Abstract
Background:
Continuous glucose monitor (CGM) systems were originally intended only for people with diabetes. Recently, there has been interest in monitoring glucose concentrations in a variety of other situations. As data accumulate to support the use of CGM systems in additional states unrelated to diabetes, the use of CGM systems is likely to increase accordingly.
Methods:
PubMed and Google Scholar were searched for articles about the use of CGM in individuals without diabetes. Relevant articles that included sufficient details were queried to identify what cohorts of individuals were adopting CGM use and to define trends of use.
Results:
Four clinical user cases were identified: (1) metabolic diseases related to diabetes with a primary dysregulation of the insulin-glucose axis, (2) metabolic diseases without a primary pathophysiologic derangement of the insulin-glucose axis, (3) health and wellness, and (4) elite athletics. Seven trends in the use of CGM systems in people without diabetes were idenfitied which pertained to both FDA-cleared medical grade products as well as anticipated future products, which may be regulated differently based on intended populations and indications for use.
Conclusions:
Wearing a CGM has been used not only for diabetes, but with a goal of improving glucose patterns to avoid diabetes, improving mental or physical performance, and promoting motivate healthy behavioral changes. We expect that clinicians will become increasingly aware of (1) glycemic patterns from CGM tracings that predict an increased risk of diabetes, (2) specific metabolic glucotypes from CGM tracings that predict an increased risk of diabetes, and (3) new genetic and genomic biomarkers in the future.
Keywords: athletes, CGM, continuous glucose monitor, healthy, obesity, prediabetes
Introduction
Continuous glucose monitoring provides detailed information about glucose fluctuations. This information is very useful for people with diabetes who have better outcomes when they bring their abnormal glucose concentrations into the target range. Recently, a trend has emerged for people without diabetes to use continuous glucose monitors (CGMs) as a personal health device to track their glucose concentrations and promote healthy habits such as increased activity, changes in nutrition, and sleep habits. The motivation behind people without diabetes wearing CGMs is to (1) improve their glucose patterns and avoid diabetes, prediabetes, or other states of glycemic fluctuations that are outside of the typical normal range; (2) optimize their blood glucose concentrations to achieve peak mental or physical performance; and (3) utilize personal data to promote/motivate sustained healthy behavioral changes.
Various CGMs are approved by the United States Food and Drug Administration (FDA) for use in adults and children with diabetes. However, little is known about the role and utility of CGMs for improving health outcomes in individuals without diabetes. Twenty-four-hour CGM profiles may detect a more detailed glycemic profile than self-monitored blood glucose and are less burdensome than checking glucose by fingerstick or by an oral glucose tolerance test. There is a growing interest in utilizing CGM technology for clinical and research purposes to augment lifestyle modification techniques and promote health outcomes across all age groups.1 -3 Continuous glucose monitoring provides an opportunity to support lifestyle modification by providing individualized biofeedback on how behavior change strategies impact biological responses and thus promote health. 4
Methods
PubMed and Google Scholar were searched for articles about the use of CGM in individuals without diabetes. Relevant articles that included sufficient details were queried to identify what cohorts of individuals were adopting CGM use and to define trends of use.
Normative Data
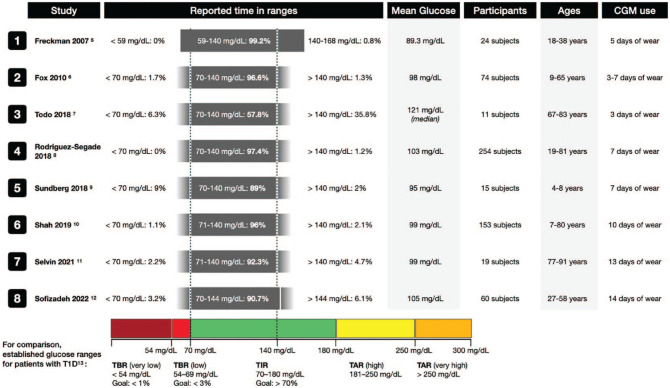
Studies of glucose concentrations using CGMs in populations of people with normal baseline fasting and glucose tolerance tests have demonstrated glucose concentrations almost completely in the tight range of 70 to 140 mg/dl.5-12 Over the past fifteen years, eight such studies from Germany in 2007, 5 the United States (US) in 2010, 6 Japan in 2018, 7 Spain in 2018, 8 Sweden in 2018, 9 the US in 2019 10 and 2021, 11 and Sweden in 2022 12 are summarized in Figure 1.
Figure 1.
Results of studies reported since 2007 using CGMs to determine times spent in various ranges of glucose concentrations in populations of people with normal baseline fasting and glucose tolerance tests. Under “reported time in ranges,” the gray bars represent the range of glucoses reported in each study. If the original study reported exact minimum or maximum values, the gray bar has hard edges; otherwise it has faded edges if only “less than” or “greater than” were used in the data reporting. The dotted lines at 70mg/dL and 140 mg/dL are not shown for studies that used different cut-off values. Established target times in ranges for patients with type 1 diabetes per the International Consensus on Time in Range. 13 Abbreviations: CGM, continuous glucose monitor; T1D, type 1 diabetes; TAR, time above range; TBR, time below range; TIR, time in range.
Effects of Diet on Glycemia Learned From Using CGM Data
It is well described that blood glucose levels change after a meal and that postprandial glucose responses (PPGR) depend on the macronutrients consumed, the time of day, and the individuals underlying beta-cell function.14,15 Postprandial glucose responses are increasingly considered a major determinant of glycemic control, yet methods for predicting PPGR to food remain elusive and of limited efficacy. 16 CGMs can be utilized to evaluate the frequency with which individuals demonstrate elevations in postprandial glucose, the types of patterns, and how patterns vary between individuals given an identical nutrient challenge.17,18 There are a growing number of scientists and physicians who are developing analytical frameworks that can group individuals according to specific patterns of glycemic responses called glucotypes.19,20 Despite the association of certain glucotypes or PPGR with increased risk of conversion to diabetes and diabetes-related complications, predicting the impact of specific foods on PPGR has been challenging because of the high variability in different individual responses to the same food. 21 Some attempts have been made to estimate PPGR using the carbohydrate content of meals or using glycemic indices. Few studies have attempted to build or test predictive models of PPGR based on specific foods eaten in individual meals. In a seminal study on personalized nutrition, Zeevi et al 16 used CGM to track the glucose response of 800 adults for one week. The study team then developed a machine learning model that could predict the glucose response of a meal for each participant based on individual factors, such as anthropometric variables, PPGR, blood panels, and gut microbiome. Subsequently, this model has been tested on several other independent cohorts and was able to generate personalized nutrition plans that resulted in improved glycemic variability. 22 Since these findings were published, several commercial companies have been working to integrate CGM technology for personalized nutrition and health.
Effects of Exercise on Glycemia Learned From Using CGM Data
Few studies have generated normative data about the effects of exercise on glycemic profiles solely on people with normal glucose tolerance. DuBose et al 23 studied 153 adults with normal glucose tolerance who performed either aerobic or resistance exercise. The mean glucose concentration during nights following exercise days was 82 mg/dl (distributed as 83 mg/dl for aerobic exercise and 76 mg/dl for resistance exercise) compared with 85 mg/dl during nights following non-exercise days (P = 0.05). 23 As measured by CGMs in adults with normal glucose tolerance and body mass index, exercise was shown to impact the metabolic effects of a high calorie diet. Greater activity resulted in lower glucose concentrations, as well as decreased visceral fat and increased cognition. 24 We expect that CGMs will be used in the future to assess the effects of exercise on glycemia in people with normal glucose tolerance. The use of CGM to identify glycemic profiles which might reflect the rate of glucose clearance has been proposed as a surrogate for evaluating resting energy expenditure, 25 although variations in the release of insulin and catecholamines can mask differences in glucose clearance. 26 Using CGM in a cohort of ten sub-elite athletes (6 hours per week), Thomas and colleagues 27 found that 4 out of 10 spent more than 70% of the total monitoring time above 108 mg/dl (even after excluding the 2-hour period after meals) and 3 out of 10 had fasting blood glucose concentrations in the prediabetes range. Although some athletes have expressed concern about hypoglycemia during exercise, 28 the small number of athletes in this study did not experience any hypoglycemia.
Effects of Stress on Glycemia Learned From Using CGM Data
The incidence of diabetes is lower among most populations living at higher altitudes than at lower altitudes. 29 However, among hikers with normal glucose tolerance wearing a CGM in Nepal, Hill et al 30 observed a significant increase in nocturnal glucose at 3600 meters and higher compared with a baseline altitude of 1100 meters. It is possible that the stress response to exercise during high altitude dominates exercise-enhanced insulin sensitivity, resulting in relative hyperglycemia. 30 Furushima et al 31 found that in a series of 40 patients in an intensive care unit with sepsis, the mean amplitude of glycemic excursions, measured by CGM, during the first 48 hours of treatment were associated with increased all-cause mortality and the concentration of urinary 8-iso-prostaglandin-F2α, which was assumed to be a surrogate of oxidative stress. The effect of the stress of an infection on glycemia was also demonstrated with CGM data in a comparison of times within target range, above target range, and below target range in a study of 31 participants with normal glucose tolerance. In this study that spanned a median time of six days per participant, 13 participants had mild COVID-19 and 18 participants did not have COVID-19. The participants with COVID-19 compared with the healthy controls experienced, respectively, significantly greater time above target ranges of both 140 mg/dl and 180 mg/dl (13.9% vs 2.3% and 1.9% vs 0%, respectively) and significantly less time within one of two target ranges (80.1% vs 93.1% for glucose 70-140 mg/dl), but not significantly less time for glucose 70-180 mg/dl (92.1% and 100%). 32 Given the evidence for an increased incidence of diabetes following a COVID-19 infection, 33 such data might be useful to identify patients with COVID-19 without diabetes who are at increased risk of developing diabetes.
Results: Potential Uses of CGM by People Without Diabetes
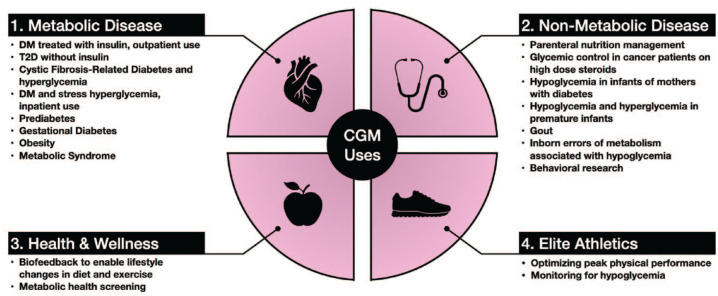
Researchers and clinicians have been interested in the monitoring capabilities of CGMs beyond diabetes since the technology became available. Broadly speaking, current and potential CGM applications can be organized into four domains (Figure 2):
Figure 2.
Clinical use case domains for continuous glucose monitors. Abbreviations: CGM, continuous glucose monitor; DM, diabetes mellitus; T2D, type 2 diabetes.
Metabolic Disease: a collection of diseases that involve the primary dysregulation of the insulin-glucose axis.
Non-Metabolic Disease: disease states where glucose concentrations are relevant to clinical management and outcomes, including secondary diabetes or hypoglycemia, but the disease process is unrelated to an intrinsic or primary pathophysiologic derangement of the insulin-glucose axis.
Health and Wellness: CGM data can be used as a biofeedback tool to drive behavior change in otherwise healthy individuals.
Elite Athletics: monitoring and optimizing the physical performance of elite or professional athletes.
Metabolic Disease
The first CGM, a professional model, was cleared by the FDA in 1999 for monitoring interstitial glucose levels in persons with diabetes mellitus.34,35 CGMs have evolved over the past two decades, but the vast majority of research (and the only FDA-cleared indication) continues to be focused on diabetes. CGMs are indicated for outpatients with diabetes who are using insulin. 36 CGMs are also being used in selected outpatients with diabetes who are not using insulin 4 as well as people with cystic fibrosis-related diabetes 37 and cystic fibrosis-related hypoglycemia. 38
CGMs have been used to achieve tight glycemic control in critically ill hospitalized patients with secondary diabetes39,40 or stress hyperglycemia in adults 41 and children. 42 This type of practice is currently investigational and requires protocols specifying sensor glucose and point of care glucose measurements for sensor validation as well as highly trained staff members and appropriate hospital policies. 43 The authors of this article believe that patients with related metabolic diseases characterized by inadequate insulin production or activity, like prediabetes, 44 gestational diabetes, 45 obesity, 46 and metabolic syndrome 47 may also benefit from CGM use, but more research is required to support and clarify these indications.
On March 1, 2022, the FDA granted breakthrough device designation to the Dexcom Hospital CGM System (Dexcom Inc., San Diego, CA). This designation will provide prioritized pre-market review and increased opportunities to interact with FDA’s experts, ultimately leading to authorization and timely access to this technology for hospitalized patients and healthcare professionals. 48
Prediabetes
For diagnosing diabetes, Hemoglobin A1c does not necessarily correlate with a fasting plasma glucose or a two-hour glucose concentration in a glucose tolerance test, and these two glucose concentrations do not necessarily correlate with each other. Hemoglobin A1c, as well as fasting, and postprandial glucose concentrations do not always cleanly differentiate between diabetes, prediabetes, and a normal metabolic state. CGMs can provide additional diagnostic information. Thus, CGM data might facilitate earlier diagnosis and treatment for people with prediabetes or at increased risk of developing type 2 diabetes (T2D). 44 A standardized method and standardized diagnostic result will be needed before CGMs will be widely accepted as a tool to diagnose diabetes, but a CGM result might provide a signal that a traditional diagnostic test for diabetes is needed. It should be noted that youth onset T2D progresses much more aggressively than adult-onset T2D and results in earlier time to onset of macro and micro vascular complications. 49 This means that early detection and treatment is key to recognition in order to prevent the early onset of these life limiting complications for youth with T2D.
Gestational diabetes
CGMs may provide information that can help predict gestational diabetes. 45 Use of CGMs for monitoring glucose concentrations during pregnancy has been beneficial in type 1 diabetes, T2D, and gestational diabetes. 50
Obesity
Obesity is the greatest risk factor for the development of diabetes. 51 CGM data can be used as biofeedback to help support behavioral and lifestyle interventions, including portion control and both timing and quality of food consumed.1,52 Obesity interventions can prevent or delay onset of T2D as well as other obesity-related diseases (e.g. cardiovascular disease, osteoarthritis, and worse outcomes with COVID-19) in those at risk. 53 Current interventions for weight management are limited, labor and time intensive, and costly. Also, they often suffer from attrition and lack of durability. New tools that leverage technology to support behavior modification could be helpful in preventing the morbidity and mortality associated with obesity. 54
Non-Metabolic Disease
The literature contains a very limited number of case reports of using CGM in a variety of primarily non-metabolic diagnoses. These uses include to manage glycemia during parenteral nutrition for inpatients, 55 monitor cancer patients with hyperglycemia due to steroid therapy,56,57 detect neonatal hypoglycemia in infants of mothers with diabetes, 58 prevent hyperglycemia and hypoglycemia in premature infants, 59 assess the relationship between glycemic fluctuations and serum uric acid concentrations in gout patients, 60 assess glucose management and optimize treatment in patients with inborn errors of metabolism associated with hypoglycemia, 61 and facilitate behavioral research. 62
Health and Wellness
Healthy behavior (e.g. portion control, increased activity and sleep, and avoidance of processed foods and emotional eating) can delay or prevent onset of T2D. If people without diabetes can see their glucose concentrations in real time and respond with healthy behavior when the concentrations are above a physician-determined target or above some concentration that is currently not standardized, then a feedback loop can be developed. Therefore, CGM information may promote healthier behaviors and prevent onset of T2D. 63 A small amount of evidence in the literature supports the idea that knowing one’s data from a CGM can lead to sustained behavior change across all activities of daily living. 44
CGMs have the potential to be used to promote behavior modifications for health benefits. 64 Liao and Schembre 65 studied a cohort of 30 people with normal glucose tolerance who wore a CGM and used a mobile app to track exercise and diet. The people found the measurement tools were easy to use and able to provide relevant information of interest to them. 65 Liao et al 66 then used CGMs along with counseling and an exercise tracker to motivate physical activity in 19 obese individuals who did not have diabetes. Participants gave high satisfaction ratings to the intervention. 66 See Table 1 for results of the acceptability survey by Liao et al.
Table 1.
Mean of Acceptability Score for Each Data Collection Tool and Comparisons of Their Mean Scores.
| Data collection tool | Acceptability score, mean (SD) | Tool, absolute mean difference (SD) | ||
|---|---|---|---|---|
| CGM sensor | CGM receiver | MyFitnessPal | ||
| Dexcom G4 Platinum CGM Sensor | 4.06 (0.55) | — | 0.01 (0.21) | 0.04 (0.90) |
| Dexcom G4 Platinum CGM Receiver | 4.05 (0.58) | 0.01 (0.21) | — | 0.05 (0.90) |
| MyFitnessPal Mobile App | 4.10 (0.68) | 0.04 (0.90) | 0.05 (0.90) | — |
| ActiGraph GT3X Accelerometer | 3.73 (0.76) | 0.33 (0.55)* | 0.32 (0.55)* | 0.37 (0.98)* |
Source: Modified from Liao and Schembre. 65
The Likert scale used in the ratings was 1 = strongly disagree, 2 = disagree, 3 = neither agree nor disagree, 4 = agree, 5 = strongly agree.
Abbreviations: CGM, continuous glucose monitor; SD, standard deviation.
P < .05 based on a two-tailed paired-sample t test.
Whelan et al 67 conducted a qualitative study exploring intuitive engagement with real-time glucose from CGM data and physical activity feedback and found that accessing behavioral and physiological feedback increased self-awareness of how lifestyle impacts short-term health in adults with obesity at risk for T2D. Participants in this study reported changing what food types they selected at meals based on seeing a prominent spike on their CGM from prior consumption. In addition, they reported that motivation to avoid food that resulted in large glycemic excursions encouraged them to make healthier eating choices. 67 Other studies have utilized CGMs as behavioral modification tools both to provide real-time feedback and for hunger cue training and impulse control response. 1
The team at January AI was the first to investigate the potential benefit of using a CGM with a mobile app in people without diabetes in the Sugar Challenge study. They tested a regimen consisting of a ten-day CGM combined with a mobile app that links an individual’s glucose tracing to meal composition, heart rate, and physical activity and provides feedback in a cohort of 473 participants, who self-declared as being healthy (n = 448) or having prediabetes (n = 25). The result was a significant improvement in the time in range (defined as 54-140 mg/dl) from the first two days until the final two days of the wear period. 68 A study sponsored by Signos, Inc, is currently recruiting 20,000 people without diabetes to participate in a study that will use a CGM and a mobile health app to optimize general wellness and body weight. 69
Elite Athletics
Some clinicians have touted the idea that there is a glucose concentration which is a subset of glucose levels that are not widely considered pathological where the body functions at peak performance. These clinicians believe that maintaining normal baseline glucose levels in a narrow peak performance range will improve body function by avoiding occasional hyperglycemia that is inherently unhealthy and providing particularly protection from developing T2D. 70 There is virtually no evidence for this belief, but there has been little research in this area because CGM data is so new and almost all the CGM data in the literature are in people with diabetes. In testing the value of CGM use by athletes without diabetes, it is helpful to determine sensor accuracy both at rest and during exercise. 71 Ishihara and colleagues 72 studied CGM data on seven ultramarathon (160 km) runners and found that their glucose concentrations ranged from 61.9 to 252.0 mg/dl. Average glucose concentrations over the entire race in the runners ranged from 104 to 164 mg/dl. Running speeds correlated significantly with glucose concentrations and with energy/carbohydrate intake. The article did not specify whether the athletes were aware of their glucose concentrations. 72 In a study of sub-elite athletes wearing CGMs by Thomas and colleagues, 27 one of ten participants spent a significant amount of time (between 10% and 20%) with glucose concentrations below 72 mg/dl. Olsson reported in an unpublished white paper that among four Swedish national elite swimmers monitored with a CGM for six days, three had occurrences of hypoglycemia (below 70 mg/dl). These three swimmers were hypoglycemic for an average of 224 minutes per week, which represented 2.59% of the entire week. 73 No study has been reported in the medical literature as to (1) whether an otherwise healthy person with normal glucose tolerance or an elite athlete can use CGM to attain a narrow glucose range generally accepted as peak performance level of glycemia and (2) whether increased time living at such a level indeed improves performance at any task. Are the potential benefits of CGM real for people without diabetes, including elite athletes? At this time, the answer is unproven.
Discussion: Summary of Benefits
The evidence for CGM use is scant beyond the metabolic and non-metabolic disease domains, and consensus is virtually non-existent outside of diabetes. Since using CGMs is not without cost, most endocrinologists are awaiting data before recommending CGM to people without diabetes. On the other hand, some people are choosing to self-pay for CGM equipment because of the appeal of knowing their glucose concentrations under various circumstances, including following ingestion of specific foods and performance of specific activities. Some endocrinologists are advocating use of CGM to prevent T2D without evidence in the medical literature based on the idea that absence of proof is not proof of absence. 74 Many CGM users without diabetes are advocates of the quantified self-movement, which advocates self-monitoring and self-management of multiple types of physiological data streams. 75
Potential Drawbacks of CGM Use by People Without Diabetes
There is virtually no risk to using a CGM other than (1) cost, which is currently not paid by insurance companies outside of an evidence-based covered wellness program, (2) tape allergy, which is infrequent, (3) skin trauma, which is rare, and (4) stress or distress, which could be associated with having access to the data. Two drawbacks to the use of CGMs by people without diabetes are not specifically risks but rather problems with using the data and these include (1) an absence of consensus standards for defining abnormal values and (2) an absence of consensus standards for how to respond to abnormal values. Finally, regardless of the use case, a CGM system may provide inaccurate readings if it is applied incorrectly or malfunctions, which could potentially lead to inappropriate or dangerous corrective action. It is important for a patient to understand the product’s indications and limitations. At the high end of the target range for most people (70-180 mg/dl) measured by CGMs, most of these products on the market function at approximately their published levels of accuracy, but at the low end of the target range, they are usually less accurate. 76 Several questions are currently unanswered (Table 2). With further research, it is hoped that the indications, benefits, and drawbacks of CGM for people without diabetes will become clearer.
Table 2.
Questions About CGM in People Without Diabetes.
| 1 | Does CGM improve diet and does improved diet lead to less diabetes? |
| 2 | Does CGM improve exercise and does improved exercise lead to less diabetes? |
| 3 | Does CGM improve activity and does improved activity lead to less diabetes? |
| 4 | Does CGM improve sleep and does improved sleep lead to less diabetes? |
| 5 | What kind of improvement in glucose is needed to achieve the improved outcomes in questions 1-4? Is the most important glycemic target to address improved fasting, postprandial, or mean blood glucose or is it TIR or GRI? |
| 6 | What is the role of improved GV in improved outcomes? |
| 7 | Is this information more or less useful for athletes? |
Abbreviation: CGM, continuous glucose monitoring; GRI, Glycemia Risk Index; GV, glycemic variability; TIR, time in range.
Regulation of CGM Systems Used for People Without Diabetes
Four manufacturers produce all the FDA authorized CGM systems in the United States. They include Abbott Diabetes Care (Alameda, CA), Dexcom Inc. (San Diego, CA), Medtronic Diabetes (Northridge, CA), and Senseonics (Germantown, MD). All of those systems have either been cleared or approved as prescription use only devices for the management of patients with diabetes. Currently, therefore, manufacturers are restricted in making any claims for the use of their product on people without diabetes, although clinicians are able to prescribe them for such use.
Will the FDA regulate the use of CGM systems in use for people without diabetes? The FDA has broad authority to regulate diagnostic devices, yet it has recognized that the use of such devices to promote healthy living or for sport performance is low risk, so it has concluded that is not worth spending its limited resources regulating such devices. On September 27, 2019, FDA issued a final guidance on “General Wellness: Policy for Low Risk Devices.” 77 Two of the possible uses mentioned, which are to inspire healthy behavior or to function at peak performance, fit squarely within the FDA stated policy. A claim for detecting prediabetes does not. Besides making general wellness claims, a device must be non-invasive. The guidance defines invasive as: “penetrates or pierces the skin or mucous membranes of the body.” As such, CGM devices that are non-invasive will likely not be regulated by the FDA, but devices that are implanted, as most of currently approved and cleared devices are, will have to make a filing with the Agency before such devices can be placed in the market. Even for non-invasive CGMs, the FDA will be concerned with the risks associated with an over-the-counter device that has wellness claims. Chief among those will be the use of such devices by people with diabetes to manage their condition. Given that the FDA still has jurisdiction over such devices, it can issue safety alerts or develop labeling or other controls to mitigate the risks those devices may pose, or change the policy for those devices that pose an increased risk to health.
Commercial Health and Wellness CGM Programs
In the US and Canada, CGMs require a medical prescription and are only approved for use in patients with diabetes. In recent years, several digital health companies (Table 3) have started offering factory calibrated CGMs from Abbott Diabetes Care or Dexcom to consumers paired with a mobile app and often coaching or dietician services to “improve general health.” Most of these companies do not detail on their website how they provide CGMs to people without diabetes and they do not claim to be recruiting for clinical trials of CGM. However, the fact that these digital health companies do not accept insurance and yet they offer to provide prescriptions suggests that the companies employ or contract with physicians that write off-label prescriptions for CGMs that the patients pay for out of pocket. The basic premise of these services is not dissimilar to the role of CGMs in more traditional clinical and research settings: to provide real-time biofeedback to help individuals make lifestyle changes. However, whether CGMs are effective behavior change tools in people without diabetes is not known. It is also not known who is most likely to benefit from using a CGM and how often diabetes can be prevented or delayed. Finally, it is unclear what, if any, is the impact of glycemic variability in individuals with normal glucose homeostasis. Research is needed to define the best role for CGMs in populations without diabetes. Several consumer health product companies are developing noninvasive glucose monitors (mostly with a wrist watch form factor) for people without diabetes to measure glucose optically from the skin in the wrist.78-81 It remains to be determined how accurate these new devices will be and how they will be regulated.
Table 3.
Companies That Market CGMs for Health and Wellness Applications.
| Company | Product | Services | Cost |
|---|---|---|---|
| Abbott82,83 | Lingo | CGM (available only in EU), CKM, and lactate biosensors to be launched in the future, Lingo line of products is not intended for medical use | Unknown |
| Abbott EU84-86 | Abbott Libre Sense | CGM for athletes but not intended for medical use, data can be read using compatible smartphone app, up to 14-day wear, can be worn while swimming, partnered with Supersapiens | Unknown |
| Ageless Rx 87 | Freestyle Libre | CGM can be worn up to 14 days, can be worn while swimming, showering, and exercising, readings can be found in the LibreLink app | $85-$95/CGM |
| January88,89 | Freestyle Libre 2 or Dexcom G6 | A pair of CGMs, Telehealth Assessment, and January App access | $144/CGM |
| Levels89,90 | Freestyle Libre | Telemedicine Consultation, Levels App, and a pair of CGMs | $197.5/CGM |
| NutriSense89,91 | Abbott CGM | CGM, NutriSense App (includes CGM tracking, meal composition data, fasting/meal timing, exercise routines, stress and sleep, habits and routines), access to Dietitian Team | Packages range from $175 for two weeks or $160 a month for a 18-month commitment |
| Signos 92 | Not specified | CGM, Signos App access | Unknown |
| Supersapiens 93 | Abbott Libre Sense | (available only in EU) CGM, Supersapiens App, Glucose response simulator, training guides | Packages range from 150 Euros for 2 CGMs up to 700 Euros for 9 CGMs |
| Ultrahuman Cyborg94,95 | Freestyle Libre 2 | (Currently in private beta) CGM, Ultrahuman: Metabolic Fitness App | Unknown |
Abbreviation: CGM, continuous glucose monitor; CKM, continuous ketone monitor; EU, European Union.
Conclusions: The Future of CGM Use by People Without Diabetes
We expect increasing adoption of CGM use in four areas: metabolic diseases other than diabetes, non-metabolic diseases, health and wellness, and elite athletics. We see seven trends in the use of CGM systems in people without diabetes (Table 4). These trends can pertain to both FDA-cleared medical grade products as well as anticipated future products, which may be regulated differently based on intended populations and indications for use. 64 We expect that clinicians will become increasingly aware of (1) glycemic patterns from CGM tracings that predict an increased risk of diabetes, (2) specific metabolic glucotypes from CGM tracings that predict an increased risk of diabetes, and (3) new genetic and genomic biomarkers in the future that are linked to an increased risk of developing diabetes in the presence of any atypical CGM readings. It is likely that a role for CGMs will be discovered for people without diabetes who are (1) at increased risk of developing diabetes based on emerging biomarkers and glucotype classifications, (2) in an overweight or obese state, or (3) elite athletes at risk of hypoglycemia. Although little data has been reported to date on the benefits of CGM for people without diabetes, a groundswell of interest in defining the role of this type of sensor is now beginning. We expect that well designed clinical trials will demonstrate a specific role for CGM use in people without diabetes, and that consensus guidelines will establish how to interpret and respond to this type of data. As our understanding of this technology and the data it generates improves through rigorous science, more and more people without diabetes will be able to benefit from CGMs.
Table 4.
Future Uses of CGM Systems in People Without Diabetes.
| 1 | Greater use of CGM systems by people without diabetes |
| 2 | Predictions for developing type 2 diabetes and gestational diabetes |
| 3 | Predictions for future short-term glucose concentrations including hypoglycemia and hyperglycemia |
| 4 | Integration of CGM data into the electronic health record |
| 5 | Widespread adoption of sound diabetes device-specific cybersecurity standards for CGM systems |
| 6 | Use of dashboards and platforms to assemble and interpret CGM data along with other physiologic sensor data |
| 7 | CGM data combined with other sensor data and analyzed by artificial intelligence could become the linchpin in the personalized management of diabetes |
Abbreviation: CGM, continuous glucose monitor.
Acknowledgments
The authors thank Lynn Kysh for her help in designing and conducting the background literature search, and Annamarie Sucher-Jones for her expert editorial assistance.
Footnotes
Abbreviations: CGM, continuous glucose monitor; CKM, continuous ketone monitor; COVID-19, coronavirus disease 2019; DM, diabetes mellitus; EU, European Union; FDA, United States Food and Drug Administration; GRI, Glycemia Risk Index; GV, glycemic variability; PPGR, postprandial glucose responses; TAR, time above range; TBR, time below range; TIR, time in range; T1D, type 1 diabetes; T2D, type 2 diabetes; US, United States.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: DCK is a consultant for Eoflow, Eli Lilly, Lifecare, Integrity, Medtronic, Roche Diagnostics, Rockley Photonics, and Thirdwayv. KTN and NYX are consultants for Abbott Diabetes Care. AG is a contract consultant for the ProPharma Group and helps medical device companies with regulatory issues. JCE is a paid consultant for AI Health. JCE’s time is supported in part by the Food and Drug Administration under award number P50FD006425 for The West Coast Consortium for Technology & Innovation in Pediatrics (PI: Espinoza). APV receives CGM product in kind from Dexcom for research conduct. The content is solely the responsibility of the authors and does not necessarily represent the official views of the FDA.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: David C. Klonoff  https://orcid.org/0000-0001-6394-6862
https://orcid.org/0000-0001-6394-6862
Kevin T. Nguyen  https://orcid.org/0000-0001-9102-6537
https://orcid.org/0000-0001-9102-6537
Nicole Y. Xu  https://orcid.org/0000-0001-9353-8819
https://orcid.org/0000-0001-9353-8819
Alberto Gutierrez  https://orcid.org/0000-0002-9573-8379
https://orcid.org/0000-0002-9573-8379
Juan C. Espinoza  https://orcid.org/0000-0003-0513-588X
https://orcid.org/0000-0003-0513-588X
Alaina P. Vidmar  https://orcid.org/0000-0003-3790-6255
https://orcid.org/0000-0003-3790-6255
References
- 1. Hegedus E, Salvy SJ, Wee CP, et al. Use of continuous glucose monitoring in obesity research: a scoping review. Obes Res Clin Pract. 2021;15(5):431-438. doi: 10.1016/j.orcp.2021.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rasmussen L, Christensen ML, Poulsen CW, et al. Effect of high versus low carbohydrate intake in the morning on glycemic variability and glycemic control measured by continuous blood glucose monitoring in women with gestational diabetes mellitus—a randomized crossover study. Nutrients. 2020;12(2):475. doi: 10.3390/nu12020475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Quaglia S. What I Learned From Wearing a Blood Sugar Monitor. Slate. https://slate.com/technology/2022/04/continuous-blood-sugar-monitors-diabetes-health-wellness.html. Published online April 14, 2022. Accessed April 28, 2022.
- 4. Cowart K, Updike WH, Franks R. Continuous glucose monitoring in persons with type 2 diabetes not using insulin. Expert Rev Med Devices. 2021;18(11):1049-1055. doi: 10.1080/17434440.2021.1992274. [DOI] [PubMed] [Google Scholar]
- 5. Freckmann G, Hagenlocher S, Baumstark A, et al. Continuous glucose profiles in healthy subjects under everyday life conditions and after different meals. J Diabetes Sci Technol. 2007;1(5):695-703. doi: 10.1177/193229680700100513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group, Fox LA, Beck RW, Xing D. Variation of interstitial glucose measurements assessed by continuous glucose monitors in healthy, nondiabetic individuals. Diabetes Care. 2010;33(6):1297-1299. doi: 10.2337/dc09-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Todo H. Continuous glucose monitoring can disclose glucose fluctuation in advanced Parkinsonian syndromes. Neurol Int. 2018;10(4):7921. doi: 10.4081/ni.2018.7921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rodriguez-Segade S, Rodriguez J, Camiña F, et al. Continuous glucose monitoring is more sensitive than HbA1c and fasting glucose in detecting dysglycaemia in a Spanish population without diabetes. Diabetes Res Clin Pract. 2018;142:100-109. doi: 10.1016/j.diabres.2018.05.026. [DOI] [PubMed] [Google Scholar]
- 9. Sundberg F, Forsander G. Continuous glucose monitoring in healthy children aged 2-8 years. Diabetes Technol Ther. 2018;20(2):113-116. doi: 10.1089/dia.2017.0270. [DOI] [PubMed] [Google Scholar]
- 10. Shah VN, DuBose SN, Li Z, et al. Continuous glucose monitoring profiles in healthy nondiabetic participants: a multicenter prospective study. J Clin Endocrinol Metab. 2019;104(10):4356-4364. doi: 10.1210/jc.2018-02763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Selvin E, Wang D, Tang O, Minotti M, Echouffo-Tcheugui JB, Coresh J. Glucose patterns in very old adults: a pilot study in a community-based population. Diabetes Technol Ther. 2021;23(11):737-744. doi: 10.1089/dia.2021.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sofizadeh S, Pehrsson A, Ólafsdóttir AF, Lind M. Evaluation of reference metrics for continuous glucose monitoring in persons without diabetes and prediabetes. J Diabetes Sci Technol. 2022;16(2):373-382. doi: 10.1177/1932296820965599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the International Consensus on Time in Range. Diabetes Care. 2019;42(8):1593-1603. doi: 10.2337/dci19-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fechner E, Op’t Eyndt C, Mulder T, Mensink RP. Diet-induced differences in estimated plasma glucose concentrations in healthy, non-diabetic adults are detected by continuous glucose monitoring—a randomized crossover trial. Nutr Res. 2020;80:36-43. doi: 10.1016/j.nutres.2020.06.001. [DOI] [PubMed] [Google Scholar]
- 15. Roux de, Bézieux H, Bullard J, Kolterman O, Souza M, Perraudeau F. Medical food assessment using a smartphone app with continuous glucose monitoring sensors: proof-of-concept study. JMIR Form Res. 2021;5(3):e20175. doi: 10.2196/20175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zeevi D, Korem T, Zmora N, et al. Personalized nutrition by prediction of glycemic responses. Cell. 2015;163(5):1079-1094. doi: 10.1016/j.cell.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 17. Chekima K, Wong BTZ, Noor MI, Ooi YBH, Yan SW, Chekima B. Use of a continuous glucose monitor to determine the glycaemic index of rice-based mixed meals, their effect on a 24 h glucose profile and its influence on overweight and obese young adults’ meal preferences. Foods. 2022;11(7):983. doi: 10.3390/foods11070983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pallayova M, Zaghloul HB, Arora T, et al. Investigating physiological glucose excursions before, during, and after Ramadan in adults without diabetes mellitus. Physiol Behav. 2017;179:110-115. doi: 10.1016/j.physbeh.2017.05.032. [DOI] [PubMed] [Google Scholar]
- 19. Hall H, Perelman D, Breschi A, et al. Glucotypes reveal new patterns of glucose dysregulation. PLoS Biol. 2018;16(7):e2005143. doi: 10.1371/journal.pbio.2005143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hulman A, Foreman YD, Brouwers MCGJ, et al. Towards precision medicine in diabetes? A critical review of glucotypes. PLoS Biol. 2021;19(3):e3000890. doi: 10.1371/journal.pbio.3000890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jenkins DJ, Wolever TM, Taylor RH, et al. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr. 1981;34(3):362-366. doi: 10.1093/ajcn/34.3.362. [DOI] [PubMed] [Google Scholar]
- 22. Mortazavi BJ, Gutierrez-Osuna R. A review of digital innovations for diet monitoring and precision nutrition. J Diabetes Sci Technol. 2021:19322968211041356. doi: 10.1177/19322968211041356. Published online September 1, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. DuBose SN, Li Z, Sherr JL, Beck RW, Tamborlane WV, Shah VN. Effect of exercise and meals on continuous glucose monitor data in healthy individuals without diabetes. J Diabetes Sci Technol. 2021;15(3):593-599. doi: 10.1177/1932296820905904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Krogh-Madsen R, Pedersen M, Solomon TPJ, et al. Normal physical activity obliterates the deleterious effects of a high-caloric intake. J Appl Physiol (1985). 2014;116(3):231-239. doi: 10.1152/japplphysiol.00155.2013. [DOI] [PubMed] [Google Scholar]
- 25. Gribok A, Rumpler W, Hines W, Hoyt R, Buller M. Subcutaneous glucose concentration as a predictor variable for energy expenditure during resistance exercise in humans. In: Proceedings of the 2014 11th International Conference on Wearable and Implantable Body Sensor Networks. BSN ‘14. IEEE Computer Society; 2014:16-21. doi: 10.1109/BSN.2014.11. [DOI] [Google Scholar]
- 26. Coker RH, Kjaer M. Glucoregulation during exercise : the role of the neuroendocrine system. Sports Med. 2005;35(7):575-583. doi: 10.2165/00007256-200535070-00003. [DOI] [PubMed] [Google Scholar]
- 27. Thomas F, Pretty CG, Desaive T, Chase JG. Blood glucose levels of subelite athletes during 6 days of free living. J Diabetes Sci Technol. 2016;10(6):1335-1343. doi: 10.1177/1932296816648344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Felig P, Cherif A, Minagawa A, Wahren J. Hypoglycemia during prolonged exercise in normal men. N Engl J Med. 1982;306(15):895-900. doi: 10.1056/NEJM198204153061503. [DOI] [PubMed] [Google Scholar]
- 29. Woolcott OO, Castillo OA, Gutierrez C, Elashoff RM, Stefanovski D, Bergman RN. Inverse association between diabetes and altitude: a cross-sectional study in the adult population of the United States. Obesity (Silver Spring). 2014;22(9):2080-2090. doi: 10.1002/oby.20800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hill NE, Deighton K, Matu J, et al. Continuous glucose monitoring at high altitude—effects on glucose homeostasis. Med Sci Sports Exerc. 2018;50(8):1679-1686. doi: 10.1249/MSS.0000000000001624. [DOI] [PubMed] [Google Scholar]
- 31. Furushima N, Egi M, Obata N, Sato H, Mizobuchi S. Mean amplitude of glycemic excursions in septic patients and its association with outcomes: a prospective observational study using continuous glucose monitoring. J Crit Care. 2021;63:218-222. doi: 10.1016/j.jcrc.2020.08.021. [DOI] [PubMed] [Google Scholar]
- 32. Shen Y, Zhang L, Fan X, Zhou J. Glycemic fluctuations caused by COVID-19: results from continuous glucose monitoring. Obes Med. 2021;22:100328. doi: 10.1016/j.obmed.2021.100328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xie Y, Al-Aly Z. Risks and burdens of incident diabetes in long COVID: a cohort study. Lancet Diabetes Endocrinol. 2022;10(5):311-321. doi: 10.1016/S2213-8587(22)00044-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Reddy N, Verma N, Dungan K. Monitoring technologies—continuous glucose monitoring, mobile technology, biomarkers of glycemic control. In: Feingold KR, Anawalt B, Boyce A, et al. eds. Endotext. MDText.com, Inc.; 2000. http://www.ncbi.nlm.nih.gov/books/NBK279046/. Accessed April 21, 2022. [Google Scholar]
- 35. Premarket Approval (PMA). https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P980022. Accessed April 21, 2022.
- 36. Office of the Commissioner. FDA authorizes first fully interoperable continuous glucose monitoring system, streamlines review pathway for similar devices. U.S. Food and Drug Administration. https://www.fda.gov/news-events/press-announcements/fda-authorizes-first-fully-interoperable-continuous-glucose-monitoring-system-streamlines-review. Published March 27, 2018. Accessed April 28, 2022.
- 37. Scully KJ, Sherwood JS, Martin K, et al. Continuous glucose monitoring and HbA1c in cystic fibrosis: clinical correlations and implications for CFRD diagnosis. J Clin Endocrinol Metab. 2022;107(4):e1444-e1454. doi: 10.1210/clinem/dgab857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hicks R, Marks BE, Oxman R, Moheet A. Spontaneous and iatrogenic hypoglycemia in cystic fibrosis. J Clin Transl Endocrinol. 2021;26:100267. doi: 10.1016/j.jcte.2021.100267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Faulds ER, Boutsicaris A, Sumner L, et al. Use of continuous glucose monitor in critically ill COVID-19 patients requiring insulin infusion: an observational study. J Clin Endocrinol Metab. 2021;106(10):e4007-e4016. doi: 10.1210/clinem/dgab409. [DOI] [PubMed] [Google Scholar]
- 40. Agarwal S, Mathew J, Davis GM, et al. Continuous glucose monitoring in the intensive care unit during the COVID-19 pandemic. Diabetes Care. 2021;44(3):847-849. doi: 10.2337/dc20-2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bode B, Garrett V, Messler J, et al. Glycemic characteristics and clinical outcomes of COVID-19 patients hospitalized in the United States. J Diabetes Sci Technol. 2020;14(4):813-821. doi: 10.1177/1932296820924469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhao Y, Wu Y, Xiang B. Tight glycemic control in critically ill pediatric patients: a meta-analysis and systematic review of randomized controlled trials. Pediatr Res. 2018;84(1):22-27. doi: 10.1038/s41390-018-0002-3. [DOI] [PubMed] [Google Scholar]
- 43. Galindo RJ, Umpierrez GE, Rushakoff RJ, et al. Continuous glucose monitors and automated insulin dosing systems in the hospital consensus guideline. J Diabetes Sci Technol. 2020;14(6):1035-1064. doi: 10.1177/1932296820954163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ehrhardt N, Al Zaghal E. Behavior modification in prediabetes and diabetes: potential use of real-time continuous glucose monitoring. J Diabetes Sci Technol. 2019;13(2):271-275. doi: 10.1177/1932296818790994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tartaglione L, di Stasio E, Sirico A, et al. Continuous glucose monitoring in women with normal OGTT in pregnancy. J Diabetes Res. 2021;2021:9987646. doi: 10.1155/2021/9987646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wright EE, Subramanian S. Evolving use of continuous glucose monitoring beyond intensive insulin treatment. Diabetes Technol Ther. 2021;23(S3):S12-S18. doi: 10.1089/dia.2021.0191. [DOI] [PubMed] [Google Scholar]
- 47. Buscemi S, Verga S, Cottone S, et al. Glycaemic variability and inflammation in subjects with metabolic syndrome. Acta Diabetol. 2009;46(1):55-61. doi: 10.1007/s00592-008-0061-8. [DOI] [PubMed] [Google Scholar]
- 48. DexCom, Inc. FDA Grants Breakthrough Device Designation for Dexcom Hospital CGM System. https://investors.dexcom.com/news-releases/news-release-details/fda-grants-breakthrough-device-designation-dexcom-hospital-cgm. Accessed April 21, 2022.
- 49. Shah AS, Nadeau KJ, Dabelea D, Redondo MJ. Spectrum of phenotypes and causes of type 2 diabetes in children. Annu Rev Med. 2022;73:501-515. doi: 10.1146/annurev-med-042120-012033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. O’Malley G, Wang A, Ogyaadu S, Levy CJ. Assessing glycemic control using CGM for women with diabetes in pregnancy. Curr Diab Rep. 2021;21(11):44. doi: 10.1007/s11892-021-01415-2. [DOI] [PubMed] [Google Scholar]
- 51. Aras M, Tchang BG, Pape J. Obesity and diabetes. Nurs Clin North Am. 2021;56(4):527-541. doi: 10.1016/j.cnur.2021.07.008. [DOI] [PubMed] [Google Scholar]
- 52. Schembre SM, Liao Y, Jospe MR. Continuous glucose monitors as wearable lifestyle behavior change tools in obesity and diabetes. In: Faintuch J, Faintuch S, eds. Obesity and Diabetes: Scientific Advances and Best Practice. Cham: Springer International Publishing; 2020:591-603. doi: 10.1007/978-3-030-53370-0_43. [DOI] [Google Scholar]
- 53. American Diabetes Association Professional Practice Committee, Draznin B, Aroda VR, et al. 8. Obesity and weight management for the prevention and treatment of type 2 diabetes: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45(suppl 1):S113-S124. doi: 10.2337/dc22-S008. [DOI] [PubMed] [Google Scholar]
- 54. Du Y, Dennis B, Rhodes SL, et al. Technology-assisted self-monitoring of lifestyle behaviors and health indicators in diabetes: qualitative study. JMIR Diabetes. 2020;5(3):e21183. doi: 10.2196/21183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chow KW, Kelly DJ, Gupta R, Miller JD. Use of continuous glucose monitoring to assess parenteral nutrition-induced hyperglycemia in an adult patient with severe COVID-19. JPEN J Parenter Enteral Nutr. 2021;45(1):208-211. doi: 10.1002/jpen.2032. [DOI] [PubMed] [Google Scholar]
- 56. Véber O, Wilde A, Demeter J, Tamás G, Mucsi I, Tabák AG. The effect of steroid pulse therapy on carbohydrate metabolism in multiple myeloma patients: a randomized crossover observational clinical study. J Endocrinol Invest. 2014;37(4):345-351. doi: 10.1007/s40618-013-0027-8. [DOI] [PubMed] [Google Scholar]
- 57. Zhang F, Karam JG. Glycemic profile of intravenous dexamethasone-induced hyperglycemia using continuous glucose monitoring. Am J Case Rep. 2021;22:e930733. doi: 10.12659/AJCR.930733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nally LM, Bondy N, Doiev J, Buckingham BA, Wilson DM. A feasibility study to detect neonatal hypoglycemia in infants of diabetic mothers using real-time continuous glucose monitoring. Diabetes Technol Ther. 2019;21(4):170-176. doi: 10.1089/dia.2018.0337. [DOI] [PubMed] [Google Scholar]
- 59. Beardsall K, Thomson L, Guy C, et al. Real-time continuous glucose monitoring in preterm infants (REACT): an international, open-label, randomised controlled trial. Lancet Child Adolesc Health. 2021;5(4):265-273. doi: 10.1016/S2352-4642(20)30367-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mu Z, Wang J, Wang W, et al. Blood glucose fluctuations detected by continuous glucose monitoring system in gout patients with normal glucose tolerance and the effect of urate-lowering therapy. Int J Rheum Dis. 2020;23(9):1145-1151. doi: 10.1111/1756-185X.13862. [DOI] [PubMed] [Google Scholar]
- 61. Peeks F, Hoogeveen IJ, Feldbrugge RL, et al. A retrospective in-depth analysis of continuous glucose monitoring datasets for patients with hepatic glycogen storage disease: recommended outcome parameters for glucose management. J Inherit Metab Dis. 2021;44(5):1136-1150. doi: 10.1002/jimd.12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wagner J, Tennen H, Wolpert H. Continuous glucose monitoring: a review for behavioral researchers. Psychosom Med. 2012;74(4):356-365. doi: 10.1097/PSY.0b013e31825769ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ziegler R, Heinemann L, Freckmann G, Schnell O, Hinzmann R, Kulzer B. Intermittent use of continuous glucose monitoring: expanding the clinical value of CGM. J Diabetes Sci Technol. 2021;15(3):684-694. doi: 10.1177/1932296820905577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Holzer R, Bloch W, Brinkmann C. Continuous glucose monitoring in healthy adults—possible applications in health care, wellness, and sports. Sensors. 2022;22(5):2030. doi: 10.3390/s22052030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Liao Y, Schembre S. Acceptability of continuous glucose monitoring in free-living healthy individuals: implications for the use of wearable biosensors in diet and physical activity research. JMIR Mhealth Uhealth. 2018;6(10):e11181. doi: 10.2196/11181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Liao Y, Basen-Engquist KM, Urbauer DL, Bevers TB, Hawk E, Schembre SM. Using continuous glucose monitoring to motivate physical activity in overweight and obese adults: a pilot study. Cancer Epidemiol Biomarkers Prev. 2020;29(4):761-768. doi: 10.1158/1055-9965.EPI-19-0906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Whelan ME, Denton F, Bourne CLA, et al. A digital lifestyle behaviour change intervention for the prevention of type 2 diabetes: a qualitative study exploring intuitive engagement with real-time glucose and physical activity feedback. BMC Public Health. 2021;21(1):130. doi: 10.1186/s12889-020-09740-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dehghani Zahedani A, Shariat Torbaghan S, Rahili S, et al. Improvement in glucose regulation using a digital tracker and continuous glucose monitoring in healthy adults and those with type 2 diabetes. Diabetes Ther. 2021;12(7):1871-1886. doi: 10.1007/s13300-021-01081-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Signos Launches a First-of-Its-Kind 20,000 Person Study on CGM Use Paired With an AI-Powered Health App to Unlock the Role of Glucose Responses in Health Outcomes for All. https://www.businesswire.com/news/home/20220216005221/en/Signos-Launches-a-First-of-its-Kind-20000-Person-Study-on-CGM-Use-Paired-With-an-AI-Powered-Health-App-to-Unlock-the-Role-of-Glucose-Responses-in-Health-Outcomes-for-All. Published February 16, 2022. Accessed March 22, 2022.
- 70. Cermak NM, van Loon LJ. The use of carbohydrates during exercise as an ergogenic aid. Sports Med. 2013;43(11):1139-1155. doi: 10.1007/s40279-013-0079-0. [DOI] [PubMed] [Google Scholar]
- 71. Clavel P, Tiollier E, Leduc C, Fabre M, Lacome M, Buchheit M. Concurrent validity of a continuous glucose-monitoring system at rest and during and following a high-intensity interval training session. Int J Sports Physiol Perform. 2022;17(4):627-633. doi: 10.1123/ijspp.2021-0222. [DOI] [PubMed] [Google Scholar]
- 72. Ishihara K, Uchiyama N, Kizaki S, Mori E, Nonaka T, Oneda H. Application of continuous glucose monitoring for assessment of individual carbohydrate requirement during ultramarathon race. Nutrients. 2020;12(4):E1121. doi: 10.3390/nu12041121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Olsson J. Swedish Elite Swimmers Blood Glucose Levels During Recovery : A Descriptive Study Using Continuous Glucose Monitoring Systems. 2017. http://urn.kb.se/resolve?urn=urn:nbn:se:gih:diva-4770. Accessed April 21, 2022.
- 74. Ommen ES, Winston JA, Murphy B. Medical risks in living kidney donors: absence of proof is not proof of absence. Clin J Am Soc Nephrol. 2006;1(4):885-895. doi: 10.2215/CJN.00840306. [DOI] [PubMed] [Google Scholar]
- 75. Swan M. The quantified self: fundamental disruption in big data science and biological discovery. Big Data. 2013;1(2):85-99. doi: 10.1089/big.2012.0002. [DOI] [PubMed] [Google Scholar]
- 76. Moser O, Sternad C, Eckstein ML, et al. Performance of intermittently scanned continuous glucose monitoring systems in people with type 1 diabetes: a pooled analysis. Diabetes Obes Metab. 2022;24(3):522-529. doi: 10.1111/dom.14609. [DOI] [PubMed] [Google Scholar]
- 77. Center for Devices and Radiological Health. General Wellness: Policy for Low Risk Devices—Guidance for Industry and Food and Drug Administration Staff. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/general-wellness-policy-low-risk-devices. Published online September 27, 2019. Accessed June 29, 2022.
- 78. Webster S. Rockley photonics, an Apple Partner, is developing a wearable that can monitor blood sugar levels. Tech Times. https://www.techtimes.com/articles/272895/20220311/apple-partner-rockley-photonics-working-wearable-tech-monitor-blood-sugar.htm. Published March 11, 2022. Accessed March 22, 2022.
- 79. Alonzo I. Apple Watch Series 7, Samsung Galaxy Watch 4 to feature blood sugar monitors! Tech Times. https://www.techtimes.com/articles/256327/20210125/apple-watch-series-7-samsung-galaxy-4-soon-monitor-blood-sugar.htm. Published January 25, 2021. Accessed March 22, 2022.
- 80. Deakin DR. Wrist-worn non-invasive continuous glucose monitor from Movano takes step closer to FDA clearance after type 1 diabetes pilot study completion. Notebookcheck. https://www.notebookcheck.net/Wrist-worn-non-invasive-continuous-glucose-monitor-from-Movano-takes-step-closer-to-FDA-clearance-after-type-1-diabetes-pilot-study-completion.604453.0.html. Accessed March 22, 2022.
- 81. Know Labs Provides 2022. Outlook, Outlines Preparations for Pre-Submission Meeting with FDA. https://finance.yahoo.com/news/know-labs-provides-2022-outlook-140000070.html. Accessed March 22, 2022.
- 82. TechCrunch. Abbott tells CES it’s getting into consumer biowearables. https://techcrunch.com/2022/01/07/abbott-lingo/. Accessed March 29, 2022.
- 83. Abbott Announces Future of Biowearables at Consumer Electronics Show. January 6, 2022. https://abbott.mediaroom.com/2022-01-06-Abbott-Announces-Future-of-Biowearables-at-Consumer-Electronics-Show. Accessed March 29, 2022.
- 84. Libre Sense Glucose Sport Biosensor. Real-Time Monitoring. https://www.libresense.abbott/en/home.html. Accessed March 29, 2022.
- 85. Abbott Launches First Glucose Sport Biosensor for Athletes. Abbott. https://www.abbott.com/corpnewsroom/strategy-and-strength/abbott-launches-first-glucose-sport-biosensor-for-athletes.html. Accessed March 29, 2022.
- 86. Abbott Introduces Libre Sense Glucose Sport Biosensor in Europe, World’s First Glucose Biosensor Designed for Athletes. Abbott MediaRoom. https://abbott.mediaroom.com/2020-09-17-Abbott-Introduces-Libre-Sense-Glucose-Sport-Biosensor-in-Europe-Worlds-First-Glucose-Biosensor-Designed-for-Athletes. Accessed March 29, 2022.
- 87. Longevity Prescriptions—Buy Metformin, NAD+, LDN, & More Online. AgelessRx. https://www.agelessrx.com. Accessed March 29, 2022.
- 88. January AI. Optimize your blood sugar intelligently. January.ai. https://www.january.ai/. Accessed March 29, 2022.
- 89. O’Connor A. Can technology help us eat better? The New York Times. https://www.nytimes.com/2021/02/08/well/diet-glucose-monitor.html. Published February 8, 2021. Accessed March 29, 2022.
- 90. Levels—Metabolic Fitness Program. Levels. https://www.levelshealth.com/. Accessed March 29, 2022.
- 91. NutriSense. https://www.nutrisense.io/. Accessed March 29, 2022.
- 92. Signos—Continuous Glucose Monitor Device for Weight Loss. https://www.signos.com/. Accessed March 29, 2022.
- 93. Supersapiens. https://www.supersapiens.com. Accessed March 29, 2022.
- 94. Ultrahuman Cyborg . https://ultrahuman.com/. Accessed April 29, 2022.
- 95. Lomas N. Four weeks as an Ultrahuman “Cyborg.” TechCrunch. https://social.techcrunch.com/2022/01/06/four-weeks-as-an-ultrahuman-cyborg/. Published January 6, 2022. Accessed April 29, 2022.