Summary
Background
We aimed to evaluate and compare papillary muscle free strain in hypertrophic cardiomyopathy (HCMP) and hypertensive (HT) patients.
Methods
Global longitudinal strain (GLS), and longitudinal myocardial strain of the anterolateral (ALPM) and posteromedial papillary muscles (PMPM) were obtained in 46 HCMP and 50 HT patients.
Results
Interventricular septum (IVS)/posterior wall (PW) thickness ratio, left ventricular mass index (LVMI), left atrial anteroposterior diameter (LAAP) and mitral E/E′ were found to be increased in patients with HCMP compared to HT patients. Left ventricular cavity dimensions were smaller in HCMP patients. GLS of HCMP and HT patients were –14.52 ± 3.01 and –16.85 ± 1.36%, respectively (p < 0.001). Likewise, ALPM and PMPM free strain values were significantly reduced in HCMP patients over HT patients [–14.00% (–22 to –11%) and –15.5% (–24.02 to –10.16%) vs –23.00% (–24.99 to –19.01%) and –22.30% (–26.48 to –15.95%) (p = 0.016 and p = 0.010)], respectively. ALPM free strain showed a statistically significant correlation with GLS, maximal wall thickness, IVS thickness and LVMI. PMPM free strain showed a significant correlation with GLS, IVS thickness and LAAP. The GLS value of –13.05 had a sensitivity of 61.9% and a specificity of 97.4% for predicting HCMP. ALPM and PMPM free strain values of –15.31 and –17.17% had 63 and 76.9% sensitivity and 85.7 and 76.9% specificity for prediction of HCMP.
Conclusion
Besides other echocardiographic variables, which were investigated in earlier studies, papillary muscle free strain also could be used in HCMP to distinguish HCMP- from HT-associated hypertrophy.
Keywords: hypertrophic cardiomyopathy, hypertension, papillary muscle, strain
Hypertrophic cardiomyopathy (HCMP) is an autosomal dominantly inherited disease with a wide spectrum of clinical phenotypes. Its estimated prevalence has been reported to be one in 500 persons in the general population.1 With the advancements in understanding the molecular and genetic bases of the disease and technological improvements in cardiac imaging modalities, it seems that the prevalence of HCMP has been underestimated. In addition, prevalence estimates have not included genotypepositive and phenotype-negative patients who are clinically normal but at risk of developing the disease in the future.2
Diagnosis of HCMP has the utmost importance in terms of both management of the disorder and screening of patients’ family members. The histopathological features of the disease include myocyte disarray and interstitial fibrosis with heterogeneous involvement of the heart.3 The current guidelines recommend that if one of the myocardial segments has an end-diastolic wall thickness greater than 15 mm, then HCMP must be considered.4 Transthoracic echocardiography (TTE) is usually the first-line imaging modality used to assess patients with left ventricular hypertrophy (LVH). Cardiac anatomy and function can be delineated via two- (2D) or three-dimensional echocardiography, tissue Doppler imaging (TDI) and speckle-tracking echocardiography (STE). In addition to HCMP, LVH constitutes an adaptation response to exercise or hypertension. Clinicians frequently encounter it in clinical practice.
Differentiation of the aetiology of LVH may be challenging in some cases. It has been suggested that the pattern and degree of LVH help distinguish HCMP from HT hypertrophy. Hypertrophy associated with HCMP tends to be more severe than HT hypertrophy.5 Although concentric LVH has been thought to be associated with hypertension, asymmetric involvement of the left ventricle has also been reported.5 Myocardial strain analysis of the left ventricle has shown that increased endocardial to epicardial myocardial strain values can be used to distinguish HCMP from HT LVH.6
In most HCMP patients, the predominant phenotypic expression of the disease is characterised by LVH. However, in one study, the papillary muscles of four to 13% of the patients had morphological abnormalities, such as papillary muscle hypertrophy, anomalous insertion of papillary muscles, and hypermobile or bifid papillary muscles.7,8 It has been found that antero-apical displacement of the anterolateral papillary muscles (ALPM) was associated with a higher incidence of systolic anterior motion of the mitral valve. Moreover, increased papillary muscle thickness resulted in reduced distance between the ALPM and the interventricular septum (IVS) and smaller left ventricular cavity volume.8
Cardiac magnetic resonance imaging (MRI) studies have shown that the severity of symptoms, cardiac dysfunction and arrhythmias tend to increase in cases of co-existing papillary muscle abnormalities.9 Papillary muscle hypertrophy and fibrosis could also be seen in hypertension, which is proportional to LVH.10 Therefore, we aimed to evaluate papillary muscle function by measuring papillary muscle free strain in HCMP and HT patients to find any differences that might exist. In addition, we investigated the predictive value of papillary muscle free strain for the diagnosis of HT hypertrophy and HCMPassociated hypertrophy.
Methods
We enrolled 46 HCMP patients and 50 HT patients in this retrospective, comparative study. Echocardiographic recordings, and clinical and demographical characteristics of the patients were obtained from the hospital database system. Echocardiographic images of the patients were re-evaluated and the strain analysis was performed on stored images. Participants with systemic diseases, ischaemic heart disease, primary valvular disease, malignancy, thyroid abnormalities, hepatic and/or renal failure, poor image quality, Fabry disease, amyloidosis, or Noonan’s syndrome were excluded. Conventional echocardiography, TDI and 2D speckletracking imaging (2D-STI) of each patient were evaluated.
The local ethics committee approved the study, which was compiled in accordance with the Declaration of Helsinki. All patients’ written informed consents were acquired before study enrollment.
Diagnosis of HCMP was done according to the current guidelines, which state that HCMP diagnosis can be done in the presence of left ventricular maximum wall thickness greater than or equal to 15 mm without underlying secondary causes.4 Diagnosis of HT was done if a patient used antihypertensive medication or her/his blood pressure was greater than 140/90 mmHg. All echocardiographic examinations were performed by iE33 and Q-lab version 8.1 (CMQ, Philips Inc) according to current guidelines.11 Conventional cardiac structural and functional assessment was done by 2D echocardiography. IVS and posterior wall (PW) thickness, maximal wall thickness, left ventricular end-diastolic diameter (LVEDD), left ventricular end-systolic diameter (LVESD), left atrial anteroposterior diameter (LAAP), left ventricular mass index (LVMI), aortic annulus, and tricuspid annular systolic excursion (TAPSE) were measured. Left ventricular ejection fraction (LVEF) was measured using the modified Simpson method. Early (E) and late (A) mitral diastolic inflow velocities and deceleration time (DT) were obtained by pulsed-wave Doppler sample volume, which was placed at the tips of the mitral and tricuspid valves. Mitral annular septal velocities were taken by TDI.
Besides 2D echocardiographic parameters, 2D-STI was also used in order to measure longitudinal systolic strain from the apical four-, two- and three-chamber views with an increased frame rate of 50 to 70 frames per second. Three to four cardiac cycles from acceptable images were digitally stored for offline analysis in the Q-lab software package. Two basal and one apical anchor point was identified manually, after which the program traced the endocardial border automatically. In the case of inappropriate endocardial tracking, endocardial surfaces were manually corrected by the operator. Apical four-chamber (4C), three-chamber (3C) and two-chamber (2C) longitudinal strain analyses of each patient were performed. The 17-segment left ventricular model was used for calculation of segmental longitudinal strain values that were obtained from three views. Global longitudinal strain (GLS) was the average strain value measured by STI, with more negative values indicating higher contractility. Longitudinal myocardial strain of the ALPM and posteromedial papillary muscles (PMPM) were obtained with the free-strain method, which evaluates strain values within a myocardial region. This method enables us to measure myocardial deformation in a quick and practical way.
In order to calculate papillary muscle strain, the base and tip of the papillary muscle were selected manually. The first point was the base of the papillary muscle where it attaches to the left ventricular wall. The second point was the tip of the papillary muscle where it attaches to the chorda tendinea. ALPM and PMPM free strains were measured in the apical 4C and 3C views, respectively. Fig. 1 depicts the measurement of ALPM and PMPM free strain. Patients who did not have adequate echocardiographic images for assessment of systolic and diastolic papillary muscle free strain were excluded from the study.
Fig. 1.
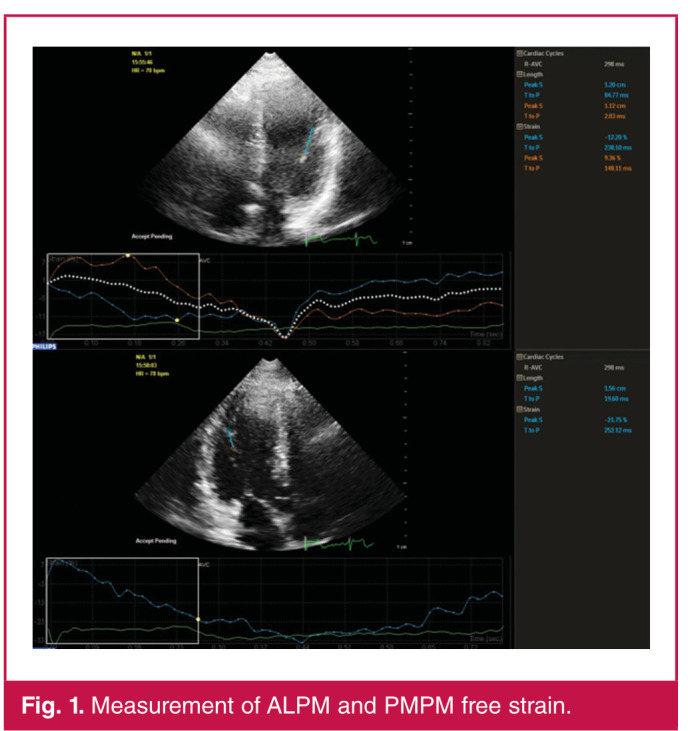
Measurement of ALPM and PMPM free strain.
All echocardiographic examinations were performed by the same cardiologist who was experienced in echocardiographic imaging. In order to evaluate intra-observer reliability, recordings of the 15 patients were re-evaluated one week later.
Statistical analysis
Normality of data was examined with the Kolmogorov–Smirnov test. Data with Gaussian and non-Gaussian distribution are expressed as mean ± standard deviation (SD) or median and interquartile range (IQR), respectively. Two-group comparisons were done with the independent sample’s t-test or Mann– Whitney U-test. Correlation of papillary muscle strain with other parameters was done with Spearman’s correlation analysis. Receiver operating characteristic (ROC) curve analysis was conducted in order to find ALPM and PMPM strain values for the prediction of HCMP. A two-tailed p-value < 0.05 was accepted as significant. An intraclass correlation coefficient model was used to examine intra-observer variability.
Results
The mean ages of HCMP and HT patients were 53.85 ± 15.46 and 56.60 ± 12.05 years, respectively. Of the 46 HCMP patients, 30 (65.2%) were male and 16 (34.8) were female. Of the 40 hypertensive patients, 33 (82.5%) were male and seven (17.5) were female. We did not find any differences with regard to age, gender ratio, body mass index and body surface area of the two groups.
Although the HT patients had slightly higher systolic and diastolic blood pressures, it did not reach statistical significance. Maximal wall thickness and IVS thickness were significantly higher in the HCMP patients compared to the HT patients. Similarly, IVS/PW ratio, LVMI and LAAP diameter were found to be more increased in patients with HCMP than in the HT patients. Left ventricular cavity dimensions were smaller in the HCMP patients: LVEDD were 45 (40–47.2) and 48 (46–50) mm, and LVESD were 24.97 ± 5.28 and 28.17 ± 3.58 mm in the HCMP and HT patients, respectively.
Mitral E/E′ was significantly higher in the HCMP patients compared to the HT patients (15.96 ± 7.51 vs 11.02 ± 2.79, respectively, p = 0.032). There were no significant differences in pulsed-wave Doppler recordings of the mitral and tricuspid valves, except for the higher value of the mitral E velocity that reached statistical significance in patients with HCMP (77.47 ± 20.33 and 64.48 ± 13.11 ms, respectively; p = 0.002).
Apical 4C, 2C, 3C longitudinal strain were significantly reduced in HCMP patients compared to the HT patients. GLS of HCMP and HT patients were –14.52 ± 3.01 and –16.85 ± 1.36%, respectively (p < 0.001). Likewise, ALPM and PMPM free strain values were significantly more reduced in the HCMP patients than in HT patients [–14.00 (–22 to –11%) and –15.5 (–24.02 to –10.16%) vs –23.00 (–24.99 to –19.01%) and –22.30 (–26.48 to –15.95%), p = 0.016 and p = 0.010], respectively. Table 1 shows a comparison of variables of the two groups and Fig. 2 depicts the GLS, and ALPM and PMPM free strain values of the two groups.
Fig. 2.
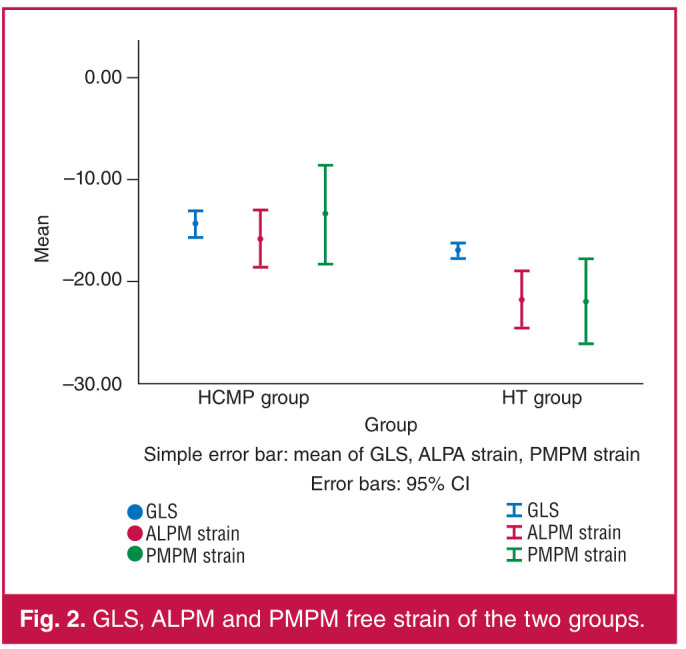
GLS, ALPM and PMPM free strain of the two groups.
Table 1. Clinical and echocardiographic findings of HCMP and HT patients.
| Variables | HCMP patients (n = 46) | HT patients (n = 50) | p-value |
| Age (years) | 53.85 + 15.46 | 56.60 + 12.05 | 0.365 |
| Gender, n (%) | 0.068 | ||
| Male | 30 (65.2) | 33 (82.5) | |
| Female | 16 (34.8) | 7 (17.5) | |
| Body mass index (kg/m²) | 27.27 + 4.77 | 27.38 + 2.77 | 0.892 |
| Body surface area (m ² | 1.85 + 0.32 | 1.88 + 0.30 | 0.704 |
| SBP (mmHg) | 127.21 + 23.37 | 131.05 + 12.75 | 0.334 |
| DBP (mmHg) | 79.18 + 13.01 | 84.65 + 10.53 | 0.083 |
| HR (bpm) | 76.42 + 13.77 | 73.88 + 10.59 | 0.506 |
| Maximal wall thickness (mm) | 25.02 + 5.84 | 15.33 + 3.21 | < 0.001 |
| IVS thickness (mm) | 2.35 (2.1-2.8) | 1.5 (1.4-1.7) | < 0.001 |
| PW thickness (mm) | 1.3 (1.1-1.4) | 1.3 (1.2-1.5) | 0.156 |
| IVS/PW | 1.85 + 0.50 | 1.18 + 0.24 | < 0.001 |
| LVMI (gr/m²) | 202.82 (162.41-249.99) | 161.26 (128.09-195.70) | <0.001 |
| LVEDD (mm) | 45 (40-47.2) | 48 (46-50) | <0.001 |
| LVESD (mm) | 24.97 + 5.28 | 28.17 + 3.58 | 0.002 |
| Aortic annulus (mm) | 24 (22-26) | 23 (22-24) | 0.137 |
| LAAP (mm) | 40.25 + 6.51 | 36.73 + 2.73 | 0.005 |
| TAPSE (mm) | 20 (18.05-26.35) | 16 (23-30) | 0.006 |
| Mitral E velocity (cm/s) | 77.47 + 20.33 | 64.48 + 13.11 | 0.002 |
| Mitral A velocity (cm/s) | 69.10 (55.90-93.00) | 64.00 (60.00-83.25) | 0.970 |
| E/A | 1.0 (0.74-1.42) | 0.84 (0.79-1.11) | 0.180 |
| DT (ms) | 197.88 + 72.19 | 232.33 + 50.69 | 0.113 |
| MPI | 0.52 + 0.12 | 0.46 + 0.06 | 0.121 |
| Tricuspid E velocity (cm/s) | 52.50 + 8.6 | 46.70 + 11.94 | 0.174 |
| Tricuspid A velocity (cm/s) | 49.01 + 10.83 | 42.73 + 5.90 | 0.186 |
| Tricuspid DT (ms) | 224.58 + 61.57 | 221.57 + 53.44 | 0.916 |
| Tricuspid E/A | 0.94 (0.85-1.31) | 1.08 (0.92-1.35) | 0.655 |
| Mitral S' velocity (cm/s) | 7.08 + 1.93 | 7.05 + 1.07 | 0.950 |
| Mitral E' velocity (cm/s) | 5.62 + 2.05 | 6.25 + 1.39 | 0.326 |
| Mitral A' velocity (cm/s) | 7.47 + 2.51 | 9.09 + 1.73 | 0.041 |
| Mitral E'/A' | 0.71 (0.50-1.00) | 0.69 (0.60-0.87) | 0.967 |
| Mitral E/E' | 15.96 + 7.51 | 11.02 + 2.79 | 0.032 |
| Apical 4C longitudi- nal strain (%) | -14.36 + 3.21 | -16.94 + 1.64 | < 0.001 |
| Apical 2C longitudi- nal strain (%) | -15.10 (-16.80 to -12.30) | -17.10 (-17.80 to -16.00) | 0.001 |
| Apical 3C longitudi- nal strain (%) | -14.33 + 3.77 | -16.30 + 1.49 | 0.003 |
| Global longitudinal strain (%) | -14.52 + 3.01 | -16.85 + 1.36 | 0.001 |
| ALPM strain (%) | -14.00 (-22 to -11) | -23.00 (-24.99 to -19.01) | 0.016 |
| AL peak strain | 0.33 + 0.13 | 0.44 + 0.11 | 0.010 |
| PMPM strain (%) | -15.5 (-24.02 to -10.16) | -22.30(-26.48to-15.95) | 0.010 |
| PM peak strain | 0.34 + 0.12 | 0.45 + 0.09 | 0.007 |
SBP: systolic blood pressure, DBP: diastolic blood pressure, HR: heart rate, IVS: interventricular septum, PW: posterior wall, LVMI: left ventricular mass index, LVEDD: left ventricular end-diastolic diameter, LVESD: left ventricular endsystolic
diameter, LAAP: left atrial anteroposterior diameter, TAPSE: tricuspid annular systolic excursion, DT: deceleration time, MPI: myocardial performance
index, ALPM: anterolateral papillary muscle, PMPM: posteromedial papillary muscle.
Five (10.86%) patients with HCMP underwent implantable cardioverter-defibrillator (ICD) implantation, 12 (26.08%) had a family history of HCMP, and eight (17.39%) had a family history of sudden death. In patients with HCMP, the type of the hypertrophy was asymmetrical septal hypertrophy in 34 (73.91%) patients, concentric in 11 (23.91%) patients, and apical in one (2.18%) patient. One patient had apical aneurysm formation. Ten (21.73%), 14 (30.43%) and five (10.86%) patients had minimal, mild and moderate mitral regurgitation, respectively. Twenty-two (47.82%) patients had left ventricular outflow obstruction with a mean gradient of 80.50 ± 30.62 mmHg.
ALPM free strain showed a statistically significant correlation with GLS (r = 0.604, p < 0.001), maximal wall thickness (r = 0.407, p = 0.032), IVS thickness (r = 0.425, p = 0.006) and LVMI (r = 0.465, p = 0.002) (Table 2). PMPM free strain showed a significant correlation with GLS (r = 0.459, p = 0.004), IVS thickness (r = 0.319, p = 0.045) and LAAP (r = 0.430, p = 0.018) (Table 3). Figs 3 and 4 show a correlation of ALPM and PMPM with IVS thickness, respectively.
Fig. 3.
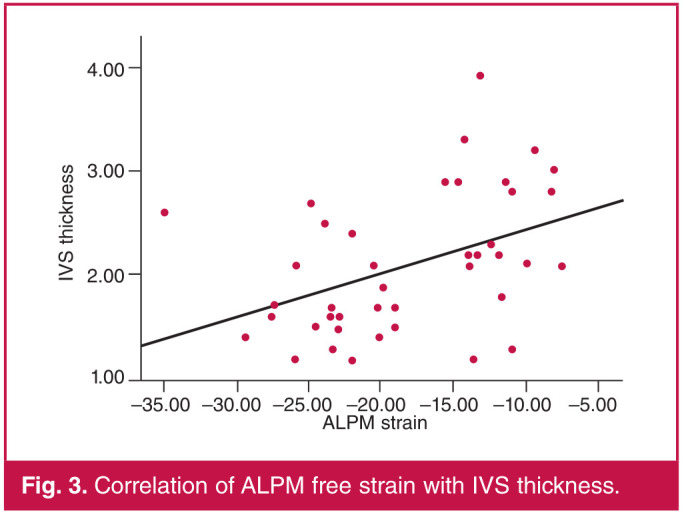
Correlation of ALPM free strain with IVS thickness.
Fig. 4.
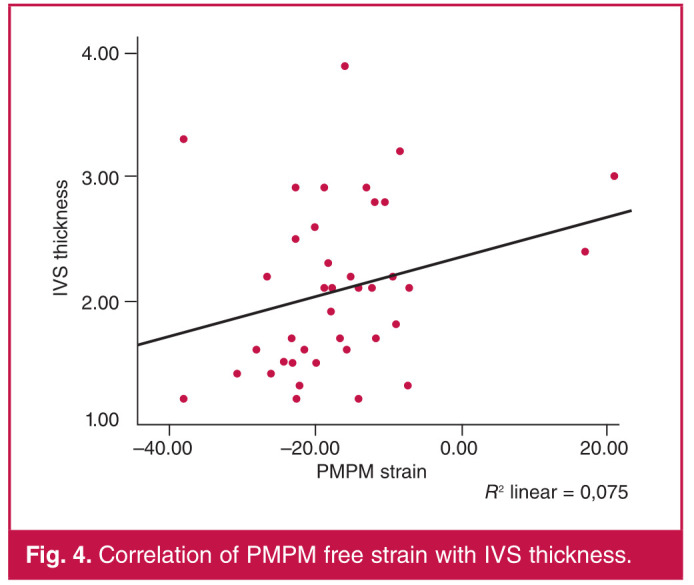
Correlation of PMPM free strain with IVS thickness.
Table 2. Correlation of ALPM with other parameters.
| Variables | r | p-value |
| Global longitudinal strain | 0.604 | < 0.001 |
| Maximal wall thickness | 0.407 | 0.032 |
| IVS thickness | 0.425 | 0.006 |
| LVMI | 0.465 | 0.002 |
| LVEDD | -0.135 | 0.399 |
| LVESD | 0.008 | 0.958 |
| LAAP | 0.277 | 0.131 |
| Mitral S' velocity | -0.172 | 0.317 |
IVS: interventricular septum, LVMI: left ventricular mass index, LVEDD: left ventricular end-diastolic diameter, LVESD: left ventricular end-systolic diameter, LAAP: left atrial anteroposterior diameter.
Table 3. Correlation of PMPM with other parameters.
| Variables | r | p-value |
| Global longitudinal strain | 0.459 | 0.004 |
| Maximal wall thickness | 0.071 | 0.725 |
| IVS thickness | 0.319 | 0.045 |
| LVMI | 0.135 | 0.405 |
| LVEDD | -0.048 | 0.767 |
| LVESD | 0.052 | 0.752 |
| LAAP | 0.430 | 0.018 |
| Mitral S' velocity | -0.057 | 0.743 |
IVS: interventricular septum, LVMI: left ventricular mass index, LVEDD: left ventricular end-diastolic diameter, LVESD: left ventricular end-systolic diameter, LAAP: left atrial anteroposterior diameter.
In order to determine the cut-off values of GLS, and ALPM and PMPM strain, ROC curve analysis was done. A GLS value of –13.05 had a sensitivity of 61.9% and specificity of 97.4% for predicting HCMP. ALPM and PMPM free strain values of –15.31 and –17.17% had 63 and 76.9% sensitivity and 85.7 and 76.9% specificity for prediction of HCMP. Table 4 and Fig. 5 show the ROC curve analysis for predicting HCMP.
Fig. 5.
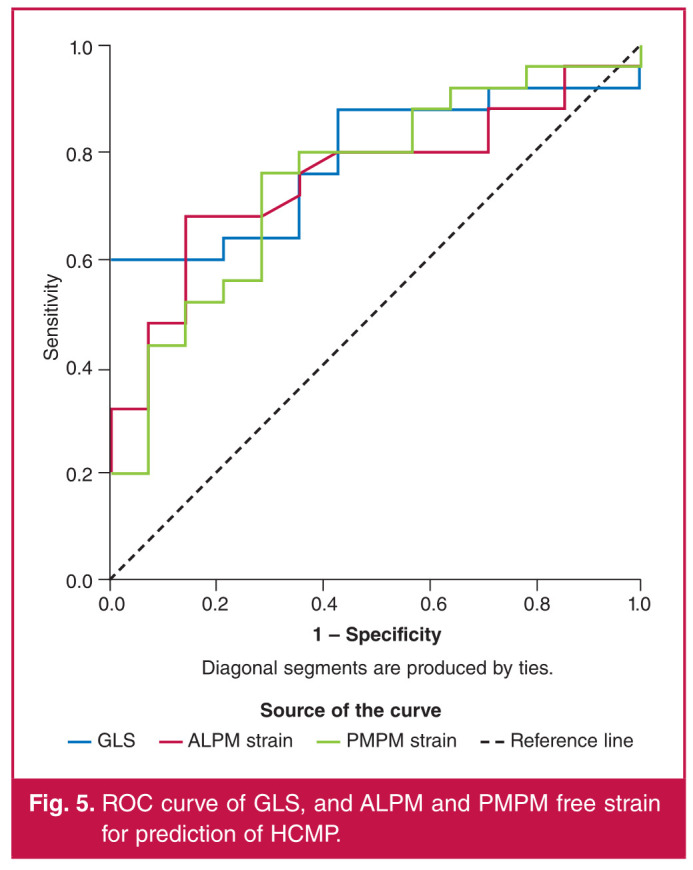
ROC curve of GLS, and ALPM and PMPM free strain for prediction of HCMP.
Table 4. ROC curve analysis of strain values for predicting HCMP.
| Strain | AUC | p- value | 95% CI | Value | Sensi- tivity | Specifi- city |
| GLS | 0.789 | 0.003 | 0.647-0.931 | -13.05 | 61.9 | 97.4 |
| ALPM strain | 0.751 | 0.010 | 0.598-0.905 | -15.31 | 63 | 85.7 |
| PMPM strain | 0.749 | 0.011 | 0.590-0.908 | -17.17 | 76.9 | 71.4 |
GLS, global longitudinal strain; ROC, receiver operating characteristic;
AUC, area under the curve; CI, confidence interval.
The intraclass correlation coefficient of two echocardiographic measurements was found to be 0.837 (95% confidence interval: 0.635–0.926), indicating a good agreement between the two measurements.
Discussion
Our study showed that, in addition to GLS, papillary muscle free strain was reduced in HCMP, implying functional abnormality in the papillary muscle. The papillary muscle free strain reduction was greater in the HCMP patients than in the HT patients and had a predictive value in distinguishing HCMP from HT. Other echocardiographic parameters that were helpful in differentiating HCMP from HT were IVS thickness, LVEDD, LVESD, LVMI, IVS/PW, LAAP diameter and mitral E/E′ ratio.
The differential diagnosis of HT hypertrophy from HCMP hypertrophy can be cumbersome. Histologically, myocyte hyperplasia and interstitial fibrosis are predominant findings in HT hypertrophy, whereas HCMP hypertrophy is linked with myocyte disarray and fibrosis that is unproportional to the hypertrophy.12,13 The amount of fibrosis, regardless of its cause, can be used for risk stratification of patients.14
Echocardiography is usually the first imaging method used for evaluating patients with LVH. Generally concentric hypertrophy is associated with hypertension but about 4–47% of patients with hypertension have asymmetrical hypertrophy, whereas 13–31% of patients with HCMP have concentric hypertrophy.15
Several studies have been conducted to differentiate HT hypertrophy from HCMP-associated hypertrophy. Kator et al. studied conventional echocardiographic and TDI strain parameters in HCMP and HT patients. Their study showed that septum-to-posterior wall thickness ratio and mean systolic strain were independently associated with HCMP, with a cut-off systolic strain value of –10.6%.15
Ozer et al. investigated the prognostic utility of left ventricular GLS in HCMP, HT and athlete’s heart. The HCMP patients had the worst left ventricular GLS values, and a cut-off value of –12.5% predicted mortality in HCMP patients with 64% sensitivity and 70% specificity.16 Other studies also confirmed the diagnostic value of strain measurements in differentiating pathological from physiological LVH.17-19
In a study by Yang et al., the degree of reduction in strain value was greater in the hypertrophied cardiac segments and correlated with the amount of histopathological abnormalities.20 Our findings with regard to GLS values were in concordance with previous data. The HCMP patients in our study had a greater GLS reduction than the HT patients.
In the last few decades, improvements in imaging techniques have shed light on papillary muscle anatomy and function in HCMP patients. It has been suggested that papillary muscle abnormalities are among the causal factors underlying the mechanism of left ventricular outflow obstruction, which was traditionally attributed to septal hypertrophy and the Venturi effect.21-25 Studies have shown that left ventricular obstruction and systolic anterior motion of the mitral valve are independent from interventricular septal hypertrophy, which persisted in almost 25% of the patients after septal myectomy.8 Cardiac MRI studies revealed papillary muscle hypertrophy that was in correlation with LVH, and abnormal insertion of papillary and accessory papillary muscles in HCMP.10
In one study, delayed gadolinium-enhanced areas were reported in up to 6% of the HCMP patients and increased papillary muscle mass was found to be associated with greater LVMI.26 In the present study, the papillary muscle free strain of both papillary muscles in HCMP patients was reduced compared to the HT patients. Our results confirm that pathological hypertrophy is associated with a more severe impairment of left ventricular function, including papillary muscles. In addition, both papillary muscle strains predicted the presence of HCMP over HT. Whether this impairment was due to fibrosis, perfusion abnormalities or myocyte dysfunction needs to be clarified.
Other findings of our study merit some comments. In addition to the 2D-STE strain parameters, the IVS thickness, LVEDD, LVESD, LVMI, IVS/PW, LAAP diameter and mitral E/E′ ratio were significantly different between the two groups. Previous reports have shown that left ventricular cavity dimensions are good predictors of pathological hypertrophy, characterised by unproportioned hypertrophy compared to left ventricular cavity size.27 We found smaller LVEDD and LVESD in patients with HCMP.
A high IVS/PW ratio, although not specific for HCMP, was suggested as a criterion for the diagnosis of HCMP.28 Similarly, IVS/PW ratio was found to be significantly higher in HCMP patients than in HT patients (1.85 ± 0.50 vs 1.18 ± 0.24, p < 0.001). In our study, mitral E/E′ ratio and LAAP diameter were higher in HCMP patients compared to HT patients. These findings support earlier reports that stated HCMP was associated with increased left atrial filling pressures.14 Limitations of the study are as follows. It was a single-centre study and the sample size was relatively small. Long-term follow up of patients was not done and the prognostic value of papillary free strain was not evaluated. Since coronary angiograms of the patients were not done, asymptomatic coronary artery disease was not excluded.
Conclusions
The novelty of our study lies in assessing the papillary muscle free strain in HCMP and HT patients and exploring whether it had a value in differentiation between LVH associated with HCMP and HT. Besides other echocardiographic variables, which have been investigated in earlier studies, papillary muscle free strain also could be used in HCMP patients to distinguish HCMP-associated from HT-associated hypertrophy. Further studies are needed to explore the prognostic use of papillary muscle free strain in this group of subjects.
Contributor Information
Cennet Yildiz, Email: cennet_yildiz@live.com.
Lutfi Ocal, Email: cindelen@gmail.com.
Mustafa Ozan Gursoy, Department of Cardiology, Izmir Ataturk Education and Research Hospital, Izmir, Turkey.
Gokhan Kahveci, Department of Cardiology, Istinye University, Liv Hospital, Istanbul, Turkey.
References
- 1.Semsarian C, Ingles J, Maron MS, Maron BJ. New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2015;65(12):1249–1254. doi: 10.1016/j.jacc.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 2.Maron BJ, Gardin J, Flack JM, Gidding SS, Kurosaki TT, Bild DE. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA study. Coronary artery risk development in (young) adults. Circulation. 1995;92(4):785–789. doi: 10.1161/01.cir.92.4.785. [DOI] [PubMed] [Google Scholar]
- 3.Diez-Lopez C, Salazar-Mendiguchia J. Clinical presentations of hypertrophic cardiomyopathy and implications for therapy. Glob Cardiol Sci Pract. 2018;2018(3):19. doi: 10.21542/gcsp.2018.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ommen SR, Mital S, Burke MA, Day SM, Deswal A, Elliott P. et al. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: executive summary: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. J Am Coll Cardiol. 2020;76(25):3022–3055. doi: 10.1016/j.jacc.2020.08.044. [DOI] [PubMed] [Google Scholar]
- 5.Cardim N, Galderisi M, Edvardsen T, Plein S, Popescu BA, D’Andrea A. et al. Role of multimodality cardiac imaging in the management of patients with hypertrophic cardiomyopathy: an expert consensus of the European Association of Cardiovascular Imaging endorsed by the Saudi Heart Association. Eur Heart J Cardiovasc Imaging. 2015;16(3):280. doi: 10.1093/ehjci/jeu291. [DOI] [PubMed] [Google Scholar]
- 6.Sun JP, Ni X, Xu T, Hu J, Yang XS, Wang S. et al. Differentiating hypertrophic cardiomyopathy from hypertensive left ventricular hypertrophy using quantitative layer-specific speckle-tracking echocardiography. Circulation. 2016;134:A11644. [Google Scholar]
- 7.Klues HG, Roberts WC, Maron BJ. Anomalous insertion of papillary muscle directly into anterior mitral leaflet in hypertrophic cardiomyopathy. Significance in producing left ventricular outflow obstruction. Circulation. 1991;84(3):1188–1197. doi: 10.1161/01.cir.84.3.1188. [DOI] [PubMed] [Google Scholar]
- 8.Kwon DH, Setser RM, Thamilarasan M, Popovic ZV, Smedira NG, Schoenhagen P. et al. Abnormal papillary muscle morphology is independently associated with increased left ventricular outflow tract obstruction in hypertrophic cardiomyopathy. Heart. 2008;94(10):1295–1301. doi: 10.1136/hrt.2007.118018. [DOI] [PubMed] [Google Scholar]
- 9.Teraoka K, Hirano M, Ookubo H, Sasaki K, Katsuyama H, Amino M. et al. Delayed contrast enhancement of MRI in hypertrophic cardiomyopathy. Magn Reson Imaging. 2004;22:155–161. doi: 10.1016/j.mri.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Rajiah P, Fulton NL, Bolen M. Magnetic resonance imaging of the papillary muscles of the left ventricle: normal anatomy, variants, and abnormalities. Insights Imaging. 2019;10(1):83. doi: 10.1186/s13244-019-0761-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L. et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Coelho-Filho OR, Shah RV, Mitchell R, Neilan TG, Moreno Jr H, Simonson B. et al. Quantification of cardiomyocyte hypertrophy by cardiac magnetic resonance: Implications for early cardiac remodeling. Circulation. 2013;128(11):1225–1233. doi: 10.1161/CIRCULATIONAHA.112.000438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanaka M, Fujiwara H, Onodera T, Wu DJ, Hamashima Y, Kawai C. Quantitative analysis of myocardial fibrosis in normals, hypertensive hearts, and hypertrophic cardiomyopathy. Heart. 1986;55(6):575–581. doi: 10.1136/hrt.55.6.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rudolph A, Abdel-Aty H, Bohl S, Boye P, Zagrosek A, Dietz R. et al. Noninvasive detection of fibrosis applying contrast-enhanced cardiac magnetic resonance in different forms of left ventricular hypertrophy. Relation to remodeling . J Am Coll Cardiol. 2009;53(3):284–291. doi: 10.1016/j.jacc.2008.08.064. [DOI] [PubMed] [Google Scholar]
- 15.Kator TS, Noda A, Izawa H, Yamada A, Obata K, Nagata K. et al. Discrimination of nonobstructive hypertrophic cardiomyopathy from hypertensive left ventricular hypertrophy on the basis of strain rate imaging by tissue Doppler ultrasonography. Circulation. 2004;110(25):3808–3814. doi: 10.1161/01.CIR.0000150334.69355.00. [DOI] [PubMed] [Google Scholar]
- 16.Ozer PK, Govdeli EA, Engin B, Atıcı A, Baykız D, Orta H. et al. Role of global longitudinal strain in discriminating variant forms of left ventricular hypertrophy and predicting mortality. Anatol J Cardiol. 2021;25(12):863–871. doi: 10.5152/AnatolJCardiol.2021.21940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Butz T, van Buuren F, Mellwig KP, Langer C, Plehn G, Meissner A. et al. Two-dimensional strain analysis of the global and regional myocardial function for the differentiation of pathologic and physiologic left ventricular hypertrophy: a study in athletes and in patients with hypertrophic cardiomyopathy. Int J Cardiovasc Imaging. 2011;27:91–100. doi: 10.1007/s10554-010-9665-5. [DOI] [PubMed] [Google Scholar]
- 18.Ishizu T, Seo Y, Kameda Y, Kawamura R, Kimura T, Shimojo N. et al. Left ventricular strain and transmural distribution of structural remodeling in hypertensive heart disease. Hypertension. 2014;63:500–506. doi: 10.1161/HYPERTENSIONAHA.113.02149. [DOI] [PubMed] [Google Scholar]
- 19.Afonso L, Kondur A, Simegn M, Niraj A, Hari P, Kaur R. et al. Two-dimensional strain profiles in patients with physiological and pathological hypertrophy and preserved left ventricular systolic function: a comparative analyses. Br Med J Open. 2012;2:e001390. doi: 10.1136/bmjopen-2012-001390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang H, Sun JP, Lever HM, Popovic ZB, Drinko JK, Greenberg NL. et al. Use of strain imaging in detecting segmental dysfunction in patients with hypertrophic cardiomyopathy. J Am Soc Echocardiogr. 2003;16:233–239. doi: 10.1067/mje.2003.60. [DOI] [PubMed] [Google Scholar]
- 21.Henry WL, Clark CE, Epstein SE. Asymmetrical septal hypertrophy (ASH): the unifying link in the IHSS spectrum. Observations regarding its pathogenesis, pathophysiology, and course. Circulation. 1973;47:827–832. doi: 10.1161/01.cir.47.4.827. [DOI] [PubMed] [Google Scholar]
- 22.Spirito P, Maron BJ, Rosing DR. Morphologic determinants of hemodynamic state after ventricular septal myotomy–myectomy in patients with obstructive hypertrophic cardiomyopathy: M mode and two-dimensional echocardiographic assessment. Circulation. 1984;70:984–995. doi: 10.1161/01.cir.70.6.984. [DOI] [PubMed] [Google Scholar]
- 23.Henry WL, Clark CE, Roberts WC, Morrow AG, Epstein SE. Differences in distribution of myocardial abnormalities in patients with obstructive and nonobstructive asymmetric septal hypertrophy (ASH). Echocardiographic and gross anatomic findings. Circulation. 1974;50:447–455. doi: 10.1161/01.cir.50.3.447. [DOI] [PubMed] [Google Scholar]
- 24.Yock PG, Hatle L, Popp RL. Patterns and timing of Doppler-detected intracavitary and aortic flow in hypertrophic cardiomyopathy. J Am Coll Cardiol. 1986;8:1047–1058. doi: 10.1016/s0735-1097(86)80381-3. [DOI] [PubMed] [Google Scholar]
- 25.Sasson Z, Yock PG, Hatle LK, Alderman EL, Popp RL. Doppler echocardiographic determination of the pressure gradient in hypertrophic cardiomyopathy. J Am Coll Cardiol. 1988;11:752–756. doi: 10.1016/0735-1097(88)90207-0. [DOI] [PubMed] [Google Scholar]
- 26.Harrigan CJ, Appelbaum E, Maron BJ, Buros JL, Gibson CM, Lesser JR. et al. Significance of papillary muscle abnormalities identified by cardiovascular magnetic resonance in hypertrophic cardiomyopathy. Am J Cardiol. 2008;101(5):668–673. doi: 10.1016/j.amjcard.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 27.Caselli S, Maron MS, Urbano-Moral JA, Pandian NG, Maron BJ, Pelliccia A. Differentiating left ventricular hypertrophy in athletes from that in patients with hypertrophic cardiomyopathy. Am J Cardiol. 2014;114:1383–1389. doi: 10.1016/j.amjcard.2014.07.070. [DOI] [PubMed] [Google Scholar]
- 28.Mandeş L, Roşca M, Ciuperca D, Popescu BA. The role of echocardiography for diagnosis and prognostic stratification in hypertrophic cardiomyopathy. J Echocardiogr. 2020;18(3):137–148. doi: 10.1007/s12574-020-00467-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


