Abstract
Objectives:
To compare the onset and trajectory of multimorbidity between individuals with and without rheumatoid arthritis (RA).
Methods:
A matched, retrospective cohort study was completed in a large, U.S., commercial insurance database (MarketScan®) from 2006-2015. Using validated algorithms, RA patients (overall and incident) were age- and sex-matched to non-RA patients. Diagnostic codes for 44 pre-identified chronic conditions were selected to determine the presence (≥2 conditions) and burden (count) of multimorbidity. Cross-sectional comparisons were completed using the overall RA cohort and conditional logistic and negative binomial regression models. Trajectories of multimorbidity were assessed within the incident RA sub-cohort using generalized estimating equations.
Results:
The overall cohort (n=277,782) and incident sub-cohort (n=61,124) were female predominant (76.5%, 74.1%) with a mean age of 55.6 and 54.5 years. The cross-sectional prevalence (odds ratio [OR] 2.29, 95% CI 2.25-2.34) and burden (ratio of conditions 1.68, 95% CI 1.66-1.70) of multimorbidity were significantly higher in RA than non-RA in the overall cohort. Within the incident RA cohort, RA patients had more chronic conditions than non-RA (β 1.13, 95% CI 1.10-1.17), and the rate of accruing chronic conditions was significantly higher in RA compared to non-RA (RA x follow-up year, β 0.21, 95%CI 0.20-0.21, P<0.001). Results were similar when including the pre-RA period and in several sensitivity analyses.
Conclusions:
Multimorbidity is highly prevalent in RA and progresses more rapidly in RA than in non-RA patients during and immediately following RA onset. Therefore, multimorbidity should be aggressively identified and targeted early in the RA disease course.
Keywords: rheumatoid arthritis, multimorbidity, comorbidity, chronic disease
Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune disease that predisposes to physical impairment and premature mortality(1-3). While extra-articular involvement is well recognized to complicate the RA disease course(4), links between RA and other chronic diseases, including osteoporosis(5), cardiovascular disease(6), malignancy(7), and mental health disorders(8), have also been identified. Additionally, therapies used to treat RA may have unintended consequences that predispose to the development of chronic diseases. For example, glucocorticoid use is associated with numerous adverse effects including bone loss, elevated blood glucose and blood pressure, and the development of cataracts and glaucoma, among other potential toxicities(9).
The study of chronic conditions occurring in individuals with RA has primarily focused on select conditions co-occurring with RA, under the framework and terminology of “comorbidity”. Multimorbidity, which considers the burden of conditions to the patient rather than to an index condition, has become the preferred terminology to describe the co-occurrence of multiple chronic conditions in the general population and rheumatic diseases(10). Multimorbidity has been the subject of only limited investigation in RA to date. Initial studies in RA have shown lower rates of biologic disease-modifying anti-rheumatic drug (bDMARD) use and poorer response to treatment among those with multimorbidity(11-13), as well as multimorbidity contributing to excess mortality occurring in RA(14).
The world’s population is aging, with the World Health Organization estimating the number of individuals aged 65 or older to increase from 524 million in 2010 to 1.5 billion in 2050(15). As a result of this increased longevity and a rising frequency of chronic disease risk factors, chronic disease prevalence is projected to rise steadily with over 170 million individuals estimated to have ≥1 chronic condition by 2030 in the U.S. alone(16). Accompanying the growing prevalence of chronic disease is the development of multiple chronic conditions, i.e. multimorbidity. In 2014, over 40% of U.S. adults had multiple chronic conditions(17). The consequences of multimorbidity include death and disability, reduced quality of life, and increased healthcare utilization and costs(18). Thus, multimorbidity is a critically important public health concern that needs to be aggressively targeted. This is especially true in RA, a disease perhaps of accelerated aging(19, 20) that portends poor long-term outcomes(1-3) and carries an enormous economic impact(21).
Targeting multimorbidity with interventions requires understanding its onset and rate of progression.The purpose of this study was to compare the burden and trajectory of multimorbidity between individuals with and without RA. We hypothesized that the burden of multimorbidity and rate of accruing chronic conditions would be greater in RA.
Methods
Study design and patient selection
We performed a matched, retrospective cohort study within the Truven MarketScan® commercial claims and encounters database from January 1, 2006 to September 30, 2015. MarketScan® is a US-wide database of commercially insured individuals with medical and pharmacy claims data that has been used extensively for rheumatic disease research(22-25). This study was reviewed by the institutional review boards at the University of Nebraska Medical Center and University of Alabama at Birmingham. Patients and the public were not involved in this study.
We constructed two RA cohorts (all RA and incident RA) that were matched (1:1) to non-RA patients from January 1, 2006 to December 31, 2014. We required patients to have 12 months of continuous enrollment during our study window to be eligible for analyses. We used validated RA algorithms that required ≥2 RA diagnostic codes (International Classification of Diseases 9th edition, clinical modification [ICD-9-CM]: 714.0, 714.1, 714.2, and 714.8) between 30-365 days apart, including ≥1 diagnostic code from a rheumatology provider, and a DMARD prescription. Similar algorithms have a positive predictive value (PPV) for RA >90%(26). Within this overall RA cohort, we identified a sub-cohort of incident RA patients using an administrative algorithm requiring ≥12 months of continuous enrollment without RA diagnostic codes or DMARD prescription (PPV of 70-80%)(27). The date patients fulfilled the algorithm was considered RA index date. We then selected patients without diagnostic codes for RA and matched them 1:1 with RA patients on sex, year of birth, and year entering the database during our study window. We required controls to be enrolled on the index date of the accompanying RA patient and assigned the same index date. Patients were followed until disenrollment, death, or end of the study observation period (September 30, 2015 due to transition to ICD-10).
Chronic conditions and multimorbidity
In addition to using established comorbidity indices (see below), we manually assembled a list of 44 chronic conditions based on their prevalence and importance in the general population and RA, informed by prior studies including systematic reviews of multimorbidity(4, 28-30). Diagnostic codes for these conditions were adapted from enhanced definitions for established comorbidity indices and the Healthcare Cost and Utilization Project Clinical Classification Software codes (https://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp) (provided in Table S1). We queried these conditions from January 1, 2006 to September 30, 2015, a period utilizing only ICD-9-CM codes, within inpatient and outpatient encounters. To minimize misclassification of these conditions (e.g. unconfirmed or rule-out diagnoses), we required ≥2 diagnoses for these chronic conditions to be considered present, with the date of the second diagnostic code considered the date of onset. Once a condition occurred, we considered the condition prevalent throughout the remainder of follow-up.
We defined multimorbidity as the presence of ≥2 conditions from the aforementioned list. We did not include RA as one of the conditions, as this would inherently bias our results towards greater multimorbidity in RA. We also used a more stringent definition of multimorbidity, requiring the presence of ≥3 conditions from the list. The total count of chronic conditions present (possible range of 0-43, as two conditions were sex-specific) was considered to represent the burden of multimorbidity. To ensure results were not dependent upon these definitions of multimorbidity, we also utilized established comorbidity indices. This included the Charlson-Deyo comorbidity index(31), which has been extensively used in health services research, and the Rheumatic Disease Comorbidity Index(32), which has specifically been validated in individuals with rheumatic diseases.
Statistical analyses
We compared the cross-sectional prevalence of multimorbidity and individual chronic conditions between RA and non-RA in the overall cohort using conditional logistic regression models, conditioning on the matched pair. Comparisons of multimorbidity burden were completed using conditional negative binomial regression. In primary analyses, these comparisons were completed at the index date, while in secondary analyses we performed these comparisons at 1 year of follow-up to ensure all RA patients had prevalent, rather than incident, disease.
The trajectory of multimorbidity burden in RA vs non-RA in the incident sub-cohort was assessed using generalized estimating equations with an interaction term between RA status and year of follow-up (to assess differences in the rate of accruing chronic conditions over time) and an autoregressive covariance matrix. The burden of multimorbidity (count of chronic conditions) was specified using a Gaussian distribution for clinical relevance. Skewness and residuals were similar to models generated using a negative binomial distribution (Supplementary Figure 1), and observed means suggested a linear relationship between multimorbidity burden and RA status on the raw scale (Supplementary Figure 2). In our primary approach, we censored individuals, but not the pair, who disenrolled from the insurance plan to maximize follow-up time. To account for differences that developed between RA and non-RA patients over follow-up periods, models included adjustments for age (updated at each year of follow-up) and sex. To investigate multimorbidity trajectory specifically during the period of RA onset, we performed secondary analyses restricting the sample to individuals with ≥3 years of observation before the index date (date classified as RA) and started follow-up at 2 years prior to the index date.
To ensure robustness of our results, we performed several sensitivity analyses. These were: 1) removing chronic conditions that are known to be closely associated with RA or may be misclassified as RA (anemia, osteoarthritis, fibromyalgia, interstitial lung disease, chronic back pain, gout, osteoporosis, inflammatory skin disorders), 2) restricting our sample to patients with ≥1 year of follow-up, 3) censoring the pair when one patient in the pair disenrolled, 4) using a stricter 2-year period without RA diagnostic codes or DMARD prescription for incident RA(27), 5) requiring only ≥1 ICD-9 code to be present for a condition, 6) adjusting for multimorbidity burden at the index date, and 7) removing “silent chronic conditions” that could be subject to surveillance bias. We assessed adjustment for geographic region but this did not confound results and was not included in the final models (data not shown). Analyses were completed using SAS v9.4. Data are available upon reasonable request and ethical approval.
Results
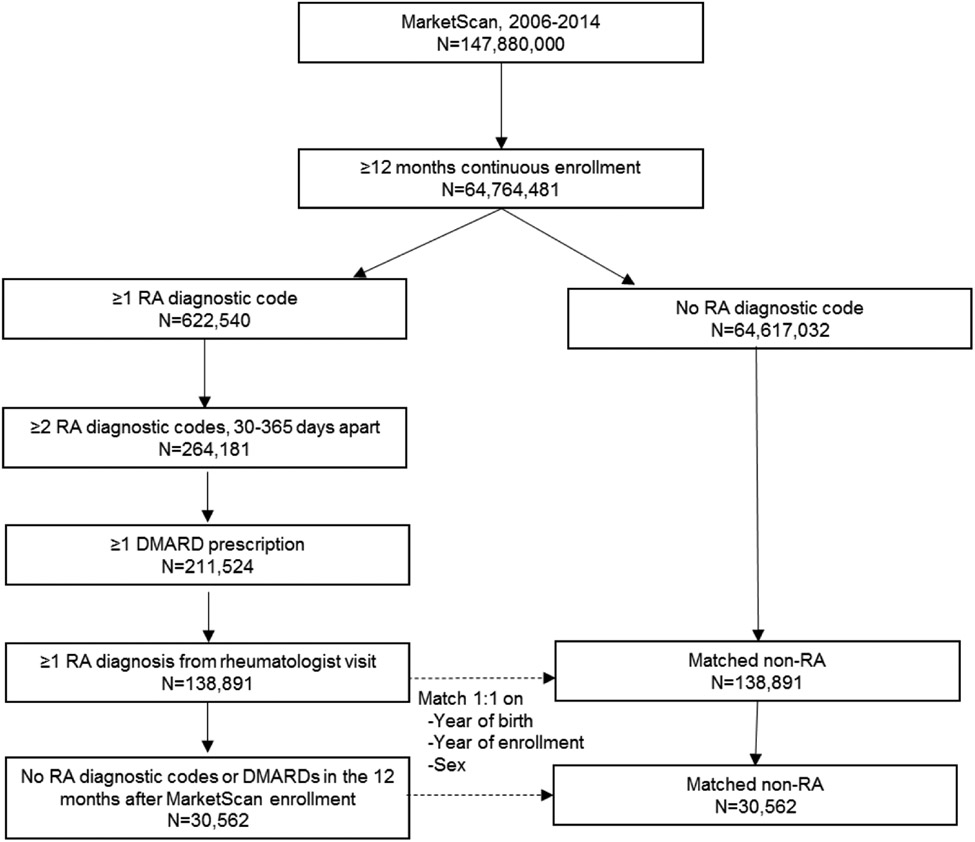
Of the >147 million individuals enrolled in MarketScan® between 2006 and 2014, we identified 138,891 who fulfilled our eligibility criteria and the RA algorithm, including 30,562 with incident RA (Figure 1). After matching (1:1), there were 277,782 patients in the overall cohort and 61,124 patients in the incident sub-cohort. Baseline characteristics of these patients are shown in Table 1. The study sample was female predominant (76.5% overall and 74.1% incident sub-cohort) with a mean age of 55.6 and 54.5 years (overall and incident). Time elapsed from entering the database to the index date was 1.5 years (SD 1.7) in the overall cohort and 3.5 years (SD 1.8) in the incident sub-cohort. Biologic DMARD use was significantly less frequent in the incident cohort (10.1%) compared to the overall cohort (24.6%).
Figure 1. Study flow diagram.
Overview of study cohort derivation. Rheumatoid arthritis (RA) patients were identified within MarketScan commercial claims and encounters database between 2006 and 2014. A sub-cohort of incident RA was identified within the overall RA cohort. RA patients were matched 1:1 with non-RA patients on sex, year of birth, and year of enrollment.
Abbreviations: RA, rheumatoid arthritis; DMARD, disease-modifying anti-rheumatic drug
Table 1.
Characteristics of rheumatoid arthritis (RA) and non-RA patients
| RA (overall) | Non-RA | RA (incident) | Non-RA | |
|---|---|---|---|---|
| N | 138,891 | 138,891 | 30,562 | 30,562 |
| Age, years | 55.6 (13.3) | 55.6 (13.3) | 54.5 (13.5) | 54.5 (13.5) |
| Female sex, % | 106,254 (76.5) | 106,254 (76.5) | 22,649 (74.1) | 22,649 (74.1) |
| Year of entrya, % | ||||
| 2006 | 43,543 (31.4) | 43,543 (31.4) | 12,636 (41.4) | 12,636 (41.4) |
| 2007 | 11,858 (8.5) | 11,858 (8.5) | 2,916 (9.5) | 2,916 (9.5) |
| 2008 | 25,187 (18.1) | 25,187 (18.1) | 5,903 (19.3) | 5,903 (19.3) |
| 2009 | 17,284 (12.4) | 17,284 (12.4) | 3,596 (11.8) | 3,596 (11.8) |
| 2010 | 16,569 (11.9) | 16,569 (11.9) | 2,994 (9.8) | 2,994 (9.8) |
| 2011 | 11,624 (8.4) | 11,624 (8.4) | 1,653 (5.4) | 1,653 (5.4) |
| 2012 | 7,589 (5.5) | 7,589 (5.5) | 616 (2.0) | 616 (2.0) |
| 2013 | 5,237(3.8) | 5,237(3.8) | 248 (0.8) | 248 (0.8) |
| Time from entry to index date, years | 1.5 (1.7) | 1.5 (1.7) | 3.5 (1.8) | 3.5 (1.8) |
| U.S. region, % | ||||
| Northeast | 23,393 (16.8) | 23,487 (16.9) | 4,806 (15.7) | 4,517 (14.8) |
| North Central | 29,559 (21.3) | 31,941 (23.0) | 6,007 (19.7) | 6,435 (21.1) |
| South | 62,618 (45.1) | 53,884 (38.8) | 14,732 (48.2) | 12,854 (42.1) |
| West | 21,280 (15.3) | 25,669 (18.5) | 4,707 (15.4) | 6,488 (21.2) |
| Unknown | 2,041 (1.5) | 3,910 (2.8) | 310 (1.0) | 268 (0.9) |
| RA medicationsb, % | ||||
| Methotrexate | 86,895 (62.6) | 530 (0.4) | 20,230 (66.2) | 172 (0.6) |
| Hydroxychloroquine | 42,288 (30.5) | 629 (0.5) | 11,252 (36.8) | 234 (0.8) |
| Sulfasalazine | 11,545 (8.3) | 176 (0.1) | 2,879 (9.4) | 69 (0.2) |
| Leflunomide | 13,611 (9.8) | 60 (0.04) | 1,892 (6.2) | 19 (0.06) |
| b/tsDMARDs | 34,177 (24.6) | 265 (0.2) | 3,070 (10.1) | 84 (0.3) |
Values mean (standard deviation) or n (%) of variables at the index date
Abbreviations: RA, rheumatoid arthritis; b/tsDMARDs, biologic or targeted synthetic disease-modifying anti-rheumatic drugs
year entering the database during study window
RA medications received prior to, or on, the index date
Multimorbidity prevalence and burden
At baseline, 57.4% of RA and 40.8% of non-RA had ≥1 chronic condition with 33.9% and 21.1% being multimorbid, respectively. The odds of multimorbidity were 2.3-fold higher in RA than non-RA at baseline (conditional OR 2.29, 95% CI 2.25-2.34) (Table 2). Similar odds of multimorbidity for RA vs. non-RA were observed when ≥3 conditions was used to define multimorbidity or when requiring ≥1 year of follow-up (Table 2). The prevalence of multimorbidity in patients with RA was 51.8% when ≥1 year of follow-up was mandated. Of the 44 chronic conditions, 39 were over-represented in RA (Supplementary Table 2). The most over-represented chronic conditions in RA were interstitial lung disease (OR 12.62, 95% CI 10.54-15.11), fibromyalgia (OR 5.86, 95% CI 5.50-6.25), osteoarthritis (OR 5.16, 95% CI 4.98-5.35), and osteoporosis (OR 4.54, 95% CI 4.19-4.92).
Table 2.
Comparison of multimorbidity prevalence between rheumatoid arthritis (RA) and non-RA patients.
| Multimorbidity ≥2 conditions | Multimorbidity ≥3 conditions | |||
|---|---|---|---|---|
| N (%) | OR (95% CI) | N (%) | OR (95% CI) | |
| Baseline (all patients, n=277,782) | ||||
| RA | 47,083 (33.9) | 2.29 (2.25, 2.34) | 29,229 (21.0) | 2.42 (2.36, 2.48) |
| Non-RA | 29,311 (21.1) | 1 | 16,083 (11.6) | 1 |
| With ≥1 year of follow-up for matched pair (n=226,850) | ||||
| RA | 58,774 (51.8) | 2.47 (2.42, 2.51) | 39,160 (34.5) | 2.55 (2.50, 2.61) |
| Non-RA | 37,372 (33.0) | 1 | 21,552 (19.0) | 1 |
RA and non-RA matched on sex, year of birth, and year of entry into the database
All P < 0.001.
Abbreviations: CI, confidence interval; OR, odds ratio; RA, rheumatoid arthritis;
Multimorbidity burden (count of chronic conditions) was significantly higher in RA than non-RA (ratio 1.68, 95% CI 1.66-1.70) (Table 3). Use of the Charlson-Deyo (ratio 1.32, 95% CI 1.29-1.35) and RDCI (ratio 1.39, 95% CI 1.37-1.41) to measure multimorbidity burden also showed a higher burden of multimorbidity in RA. Similar findings were obtained when requiring ≥1 year of follow-up after RA diagnosis.
Table 3.
Comparison of multimorbidity burden between rheumatoid arthritis (RA) and non-RA patients.
| Chronic conditionsa | Charlson-Deyob | RDCI | ||||
|---|---|---|---|---|---|---|
| Mean (SD) | Ratio (95% CI) | Mean (SD) | Ratio (95% CI) | Mean (SD) | Ratio (95% CI) | |
| Baseline (all patients, n=277,782) | ||||||
| RA | 1.47 (2.00) | 1.68 (1.66, 1.70) | 0.23 (0.58) | 1.32 (1.29, 1.35) | 0.54 (0.96) | 1.39 (1.37, 1.41) |
| Non-RA | 0.88 (1.48) | Ref | 0.18 (0.51) | Ref | 0.39 (0.82) | Ref |
| With ≥1 year of follow-up (n=226,850) | ||||||
| RA | 2.20 (2.28) | 1.66 (1.65, 1.68) | 0.37 (0.72) | 1.37 (1.35, 1.39) | 0.85 (1.15) | 1.42 (1.40, 1.44) |
| Non-RA | 1.33 (1.76) | Ref | 0.27 (0.63) | Ref | 0.60 (0.98) | Ref |
RA and non-RA matched on sex, year of birth, and year of entry into the database
All P < 0.001
N=44 chronic conditions
Connective tissue disease was not included in scoring
Abbreviations: CI, confidence interval; OR, odds ratio; RA, rheumatoid arthritis; RDCI, Rheumatic Disease Comorbidity Index
Multimorbidity trajectory
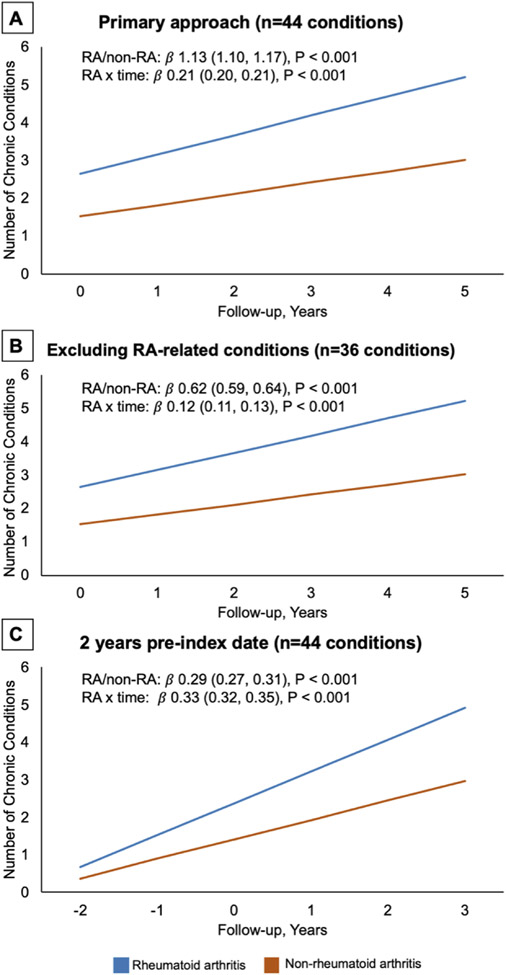
In the trajectory analyses using the incident RA sub-cohort, the mean follow-up was 2.0 (SD 1.8) years in RA and 1.8 (SD 1.8) years in non-RA. RA patients had a greater burden of multimorbidity at diagnosis and throughout follow-up (Table 4 and Figure 2A). The rate of accrual of chronic conditions was significantly higher over time in RA relative to non-RA patients (Table 4; RA x time [years] β 0.21, 95% CI 0.20-0.21, P<0.001). Other factors associated with greater multimorbidity burden were female sex, older age, and a longer duration of follow-up. The greater burden of multimorbidity throughout follow-up and higher rate of accruing chronic conditions persisted when removing conditions closely related to RA or that may be misclassified as RA (Table 4 and Figure 2B; RA x time β 0.12, 95% CI 0.11-0.13, P<0.001). The accelerated accrual of chronic conditions over time was greater in RA when restricting to individuals with pre-RA data (Table 4 and Figure 2C; RA x time β 0.33, 95% CI 0.32-0.35, P<0.001). Among those with pre-RA data, chronic conditions developed at a significantly higher rate in RA vs. non-RA after RA onset (RA status x post/pre-RA period β 0.67, 95% CI 0.63-0.71, P<0.001). All sensitivity analyses confirmed a greater burden of multimorbidity and a higher rate of accruing conditions in RA (Supplementary Figure 3).
Table 4.
Trajectory of multimorbidity in incident rheumatoid arthritis (RA) compared to non-RA patients
| A. Primary analysis (n=44 conditions) |
B. Secondary analysisa (n=36 conditions) |
C. 2 years pre-index date (n=44 conditions) |
||||
|---|---|---|---|---|---|---|
| β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | |
| RA vs. non-RA | 1.13 (1.10, 1.17) | <0.001 | 0.62 (0.59, 0.64) | <0.001 | 0.29 (0.27, 0.31) | <0.001 |
| Year of follow-up | 0.24 (0.23, 0.24) | <0.001 | 0.18 (0.18, 0.19) | <0.001 | 0.48 (0.47, 0.49) | <0.001 |
| RA x time (years) | 0.21 (0.20, 0.21) | <0.001 | 0.12 (0.11, 0.13) | <0.001 | 0.33 (0.32, 0.35) | <0.001 |
| Age (per year) | 0.06 (0.06, 0.06) | <0.001 | 0.05 (0.05, 0.05) | <0.001 | 0.04 (0.04, 0.04) | <0.001 |
| Female sex | 0.28 (0.24, 0.32) | <0.001 | 0.15 (0.12, 0.18) | <0.001 | 0.16 (0.12, 0.20) | <0.001 |
Excluded conditions known to be related to RA or could be misclassified as RA
A & B. N=61,528 patients and 173,469 observations
C. N=33,202 patients and 153,121 observations
Figure 2. Predicted burden of multimorbidity in incident rheumatoid arthritis (RA) compared to non-RA patients after diagnosis.
Predicted burden of chronic conditions from generalized estimating equation models comparing RA and non-RA patients. Shown are the number of chronic conditions in RA and non-RA as well as the interaction term between RA status and follow-up time (years). Panel A, primary analytic approach requiring 1 year in the dataset without RA diagnostic codes or medications and evaluating 44 chronic conditions. Panel B, similar analytic approach evaluating 36 chronic conditions after removing those known to be associated with RA or could be misclassified as RA. Panel C, restricting the population to individuals with at least 3 years of data prior to index date and beginning follow-up at 2 years before the index date (date fulfilling RA algorithm). Confidence intervals are shown but fall within the width of the predicted lines.
Discussion
Given an aging population and growing prevalence of chronic conditions, multimorbidity represents a major public health concern(15-17). In this study, we have evaluated the onset and trajectory of multimorbidity in individuals with RA in a large, U.S. commercial claims database during the current treatment era with robust capture of medical care. We found a substantially higher prevalence and burden of multimorbidity in individuals with RA relative to non-RA. Importantly, we identified that the heightened burden of multimorbidity in RA appears to start early in the RA disease course or even during the pre-RA period. Our findings shed important light on the natural history of multimorbidity and will help inform the future development of preventive and/or therapeutic interventions aimed at reducing multimorbidity burden in this high-risk population.
RA is known to predispose to many chronic conditions and there are ongoing efforts to better understand multimorbidity in RA(33-35). In this study, we have demonstrated that multimorbidity is highly prevalent in RA. When requiring ≥1 year of post-diagnosis follow-up in our overall cohort, >51% of RA patients were multimorbid. Moreover, the odds of multimorbidity were 2.3- to 2.5-fold higher in RA relative to non-RA patients. Similarly, the burden of multimorbidity, operationalized as the count of chronic conditions, was more than 60% higher in RA. To avoid overestimation resulting from cohort construction, we did not consider RA to be a condition contributing to the definition of multimorbidity. Therefore, our results underestimate the true prevalence and burden of multimorbidity affecting RA patients. Notably, the 44 chronic condition list we compiled from prior studies of multimorbidity in the general population and known RA-related conditions was more sensitive for assessing the burden of multimorbidity in RA than either the Charlson-Deyo index or the RDCI. While the focus of our study was on multimorbidity in whole, most individual chronic conditions were overrepresented in RA (39 of 44 conditions), as previously reported(36). As expected, extra-articular manifestations (e.g. interstitial lung disease) and other musculoskeletal conditions were the conditions most closely associated with RA.
In addition to demonstrating a higher multimorbidity burden in RA, trajectory analyses in incident RA illustrate that the rate of acquiring chronic conditions increases disproportionately compared to non-RA persons. This finding supports our proposed hypothesis and was robust to several sensitivity analyses, including analyses that excluded conditions that may be directly related to RA or misclassified as RA. It is also consistent with results from a recent study evaluating post-diagnosis conditions as predictors of mortality within the Nurses’ Health Study where scores for the Multimorbidity Weighted Index increased more rapidly among females with RA than controls(14). A novel finding from our national study of both women and men is that even during a treatment era characterized by earlier RA diagnosis and DMARD initiation, progression of multimorbidity in RA outpaced the rate in non-RA patients during the pre-RA period. There are many potential mechanisms for this accelerated progression of multimorbidity in RA. In addition to some chronic conditions being well-established extra-articular features of RA, others may result from the inflammatory processes (e.g. cardiovascular disease) and/or disease burden (e.g. mental health disorders) accompanying RA(37). Medications used to treat RA or manage RA symptoms may also contribute to the development of chronic conditions(9). Finally, the onset of RA results in an increase in health care encounters and utilization that may contribute to increased chronic disease screening and identification(38). Because chronic conditions were frequent in our non-RA patients and results were similar with adjustment for the number of chronic conditions at baseline as well as with the exclusion of “silent chronic conditions”, it is unlikely that heightened surveillance accounts for our findings.
The observation that multimorbidity occurs and progresses early in the disease course, or even preceding disease onset, has important implications for future strategies targeting multimorbidity in RA to improve long-term outcomes. The early RA period is typically characterized by establishing the diagnosis of RA, initiating DMARDs, monitoring disease activity, and adjusting DMARD regimen following a treat-to-target approach(39, 40). Other management considerations during this time include administration of vaccinations, adjunctive treatment modalities (physical and occupational therapy), and symptom management. While the rheumatologist may be focused on these important tasks, our findings illustrate the need for the early RA period to also include aggressive screening for, and management of, multimorbidity. Optimal care models for screening and managing multimorbidity in RA are not known and should be a focus of future research. Specifically, studies are needed to assess the existing patterns of screening for multimorbidity in RA patients, which providers are performing these screenings, and whether such methods are effectively identifying chronic conditions. In RA, co-management with a primary care physician improves screening for hyperlipidemia(41). Alternative care delivery models that utilize case managers and multidisciplinary teams have been tested with heterogenous results in the general population(42).
Limitations of this study include the inability to adjust for health behaviors and sociodemographics, which may result in unmeasured confounding. There may be misclassification of RA status, incident vs. prevalent RA, and chronic condition development. However, we used validated algorithms for RA and required the presence of ≥2 diagnostic codes for chronic conditions(26, 27). The sample consisted of U.S. individuals with commercial insurance and may not be generalizable outside of this setting. Because of the frequency of disenrollment from the commercial health plans, follow-up time was limited. Chronic conditions were considered independent, and future work will be needed to precisely characterize the interconnectedness of chronic conditions that defines multimorbidity in RA. The chosen “silent conditions” may cause symptoms and are not exhaustive, but were selected as those most likely to be influenced by surveillance bias. Finally, while multimorbidity differentiates itself from comorbidity by not specifying an index condition, the study of multimorbidity in a specific population, such as RA, requires anchoring on the characteristic of that population.
In conclusion, in this large cohort study using a national commercial insurance database, we found a significantly higher burden of multimorbidity in RA compared to non-RA individuals. Our trajectory analyses demonstrate that multimorbidity onset occurs early in the RA disease course, or even precedes RA onset, and RA patients experience an accelerated rate of accruing chronic conditions. Strategies aimed at managing multimorbidity to prevent its progression and complications will need delivered early in the RA disease course.
Supplementary Material
Key messages.
What is already known about this subject?
Multimorbidity is a growing public health problem with an aging population and rising rates of chronic conditions.
While select comorbid conditions are known to complicate rheumatoid arthritis (RA), the timing of onset and rate of accruing multimorbidity in RA is unknown.
What does this study add?
Using a large, commercial insurance database in the current treatment era, we have shown that multimorbidity is significantly more common in patients with RA.
Multimorbidity occurs early in the RA disease course and progresses more rapidly than in patients without RA.
How might this impact on clinical practice or future developments?
Multimorbidity should be targeted early in the RA disease course to prevent progression and achieve better long-term patient outcomes.
Funding info:
This work was supported by the Rheumatology Research Foundation Scientist Development Award (BRE), Great Plains IDeA-CTR Scholars Award (BRE), and Patient-Centered Outcomes Research Institute (JRC). Dr. Mikuls is supported by the NIH/NIGMS (U54GM115458) and NIAAA (R25AA020818). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Competing interests: The authors have no competing interests.
Ethical approval information: University of Nebraska Medical Center and University of Alabama at Birmingham Institutional Review Boards reviewed this study.
Patient and Public Involvement: We did not include patient and public involvement in this study.
Data sharing statement:
Data are available upon reasonable request and ethical approval.
References
- 1.Sherrer YS, Bloch DA, Mitchell DM, Young DY, Fries JF. The development of disability in rheumatoid arthritis. Arthritis Rheum. 1986;29(4):494–500. [DOI] [PubMed] [Google Scholar]
- 2.England BR, Sayles H, Michaud K, Caplan L, Davis LA, Cannon GW, et al. Cause-Specific Mortality in Male US Veterans With Rheumatoid Arthritis. Arthritis Care Res (Hoboken). 2016;68(1):36–45. [DOI] [PubMed] [Google Scholar]
- 3.Sparks JA, Chang SC, Liao KP, Lu B, Fine AR, Solomon DH, et al. Rheumatoid Arthritis and Mortality Among Women During 36 Years of Prospective Follow-Up: Results From the Nurses' Health Study. Arthritis Care Res (Hoboken). 2016;68(6):753–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young A, Koduri G. Extra-articular manifestations and complications of rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2007;21(5):907–27. [DOI] [PubMed] [Google Scholar]
- 5.Haugeberg G, Uhlig T, Falch JA, Halse JI, Kvien TK. Bone mineral density and frequency of osteoporosis in female patients with rheumatoid arthritis: results from 394 patients in the Oslo County Rheumatoid Arthritis register. Arthritis Rheum. 2000;43(3):522–30. [DOI] [PubMed] [Google Scholar]
- 6.England BR, Thiele GM, Anderson DR, Mikuls TR. Increased cardiovascular risk in rheumatoid arthritis: mechanisms and implications. BMJ. 2018;361:k1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simon TA, Thompson A, Gandhi KK, Hochberg MC, Suissa S. Incidence of malignancy in adult patients with rheumatoid arthritis: a meta-analysis. Arthritis Res Ther. 2015;17:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maloley PM, England BR, Sayles H, Thiele GM, Michaud K, Sokolove J, et al. Post-traumatic stress disorder and serum cytokine and chemokine concentrations in patients with rheumatoid arthritis(). Semin Arthritis Rheum. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDonough AK, Curtis JR, Saag KG. The epidemiology of glucocorticoid-associated adverse events. Curr Opin Rheumatol. 2008;20(2):131–7. [DOI] [PubMed] [Google Scholar]
- 10.Radner H, Yoshida K, Smolen JS, Solomon DH. Multimorbidity and rheumatic conditions-enhancing the concept of comorbidity. Nat Rev Rheumatol. 2014;10(4):252–6. [DOI] [PubMed] [Google Scholar]
- 11.Armagan B, Sari A, Erden A, Kilic L, Erdat EC, Kilickap S, et al. Starting of biological disease modifying antirheumatic drugs may be postponed in rheumatoid arthritis patients with multimorbidity: Single center real life results. Medicine (Baltimore). 2018;97(13):e9930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Radner H, Yoshida K, Frits M, Iannaccone C, Shadick NA, Weinblatt M, et al. The impact of multimorbidity status on treatment response in rheumatoid arthritis patients initiating disease-modifying anti-rheumatic drugs. Rheumatology (Oxford). 2015;54(11):2076–84. [DOI] [PubMed] [Google Scholar]
- 13.Radner H, Yoshida K, Hmamouchi I, Dougados M, Smolen JS, Solomon DH. Treatment Patterns of Multimorbid Patients with Rheumatoid Arthritis: Results from an International Cross-sectional Study. J Rheumatol. 2015;42(7):1099–104. [DOI] [PubMed] [Google Scholar]
- 14.Yoshida K, Lin TC, Wei M, Malspeis S, Chu SH, Camargo CA, et al. The roles of post-diagnosis accumulation of morbidities and lifestyle changes on excess total and cause-specific mortality risk in rheumatoid arthritis. Arthritis Care Res (Hoboken). 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization, U.S. National Institute of Aging. Global health and ageing. 2011. [Google Scholar]
- 16.Wu S-Y, Green A Projection of Chronic Illness Prevalence and Cost Inflation. RAND Corporation. 2000. [Google Scholar]
- 17.Buttorff C, Ruder T, Bauman M. Multiple Chronic Conditions in the United States. RAND Corporation. 2017. [Google Scholar]
- 18.Salive ME. Multimorbidity in older adults. Epidemiol Rev. 2013;35:75–83. [DOI] [PubMed] [Google Scholar]
- 19.Crowson CS, Liang KP, Therneau TM, Kremers HM, Gabriel SE. Could accelerated aging explain the excess mortality in patients with seropositive rheumatoid arthritis? Arthritis Rheum. 2010;62(2):378–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goronzy JJ, Shao L, Weyand CM. Immune aging and rheumatoid arthritis. Rheum Dis Clin North Am. 2010;36(2):297–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Birnbaum H, Pike C, Kaufman R, Marynchenko M, Kidolezi Y, Cifaldi M. Societal cost of rheumatoid arthritis patients in the US. Curr Med Res Opin. 2010;26(1):77–90. [DOI] [PubMed] [Google Scholar]
- 22.George MD, Baker JF, Winthrop K, Alemao E, Chen L, Connolly S, et al. Risk of Biologics and Glucocorticoids in Patients With Rheumatoid Arthritis Undergoing Arthroplasty: A Cohort Study. Ann Intern Med. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Curtis JR, Sarsour K, Napalkov P, Costa LA, Schulman KL. Incidence and complications of interstitial lung disease in users of tocilizumab, rituximab, abatacept and anti-tumor necrosis factor alpha agents, a retrospective cohort study. Arthritis Res Ther. 2015;17:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.England BR, Mikuls TR, Xie F, Yang S, Chen L, Curtis JR. Herpes Zoster as a Risk Factor for Incident Giant Cell Arteritis. Arthritis Rheumatol. 2017;69(12):2351–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim SC, Solomon DH, Rogers JR, Gale S, Klearman M, Sarsour K, et al. Cardiovascular Safety of Tocilizumab Versus Tumor Necrosis Factor Inhibitors in Patients With Rheumatoid Arthritis: A Multi-Database Cohort Study. Arthritis Rheumatol. 2017;69(6):1154–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chung CP, Rohan P, Krishnaswami S, McPheeters ML. A systematic review of validated methods for identifying patients with rheumatoid arthritis using administrative or claims data. Vaccine. 2013;31 Suppl 10:K41–61. [DOI] [PubMed] [Google Scholar]
- 27.Curtis JR, Xie F, Chen L, Greenberg JD, Zhang J. Evaluation of a Methodologic Approach to Define an Inception Cohort of Rheumatoid Arthritis Patients Using Administrative Data. Arthritis Care Res (Hoboken). 2018;70(10):1541–5. [DOI] [PubMed] [Google Scholar]
- 28.Willadsen TG, Bebe A, Koster-Rasmussen R, Jarbol DE, Guassora AD, Waldorff FB, et al. The role of diseases, risk factors and symptoms in the definition of multimorbidity - a systematic review. Scand J Prim Health Care. 2016;34(2):112–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van den Bussche H, Koller D, Kolonko T, Hansen H, Wegscheider K, Glaeske G, et al. Which chronic diseases and disease combinations are specific to multimorbidity in the elderly? Results of a claims data based cross-sectional study in Germany. BMC Public Health. 2011;11:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zulman DM, Pal Chee C, Wagner TH, Yoon J, Cohen DM, Holmes TH, et al. Multimorbidity and healthcare utilisation among high-cost patients in the US Veterans Affairs Health Care System. BMJ Open. 2015;5(4):e007771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–9. [DOI] [PubMed] [Google Scholar]
- 32.England BR, Sayles H, Mikuls TR, Johnson DS, Michaud K. Validation of the rheumatic disease comorbidity index. Arthritis Care Res (Hoboken). 2015;67(6):865–72. [DOI] [PubMed] [Google Scholar]
- 33.Radner H, Yoshida K, Mjaavatten MD, Aletaha D, Frits M, Lu B, et al. Development of a multimorbidity index: Impact on quality of life using a rheumatoid arthritis cohort. Semin Arthritis Rheum. 2015;45(2):167–73. [DOI] [PubMed] [Google Scholar]
- 34.Dougados M, Soubrier M, Antunez A, Balint P, Balsa A, Buch MH, et al. Prevalence of comorbidities in rheumatoid arthritis and evaluation of their monitoring: results of an international, cross-sectional study (COMORA). Ann Rheum Dis. 2014;73(1):62–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Norton S, Koduri G, Nikiphorou E, Dixey J, Williams P, Young A. A study of baseline prevalence and cumulative incidence of comorbidity and extra-articular manifestations in RA and their impact on outcome. Rheumatology (Oxford). 2013;52(1):99–110. [DOI] [PubMed] [Google Scholar]
- 36.Luque Ramos A, Redeker I, Hoffmann F, Callhoff J, Zink A, Albrecht K. Comorbidities in Patients with Rheumatoid Arthritis and Their Association with Patient-reported Outcomes: Results of Claims Data Linked to Questionnaire Survey. J Rheumatol. 2019;46(6):564–71. [DOI] [PubMed] [Google Scholar]
- 37.Michaud K, Wolfe F. Comorbidities in rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2007;21(5):885–906. [DOI] [PubMed] [Google Scholar]
- 38.Widdifield J, Ivers NM, Bernatsky S, Jaakkimainen L, Bombardier C, Thorne JC, et al. Primary Care Screening and Comorbidity Management in Rheumatoid Arthritis in Ontario, Canada. Arthritis Care Res (Hoboken). 2017;69(10):1495–503. [DOI] [PubMed] [Google Scholar]
- 39.Singh JA, Saag KG, Bridges SL Jr., Akl EA, Bannuru RR, Sullivan MC, et al. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Rheumatol. 2016;68(1):1–26. [DOI] [PubMed] [Google Scholar]
- 40.Smolen JS, Landewe R, Bijlsma J, Burmester G, Chatzidionysiou K, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76(6):960–77. [DOI] [PubMed] [Google Scholar]
- 41.Navarro-Millan I, Yang S, Chen L, Yun H, Jagpal A, Bartels CM, et al. Screening of Hyperlipidemia Among Patients With Rheumatoid Arthritis in the United States. Arthritis Care Res (Hoboken). 2019;71(12):1593–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith SM, Wallace E, O'Dowd T, Fortin M. Interventions for improving outcomes in patients with multimorbidity in primary care and community settings. Cochrane Database Syst Rev. 2016;3:CD006560. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request and ethical approval.