Abstract
Background:
A wave of expiring patents for first-generation insulin analogues has created opportunities in the global insulin market for highly similar versions of these products, biosimilar insulins. Biologics are generally large, complex molecules produced through biotechnology in a living system, such as a microorganism, plant cell, or animal cell. Since manufacturing processes of biologics vary, biosimilars cannot be exact copies of their reference product but must exhibit a high degree of functional and structural similarity. Biosimilarity is proven by analytical approaches in comparative assessments, preclinical cell-based and animal studies, as well as clinical studies in humans facilitating the accumulation of evidence across all assessments. The approval of biosimilars follows detailed regulatory pathways derived from those of their reference products and established by agencies such as the European Medicines Agency and the US Food and Drug Administration. Regulatory authorities impose requirements to ensure that biosimilars meet high standards of quality, safety, and efficacy and are highly similar to their reference product.
Purpose:
This review aims to aid clinical understanding of the high standards of development, manufacturing, and regulation of biosimilar insulins.
Methods:
Recent relevant studies indexed by PubMed and regulatory documents were included.
Conclusions:
Driven by price competition, the emergence of biosimilar insulins may help expand global access to current insulin analogues. To maximize the impact of the advantage for falling retail costs of biosimilar insulins compared with that of reference insulins, healthcare professionals and insulin users must gain further awareness and confidence.
Keywords: biologics, biosimilar, clamp, clinical trials, diabetes, insulin, insulin therapy, manufacture, pharmacokinetic, pharmacodynamic, regulation
Introduction
Insulin has been available for use in the management of people with diabetes for a century. In the 1920s, patients with diabetes mellitus typically took three to four doses of insulin per day, creating a clear need for longer acting insulins. 1 The nature of insulin was successively determined as a polypeptide in 1928, by the amino acid sequence in 1955, and in 1969 by three-dimensional structure, laying the foundations for the development of novel insulins. Because early insulin preparations were derived from animal sources (porcine, bovine), adequate purification of insulin preparations was a problem for many years but was resolved in the 1970s. 2 In 1978, recombinant DNA techniques were introduced to produce the first genetically engineered synthetic “human” insulin in Escherichia coli. Furthermore, understanding the nature of insulin preparations, coupled with advances in manufacturing techniques, created the opportunity of developing and producing analogues of human insulin. In analogues, the structure of the insulin molecule is slightly modified to alter the pharmacokinetic (PK) properties of insulin, which primarily affects the uptake of the drug from subcutaneous tissue. Recent developments include ultra-rapid and ultra-long-acting insulin analogues.3,4 Expiration of the patents for three widely used insulin analogues (insulins glargine, lispro, and aspart) allows other manufacturers to seek regulatory approval to market an insulin analogue that is highly similar in structure, safety, tolerability, and efficacy to the original biological product—known as a biosimilar. 5
Globally, one in two people needing insulin lack access. 6 High prices and poor availability are considered the major contributors to poor access to insulin. 7 Macroeconomic conditions have often been shown to be the main cause of inequalities in the use of biologics such as insulins. As a result, access to expensive biologic treatments is often particularly poor in low-income countries. 8 Biosimilars typically have a shorter development period of around 8 years compared with 12 years for innovative medicines and costs can be 10-20% of the price of developing a new biological entity. 9 This may allow more patients who are eligible for biologics such as long-acting insulin analogues to be treated with these effective medications. 10 As health care expenditures continue to rise, the emergence of biosimilars offers, in principle, an approach to improving access to these complex products and potentially reducing the economic and social burden if offered at a lower price.
The global market for biological medicines such as insulin, erythropoietin (EPO), and anti-tumor necrosis factor (anti-TNF) drugs has grown from US$46 billion in 2002 to US$390 billion in 2020, representing approximately 28% of the global pharmaceutical market. 11 In the United States, biological medicines account for 43% of invoice-level drug expenditure, reaching US$211 billion in 2019. Within this, biosimilar spending is expected to increase from US$5.2 billion in 2019 to nearly US$27 billion in 2024. 12 In Europe, biologics represent 34% of medicine spending at list prices, reaching US$78.6 billion in 2021, and growing at a 10.5% compound annual growth rate over the past five years. The total European biosimilar market reached US$8.8 billion in 2021. 13 The global human insulin market accounted for US$22.9 billion in 2020, while the global biosimilar insulin market was valued at US$2.3 billion in 2020 and is estimated to reach US$5.6 billion by the end of 2027. 14
The emergence of biosimilars has already increased competition in the market. Biosimilars offer due to lower development costs a potentially lower price and an equally efficacious alternative that may also reduce treatment costs through increased competition within the market.
Since the approval of the first biosimilar insulin in 2014, there has been considerable scientific and clinical interest. However, many clinicians are still uncertain about their safety and efficacy in clinical use. 15 This review aims to increase clinicians’ understanding of biosimilar insulins by discussing their properties, the standards of their development, the manufacturing process, and the regulatory requirements for approval, notably in the United States and European Union.
Biosimilars: Definition and Characteristics
A biosimilar is a biological medicine highly similar to another already approved biological medicine in terms of structure, biological activity, efficacy, and safety (Table 1). These products are generally large, complex molecules produced through biotechnology in a living system, such as a microorganism, plant cell, or animal cell.16,17 Owing to inherent differences in the manufacturing process of biologics, biosimilars cannot be exact copies of reference biologics, but they need to demonstrate high similarity by extensively analyzing their structure and function. A manufacturer must also demonstrate that its proposed biosimilar product has no clinically meaningful differences from the reference product in terms of safety, purity, and potency. This is generally demonstrated through human PK and pharmacodynamic (PD) studies, an assessment of clinical immunogenicity, and, if needed, additional clinical studies.16,17
Table 1.
Definitions and Terminology Used for Biosimilars and Generics.
| Biologic(al) | An approved product consisting of proteins, nucleic acids, or combinations thereof, or living entities such as cells and tissues, isolated from natural sources and produced by biotechnological processes. |
| Reference (originator/innovator) biologic product | The single, already approved biological product to which a proposed biosimilar is compared. |
| Biosimilar17,18 | A biological medicine highly similar to another already approved biological medicine in terms of structure, biological activity, efficacy, safety and immunogenicity profile |
| Generic drug | Chemically synthesized small- or low-molecular-weight compounds consist of a simple, well-defined structure that is independent of the manufacturing process and can be easily fully characterized. |
The more complex nature of biological medications makes them more sensitive to manufacturing conditions. 19 Even minor changes in manufacturing conditions (eg, expression system, downstream processing) could lead to significant differences in structure, stability, and other quality aspects of the final product. Any variations have the potential to affect the tolerability and/or efficacy of a biosimilar and increase the risk of immune reactions. 20 The approval of biosimilars follows detailed regulatory pathways derived from those of their reference products and established by agencies such as the European Medicines Agency (EMA) and the US Food and Drug Administration (FDA). These include preclinical and clinical studies aimed at sensitive testing of comparability. 21 Moreover, the pharmacological properties of insulin reference products as a class, including their immunogenicity characteristics, have led to specific guidance relating to the approval of insulin biosimilars.16,22 While the regulatory situation varies by country, the global trend has been to follow the EMA and US FDA requirements. 23
Regulatory Compliance
Global Regulatory Guidelines
In 2004, the EMA published guidelines for the development of all biosimilars. 24 This framework covered essential aspects of the development, manufacturing, and approval of biosimilars. Following these guidelines, the World Health Organization (WHO) established a general framework for biosimilars in 2009 to support local regulatory authorities and promote conformity with international standards for the manufacture of biotherapeutics. 18 The US FDA and the China National Drug Administration (CNDA) followed their first guidelines in 2012 and 2015, respectively. Since then many other countries have adopted these guidelines, some with slight adaptations. 25 The current regulatory requirements of the EMA, 26 WHO, 18 US FDA, 17 and CNDA 27 are generally consistent but with some minor differences, including specific considerations for insulin biosimilars (Table 2).16,22 Although there are many regulatory guidance documents, there is still no common global consensus on the regulatory approval pathway for biosimilars. Several countries, including Canada, Brazil, South Africa, Japan, and Korea, have used the principles for biosimilar manufacturing outlined in the WHO guidance documents as the basis for establishing their national guidelines.28,29
Table 2.
Overview of Biosimilar Insulin Regulatory Guidelines of Different Regulatory Authorities.
| Authorities | Definition | Nonclinical evaluation | Clinical evaluation |
|---|---|---|---|
| EMA 16 | “A recombinant insulin-containing product highly similar to another already approved biological medicine (the ‘reference medicinal product’)” | Target binding to both human insulin receptors; receptor autophosphorylation and metabolic activity (≥3 assays). If in vitro comparability is satisfactory, animal studies are not required | Comparability demonstrated in the stepwise process using PK and PD, followed by limited immunogenicity studies unless waived a |
| WHO 18 | “A biotherapeutic product which is similar in terms of quality, safety, and efficacy to an already licensed reference biotherapeutic product” | In vitro assays such as receptor-binding studies or cell-based assays; animal studies for relevant biological/PD activity and toxicity | PK, PD, (confirmatory PK/PD), efficacy, and safety |
| US FDA17,22 (draft) | “A biological product highly similar to the reference product notwithstanding minor differences in clinically inactive components” | Structural analyses; functional assays; animal data | Human pharmacology data; clinical immunogenicity assessment (usually unnecessary); comparative clinical studies (if residual uncertainty whether there are clinically meaningful differences) |
Abbreviations: EMA, European Medicines Agency; FDA, Food and Drug Administration; PD, pharmacodynamics; PK, pharmacokinetics; WHO, World Health Organization.
Prelicensing safety study including immunogenicity assessment may be waived if (1) biosimilarity between the biosimilar and the reference insulin can be convincingly concluded from the physicochemical and functional characterization and comparison using sensitive, orthogonal, and state-of-the-art analytical methods, and from the comparison of the PK and PD profiles, and (2) the impurity profile and the nature of excipients of the biosimilar do not give rise to concerns.
Approval Processes
A biosimilar product only receives regulatory approval based on the totality of evidence, function, purity, potency, and safety, compared with the reference product. 30 The demand for a wide array of different types of evidence reflects the observation that no one type of evidence has the accuracy and precision required to show biosimilarity for efficacy, while safety has diverse aspects including hypoglycemia and immunogenicity.
This stepwise approach to establish biosimilarity includes comparative assessments, preclinical cell-based and animal studies, and clinical studies in humans. Biosimilarity is demonstrated based on the accumulation of evidence across all assessments (“totality of evidence”). 31 The goal is to uncover any significant differences and potential undesired pharmaceutical effects between the biosimilar and its reference medicine. Deviation in receptor binding, PK/PD studies, and immunogenicity profile will have a critical impact on regulatory decisions.
The foundation of a biosimilar development program is a comprehensive structural and functional characterization that identifies quality attributes and clinically active components. The remaining uncertainty is assessed at each step. The nature and extent of clinical studies will depend on the level of remaining uncertainty regarding the biosimilarity of the two products after comprehensive structural and functional characterization and, if applicable, animal studies have been performed. Comparative clinical studies are required to provide evidence of biosimilarity when uncertainties remain as to whether there are clinically meaningful differences between the proposed biosimilar product and the reference product.
The EMA, WHO, and US FDA requirements are broadly similar (Table 2). Compared with other biologicals, insulin products are relatively small, structurally uncomplicated, and well characterized. Furthermore, extensive experience and literature confirm that the immunogenicity of currently marketed insulins has minimal or no clinical significance in the use of these products. Therefore, special guidance was produced by the EMA in 2015 for the development of insulin biosimilar products, 16 which has been cited by recent draft guidance from the US FDA (Table 2). 22 The EMA advises that common clinical efficacy endpoints (eg HbA1c, plasma glucose, hypoglycemia) are unlikely to be sufficiently sensitive to detect clinically relevant differences between two insulins, so clinical efficacy studies are not required. 16 In addition, the requirement for a prelicensing safety study including immunogenicity assessment may be waived if biosimilarity between the biosimilar and the reference insulin can be convincingly concluded from the physicochemical and functional characterization using state-of-the-art analytic methods and by comparing product PK and PD profiles. 16 In its recent draft guidance, the US FDA goes further, stating that if a comparative analytical evaluation based on state-of-the-art technology shows that the products are highly similar, there is little or no residual uncertainty regarding immunogenicity. In such cases, a comparative clinical safety study would generally be unnecessary to demonstrate biosimilarity or interchangeability. 22
Differences can also be found in how approval is applied, in areas such as specific requirements for naming, pharmacovigilance, and interchangeability (pharmacist substitution without reference to the prescriber). Thus, the EMA does not regulate the interchangeability of medicinal products, which, as for generics, is at the discretion of individual member states. 32
Production and Manufacturing
Achieving a High-Quality Product
In contrast to traditional chemical synthesis, the production of biologicals and biosimilars requires the performance of complex multistep processes, using mammalian or microbial cell cultures. 33 In general, biological products are large, relatively unstable compounds with complex, heterogeneous structures. A biologic can be described in terms of its physical, chemical, biological, and microbiological properties, which together provide a comprehensive definition of any individual product. 34 These multiple properties are used to determine the quality attributes of the biologics. A subset of these quality attributes that elicit a direct impact on the efficacy or safety of the product is termed as its critical quality attributes (CQAs).35,36
The manufacturing of pharmaceuticals follows good manufacturing practice (GMP) guidelines, 37 which provide a basis for the standards by which proper design, monitoring, and control of manufacturing processes can be assessed. As with assessments of safety and efficacy, regulatory authorities in the country where a product is to be marketed will need to ensure that manufacturing in a different country meets its own standards. Complexity is inevitable. For example, raw materials will often come from a series of other countries, and each material will need to meet defined standards. Hence, consistency in drafting and application of GMP guidelines between countries is desirable, and indeed most international organizations and regulatory authorities do adopt similar GMP principles accordingly with a high degree of global harmonization. 38 A consequence of this is that production has to be geared to the most demanding GMP principles and thus the costliest to implement.
Matching the biosimilar’s CQAs to those of the reference product as closely as possible is a major objective when developing a manufacturing process for biosimilars. 39 Biosimilars are not generally manufactured in a continuous process, but in batches determined by the size of the fermentation tanks. As the subsequent separation and purification techniques are complex, all biological medicines are subject to inherent quality variations, despite standardized procedures and ingredients. 40 Recently, this has been an issue for the COVID-19 vaccine production, with delays resulting from problems in ensuring batch-to-batch consistency. 41 Quality of manufacturing here is not only the production of the drug substance in the fermenter, but also of isolation, post-fermentation chemical processing, and purification, and, in particular for insulin, avoiding degradation in the formulation of the drug product and storage. Accordingly, to monitor the process of scaling up to commercial quantities and to ensure the consistent manufacture of a quality product, modern production facilities incorporate analytical and process development and characterization methodologies. 42
Target Definition
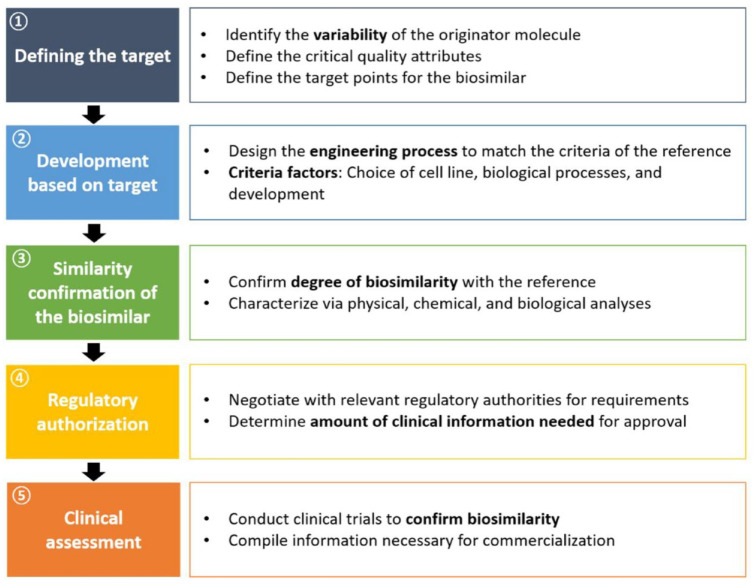
The approval-relevant development process of a biosimilar follows a structured, multistep path, which can be broken down into five intermediate goals (Figure 1). The development process starts with the definition of the target. Here, the focus includes identifying and understanding any variability in the reference target molecule. 43 Clinically relevant product attributes affecting receptor binding, cell metabolism, PK, PD, and immunogenicity can be critical factors for target definition and necessitate comprehensive characterization of as many reference CQAs as possible and the range of variation thereof. The selection of quality attributes and their classification as critical is an essential step for process development and control as well as for the design of the crucial analytical similarity study (Table 3). 44 Considering that CQAs are directly linked to the physicochemical properties of the drug, it is crucial to carry out structural analysis, for example, by amino acid composition, high-performance liquid chromatography (HPLC), and mass spectroscopy (MS), to match the reference production variability. 45
Figure 1.
The five steps in biosimilar development.
Table 3.
Quality Attributes for Analytical Similarity Assessment of a Biosimilar.
| Category | Attribute |
|---|---|
| Primary structure | Primary sequence; disulfide structure; intact mass; isoelectric point |
| Secondary and tertiary structure | Low-resolution secondary structure; indirect tertiary structure; high-resolution higher order structure |
| Dose | Protein content; deliverable volume |
| Function | Subvisible particles; biological activity; receptor and/or ligand binding |
| Product variants | High-molecular-weight species; covalent dimers; purity and impurities, C- and N-terminal modifications; micro-sequence heterogeneity |
The variability of a protein is primarily influenced by its structure, resulting from the three-dimensional folding of the polypeptide chain, which is largely determined by amino acid sequence and thus by the genes that code for them. 46 Because recombinant proteins are produced by living cells (eg, bacteria, yeast, or mammalian cells), their structure may be affected by the selection of the host cell line and the growth environment. Several enzymatic processes can influence protein expression leading to diverse post-translational modifications (PTMs). Glycosylation, the attachment of carbohydrate moieties to the protein, can directly influence the clinical properties of therapeutic proteins. 47 Post-translational modifications can result from naturally occurring processes or the manufacturing process of the biosimilar. Here, major factors include the host cell line, growth medium, temperature, and other properties in the bioreactor. For example, pH may affect protein aggregation. 48 Glycosylation, with yeast-based systems, can result in several glycoforms of the same amino acid sequence. While some problems can result from inherent batch-to-batch variability, others have resulted from planned changes to the production process implemented to yield and thus reduce costs. A minor change in the formulation of a given brand of EPO in Europe led to an increase in the incidence of pure red cell aplasia, and a number of the dialysis patients treated with the differently processed EPO product died as a consequence of the immune reaction triggered by product-related and other impurities. This case has contributed to the awareness that the consequences of introducing process changes must be evaluated and monitored very carefully. 49
Insulin has a well-defined secondary and tertiary structure. 50 Thus, even small changes in the manufacturing processes might result in structural alterations that could affect receptor signaling and the cellular process that impact potency and mitogenicity, as well as those that potentially affect physicochemical stability determining propensity to aggregation or oxidation. 51
Biologics Manufacturing Process
Step 1: Cell line selection and engineering
A core choice in the heart of the process development of a biosimilar is the selection of a suitable cell line. Two major pathways for the large-scale production of recombinant insulins are currently used. Among prokaryotes, E coli has been preferred for the production of recombinant proteins, offering advantages such as high growth rate, simple media requirements, easy handling, high yield, and high cost-effectiveness. However, using the E coli expression system for recombinant insulins has some disadvantages, notably in downstream processing from lack of PTMs, including the formation of disulfide bonds. Among yeast strains, Saccharomyces cerevisiae, Hansenula polymorpha, and Pichia pastoris are widely used (Table 4). 52 As with E coli, they grow rapidly, are easy to handle, and can be used for various genetic manipulations. Recombinant proteins produced in yeast are properly folded and may be glycosylated as with some proteins (but not insulin) expressed in mammalian cells. Using yeast instead of E coli for insulin production can therefore improve the downstream process efficiency, decrease the use of raw materials, and reduce the levels of impurities. 53
Table 4.
Commercial Insulin Products and Their Production Host System.
| Insulin type | Structure | Action | Host system |
|---|---|---|---|
| Human insulin | Identical to native human insulin | Fast/short/intermediate/long acting depending on formulation |
E coli
H polymorpha P pastoris S cerevisiae |
| Insulin glargine | A-chain: glycine instead of asparagine at position 21 B-chain: addition of two arginines at positions 31 and 32 |
Long acting |
E coli
P pastoris |
| Insulin lispro | B-chain: inversion of native B28-B29 proline-lysine sequence | Fast acting and short acting | E coli |
| Insulin aspart | B-chain: aspartic acid in place of proline in position 28 | Fast acting |
E coli
S cerevisiae |
Owing to the lack of access to the innovator’s original cell line and in-depth knowledge of the reference medicine manufacturing processes, each biosimilar producer must develop their own process to replicate the original protein product. Hence, the manufacturing process differs at least in part from that for the innovative biologic. 54 While innovators need to enter production as quickly as possible to get through clinical trials and earn a return on capital invested, biosimilars can target patent expiration years in advance. Therefore, they have more time to optimize the cell line and the manufacturing process. Improved cell lines can be established by choice and modification of promotors, and suitable culture clones can be selected by experimentation. Clone selection requires in-parallel optimization of the fermentation medium and its physical properties such as temperature and pH. Screening of hundreds to thousands of clones is needed to identify the ideal candidates for the production. 55
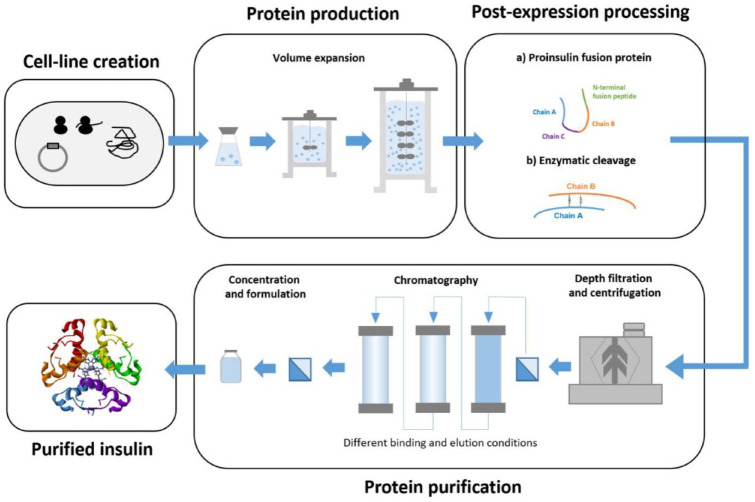
Step 2: Protein production
The next step after clone selection is cell culture upstream process development. 56 A master cell bank is created from the candidate clones that fall within the acceptable parameter range for the CQAs. From this master cell bank, a working cell bank of one vial per batch is produced. The vial contents are inoculated into shake flasks and cultured, resulting in increased cell number. The cells are then taken through serial subcultivations in expanding bioreactor volumes until the target production scale is reached (Figure 2). Quality control of cell culture conditions is essential to minitole glycosylation and contamination profiles, which may have a significant impact on the quality target product profile. For this purpose, several parameters are optimized in hundreds of experiments during process development. These parameters include oxygen levels, lactate production, pH, osmolality, and temperature. 57
Figure 2.
Manufacturing steps in the production of recombinant insulin products.
Step 3: Post-expression processing
Depending on the host cell line, the pathways of the downstream process differ. When E coli is used as an expression system, the overexpressed insulin precursor (IP) forms inclusion bodies in which the proinsulin is usually misfolded. 52 When yeast-based expression systems are used, the IP is secreted directly into the culture supernatant in its properly folded form. 58 Production of IP via the bacterial inclusion body pathway usually results in higher product concentration and productivity. Compared with soluble expression forms of bioactive therapeutic proteins in yeast-based expression systems, inclusion bodies exhibit good mechanical stability and are resistant to proteolytic degradation. However, the formation of inclusion bodies poses a significant hurdle for the production and purification of recombinant insulins: inclusion bodies require extensive processing, including solubilization, refolding, and cleavage. The refolded precursor molecule is processed into a heterodimeric insulin molecule by simultaneous removal of the connecting chain and the N-terminal fusion peptide by enzymatic digestion. 59
Step 4: Purification process
After completion of the time required for optimal product expression, purification is required (Figure 2). While the methods used will depend to some extent on the host cell line, the basic techniques used are similar in all recombinant insulin production, from human insulin, to analogues, to biosimilars. The target protein is thus harvested and separated from unwanted impurities, notably host cell proteins, membranes, and nucleic acids, but also the constituents of the fermentation medium and even viruses. 60 To separate proteins, different characteristics based on sequence and specific structure are exploited and combined with interactions between chemically functionalized resins or filters. 61 Purification steps will also be needed after any post-expression processing.
Centrifugation and size exclusion chromatography separate proteins according to their molecular mass and conformation. Affinity chromatography utilizes differences in affinity to a selective ligand, and ion-exchange chromatography is used to separate positively (acidic) or negatively (basic) charged isoforms of the biosimilar. 62 To obtain the desired biological fingerprint of the reference product, the purification process must be fine-tuned to obtain the enrichment of certain product-related attributes such as isoforms, glycans, and charged variants. 63
Step 5: Formulation
Once protein purification is completed, adjustment of the concentration and formulation of the product follows. The purpose of a stable formulation is to store the purified protein in a suitable buffer and container for long-term storage and shipment. 64 The goal is to minimize the possible degradation of the product, avoiding loss of potency and generation of immunogenic derivatives. An important aspect of this is to determine what environmental conditions cause proteins to aggregate and fall out of the solution. This includes optimizing buffer conditions with the inclusion of diverse excipients and stabilizers. 65
Studies
On completion of the developmental process for the manufacture of biosimilars, similarity has to be confirmed to the reference product (Table 5). After extensive analytical corroboration, using laboratory techniques such as HPLC and MS, in vitro comparative testing will use insulin-responsive cell lines often tweaked to be sensitive to properties such as mitogenicity. However, chemical analytical and preclinical methods are of limited value for endpoints such as immunogenicity. Preclinical (animal) testing for toxicity is usually not required for biosimilar insulin products and can be difficult owing to hypoglycemia.16,66
Table 5.
| Analytic and in vitro studies | These evaluations involve extensive in vitro characterization and comparison of the biosimilar and its reference product concerning their physicochemical and biological properties, such as amino acid sequencing and folding, the proportion of glycan and non-glycan components in their structure, stability profile, cellular mechanisms of action, cell receptor interactions, and product- and process-related impurities. Analytical evaluation reveals variations in the functional and structural properties. Analytic evaluation for insulin includes protein content, amino acid sequence, secondary structure, higher order structure, functional activity (receptor binding, dissociation, and downstream biochemical cellular action), size variants (aggregates), and conjugate variants. For metabolic endpoints, various functional assays are available including assays measuring glycogen formation, lipogenesis, inhibition of stimulated lipolysis, as well as glucose transport, which can be studied in a variety of cells. |
| Preclinical in vivo studies | Animal studies are carried out if the scientific question cannot be answered in vitro. The information obtained is used in determining the appropriateness of proceeding to human clinical trials. Limited immunogenicity testing is possible for insulin. Toxicity studies are limited by hypoglycemia if higher dose levels are used. Potency studies have high variability. |
| Clinical PD/PK studies | Comparative human PK and PD studies provide different types of information. PK studies measure how the body handles insulin, while PD studies measure how the hormone acts on the body. The objective is to evaluate the bioequivalence of PK and PD characteristics of the biosimilar to its reference product. The studies support a demonstration of biosimilarity with the assumption that similar exposure (and PD response) provides similar efficacy and safety. In the absence of specific acceptance limits for biological medicinal products in general and for insulin specifically, the conventional acceptance range for bioequivalence (80%-125% [90% CI for PK and PD]) is recommended. 16 Evaluation of clinically relevant measures of insulin action is assessed by euglycemic clamp studies. In these clamp experiments, insulin has to be administered by subcutaneous injection of insulin and the blood glucose levels are maintained (“clamped”) at a predefined level utilizing a variable glucose infusion. |
| Clinical safety studies | When necessary, safety studies should be performed with a specific focus on immunogenicity. People with T1D are the preferred population. |
Abbreviations: CI, confidence interval; PD, pharmacodynamic; PK, pharmacokinetic; T1D, type 1 diabetes.
Clinical studies that regulatory agencies require as mandatory for approval of biosimilar insulins are those that evaluate the PK and PD properties usually assessed utilizing euglycemic glucose clamp studies. 67
Clinical Studies
The aim of clinical studies is not to reconfirm safety and efficacy, but rather to detect possible unexpected differences from the reference product of clinical importance. Clinical trials are required to include a study population that is sensitive to the detection of potential differences in efficacy, safety, or immunogenicity between the biosimilar product and the reference product. For example, people with type 1 diabetes (T1D) have little endogenous insulin to buffer differences between the new and reference insulin and are inherently more likely to develop insulin antibodies. Thus, they are more sensitive and suitable for both PK/PD and immunogenicity studies. Accordingly, chosen study populations may differ from, or be a subset of, those used in pivotal regulatory clinical trials of the reference product. 68
The clinical development program for approval of a biosimilar insulin will include a comparative phase 1 PK/PD study and is typically conducted in healthy volunteers and/or people with T1D (Table 5).69,70 Where a comparative analytical assessment has not convincingly demonstrated that the biosimilar insulin and reference insulin are “highly similar,” a comparative phase 3 study in people sensitive for immunogenicity, usually also collecting data related to efficacy, tolerability, and safety, is required.16,22 Immunogenicity is the primary focus of such studies and may arise from small differences in the three-dimensional structure of the insulin molecule, and more particularly from insulin-like impurities. The primary outcome in the assessment of immunogenicity is the incidence of detectable anti-insulin antibodies in those patients who are antibody-negative at baseline, and antibody titer increase in those already positive. 22
Marketing Approvals and Evidence for Biosimilar Insulins
In 2015, the first approved biosimilar insulin was launched in the European Union and the United States under the names Abasaglar (European Union) 71 and Basaglar (United States). 72 Technically, Basaglar is not a biosimilar but rather a follow-on biologic, as it was approved according to a new drug application pathway under the US Food, Drug, and Cosmetic Act, section 505(b)(2). Since March 2020, insulin products are considered approvable in the United States under the Biologics Pathway and thus can be approved as biosimilars under the 351(k) pathway. 73 All approved insulins under the 505(b)(2) pathway were transitioned by March 2020 under section 351(a). As of April 2021, the EMA has granted marketing approval for five biosimilar insulins and the US FDA has approved one (Table 6).
Table 6.
Approved Biosimilar and Follow-on Insulins in Europe, the United States, and China.
| Follow-on insulin/biosimilar insulin | Manufacturer | Reference product | Type of insulin | Approvals |
|---|---|---|---|---|
| Abasaglar/Basaglar | Boehringer Ingelheim and Eli Lilly | Lantus | Insulin glargine | EMA 201471
US FDA 2015 as follow-on insulin 72 |
| Semglee | Viatris/Biocon | Lantus | Insulin glargine | EMA 201874
US FDA 2020 as follow-on insulin US FDA 2021 as biosimilar 75 |
| Admelog | Sanofi | Humalog | Insulin lispro | EMA 201776
US FDA 2017 as follow-on insulin 77 |
| Sar-ASP | Sanofi | NovoRapid | Insulin aspart | EMA 2020 78 |
| Kixelle | Viatris/Biocon | NovoRapid | Insulin aspart | EMA 2021 79 |
Abbreviations: EMA, European Medicines Agency; FDA, Food and Drug Administration.
Abasaglar/Basaglar (insulin glargine) was developed by Lilly as a biosimilar/follow-on biological product to the reference product Lantus (insulin glargine 100 U/mL) by Sanofi, as was Semglee (insulin glargine) by Viatris/Biocon, approved by the EMA in 2018 74 and the US FDA in 2020 as follow-on insulin, and in 2021, as a biosimilar. 75 Gan & Lee has several genetically engineered insulins on the market in China, although these have not been registered through the biosimilar regulatory processes. 27
Semglee, however, is produced in yeast, P pastoris, while the Sanofi reference glargine uses E coli. Extensive laboratory studies of Abasaglar/Basaglar and Semglee could not distinguish these biosimilar insulins in comparison with the reference insulin glargine in terms of chemical structure, purity, and biological activity. 74 Both Semglee and Abasaglar have undergone the required human PK/PD and immunogenicity studies, meeting criteria for clinical biosimilarity.80 -87
Admelog (insulin lispro) and SAR-Asp (insulin aspart) were developed by Sanofi as biosimilars to Humalog (Eli Lilly) and NovoRapid/NovoLog (NovoNordisk). Approvals were granted for the former in the European Union as a biosimilar and in the United States as follow-on insulin in 2017 and for the latter in the European Union as a biosimilar in 2020.76,77 Biosimilarity testing demonstrating the products to be indistinguishable from their reference targets has been published, covering structure, purity, biological activity, and PK/PD studies. A detailed assessment of comparative immunogenicity in phase 3 studies has been published for Admelog 88 and an additional insulin pump study for SAR-Asp. 89
With Semglee (insulin glargine), the US FDA approved the first interchangeable biosimilar insulin product. 75 Interchangeability is assessed by some regulatory authorities after the biosimilar has been approved, not simultaneously during approval. The EMA does not take responsibility for interchangeability and leaves this to the EU member states. In the United States, interchangeability is a designation that confirms the automatic substitution of an approved biosimilar with its innovator, thereby allowing an interchangeable biosimilar to be substituted for the innovator product by the pharmacist without the prescriber’s knowledge or intervention. 90 To receive an interchangeability designation in the United States, the FDA requires additional clinical trials which include at least three switches between the biosimilar candidate and the reference biologic. 91 There were concerns with different pens for biosimilar insulin glargine when first launched in the United Kingdom leading to limited use. Appropriate educational initiatives can help to overcome such issues. 92
Conclusions
Biological medications contribute to the treatment of many long-term disorders affecting quality and length of life, including diabetes. With the expiration of patent protection for insulin analogues, biosimilar insulins have emerged as an alternative to original products and are already increasing cost competition. As this market expands, more questions will arise about biosimilar insulins.
Solid standards for quality, efficacy, and safety have emerged for biosimilar products. Stringent regulatory requirements include the stepwise collection of structural, functional, nonclinical, and clinical data to demonstrate similarity to the reference product.
For biosimilar insulins, regulatory authorities require a small number of people with diabetes to be studied to confirm the quality of the product. Sufficiently powered and adequate statistical analysis of clinical trials and pharmacovigilance studies with long-term follow-up may support the decision-making regarding biosimilar use. Interchangeability is assessed on a case-by-case basis, taking into account the “totality of the evidence” and biological plausibility.
With health care expenditures continuing to rise, the emergence of biosimilar insulins provides a means to improve patient access to a core diabetes therapy. While lower costs for insulins would certainly be desirable for consumers and society, the complexity of drug pricing in various markets, particularly in the United States, poses a challenge in achieving this. For instance, the original glargine (Lantus) list price in the United States is approximately $457 for five disposable injection pens of 3 mL. Basaglar (a follow-on biologic) is $326, while the recently introduced interchangeable biosimilar Semglee has a list price of $404. Another issue with biosimilar insulins includes further development of insulin products, seemingly evergreening activities, by the originator companies. The originator company for insulin glargine, for example, has, following clinical trials showing some clinical advantages, been promoting the patented high concentration 300 IU/mL formulation of insulin glargine (Gla-300), thus protecting their share of the insulin glargine market.
There are several challenges with biosimilar insulins which have to be overcome and addressed to enhance biosimilar use. To maximize the impact of the advantage for falling retail costs of biosimilar insulins compared with that of reference insulins, health care professionals and insulin users must gain further awareness and confidence.
Acknowledgments
The authors would like to thank Martin Miszon, Sciarc GmbH, for his editorial and writing support of the article.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article:
Prof. Lutz Heinemann is member of an advisory board of Becton Dickinson, Convatec, Roche Diabetes Care, Sciarc and Member of the Board of Lifecare.
Prof. Melanie Davies has acted as consultant, advisory board member, and speaker for Boehringer Ingelheim, Lilly, Novo Nordisk, Sciarc, and Sanofi; an advisory board member and speaker for AstraZeneca; an advisory board member for Janssen, Lexicon, Pfizer, and ShouTi Pharma Inc; and a speaker for Napp Pharmaceuticals, Novartis, and Takeda Pharmaceuticals International, Inc. She has received grants in support of investigator and investigator-initiated trials from Novo Nordisk, Sanofi-Aventis, Lilly, Boehringer Ingelheim, AstraZeneca, and Janssen.
Prof. Philip Home or institutions with which he is associated have received funding for his research, lecturing, or advisory activities from Viatris, Sanofi, Gan & Lee, Sciarc, and Merck Inc in regard of biosimilar insulins, and from Ely Lilly, Novo Nordisk, and Sanofi in regard of originator insulins.
Prof. Thomas Forst is member of an advisory board of Astra Zeneca; Atrogi, Bayer; Cipla, Eli Lilly; Eyesense; Fortbildungskolleg; Novo Nordisk; Pfizer; Sanofi; Bayer; Remynd, Roche; Eyesense; Sciarc and member of the speaker Panel of Astra Zeneca; Boehringer Ingelheim, Berlin Chemie; Cipla, Daiichi-Sankyo, Eli Lilly; Fortbildungskolleg; MSD; Novartis, Novo Nordisk; Sanofi
Prof. Tina Vilsbøll has served on scientific advisory panels and/or speakers’ bureaus or has served as a consultant to and/or received research support from AstraZeneca, BMS, Boehringer Ingelheim, Eli Lilly, Gilead, MSD/Merck, Mundipharma, Novo Nordisk, Sanofi, Sciarc, and Sun Pharmaceuticals.
Prof. Oliver Schnell is a founder and CEO of Sciarc GmbH.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The publication has been funded by an unrestricted educational grant from Gan & Lee Pharmaceuticals USA Corp.
Abbreviations: Anti-TNF, anti-tumor necrosis factor; CNDA, China National Drug Administration; CQA, critical quality attribute; EMA, European Medicines Agency; EPO, erythropoietin; FDA, Food and Drug Administration; GIR, glucose infusion rate; GMP, good manufacturing practice; HPLC, high-performance liquid chromatography; IP, insulin precursor; MS, mass spectroscopy; PD, pharmacodynamic; PK, pharmacokinetic; PTM, post-translational modification; SC, subcutaneous; T1D, type 1 diabetes; WHO, World Health Organization.
ORCID iDs: Lutz Heinemann  https://orcid.org/0000-0003-2493-1304
https://orcid.org/0000-0003-2493-1304
Oliver Schnell  https://orcid.org/0000-0003-4968-2367
https://orcid.org/0000-0003-4968-2367
References
- 1. Fralick M, Zinman B. The discovery of insulin in Toronto: beginning a 100 year journey of research and clinical achievement. Diabetologia. 2021;64(5):947-953. doi: 10.1007/s00125-020-05371-6. [DOI] [PubMed] [Google Scholar]
- 2. Home P. The evolution of insulin therapy. Diabetes Res Clin Pract. 2021;175:108816. doi: 10.1016/j.diabres.2021.108816. [DOI] [PubMed] [Google Scholar]
- 3. Owens DR, Bolli GB. The continuing quest for better subcutaneously administered prandial insulins: a review of recent developments and potential clinical implications. Diabetes Obes Metab. 2020;22(5):743-754. doi: 10.1111/dom.13963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Davis CS, Fleming JW, Malinowski SS, Brown MA, Fleming LW. Ultra-long-acting insulins: a review of efficacy, safety, and implications for practice. J Am Assoc Nurse Pract. 2018;30(7):373-380. doi: 10.1097/jxx.0000000000000076. [DOI] [PubMed] [Google Scholar]
- 5. Misra M. Biosimilars: current perspectives and future implications. Indian J Pharmacol. 2012;44(1):12-14. doi: 10.4103/0253-7613.91859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Basu S, Yudkin JS, Kehlenbrink S, et al. Estimation of global insulin use for type 2 diabetes, 2018-30: a microsimulation analysis. Lancet Diabetes Endocrinol. 2019;7(1):25-33. doi: 10.1016/S2213-8587(18)30303-6. [DOI] [PubMed] [Google Scholar]
- 7. Ewen M, Joosse HJ, Beran D, Laing R. Insulin prices, availability and affordability in 13 low-income and middle-income countries. BMJ Glob Health. 2019;4(3):e001410. doi: 10.1136/bmjgh-2019-001410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baumgart DC, Misery L, Naeyaert S, Taylor PC. Biological therapies in immune-mediated inflammatory diseases: can biosimilars reduce access inequities. Front Pharmacol. 2019;10:279. doi: 10.3389/fphar.2019.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Agbogbo FK, Ecker DM, Farrand A, et al. Current perspectives on biosimilars. J Ind Microbiol Biotechnol. 2019;46(9-10):1297-1311. doi: 10.1007/s10295-019-02216-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Godman B, Haque M, Leong T, et al. The current situation regarding long-acting insulin analogues including biosimilars among African, Asian, European, and South American Countries; findings and implications for the future. Front Public Health. 2021;9:671961. doi: 10.3389/fpubh.2021.671961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. IMS Institute for Healthcare Informatics. Delivering on the Potential of Biosimilar Medicines: The Role of Functioning Competitive Markets Introduction. 2016. https://www.medicinesforeurope.com/wp-content/uploads/2016/03/IMS-Institute-Biosimilar-Report-March-2016-FINAL.pdf. Accessed August 15, 2021.
- 12. The IQVIA Institute for Human Data Science. Biosimilars in the United States 2020-2024. https://www.iqvia.com/-/media/iqvia/pdfs/institute-reports/iqvia-institute-biosimilars-in-the-united-states.pdf. Accessed March 2, 2022.
- 13. The IQVIA Institute for Human Data Science. The Impact of Biosimilar Competition in Europe. 2021. https://www.iqvia.com/-/media/iqvia/pdfs/library/white-papers/the-impact-of-biosimilar-competition-in-europe-2021.pdf. Accessed March 2, 2022
- 14. QYResearch Group. Global Biosimilar Insulin Sales Market Report 2021. https://www.marketresearch.com/QYResearch-Group-v3531/Global-Biosimilar-Insulin-Sales-14824285/. Accessed May 17, 2022.
- 15. Kabir ER, Moreino SS, Sharif Siam MK. The breakthrough of biosimilars: a twist in the narrative of biological therapy. Biomolecules. 2019;9(9):410. doi: 10.3390/biom9090410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. European Medicines Agency. Guideline on non-clinical and clinical development of similar biological medicinal products containing recombinant human insulin and insulin analogues. 2015. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-non-clinical-clinical-development-similar-biological-medicinal-products-containing_en-0.pdf. Accessed February 22, 2021.
- 17. US Food and Drug Administration. GUIDANCE DOCUMENT. Scientific Considerations in Demonstrating Biosimilarity to a Reference Product. 2015. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/scientific-considerations-demonstrating-biosimilarity-reference-product. Accessed February 22, 2022.
- 18. World Health Organization. Expert Committee on Biological Standardization: guidelines on evaluation of similar biotherapeutic products (SBPs). 2009. http://www.who.int/biologicals/areas/biological_therapeutics/BIOTHERAPEUTICS_FOR_WEB_22APRIL2010.pdf. Accessed March 15, 2021.
- 19. Shire SJ. Formulation and manufacturability of biologics. Curr Opin Biotechnol. 2009;20(6):708-714. [DOI] [PubMed] [Google Scholar]
- 20. Blandizzi C, Meroni PL, Lapadula G. Comparing originator biologics and biosimilars: a review of the relevant issues. Clin Ther. 2017;39(5):1026-1039. doi: 10.1016/j.clinthera.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 21. Wang J, Chow S-C. On the regulatory approval pathway of biosimilar products. Pharmaceuticals (Basel). 2012;5(4):353-368. doi: 10.3390/ph5040353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. US Food and Drug Administration. Clinical Immunogenicity Considerations for Biosimilar and Interchangeable Insulin Products. Guidance for Industry. 2019. https://www.fda.gov/media/133014/download. Accessed October 7, 2021.
- 23. Heinemann L, Khatami H, McKinnon R, Home P. An overview of current regulatory requirements for approval of biosimilar insulins. Diabetes Technol Ther. 2015;17(7):510-526. doi: 10.1089/dia.2014.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Official Journal of the European Union. DIRECTIVE on the Community code relating to medicinal products for human use. 2004. https://ec.europa.eu/health//sites/health/files/files/eudralex/vol-1/dir_2004_27/dir_2004_27_en.pdf. Accessed March 16, 2021.
- 25. Nellore R. Regulatory considerations for biosimilars. Perspect Clin Res. 2010;1(1):11-14. [PMC free article] [PubMed] [Google Scholar]
- 26. European Medicines Agency. Committee for Medicinal Products for Human Use (CHMP). Guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance: non-clinical and clinical issues. 2014. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-similar-biological-medicinal-products-containing-biotechnology-derived-proteins-active_en-2.pdf. Accessed March 15, 2021.
- 27. China National Drug Administration. Technical Guidelines for the Development and Evaluation of Biosimilars (for Trial Implementation). 2015. https://www.fdanews.com/ext/resources/files/03-15/03-15-China-Biosimilars.pdf?1425486653. Accessed July 7, 2021.
- 28. Kang H-N, Thorpe R, Knezevic I; Survey participants from 19 countries. The regulatory landscape of biosimilars: WHO efforts and progress made from 2009 to 2019. Biologicals. 2020;65:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kang H-N, Knezevic I. Regulatory evaluation of biosimilars throughout their product life-cycle. Bull World Health Organ. 2018;96(4):281-285. doi: 10.2471/BLT.17.206284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chow S-C, Song F, Bai H. Analytical similarity assessment in biosimilar studies. AAPS J. 2016;18(3):670-677. doi: 10.1208/s12248-016-9882-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kirchhoff CF, Wang X-ZM, Conlon HD, Anderson S, Ryan AM, Bose A. Biosimilars: key regulatory considerations and similarity assessment tools. Biotechnol Bioeng. 2017;114(12):2696-2705. doi: 10.1002/bit.26438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Crommelin DJA, Shah VP, Klebovich I, et al. The similarity question for biologicals and non-biological complex drugs. Eur J Pharm Sci. 2015;76:10-17. [DOI] [PubMed] [Google Scholar]
- 33. Morrow T, Felcone LH. Defining the difference: what makes biologics unique. Biotechnol Healthc. 2004;1(4):24-29. [PMC free article] [PubMed] [Google Scholar]
- 34. European Medicines Agency. International Conference on Harmonization. ICH guideline Q8 (R2) on pharmaceutical development Step 5. 2015. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002872.pdf. Accessed March 16, 2021.
- 35. Rathore AS, Winkle H. Quality by design for biopharmaceuticals. Nat Biotechnol. 2009;27(1):26-34. doi: 10.1038/nbt0109-26. [DOI] [PubMed] [Google Scholar]
- 36. Eon-Duval A, Broly H, Gleixner R. Quality attributes of recombinant therapeutic proteins: an assessment of impact on safety and efficacy as part of a quality by design development approach. Biotechnol Prog. 2012;28(3):608-622. doi: 10.1002/btpr.1548. [DOI] [PubMed] [Google Scholar]
- 37. Gouveia BG, Rijo P, Gonçalo TS, Reis CP. Good manufacturing practices for medicinal products for human use. J Pharm Bioallied Sci. 2015;7(2):87-96. doi: 10.4103/0975-7406.154424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sia CH, Sai MK, Chan LW. Global challenges in the manufacture, regulation and international harmonization of GMP and quality standards for biopharmaceuticals. GaBI J. 2020;9:52-63. [Google Scholar]
- 39. European Medicines Agency. Guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance: quality issues (revision 1). 2012. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-similar-biological-medicinal-products-containing-biotechnology-derived-proteins-active_en-0.pdf. Accessed March 16, 2021.
- 40. Heinemann L, Home PD, Hompesch M. Biosimilar insulins: guidance for data interpretation by clinicians and users. Diabetes Obes Metab. 2015;17(10):911-918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. European Medicines Agency. COVID-19 Vaccine Janssen: authorities in EU take steps to safeguard vaccine quality. 2021. https://www.ema.europa.eu/en/news/covid-19-vaccine-janssen-authorities-eu-take-steps-safeguard-vaccine-quality. Accessed September 7, 2021.
- 42. Gronemeyer P, Ditz R, Strube J. Trends in upstream and downstream process development for antibody manufacturing. Bioengineering (Basel). 2014;1(4):188-212. doi: 10.3390/bioengineering1040188. [DOI] [PubMed] [Google Scholar]
- 43. Declerck P, Farouk Rezk M. The road from development to approval: evaluating the body of evidence to confirm biosimilarity. Rheumatology (Oxford). 2017;56(suppl 4):iv4-iv13. doi: 10.1093/rheumatology/kex279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vandekerckhove K, Seidl A, Gutka H, et al. Rational selection, criticality assessment, and tiering of quality attributes and test methods for analytical similarity evaluation of biosimilars. AAPS J. 2018;20(4):68. doi: 10.1208/s12248-018-0230-9. [DOI] [PubMed] [Google Scholar]
- 45. Kwon O, Joung J, Park Y, Kim CW, Hong SH. Considerations of critical quality attributes in the analytical comparability assessment of biosimilar products. Biologicals. 2017;48:101-108. doi: 10.1016/j.biologicals.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 46. Vihinen M. Types and effects of protein variations. Hum Genet. 2015;134(4):405-421. doi: 10.1007/s00439-015-1529-6. [DOI] [PubMed] [Google Scholar]
- 47. Walsh G, Jefferis R. Post-translational modifications in the context of therapeutic proteins. Nat Biotechnol. 2006;24(10):1241-1252. doi: 10.1038/nbt1252. [DOI] [PubMed] [Google Scholar]
- 48. Roberts CJ. Protein aggregation and its impact on product quality. Curr Opin Biotechnol. 2014;30:211-217. doi: 10.1016/j.copbio.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Heinemann L, Hompesch M. Biosimilar insulins: how similar is similar? J Diabetes Sci Technol. 2011;5(3):741-754. doi: 10.1177/193229681100500329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Liu M, Weiss MA, Arunagiri A, et al. Biosynthesis, structure, and folding of the insulin precursor protein. Diabetes Obes Metab. 2018;20(suppl 2):28-50. doi: 10.1111/dom.13378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Heinemann L, Hompesch M. Biosimilar insulins: basic considerations. J Diabetes Sci Technol. 2014;8(1):6-13. doi: 10.1177/1932296813516958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Baeshen NA, Baeshen MN, Sheikh A, et al. Cell factories for insulin production. Microb Cell Fact. 2014;13(1):141. doi: 10.1186/s12934-014-0141-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sreenivas S, Krishnaiah SM, Govindappa N, et al. Enhancement in production of recombinant two-chain Insulin Glargine by over-expression of Kex2 protease in Pichia pastoris. Appl Microbiol Biotechnol. 2015;99(1):327-336. doi: 10.1007/s00253-014-6052-5. [DOI] [PubMed] [Google Scholar]
- 54. Tripathi NK, Shrivastava A. Recent developments in bioprocessing of recombinant proteins: expression hosts and process development. Front Bioeng Biotechnol. 2019;7:420. doi: 10.3389/fbioe.2019.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Vulto AG, Jaquez OA. The process defines the product: what really matters in biosimilar design and production? Rheumatology (Oxford). 2017;56(suppl 4):iv14-iv29. doi: 10.1093/rheumatology/kex278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gupta SK, Shukla P. Sophisticated cloning, fermentation, and purification technologies for an enhanced therapeutic protein production: a review. Front Pharmacol. 2017;8:419. doi: 10.3389/fphar.2017.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Brühlmann D, Sokolov M, Butté A, et al. Parallel experimental design and multivariate analysis provides efficient screening of cell culture media supplements to improve biosimilar product quality. Biotechnol Bioeng. 2017;114(7):1448-1458. doi: 10.1002/bit.26269. [DOI] [PubMed] [Google Scholar]
- 58. Polez S, Origi D, Zahariev S, et al. A simplified and efficient process for insulin production in Pichia pastoris. PLoS One. 2016;11(12):e0167207. doi: 10.1371/journal.pone.0167207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Siew YY, Zhang W. Downstream processing of recombinant human insulin and its analogues production from E. Coli inclusion bodies. Bioresour Bioprocess. 2021;8(1):65. doi: 10.1186/s40643-021-00419-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Liu HF, Ma J, Winter C, Bayer R. Recovery and purification process development for monoclonal antibody production. MAbs. 2010;2(5):480-499. doi: 10.4161/mabs.2.5.12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Coskun O. Separation techniques: chromatography. North Clin Istanb. 2016;3(2):156-160. doi: 10.14744/nci.2016.32757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yigzaw Y, Hinckley P, Hewig A, Vedantham G. Ion exchange chromatography of proteins and clearance of aggregates. Curr Pharm Biotechnol. 2009;10(4):421-426. doi: 10.2174/138920109788488842. [DOI] [PubMed] [Google Scholar]
- 63. Structural Genomics Consortium, China Structural Genomics Consortium, Northeast Structural Genomics Consortium, et al. Protein production and purification. Nat Methods. 2008;5(2):135-146. doi: 10.1038/nmeth.f.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wang W. Advanced protein formulations. Protein Sci. 2015;24(7):1031-1039. doi: 10.1002/pro.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mitragotri S, Burke PA, Langer R. Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nat Rev Drug Discov. 2014;13(9):655-672. doi: 10.1038/nrd4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Stebbing J, Mainwaring PN, Curigliano G, et al. Understanding the role of comparative clinical studies in the development of oncology biosimilars. J Clin Oncol. 2020;38(10):1070-1080. doi: 10.1200/JCO.19.02953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Heise T, Zijlstra E, Nosek L, Heckermann S, Plum-Mörschel L, Forst T. Euglycaemic glucose clamp: what it can and cannot do, and how to do it. Diabetes Obes Metab. 2016;18(10):962-972. doi: 10.1111/dom.12703. [DOI] [PubMed] [Google Scholar]
- 68. Declerck P, Danesi R, Petersel D, Jacobs I. The language of biosimilars: clarification, definitions, and regulatory aspects. Drugs. 2017;77(6):671-677. doi: 10.1007/s40265-017-0717-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. European Medicines Agency. Guideline on similar Biological Medicinal Products Containing Biotechnology-Derived Proteins as Active Substance: non-Clinical and Clinical Issues. 2014. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-similar-biological-medicinal-products-containing-biotechnology-derived-proteins-active_en-2.pdf. Accessed June 23, 2021.
- 70. US Food and Drug Administration. Clinical Pharmacology Data to Support a Demonstration of Biosimilarity to a Reference Product: guidance for Industry. 2016. https://www.fda.gov/media/88622/download. Accessed June 23, 2021.
- 71. European Medicines Agency. Summary of the European public assessment report for Abasaglar. 2014. https://www.ema.europa.eu/en/medicines/human/EPAR/abasaglar-previously-abasria. Accessed July 16, 2021.
- 72. US Food and Drug Administration. Basaglar (insulin glargine injection). 2016. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/205692orig1s000toc.cfm. Accessed July 16, 2021.
- 73. GaBI Online—Generics Biosimilars Initiative. FDA opens pathway to biosimilar insulin products. 2020. https://www.gabionline.net/guidelines/FDA-opens-pathway-to-biosimilar-insulin-products. Accessed July 17, 2021.
- 74. European Medicines Agency. Summary of the European public assessment report for Semglee. 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/semglee. Accessed July 16, 2021.
- 75. US Food and Drug Administration. Drug Approval Package: SEMGLEE. 2020. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/210605Orig1s000TOC.cfm. Accessed July 16, 2021.
- 76. European Medicines Agency. Summary of the European public assessment report for Admelog. 2017. https://www.ema.europa.eu/en/medicines/human/EPAR/insulin-lispro-sanofi. Accessed July 19, 2021.
- 77. US Food and Drug Administration. Drug Approval Package: Admelog (insulin lispro). 2018. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/209196Orig1s000TOC.cfm. Accessed July 19, 2021.
- 78. European Medicines Agency. Summary of the European public assessment report for SAR-Asp. 2020. https://www.ema.europa.eu/en/medicines/human/EPAR/insulin-aspart-sanofi. Accessed July 19, 2021.
- 79. European Medicines Agency. Summary of the European public assessment report for Kixelle. 2021. https://www.ema.europa.eu/en/medicines/human/EPAR/kixelle. Accessed July 22, 2021.
- 80. Blevins TC, Barve A, Sun B, Ankersen M. Efficacy and safety of MYL-1501D vs insulin glargine in patients with type 1 diabetes after 52 weeks: results of the INSTRIDE 1 phase III study. Diabetes Obes Metab. 2018;20(8):1944-1950. doi: 10.1111/dom.13322. [DOI] [PubMed] [Google Scholar]
- 81. Blevins TC, Barve A, Sun B, et al. Efficacy and safety of MYL-1501D versus insulin glargine in patients with type 2 diabetes after 24 weeks: results of the phase III INSTRIDE 2 study. Diabetes Obes Metab. 2019;21(1):129-135. doi: 10.1111/dom.13495. [DOI] [PubMed] [Google Scholar]
- 82. Blevins TC, Barve A, Raiter Y, et al. Efficacy and safety of MYL-1501D versus insulin glargine in people with type 1 diabetes mellitus: results of the INSTRIDE 3 phase 3 switch study. Diabetes Obes Metab. 2020;22(3):365-372. doi: 10.1111/dom.13904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Sun B, Sengupta N, Rao A, et al. Similar immunogenicity profiles between the proposed biosimilar MYL-1501D and reference insulin glargine in patients with diabetes mellitus: the phase 3 INSTRIDE 1 and INSTRIDE 2 studies. BMC Endocr Disord. 2021;21(1):129. doi: 10.1186/s12902-021-00797-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Rosenstock J, Hollander P, Bhargava A, et al. Similar efficacy and safety of LY2963016 insulin glargine and insulin glargine (Lantus®) in patients with type 2 diabetes who were insulin-naïve or previously treated with insulin glargine: a randomized, double-blind controlled trial (the ELEMENT 2 study). Diabetes Obes Metab. 2015;17(8):734-741. [DOI] [PubMed] [Google Scholar]
- 85. Ilag LL, Deeg MA, Costigan T, et al. Evaluation of immunogenicity of LY2963016 insulin glargine compared with Lantus® insulin glargine in patients with type 1 or type 2 diabetes mellitus. Diabetes Obes Metab. 2016;18(2):159-168. doi: 10.1111/dom.12584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Hadjiyianni I, Dahl D, Lacaya LB, Pollom RK, Chang CL, Ilag LL. Efficacy and safety of LY2963016 insulin glargine in patients with type 1 and type 2 diabetes previously treated with insulin glargine. Diabetes Obes Metab. 2016;18(4):425-429. doi: 10.1111/dom.12628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Blevins TC, Dahl D, Rosenstock J, et al. Efficacy and safety of LY2963016 insulin glargine compared with insulin glargine (Lantus®) in patients with type 1 diabetes in a randomized controlled trial: the ELEMENT 1 study. Diabetes Obes Metab. 2015;17(8):726-733. doi: 10.1111/dom.12496. [DOI] [PubMed] [Google Scholar]
- 88. Home P, Derwahl KM, Ziemen M, et al. Anti-insulin antibodies and adverse events with biosimilar insulin lispro compared with humalog insulin lispro in people with diabetes. Diabetes Technol Ther. 2018;20(2):160-170. doi: 10.1089/dia.2017.0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Thrasher J, Polsky S, Hovsepian L, et al. Safety and tolerability of insulin aspart biosimilar SAR341402 versus originator insulin aspart (NovoLog) when used in insulin pumps in adults with type 1 diabetes: a randomized, open-label clinical trial. Diabetes Technol Ther. 2020;22(9):666-673. doi: 10.1089/dia.2019.0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Halimi V, Daci A, Ancevska Netkovska K, Suturkova L, Babar Z-U-D, Grozdanova A. Clinical and regulatory concerns of biosimilars: a review of literature. Int J Environ Res Public Health. 2020;17(16):5800. doi: 10.3390/ijerph17165800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. US Food and Drug Administration. Considerations in Demonstrating Interchangeability With a Reference Product Guidance for Industry. 2019. https://www.fda.gov/media/124907/download. Accessed March 1, 2022.
- 92. Chapman SR, Fitzpatrick RW, Aladul MI. Knowledge, attitude and practice of healthcare professionals towards infliximab and insulin glargine biosimilars: result of a UK web-based survey. BMJ Open. 2017;7(6):e016730. doi: 10.1136/bmjopen-2017-016730. [DOI] [PMC free article] [PubMed] [Google Scholar]