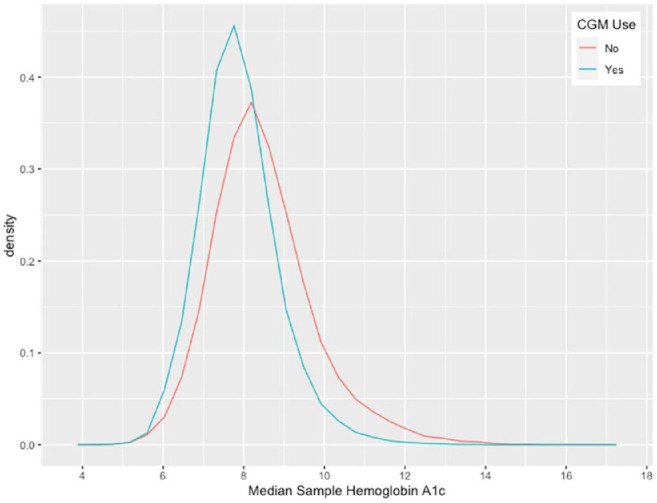
Median sample HbA1c percent comparison based on CGM use. Abbreviation: CGM, continuous glucose monitoring.
Statistical analysis
RStudio 2021.09.1+372 “Ghost Orchid” Release 22 , and R packages23-28 were used to perform statistical analysis. We compared descriptive characteristics of CGM users to non-CGM users, reporting the median (interquartile range; IQR) for continuous variables and percent for categorical variables. P-values were added based on data type, with Wilcoxon rank-sum testing used for nonparametric, continuous variables. Categorical variables used Fischer’s exact testing and chi-squared testing for goodness of fit, homogeneity, and variance. Histograms were also created for continuous variables, overall and stratified by CGM use. Bivariable analysis of statistically significant factors was conducted to evaluate CGM use. Multivariable logistic regression modeling was conducted, modeling odds of CGM use (yes/no) as the dependent variable of interest. Odds plots were generated to visualize odds of CGM use in Figure 3. Effect modification was also performed by subgroup analysis. Odds of CGM use for subsets of age group and income bracket were stratified by insurance status in Tables 3 and 4.
Table 3.
Association Between Insurance Type and CGM Use Stratified by Income.
| Subgroup analysis by reported annual income | |||
|---|---|---|---|
| Characteristic | OR | 95% CI | P-value |
| Total cohort | |||
| Type of insurance | |||
| Private insurance | – | – | |
| Government insurance | 0.58 | 0.52, 0.64 | <.001 |
| Multiple insurance | 0.84 | 0.68, 1.05 | .14 |
| Unknown | 0.76 | 0.65, 0.90 | .002 |
| Uninsured | 0.56 | 0.37, 0.84 | .007 |
| <$25 000 | |||
| Type of insurance | |||
| Private insurance | – | – | |
| Government insurance | 0.43 | 0.31, 0.58 | <.001 |
| Multiple insurance | 1.15 | 0.51, 2.39 | .7 |
| Unknown | 0.52 | 0.24, 1.03 | .075 |
| Uninsured | 0.1 | 0.01, 0.46 | .023 |
| $25 000-35 000 | |||
| Type of insurance | |||
| Private insurance | – | – | |
| Government insurance | 0.44 | 0.31, 0.62 | <.001 |
| Multiple insurance | 0.58 | 0.23, 1.28 | .2 |
| Unknown | 0.47 | 0.22, 0.92 | .038 |
| Uninsured | 0.44 | 0.07, 1.69 | .3 |
| $35 000-50 000 | |||
| Type of insurance | |||
| Private insurance | – | – | |
| Government insurance | 0.69 | 0.53, 0.89 | .005 |
| Multiple insurance | 0.75 | 0.41, 1.33 | .3 |
| Unknown | 0.56 | 0.32, 0.92 | .028 |
| Uninsured | 0.14 | 0.01, 0.69 | .057 |
| $50 000-75 000 | |||
| Type of insurance | |||
| Private insurance | – | – | |
| Government insurance | 0.64 | 0.50, 0.81 | <.001 |
| Multiple insurance | 1.03 | 0.66, 1.57 | .9 |
| Unknown | 1.23 | 0.86, 1.76 | .3 |
| Uninsured | 1.32 | 0.51, 3.20 | .5 |
| $75 000-100 000 | |||
| Type of insurance | |||
| Private insurance | – | – | |
| Government insurance | 0.67 | 0.50, 0.89 | .006 |
| Multiple insurance | 1.14 | 0.67, 1.91 | .6 |
| Unknown | 0.76 | 0.53, 1.08 | .13 |
| Uninsured | 0.45 | 0.15, 1.15 | .12 |
| >$100 000 | |||
| Type of insurance | |||
Abbreviations: CGM, continuous glucose monitoring; OR, odds ratio; CI, confidence interval.
Table 4.
Association Between Insurance Type and CGM Use Stratified by Age.
| Subgroup analysis by age group | |||
|---|---|---|---|
| Characteristic | OR | 95% CI | P-value |
| Total cohort | |||
| Type of insurance | |||
| Private insurance | – | – | |
| Government insurance | 0.57 | 0.51, 0.63 | <.001 |
| Multiple insurance | 0.83 | 0.66, 1.03 | .093 |
| Unknown | 0.77 | 0.66, 0.91 | .003 |
| Uninsured | 0.56 | 0.37, 0.84 | .007 |
| <18 years old | |||
| Type of insurance | |||
| Private insurance | – | – | |
| Government insurance | 0.75 | 0.64, 0.88 | <.001 |
| Multiple insurance | 1.06 | 0.77, 1.44 | .7 |
| Unknown | 0.74 | 0.60, 0.92 | .006 |
| Uninsured | 0.6 | 0.33, 1.03 | .072 |
| 18-64 years old | |||
| Type of insurance | |||
| Private insurance | – | – | |
| Government insurance | 0.41 | 0.33, 0.51 | <.001 |
| Multiple insurance | 0.78 | 0.48, 1.23 | .3 |
| Unknown | 0.78 | 0.58, 1.04 | .1 |
| Uninsured | 0.53 | 0.27, 0.95 | .043 |
| ≥65 years old | |||
| Type of insurance | |||
| Private insurance | – | – | |
| Government insurance | 0.56 | 0.37, 0.84 | .005 |
| Multiple insurance | 0.66 | 0.38, 1.15 | .15 |
| Unknown | 1.34 | 0.42, 4.25 | .6 |
| Uninsured | 0 | >.9 | |
Abbreviations: CGM, continuous glucose monitoring; OR, odds ratio; CI, confidence interval.
Results
Descriptive Data
Table 1 displays the descriptive characteristics for comparing CGM use. The median age of T1DX Registry participants was 18.0 (IQR = 14.0-36.0), 82% are White Non-Hispanic, 75% of households earned a college degree or higher, 47% earned $100,000 or more, and 68% were privately insured. Gender is roughly proportional (51% female vs 49% male), without significant difference based on CGM use. Self-reported incidence of SH occurred in 1.4% of the entire cohort, with 1.0% in CGM users, and 1.6% in non-CGM users. Self-reported incidence of DKA occurred in 4.5% of the entire cohort, with 2.3% in CGM users and 5.5% in non-CGM users.
Outcome Data
Table 2 and Figure 2 outline the logistic regression model used to evaluate the odds of CGM use based on co-variables, with odds ratios and 95% confidence intervals reported. Reference levels for comparison are age <18 years old, female gender, White Non-Hispanic race/ethnicity, <$25,000 annual income, Less than ninth-grade education, no self-reported episode of DKA, no self-reported episode of SH, private insurance, and insulin pump user. Compared with individuals <18 years old, adults 18-64 years old (OR = 0.89, CI = 0.82-0.96) and ≥65 years old (OR = 0.73, CI = 0.61-0.88) had lower odds of CGM use. Compared with White Non-Hispanic individuals, Black Non-Hispanic individuals had lower odds of CGM use (OR = 0.45, CI = 0.36-0.57, P < .001), with similar trends among American Indian/Alaskan Native individuals (OR = 0.33, CI = 0.14-0.71, P = .008). Individuals in higher income brackets had greater odds of CGM use as compared with people earning <$25,000 annually, with individuals earning $100,000 or more showing the greatest difference (OR = 2.06, CI = 1.75-2.45, P < .001). An increase in mean HbA1c was associated with lower odds of CGM use (OR = 0.71, CI = 0.68-0.74, P < .001). Individuals on government insurance (Medicaid, Medicare, etc.) had less likelihood of CGM use (OR = 0.59, CI = 0.52-0.66, P < .001). When adjusting for confounders, incidence of self-reported DKA events were associated with statistically significant lower odds of CGM use (OR = 0.78, CI = 0.63-0.96, P = .024).
Figure 3 outlines the type of insurance that was self-reported based on CGM use. In the overall 2016-2018 T1DX Registry study cohort, 24% of individuals self-reported having government insurance. Proportionally less CGM users (12.16%) report having government insurance, while 28.88% of non-CGM users are government insured.
Figure 4 demonstrates the median sample HbA1c for T1DX Registry participants based on CGM use. The median HbA1c for the entire 2016-2018 study cohort is 8.15 (IQR = 7.48-8.93), while CGM users had a median value of 7.80 (IQR = 7.25-8.43), and non-CGM users had a median value of 8.32 (IQR = 7.64-9.15) with a statistically significant difference indicated (P <.001).
Tables 3 and 4 demonstrate subgroup analyses for the odds of CGM use based on insurance type, as compared with self-reported annual income and age group, respectively. Compared with private insurance, government insurance consistently demonstrated decreased odds of CGM use across subgroups, with 15% increased odds for CGM use among government insured individuals ≥ 65 years old. The population with the lowest likelihood of CGM use was government insured individuals earning <$25 000 annually.
Additional Analysis
Regarding CGM use, T1DX Registry participants were asked “Which CGM device/model does/did the participant use?” during clinic exams. 18 Of the total cohort (n= 7039), 31.4% reported CGM use throughout the 2016-2018 cross-section. For CGM manufacturer, 5147 CGM users (73.1%) reported using Dexcom (San Diego, CA), 1596 CGM users (22.7%) reported using Medtronic (Minneapolis, MN), and 41 CGM users (0.6%) reported using Abbott (Abbott Park, IL). The most frequently used CGM models for Dexcom in 2016-2018 were the G5 Platinum (n = 2962) and G4 Platinum (n = 1103). The most frequently used CGM models for Medtronic were the Enlite Sensor (n = 676), Minimed 530 g (n = 280), and Minimed 670 g (n = 216). The most frequently used CGM Model for Abbot was the Freestyle Navigator (n = 28). Out of 3777 participants who reported the number of days, a CGM was used in the past month, 2493 (66.0%) reported using the CGM for all 30 days. Some CGMs reported, such as the Freestyle Navigator, were discontinued during the 2016-2018 study period, which may indicate self-report of models previously used by the participant.
Discussion
T1DX Registry is a robust sample of Persons living with T1DM across the United States. The 2016-2018 cohort demographics are 82% White Non-Hispanic, 75% of households earned a college degree or higher, 47% earned $100,000 or more, and 68% were privately insured. These estimates for the 2016-2018 T1DX Registry cohort differ from the estimated national prevalence from the SEARCH for Diabetes in Youth Study 29 conducted by the Center for Disease Control (CDC), with estimated T1DM prevalence for 2001-2016 per 1000 youth <19 y/o is 0.93 for white, 0.59 for Hispanic, 0.89 for black, 0.25-0.26 for American Indian/Pacific Islander/Asian. This may reflect differences in sampling methodology among United States endocrinology centers, as well as varying access to subspecialty care for adult versus pediatric patients with T1DM.
Compared with initial descriptions of the T1DX Registry Database, 21 CGM use in the cohort increased from 6% in 2012 to 31.4% in the 2016-18 cohort. This demonstrates a significant increase in diffusion and uptake over time. The current study demonstrates that White Non-Hispanic, age <18 years, privately insured and higher income bracket groups had the greatest odds of CGM use based on logistic regression modeling.
These findings demonstrate that populations with increased access to resources have the highest odds of utilizing CGM technology. This is in line with a previously described trend known as the inverse care law,30,31 where health technology diffusion and uptake is inversely related to disease burden. The social determinants leading to these disparities are complex. Cost has been identified as one of the most important factors for persons with T1DM to utilize CGM technology.32,33 However, studies within health systems that provide universal coverage found that disparities still exist across racial-ethnic subgroups, independent of insurance status or household income.15,34 A 2015-2018 study found that CGMs are initiated at higher rates in Non-Hispanic White children, and Non-Hispanic Black children were more likely to discontinue CGM use within the first year, when controlling for insurance status. 35 Similarly, a 2019 study on children and adults with T1DM found that racial-ethnic disparities in CGM use persisted independent of household income. 16 The Young Adult Racial Disparities in Type 1 Diabetes (YARDD) study 34 found that SES has a significant effect, but is not the main driver, of disparities in CGM use. While these social determinants likely have complex inter-dependent influences on the ability to utilize CGM technology, clear modifiable risk factors are also demonstrated within the data.
Data from our research study presented in Tables 3 and 4 demonstrate that CGM use is significantly impacted by insurance status in the United States. Government insurance consistently showed a negative correlation with CGM use when compared across age groups and income status. Proportionally less CGM users (12.16%) report having government insurance, while 28.88% of non-CGM users are government insured. Individuals ≥65 years old on government insurance had 15% higher odds of CGM use when compared with adults 18-64 years old on government insurance, which is at least partially explained by the emerging role of Medicare coverage for CGM technology during the study period. These data support focusing on the actionable goal of advocating for increased access to CGM use across third-payer insurance plans.
This study has several limitations. First, the observational, cross-sectional design prevents analysis of temporality trends and would require additional timepoint comparisons. Furthermore, data quality in a rapidly evolving biomedical device market from the 2016-2018 study period includes discontinuation of many products and introduction of emerging devices and software, such as closed-loop insulin pump/CGM systems. Self-reported data indicate whether research participants currently or previously used CGMs, without a way to identify exactly when the CGM was utilized during the study period. In addition, the shifting insurance coverage during the study period for government programs, such as Medicaid and Medicare, cannot be fully represented within the data alone. Data granularity of the database determined the ability to draw correlations, especially for variables, such as income bracket and insurance type (Medicaid and Medicare are categorized together under government insurance). Possible sources of bias include recall bias, as well as the potential for systematic bias due to data missingness. In addition, pediatric T1DX Registry participants report household education and income instead of individual measures. These variables may change across T1DX Registry yearly cross-sections as participants report individual data instead of their parent’s income and education.
Study strengths include the utilization of a public database with a large sample size of confirmed T1DM cases almost exclusively involving insulin management. T1DX Registry data also provide robust information not collected in National Health and Nutrition Examination Survey (NHANES), allowing for unique insights on a large amount and diversity of information collected with a standardized methodology. The T1DX Registry database provides a unique opportunity to test the implementation of the 2022 ADA Standards of Care as CGM utilization evolves. Once newer data become available, further research would provide additional insight about CGM use trends.
Conclusion
The results demonstrate that utilization of CGM technology follows the inverse care law,30,31 where CGM utilization is inversely related to disease burden. While all patients with insulin-managed DM should have access to CGM technology, disparities exist socioeconomically, racial-ethnically, and by third-payer insurance. 13 There is a critical need to increase access to DM technology to decrease the risk of diabetes-related complications and reduce healthcare costs among racial-ethnic minority, low-income, and marginalized communities within the United States.15,35,36 Promoting federal government policies that counter prevailing barriers to CGM utilization will foster health equity and reduce disparities. Some policy stances that can address the issue are to increase Medicaid and Medicare coverage of CGM technology. Additionally, prescriber training on the impact of insurance coverage and reimbursement for diabetes technology 33 allows clinicians to become advocates for CGM utilization.
This study provides insights on the rapidly evolving field of CGM technology, highlighting the importance of equitable access for all patients with insulin-managed DM. T1DX Registry data can guide policies that promote health equity and reduce chronic disease burden in the United States. The improving standard of care for diabetes management provides an optimistic outlook, with progress occurring despite tenacious disparities that must be addressed. Proactively working on a population health level against prevailing socioeconomic, racial, and cultural barriers to increase CGM utilization can improve health outcomes for underserved populations who need care most.
Footnotes
Abbreviations: ADA, American Diabetes Association; BMI, body mass index; CDC, Center for Disease Control; CGM, continuous glucose monitor; DKA, diabetic ketoacidosis; DM, diabetes mellitus; GED, general educational development; HbA1c, hemoglobin A1c; IQR, interquartile range; NHANES, National Health and Nutrition Examination Survey; RCT, randomized controlled trial; RWE, real-world evidence; SES, socioeconomic status; SH, severe hypoglycemia; SMBG, self-monitored blood glucose; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; T1DX Registry, Type 1 Diabetes Exchange Registry; YARDD, Young Adult Racial Disparities in Type 1 Diabetes study.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Disclaimer: The source of the data is the Jaeb Center for Health Research, but the analyses, content, and conclusions presented herein are solely the responsibility of the authors and have not been reviewed or approved by the Jaeb Center for Health Research. Data are available from: https://public.jaeb.org/datasets/diabetes.
ORCID iDs: Richard Bailey  https://orcid.org/0000-0002-6393-8878
https://orcid.org/0000-0002-6393-8878
Sriya Donthi  https://orcid.org/0000-0002-3283-7281
https://orcid.org/0000-0002-3283-7281
Colin Drummond  https://orcid.org/0000-0002-9053-3649
https://orcid.org/0000-0002-9053-3649
References
- 1. Azhar A, Gillani S, Mohiuddin G, Majeed R. A systematic review on clinical implication of continuous glucose monitoring in diabetes management. J Pharm Bioallied Sci. 2020;12(2):102-111. doi: 10.4103/jpbs.JPBS_7_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bergenstal RM. Continuous glucose monitoring data as an adjunct to A1C. In: Role of Continuous Glucose Monitoring in Diabetes Treatment. American Diabetes Association; 2018. http://www.ncbi.nlm.nih.gov/books/NBK538973/. Accessed April 8, 2022. [PubMed] [Google Scholar]
- 3. Ajjan R, Slattery D, Wright E. Continuous glucose monitoring: a brief review for primary care practitioners. Adv Ther. 2019;36(3):579-596. doi: 10.1007/s12325-019-0870-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lind M, Polonsky W, Hirsch IB, et al. Continuous glucose monitoring vs conventional therapy for glycemic control in adults with type 1 diabetes treated with multiple daily insulin injections: the GOLD randomized clinical trial. JAMA. 2017;317(4):379-387. doi: 10.1001/jama.2016.19976. [DOI] [PubMed] [Google Scholar]
- 5. Faulds ER, Zappe J, Dungan KM. Real-world implications of hybrid close loop (Hcl) insulin delivery system. Endocrine Practice. 2019;25(5):477-484. doi: 10.4158/EP-2018-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McAuley SA, Lee MH, Paldus B, et al. ; Australian JDRF Closed-Loop Research Group. Six months of hybrid closed-loop versus manual insulin delivery with fingerprick blood glucose monitoring in adults with type 1 diabetes: a randomized, controlled trial. Diabetes Care. 2020;43(12):3024-3033. doi: 10.2337/dc20-1447. [DOI] [PubMed] [Google Scholar]
- 7. Laffel LM, Kanapka LG, Beck RW, et al. ; CGM Intervention in Teens and Young Adults with T1D Study Group. Effect of continuous glucose monitoring on glycemic control in adolescents and young adults with type 1 diabetes: a randomized clinical trial. JAMA. 2020;323(23):2388-2396. doi: 10.1001/jama.2020.6940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gavin JR, Bailey CJ. Real-world studies support use of continuous glucose monitoring in type 1 and type 2 diabetes independently of treatment regimen. Diabetes Technol Ther. 2021;23(suppl 3):S19-S27. doi: 10.1089/dia.2021.0211. [DOI] [PubMed] [Google Scholar]
- 9. Pratley RE, Kanapka LG, Rickels MR, et al. ; Wireless Innovation for Seniors With Diabetes Mellitus Study Group. Effect of continuous glucose monitoring on hypoglycemia in older adults with type 1 diabetes: a randomized clinical trial. JAMA. 2020;323(23):2397-2406. doi: 10.1001/jama.2020.6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Beck RW, Riddlesworth T, Ruedy K, et al. ; for the DIAMOND Study Group. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. JAMA. 2017;317(4):371-378. doi: 10.1001/jama.2016.19975. [DOI] [PubMed] [Google Scholar]
- 11. Brown SA, Kovatchev BP, Raghinaru D, et al. Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med. 2019;381(18):1707-1717. doi: 10.1056/NEJMoa1907863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Galindo RJ, Parkin CG, Aleppo G, et al. What’s wrong with this picture? A critical review of current centers for Medicare & Medicaid services coverage criteria for continuous glucose monitoring. Diabetes Technol Ther. 2021;23(9):652-660. doi: 10.1089/dia.2021.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. American Diabetes Association Professional Practice Committee. 7. Diabetes technology: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S97-S112. doi: 10.2337/dc22-S007. [DOI] [PubMed] [Google Scholar]
- 14. CMS updates CGM eligibility to broaden coverage for beneficiaries. American Journal of Managed Care. June 21, 2021. https://www.ajmc.com/view/cms-updates-cgm-eligibility-to-broaden-coverage-for-beneficiaries. Accessed February 15, 2022.
- 15. Agarwal S, Schechter C, Gonzalez J, Long JA. Racial-ethnic disparities in diabetes technology use among young adults with type 1 diabetes. Diabetes Technol Ther. 2021;23(4):306-313. doi: 10.1089/dia.2020.0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016-2018. Diabetes Technol Ther. 2019;21(2):66-72. doi: 10.1089/dia.2018.0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Willi SM, Miller KM, DiMeglio LA, et al. ; T1D Exchange Clinic Network. Racial-ethnic disparities in management and outcomes among children with type 1 diabetes. Pediatrics. 2015;135(3):424-434. doi: 10.1542/peds.2014-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. JAEB Center for Health Research. Public study websites. n.d. https://public.jaeb.org/datasets/diabetes. Accessed October 7, 2021.
- 19. JAEB Center for Health Research. Type 1 diabetes exchange registry. Public dataset: read me current reg. www.t1dexchange.org. Published 2019. Accessed April 8, 2022.
- 20. JAEB Center for Health Research. Datasets and documents. n.d. https://public.jaeb.org/datasets/diabetes. Accessed April 8, 2022.
- 21. Beck RW, Tamborlane WV, Bergenstal RM, et al. ; for T1D Exchange Clinic Network. The T1D exchange clinic registry. J Clin Endocrinol Metab. 2012;97(12):4383-4389. doi: 10.1210/jc.2012-1561. [DOI] [PubMed] [Google Scholar]
- 22. R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2021. https://www.R-project.org/. Accessed April 8, 2022. [Google Scholar]
- 23. Hutson G. OddsPlotty: Odds Plot to Visualise a Logistic Regression Model (R package version 1.0.2). https://CRAN.R-project.org/package=OddsPlotty. Published 2021. Accessed April 8, 2022.
- 24. Wickham H, François R, Henry L, Müller K. Dplyr: A Grammar of Data Manipulation (R package version 1.0.7). https://CRAN.R-project.org/package=dplyr. Published 2021. Accessed April 8, 2022
- 25. The Comprehensive R Archive Network. n.d. https://cran.r-project.org/. Accessed November 21, 2021.
- 26. Wickham H. ggplot2: Elegant Graphics for Data Analysis. New York: Springer-Verlag. 2016. [Google Scholar]
- 27. Sjoberg DD, Whiting K, Curry M, Lavery JA, Larmarange J. Reproducible summary tables with the gtsummary package. R J. 2021;13(1):570-580. doi: 10.32614/RJ-2021-053. [DOI] [Google Scholar]
- 28. Harrell FE, DuPont C. Hmisc: Harrell Miscellaneous (R package version 4.5-0). https://CRAN.R-project.org/package=Hmisc. Published 2020. Accessed April 8, 2022.
- 29. Lawrence JM, Divers J, Isom S, et al. ; SEARCH for Diabetes in Youth Study Group. Trends in prevalence of type 1 and type 2 diabetes in children and adolescents in the US, 2001-2017. JAMA. 2021;326(8):717-727. doi: 10.1001/jama.2021.11165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Weiss D, Sund ER, Freese J, Krokstad S. The diffusion of innovative diabetes technologies as a fundamental cause of social inequalities in health. The Nord-Trøndelag Health Study, Norway. Sociol Health Illn. 2020;42(7):1548-1565. doi: 10.1111/1467-9566.13147. [DOI] [PubMed] [Google Scholar]
- 31. Hart JT. THE INVERSE CARE LAW. Lancet. 1971;297(7696):405-412. doi: 10.1016/S0140-6736(71)92410-X. [DOI] [PubMed] [Google Scholar]
- 32. Addala A, Suttiratana SC, Wong JJ, et al. Cost considerations for adoption of diabetes technology are pervasive: a qualitative study of persons living with type 1 diabetes and their families. Diabet Med. 2021;38:e14575. doi: 10.1111/dme.14575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bartelme A, Bridger P. The role of reimbursement in the adoption of continuous glucose monitors. J Diabetes Sci Technol. 2009;3(4):992-995. doi: 10.1177/193229680900300449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Auzanneau M, Rosenbauer J, Maier W, et al. Heterogeneity of access to diabetes technology depending on area deprivation and demographics between 2016 and 2019 in Germany. J Diabetes Sci Technol. 2021;15(5):1059-1068. doi: 10.1177/19322968211028608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lai CW, Lipman TH, Willi SM, Hawkes CP. Racial and ethnic disparities in rates of continuous glucose monitor initiation and continued use in children with type 1 diabetes. Diabetes Care. 2020;44(1):255-257. doi: 10.2337/dc20-1663. [DOI] [PubMed] [Google Scholar]
- 36. Peek ME, Thomas CC. Broadening access to continuous glucose monitoring for patients with type 2 diabetes. JAMA. 2021;325(22):2255-2257. doi: 10.1001/jama.2021.6208. [DOI] [PMC free article] [PubMed] [Google Scholar]