Abstract
Objective
Sepsis caused by severe acute cholangitis requires biliary drainage to decrease the intra-biliary pressure. Furthermore, several studies showed that anticoagulant treatment can improve the outcomes of patients with sepsis-associated disseminated intravascular coagulation (DIC). There were reports examining the efficacy of anti-DIC drugs in patients undergoing biliary drainage with sepsis-associated DIC, and no reports compared the efficacy of DIC treatments when no drainage is performed. In this study, the influence of antithrombin (AT) replacement therapy and recombinant thrombomodulin (rTM) preparations on the overall survival (OS) of patients with and without biliary drainage was analyzed.
Patients and Methods
This retrospective cohort study in a single institution involved patients with sepsis-associated DIC caused by severe biliary tract infection. In total, 71 patients treated by either AT replacement therapy or rTM preparation were assessed for inclusion. The two groups were patients with biliary drainage (n = 45) and without drainage (n = 26). To assess the clinical efficacy of anti-DIC drugs in each group, the 60-day OS was determined through estimated survival analysis.
Results
Focusing on the effects of different therapeutic agents for DIC, in the group of patients with biliary drainage, OS showed no difference between patients treated by rTM and AT. However, in patients without biliary drainage, the survival curves of patients treated with AT replacement were lower than those of patients with rTM preparation.
Conclusion
This study revealed that the OS of patients without biliary drainage differed depending on the DIC therapeutic agent for sepsis-associated DIC caused by acute cholangitis. We would recommend the use of rTM preparation over AT replacement therapy for patients who cannot undergo biliary drainage.
Keywords: sepsis, antithrombin, recombinant thrombomodulin, DIC treatment, acute cholangitis
Introduction
Sepsis is a state of organ damage associated with infection; it requires immediate antimicrobial, organ support, and source control when appropriate. The prognosis of patients with sepsis-associated disseminated intravascular coagulation (DIC) is poor,1 but the drug therapy for DIC itself is still controversial. The 2021 Surviving Sepsis Campaign’s international guidelines for the management of sepsis and septic shock, jointly developed by the Society of Critical Care Medicine and the European Society of Intensive Care Medicine, do not describe DIC treatment.2 On the other hand, the Japanese Clinical Practice Guidelines for the Management of Sepsis and Septic Shock 2020 (J-SSCG 2020)3 recommend the use of antithrombin (AT) replacement therapy or recombinant thrombomodulin (rTM) preparation for sepsis-associated DIC. However, no mention was made as to which drug should be selected for which condition. Previous analyses have revealed that different anti-DIC drugs show different outcomes depending on the cause of infection, and rTM preparation is superior to AT replacement therapy for DIC caused by biliary tract infection.4 Although another report pointed out the usefulness of rTM for DIC due to acute severe cholangitis, the validation was limited to patients who underwent biliary drainage.5 In severe biliary infections, Tokyo Guidelines 2018 also call for addressing the elevated intra-biliary pressure;6 however, according to national statistics, the actual rate of biliary drainage in clinical practice is about 57%.7 This presents the current situation in Japan, where biliary drainage is not performed on nearly half of the patients, but the reasons for not performing it are not specified in this report. At this time, there are no reports on the efficacy of anti-DIC drugs in patients who have not undergone biliary drainage. Therefore, this is the first study that examines whether the use of biliary drainage for severe cholangitis makes a difference in the use of DIC medications.
Methods
The ethics committee of Hakodate Goryokaku Hospital approved this study protocol (Approval No. 2020–049). Informed consent was waived because of the retrospective study design, but the data remained confidential by ensuring the privacy of patients and complying with the Declaration of Helsinki.
Patient Selection
This was a retrospective cohort study of patients with sepsis-associated DIC who were admitted to Hakodate Goryokaku Hospital during the period of May 2008–March 2023. The race of all participants was Japanese. In total, 71 patients who suffered from sepsis-associated DIC caused by acute biliary infection and were treated by either AT replacement therapy or rTM preparation were assessed for inclusion. These patients were categorized into two groups: patients with drainage (n = 45) and without drainage (n = 26).
Definitions
Sepsis was diagnosed based on the Sepsis-3 criteria,8 and DIC was defined as a total score of ≥4 according to the Japanese Association for Acute Medicine (JAAM) DIC scoring system.9 DIC improvement was considered when the JAAM DIC score decreased within seven days after starting the anti-DIC drug administration. The Acute Physiology and Chronic Health Evaluation II (APACHE-II)10 and Sequential Organ Failure Assessment (SOFA)11 were evaluated when DIC treatment was planned. All patients were followed up for 60 days after the diagnosis of sepsis-associated DIC.
Treatment Management
Antibacterial drugs against each cause of infection were first administered to all participants. A biliary drain was applied for biliary obstruction as needed, and once a diagnosis of DIC was made, anticoagulant therapy was started using by AT replacement therapy or rTM preparations. For AT replacement therapy, the supplementation dose of AT was 1500 IU/day for three days; rTM was administrated at a dose of 380 U/kg, but this was reduced to 130 U/kg in patients with impaired renal function requiring hemodialysis. Polymyxin B hemoperfusion (PMX-HP) and intravenous immunoglobulin were administered at the discretion of the attending physician.
Statistical Analysis
Results are presented as median or mean ± standard deviation. Fisher’s exact test, Mann–Whitney U-test, and chi-square test were used to compare patient’s characteristics between the two groups. Antimicrobial use between groups was cross tabulated and statistically analyzed with Pearson’s chi-square test. Overall survival (OS) was defined as the time from DIC diagnosis to death within 60 days. Kaplan–Meier method was used to estimate survival rates based on the presence or absence of biliary drainage, and DIC therapies were compared using Log rank test. To assess the influence of factors of patient’s severity and treatment effects, a Cox proportional hazards model was conducted to determine the most significant variables contributing to the 60-day OS. Hazard ratios (HRs) and 95% confidence intervals (CIs) were determined for these variables. In all tests, P < 0.05 was considered statistically significant. Statistical analysis was performed using IBM SPSS Statistics for Macintosh, Version 21.0. (IBM, Armonk, NY, USA).
Results
Influence of Biliary Drainage Implementation on the 60-Day Overall Survival
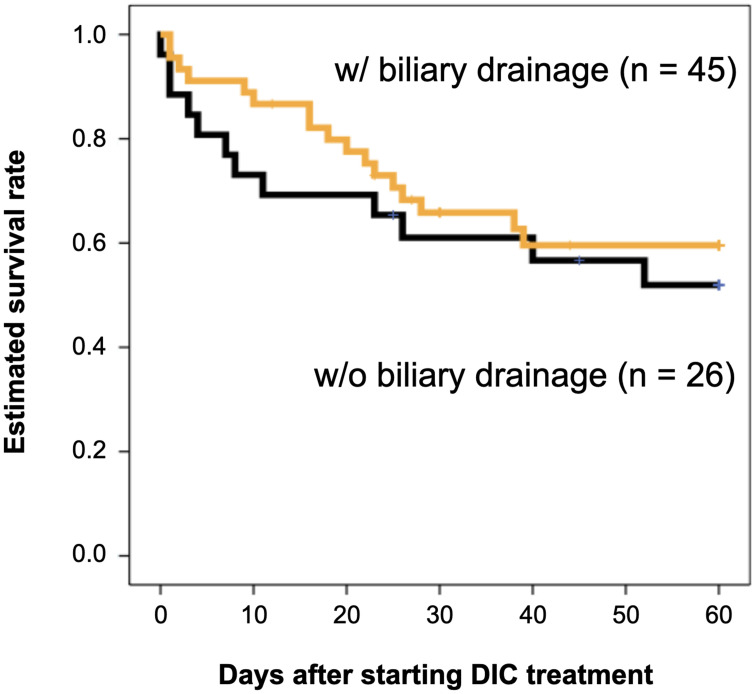
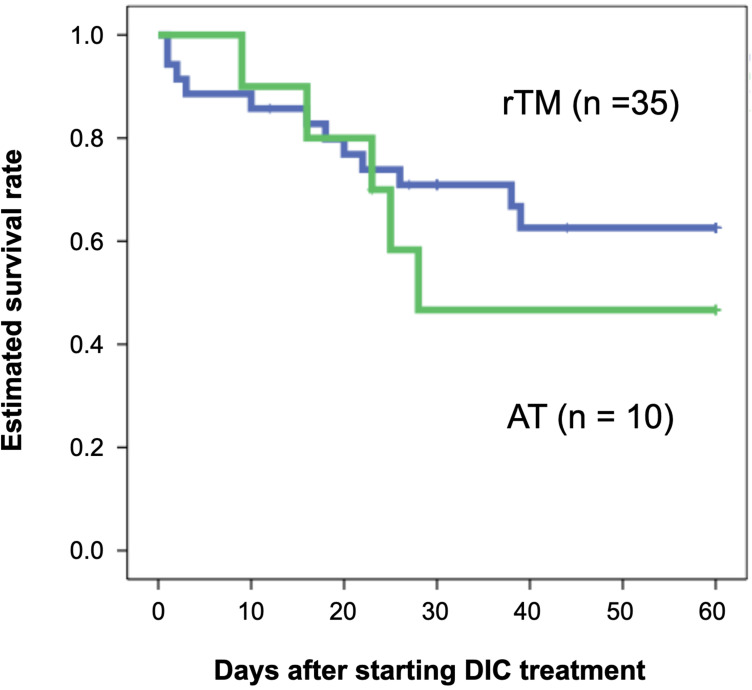
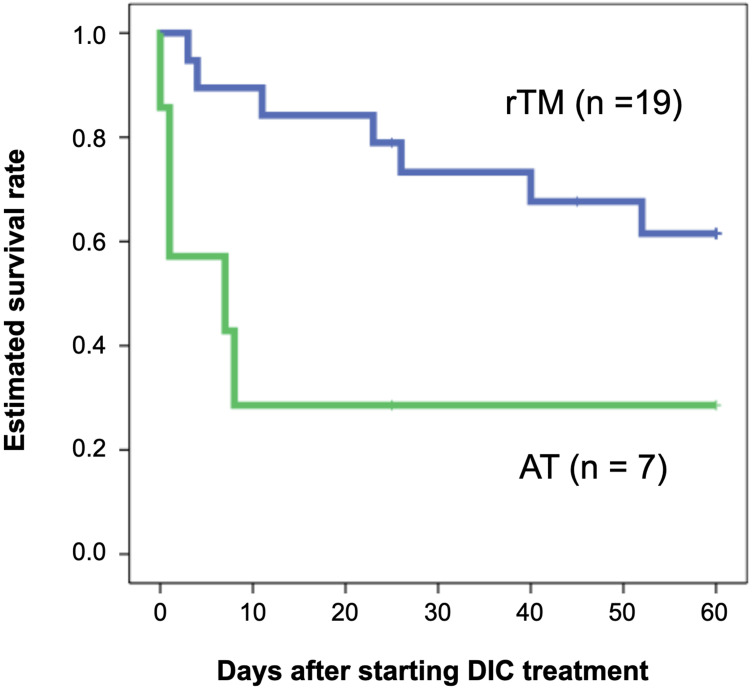
In this study, all patients had DIC and received DIC treatment for the first time. They were treated in an intensive care unit during the onset of DIC. Table 1 shows the patients’ characteristics categorized into two groups: patients with biliary drainage and without biliary drainage. There were no differences between their laboratory data, disease severity, and DIC improvement rate. There was also no difference between the two groups in terms of estimated survival rate from the start of DIC treatment to 60 days (P = 0.533) (Figure 1). Focusing on the effects of different therapeutic agents for DIC, in the biliary drainage group (Figure 2), no difference in the 60-day OS was found between patients treated with rTM or AT (P = 0.219). However, in patients without biliary drainage (Figure 3), the survival curves for patients treated with AT replacement were lower than those of patients with rTM preparation (P = 0.011).
Table 1.
Baseline Clinical Characteristics of Patient Categorized into Two Groups with and without Biliary Drainage
| Biliary Drainage | P value | ||
|---|---|---|---|
| Presence (n = 45) | Absence (n = 26) | ||
| Age, median (range) | 79 (50–96) | 76 (48–102) | 0.830 |
| Sex, male, n (%) | 26 (58) | 12 (46) | 0.344 |
| APACHE-II score, mean ± SD | 16 ± 5.4 | 15 ± 4.6 | 0.256 |
| SOFA score, mean ± SD | 7.7 ± 2.9 | 7.3 ± 3.3 | 0.607 |
| JAAM DIC score, mean ± SD | 6.0 ± 1.2 | 5.6 ± 1.2 | 0.756 |
| Albumin (g/dL), mean ± SD | 2.5 ± 0.7 | 2.4 ± 0.8 | 0.507 |
| Total bilirubin (mg/dL), mean ± SD | 3.8 ± 3.0 | 3.7 ± 4.2 | 0.882 |
| AST(U/L), mean ± SD | 197 ± 251 | 298 ± 620 | 0.337 |
| ALT(U/L), mean ± SD | 152 ± 179 | 181 ± 240 | 0.557 |
| γ-GTP(IU/L), mean ± SD | 260 ± 208 | 224 ± 231 | 0.502 |
| ALP(IU/mL), mean ± SD | 998 ± 803 | 1052 ± 1468 | 0.841 |
| CRP (mg/dl), mean ± SD | 22 ± 8.0 | 18 ± 10 | 0.069 |
| rTM preparation, n (%) | 35 (78) | 19 (73) | 0.655 |
| AT replacement therapy, n (%) | 10 (22) | 7 (27) | 0.655 |
| Intravenous immunoglobulin, n (%) | 12 (27) | 3 (12) | 0.132 |
| PMX-HP, n (%) | 3 (6.7) | 1 (3.8) | 0.619 |
| Mechanical ventilation, n (%) | 4 (8.9) | 3 (12) | 0.718 |
| Improvement of DIC, n (%) | 30 (67) | 19 (73) | 0.574 |
| 28-day mortality, n (%) | 16 (36) | 11 (42) | 0.487 |
| Antibiotics, n (%) | 0.312 | ||
| SBT/CPZ | 23 (51) | 10 (38) | |
| TAZ/PICC | 8 (18) | 5 (19) | |
| MEPM | 9 (20) | 3 (12) | |
| DRPM | 3 (7) | 5 (19) | |
| CZOP | 2 (4) | 3 (12) | |
Abbreviations: APACHE-II, Acute Physiology and Chronic Health Evaluation II; SOFA, Sequential Organ Failure Assessment; JAAM, Japanese Association for Acute Medicine; AST, aspartate aminotransferase; ALT, alanine aminotransferase; γ-GTP, γ-glutamyl transpeptidase; ALP, alkaline phosphatase; CRP, C-reactive protein; rTM, recombinant thrombomodulin; AT, antithrombin; PMX-HP, Polymyxin B hemoperfusion; DIC, disseminated intravascular coagulation; SBT/CPZ, Sulbactam/Cefoperazone; TAZ/PIPC, Tazobactam/Piperacillin; MEPM, Meropenem; DRPM, Doripenem; CZOP, Cefozopran.
Figure 1.
Estimated overall survival rates for patients categorized into two groups with and without biliary drainage.
Notes: An orange line and a black line show survival curve for patients treated with biliary drainage (n = 45) and without drainage (n = 26), respectively. Log rank test resulted that there was no significant difference between two groups (P = 0.533).
Figure 2.
Estimated overall survival rates for patients with biliary drainage.
Notes: A blue and a green line show survival curve for patients treated by rTM preparation (n = 35) and AT replacement therapy (n = 10), respectively. There was no statistically significant difference between rTM preparation and AT replacement therapy in the 60-day OS (P = 0.219).
Abbreviations: rTM, recombinant thrombomodulin; AT, antithrombin; OS, overall survival.
Figure 3.
Estimated overall survival rates for patients without biliary drainage.
Notes: A blue and a green line show survival curve for patients treated by rTM preparation (n = 19) and AT replacement therapy (n = 7), respectively. There was a statistically significant difference between rTM preparation and AT replacement therapy in the 60-day OS (P = 0.011).
Abbreviations: rTM, recombinant thrombomodulin; AT, antithrombin; OS, overall survival.
Influence of Anti-DIC Drug on the 60-Day Overall Survival
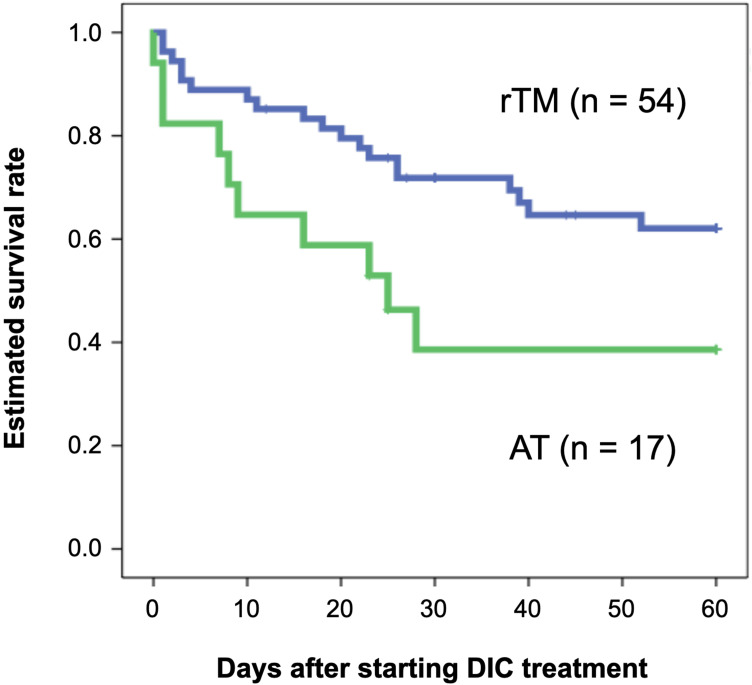
Table 2 shows the background data of the rTM preparation and AT replacement therapy group. JAAM DIC score, blood albumin concentration, and ventilation use rate were significantly different between patients treated by rTM and AT. Preparation of rTM also led to a significantly better OS than AT replacement therapy in patients with biliary tract infection (P = 0.022) (Figure 4). Based on multivariate analysis, the variables with significant effect on OS were aging, JAAM DIC score, APACHE-II score, SOFA score, biliary drainage implementation, and anti-DIC treatment type. With stepwise variable selection for Cox proportional hazards model, rTM administration was only selected as a significant factor for 60-day OS (HR 2.690, 95% CI 1.301–5.561, P = 0.008).
Table 2.
Clinical Characteristics of Patient’s Background Data of the rTM Preparation and at Replacement Therapy Group
| Anti-DIC Treatment | P value | ||
|---|---|---|---|
| rTM (n = 54) | AT (n = 17) | ||
| Age, median (range) | 79(48–102) | 77(60–91) | 0.441 |
| Sex, male, n (%) | 32(59) | 6(35) | 0.084 |
| APACHE-II score, mean ± SD | 15 ± 4.8 | 16 ± 6.4 | 0.835 |
| SOFA score, mean ± SD | 7.5 ± 2.8 | 8.0 ± 3.6 | 0.527 |
| JAAM DIC score, mean ± SD | 5.7 ± 1.2 | 6.6 ± 0.8 | 0.002 |
| Albumin (g/dL), mean ± SD | 2.6 ± 0.7 | 2.0 ± 0.6 | 0.003 |
| Total bilirubin (mg/dL), mean ± SD | 4.0 ± 3.7 | 3.1 ± 2.5 | 0.325 |
| AST(U/L), mean ± SD | 210 ± 256 | 313 ± 748 | 0.386 |
| ALT(U/L), mean ± SD | 173 ± 203 | 129 ± 200 | 0.437 |
| γ-GTP(IU/L), mean ± SD | 263 ± 217 | 196 ± 211 | 0.262 |
| ALP(IU/mL), mean ± SD | 919 ± 697 | 1330 ± 1844 | 0.174 |
| CRP (mg/dl), mean ± SD | 21 ± 9.5 | 21 ± 8.1 | 0.833 |
| Biliary drainage, n (%) | 35(65) | 10(59) | 0.720 |
| Intravenous immunoglobulin, n (%) | 13(24) | 2(12) | 0.278 |
| PMX-HP, n (%) | 2(3.7) | 2(12) | 0.209 |
| Mechanical ventilation, n (%) | 3(5.6) | 4(24) | 0.030 |
| Improvement of DIC, n (%) | 41(74) | 8(47) | 0.025 |
| 28-day mortality, n (%) | 15(28) | 12(71) | 0.002 |
| Antibiotics | 0.015 | ||
| SBT/CPZ | 20 (37) | 13 (76) | |
| TAZ/PICC | 13 (24) | 0 | |
| MEPM | 11 (20) | 1 (6) | |
| DRPM | 5 (9) | 3 (18) | |
| CZOP | 5 (9) | 0 | |
Abbreviations: APACHE-II, Acute Physiology and Chronic Health Evaluation II; SOFA, Sequential Organ Failure Assessment; JAAM, Japanese Association for Acute Medicine; AST, aspartate aminotransferase; ALT, alanine aminotransferase; γ-GTP, γ-glutamyl transpeptidase; ALP, alkaline phosphatase; CRP, C-reactive protein; PMX-HP, Polymyxin B hemoperfusion; DIC, disseminated intravascular coagulation; SBT/CPZ, Sulbactam/Cefoperazone; TAZ/PIPC, Tazobactam/Piperacillin; MEPM, Meropenem; DRPM, Doripenem; CZOP, Cefozopran.
Figure 4.
Estimated overall survival rates for patients with cholangitis-associated DIC treated by rTM preparation or AT replacement therapy.
Notes: A blue and a green line show survival curve for patients treated by rTM preparation (n = 54) and AT replacement therapy (n = 17), respectively. There was a statistically significant difference between rTM preparation and AT replacement therapy in the 60-day OS (P = 0.022).
Abbreviations: DIC, disseminated intravascular coagulation; rTM, recombinant thrombomodulin; AT, antithrombin; OS, overall survival.
Discussion
This study examined the differences in the impact of anti-DIC drugs depending on biliary drainage implementation for severe cholangitis. The results showed that when biliary drainage was performed, there was no difference in survival rates between rTM preparation and AT replacement therapy. In contrast, rTM preparation gave better results when biliary drainage was not performed.
In severe biliary infections, an increased intra-biliary pressure can easily induce septic shock, so biliary tract decompression by appropriate drainage is the principle of treatment.6 However, according to a report on the Japanese Nationwide Database in 2020,7 the proportion of cases in which any biliary drainage was not performed even in patients with severe acute cholangitis was about 43% (1243/2865). This indicates that there are many cases in which the effective treatment for elevated biliary pressure is not available in clinical practice. Our data also showed that 37% (26/71) of patients did not undergo drainage. In our series, a comparison of background factors in the two groups, divided by whether drainage was performed or not, showed no difference in patient severity, laboratory data, or percentage of treatment other than drainage. Although the 60-day OS after the onset of DIC was better in patients who underwent drainage, the difference was not statistically significant. The reasons why drainage was not performed at our hospital were: biliary stent was already placed and replacement was difficult (23%), the patient was in poor condition (15%), and the approach was difficult after biliary system surgery (15%). However, the importance of aggressive biliary drainage remains to avoid the risk of increased intra-biliary pressure leading to circulatory failure and exacerbation of sepsis.12 Recently, an ultrasound endoscopic (EUS)-based drainage method has been developed, providing an additional treatment option for patients who have difficulty using the conventional drainage method of the biliary tract. This method allows the drainage of the target bile duct via a trans-gastric or trans-duodenal approach, and it is expected to expand the range of indications.13
In 2016, a nationwide multicenter registry in Japan14 was analyzed and first reported that aggressive therapy of DIC is important to improve the patient outcomes and to treat the underlying disease. To manage DIC, AT replacement therapy and rTM preparations were suggested by J-SSCG 2020;3 however, this guideline does not provide any comment on selecting drugs based on individual causes of infection. A previous study on biliary and respiratory infections in 2022 showed a difference in survival rates with different DIC drugs, confirming the importance of appropriate drug selection.15 For DIC associated with severe cholangitis, Tokyo Guidelines 20186 stated that the value of anticoagulants was still unclear, but rTM administration might be considered. A multicenter study evaluating the clinical effectiveness of rTM in 284 patients who underwent biliary drainage5 concluded that the 28-day survival rate was significantly higher in the rTM group than that in the non-rTM group. However, there have been no previous reports examining the effect of anti-DIC drugs on the absence of biliary drainage.
As mentioned earlier, the most important thing in sepsis is to eliminate the cause of infection as soon as possible. However, the cause of infection in some conditions is not easy to treat. For example, in peritonitis due to intestinal necrosis, the cause of infection can be removed by bowel resection and drainage. In urinary tract infections caused by urinary tract obstruction, the placement of an indwelling ureteral catheter decreases the elevated internal pressure in the urinary tract system. For bloodstream infections caused by intravascular catheters, the source of infection can be removed by removing the indwelling catheter. Our previous studies4 showed no differences in efficacy between rTM preparation and AT replacement therapy for concomitant DIC in peritonitis, urinary tract infection, and catheter-related bloodstream infection, where the underlying causes of infection can be treated. These results suggest that in sepsis-associated DIC, where the causative disorder can be eliminated, differences in DIC treatments may not affect the outcome. In severe acute biliary inflammation, biliary decompression is fundamental to prevent the exacerbation of the disease, but there are many cases where drainage could not be performed. Based on these results, it is meaningful to focus on biliary tract infections and examine them in terms of the presence or absence of biliary drainage therapy. No previous studies examined the efficacy of DIC treatments with or without biliary drainage, and this is the first study that refers to the selectivity of drugs. Hence, an appropriate selection of anti-DIC drugs was found to be more important depending on whether or not an effective procedure was performed in sepsis-associated DIC.
Limitations
Several limitations must be considered in the present study. First, this was a single-center retrospective study with a long enrollment period for eligible patients. However, we could not find significant influence on treatment outcomes by time in multivariate analysis (HR 0.764, 95% CI 0.445–1.311, P = 0.329). Second, the choice of drug is left to the judgment of the attending physician, and there may be a bias between the groups to be compared. Third, the post-treatment OS of patients treated with AT replacement at our hospital was very low. When patient background data were compared with those of rTM-treated patients, there were clear differences in DIC scores, blood albumin concentration and selection of antimicrobial agents. This may be the reason for the inadequate efficacy of AT replacement therapy in patients with biliary tract infection. Lastly, the survival analysis in our study included five patients who died in one day after starting treatment for DIC. The mortality rate for septic patients at our hospital is rather higher than at other intensive care facilities in Japan. Therefore, additional multicenter studies with a larger sample size should be needed to confirm our results.
Conclusion
A prompt and aggressive approach to underlying diseases is crucial in patients with sepsis-associated DIC. If the causative pathology of a disease is difficult to eliminate, the choice of which anti-DIC drug to use will have important consequences. Although it needs further validation in a study with a large number of cases, we would recommend the use of rTM preparations for patients with cholangitis-associated DIC who cannot undergo biliary drainage.
Acknowledgments
The authors would like to thank Enago (Crimson Interactive Japan Co., Ltd, Japan) for the English language review.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Rivers E, Nguyen B, Havstad S., et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368–1377. doi: 10.1056/NEJMoa010307 [DOI] [PubMed] [Google Scholar]
- 2.Evans L, Rhodes A, Alhazzani W, et al. Surviving Sepsis Campaign: international Guidelines for Management of Sepsis and Septic Shock 2021. Crit Care Med. 2021;49(11):e1063–e1143. doi: 10.1097/CCM.0000000000005337 [DOI] [PubMed] [Google Scholar]
- 3.Egi M, Ogura H, Yatabe T, et al. The Japanese clinical practice guidelines for management of sepsis and septic shock 2020 (J-SSCG 2020). J Intensive Care. 2021;9(1):53. doi: 10.1186/s40560-021-00555-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kobayashi M, Asakura R, Ehama Y, et al. Impact of anticoagulant therapy on mortality for sepsis-associated disseminated intravascular coagulation depending on the source of infection. J Intensive Crit Care. 2021;7(3):62. doi: 10.36648/2471-8505.21.7.37 [DOI] [Google Scholar]
- 5.Ogura T, Eguchi T, Nakahara K, et al. Clinical impact of recombinant thrombomodulin administration on disseminated intravascular coagulation due to severe acute cholangitis (Recover-AC study). J Hepatobiliary Pancreat Sci. 2023;30(2):221–228. doi: 10.1002/jhbp.998 [DOI] [PubMed] [Google Scholar]
- 6.Miura F, Okamoto K, Takada T, et al. Tokyo Guidelines 2018: initial management of acute biliary infection and flowchart for acute cholangitis. J Hepatobiliary Pancreat Sci. 2018;25(1):31–40. doi: 10.1002/jhbp.509 [DOI] [PubMed] [Google Scholar]
- 7.Tarasawa K, Fujimori K, Fushimi K. Recombinant human soluble thrombomodulin contributes to a reduction in-hospital mortality of acute cholangitis with disseminated intravascular coagulation: a propensity score analyses of a Japanese nationwide database. Tohoku J Exp Med. 2020;252(1):53–61. doi: 10.1620/tjem.252.53 [DOI] [PubMed] [Google Scholar]
- 8.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gando S, Saitoh D, Ogura H, et al. Natural history of disseminated intravascular coagulation diagnosed based on the newly established diagnostic criteria for critically ill patients: results of a multicenter, prospective survey. Crit Care Med. 2008;36(1):145–150. [DOI] [PubMed] [Google Scholar]
- 10.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. doi: 10.1097/00003246-198510000-00009 [DOI] [PubMed] [Google Scholar]
- 11.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751 [DOI] [PubMed] [Google Scholar]
- 12.Kiriyama S, Takada T, Hwang TL, et al. Clinical application and verification of the TG13 diagnostic and severity grading criteria for acute cholangitis: an international multicenter observational study. J Hepatobiliary Pancreat Sci. 2017;24(6):329–337. doi: 10.1002/jhbp.458 [DOI] [PubMed] [Google Scholar]
- 13.van der Merwe SW, van Wanrooij RLJ, Bronswijk M, et al. Therapeutic endoscopic ultrasound: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2022;54(2):185–205. doi: 10.1055/a-1717-1391 [DOI] [PubMed] [Google Scholar]
- 14.Yamakawa K, Umemura Y, Hayakawa M, et al. Benefit profile of anticoagulant therapy in sepsis: a nationwide multicentre registry in Japan. Crit Care. 2016;20:229. doi: 10.1186/s13054-016-1415-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobayashi M, Ehama Y, Hirayama S. The Necessity of Individualized Treatment for Sepsis-Associated Disseminated Intravascular Coagulation by Infected Organ. Open Access Emerg Med. 2022;14:133–140. doi: 10.2147/OAEM.S359216 [DOI] [PMC free article] [PubMed] [Google Scholar]