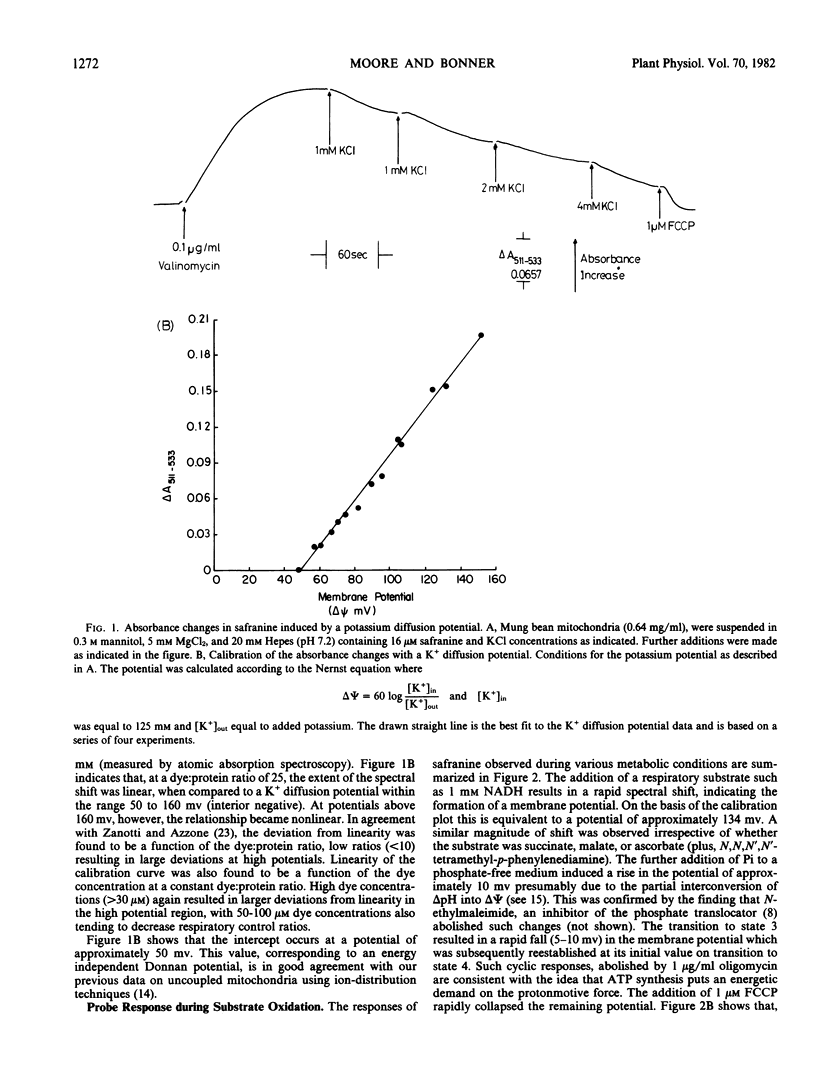
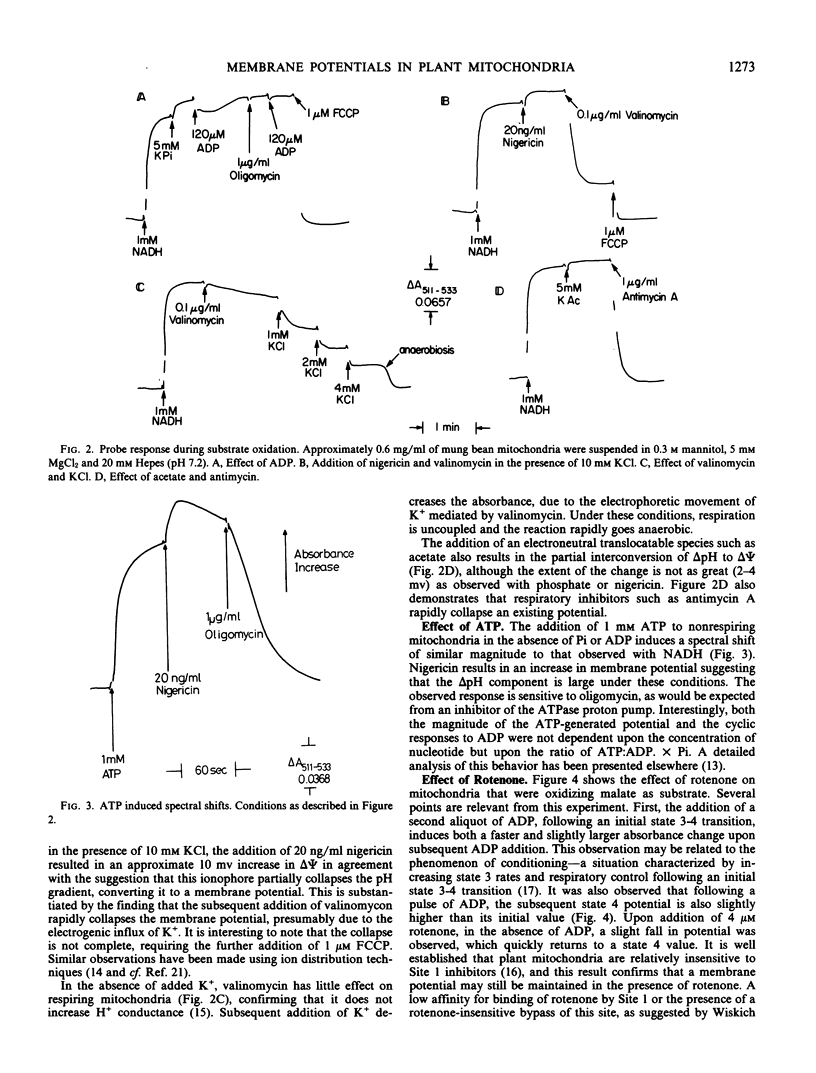
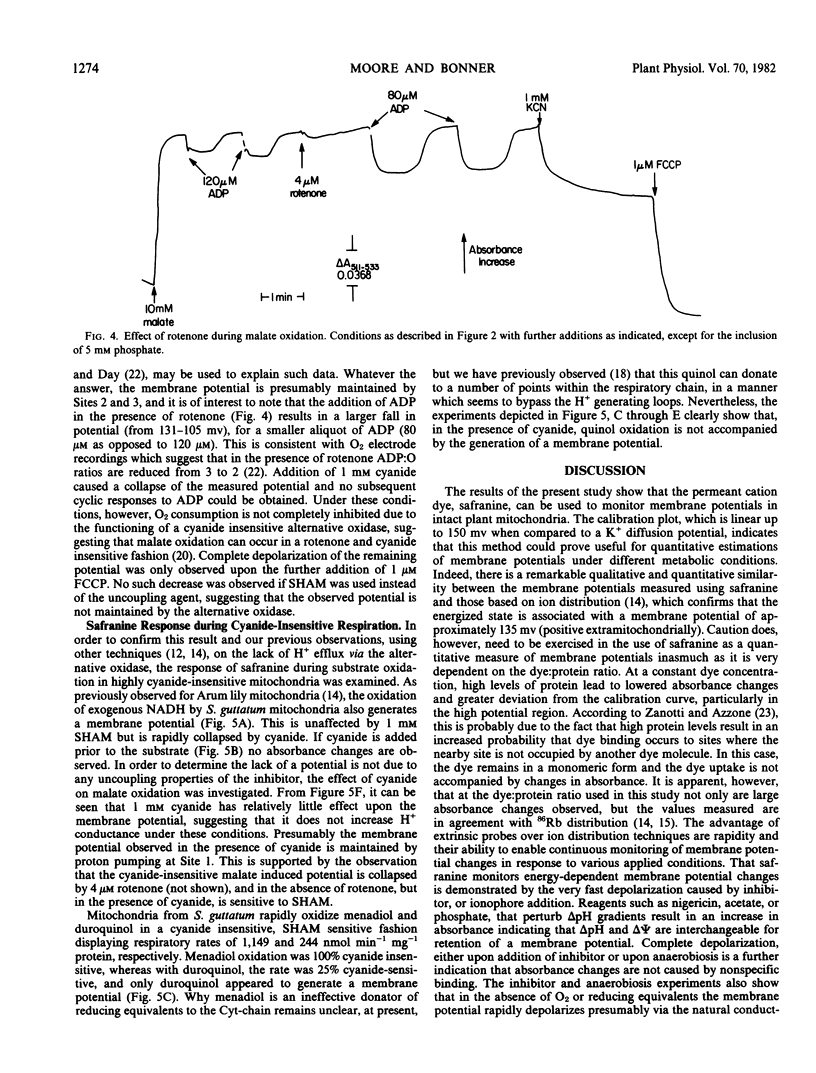
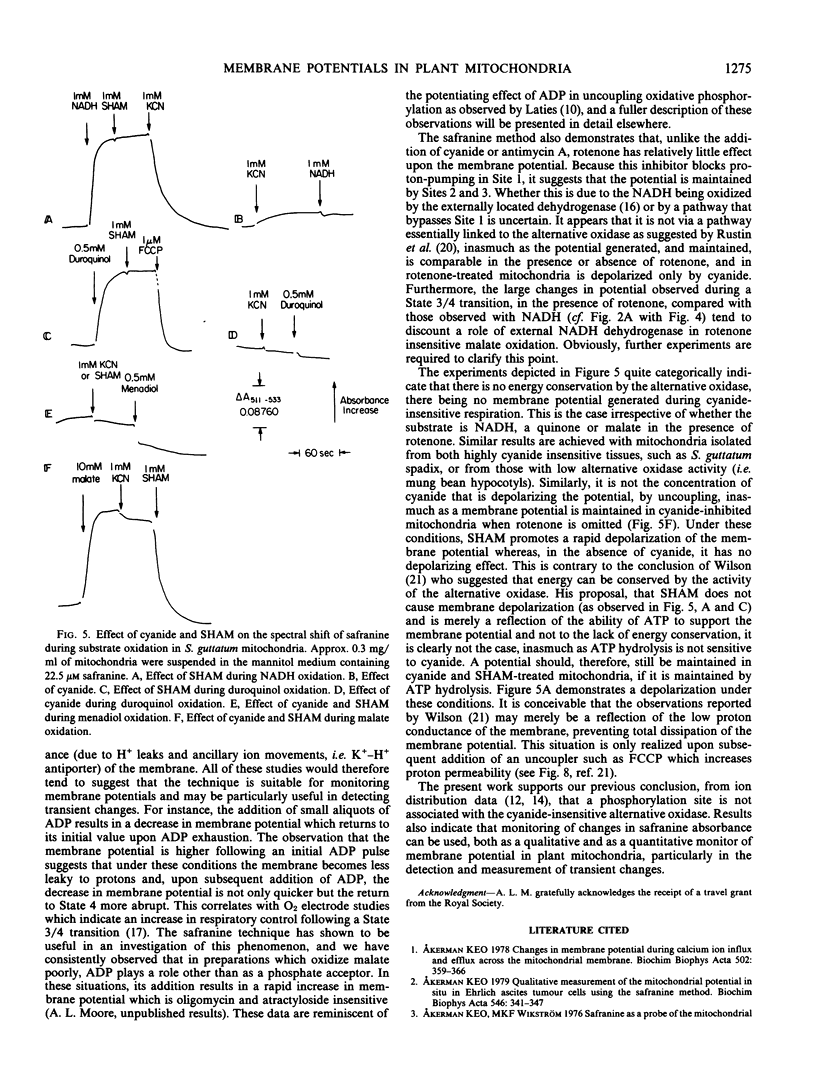
Abstract
The positively charged dye, safranine, has been used as an indicator of membrane potentials in mung bean (Phaseolus aureus) and Voodoo lily (Sauromatum guttatum) mitochondria under a variety of metabolic conditions. The spectral response of safranine has been calibrated with respect to a K+ diffusion potential and was found to be linearly related to the developed potential within the range of 50 to 160 millivolts. Both respiration and ATP hydrolysis gave rise to a membrane potential of approximately 135 millivolts. Respiratory inhibitors such as cyanide and antimycin depolarized the potential, whereas rotenone has little effect. No potentials were developed during NADH supported cyanide insensitive respiration. It is concluded that safranine may be a useful spectrophotometric probe of the mitochondrial membrane potential.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerman K. E. Changes in membrane potential during calcium ion influx and efflux across the mitochondrial membrane. Biochim Biophys Acta. 1978 May 10;502(2):359–366. doi: 10.1016/0005-2728(78)90056-7. [DOI] [PubMed] [Google Scholar]
- Akerman K. E. Qualitative measurements of the mitochondrial membrane potential in situ in Ehrlich ascites tumour cells using the safranine method. Biochim Biophys Acta. 1979 May 9;546(2):341–347. doi: 10.1016/0005-2728(79)90051-3. [DOI] [PubMed] [Google Scholar]
- Bashford C. L., Smith J. C. The use of optical probes to monitor membrane potential. Methods Enzymol. 1979;55:569–586. doi: 10.1016/0076-6879(79)55067-8. [DOI] [PubMed] [Google Scholar]
- Colnna R., Massari S., Azzone G. F. The problem of cation-binding sites in the energized membrane of intact mitochondria. Eur J Biochem. 1973 May 2;34(3):577–585. doi: 10.1111/j.1432-1033.1973.tb02798.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laties G. G. The potentiating effect of adenosine diphosphate in the uncoupling of oxidative phosphorylation in potato mitochondria. Biochemistry. 1973 Aug 14;12(17):3350–3355. doi: 10.1021/bi00741a032. [DOI] [PubMed] [Google Scholar]
- Moore A. L., Bonner W. D., Jr, Rich P. R. The determination of the proton-motive force during cyanide-insensitive respiration in plant mitochondria. Arch Biochem Biophys. 1978 Mar;186(2):298–306. doi: 10.1016/0003-9861(78)90439-3. [DOI] [PubMed] [Google Scholar]
- Raison J. K., Lyons J. M., Campbell L. C. Inhibition of the state 3 respiration of isolated mitochondria and its implications in comparative studies. J Bioenerg. 1973;4(4):397–408. doi: 10.1007/BF01648967. [DOI] [PubMed] [Google Scholar]
- Rustin P., Moreau F., Lance C. Malate Oxidation in Plant Mitochondria via Malic Enzyme and the Cyanide-insensitive Electron Transport Pathway. Plant Physiol. 1980 Sep;66(3):457–462. doi: 10.1104/pp.66.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater E. C. Mechanism of oxidative phosphorylation. Annu Rev Biochem. 1977;46:1015–1026. doi: 10.1146/annurev.bi.46.070177.005055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S. B. Energy conservation by the plant mitochondrial cyanide-insensitive oxidase. Some additional evidence. Biochem J. 1980 Aug 15;190(2):349–360. doi: 10.1042/bj1900349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanotti A., Azzone G. F. Safranine as membrane potential probe in rat liver mitochondria. Arch Biochem Biophys. 1980 Apr 15;201(1):255–265. doi: 10.1016/0003-9861(80)90510-x. [DOI] [PubMed] [Google Scholar]
- de la Roche A. I. Increase in linolenic Acid is not a prerequisite for development of freezing tolerance in wheat. Plant Physiol. 1979 Jan;63(1):5–8. doi: 10.1104/pp.63.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]


