Abstract
Research in hearing sciences has provided extensive knowledge about how the human auditory system processes speech and assists communication. In contrast, little is known about how this system processes “natural soundscapes,” that is the complex arrangements of biological and geophysical sounds shaped by sound propagation through non-anthropogenic habitats [Grinfeder et al. (2022). Frontiers in Ecology and Evolution. 10: 894232]. This is surprising given that, for many species, the capacity to process natural soundscapes determines survival and reproduction through the ability to represent and monitor the immediate environment. Here we propose a framework to encourage research programmes in the field of “human auditory ecology,” focusing on the study of human auditory perception of ecological processes at work in natural habitats. Based on large acoustic databases with high ecological validity, these programmes should investigate the extent to which this presumably ancestral monitoring function of the human auditory system is adapted to specific information conveyed by natural soundscapes, whether it operate throughout the life span or whether it emerges through individual learning or cultural transmission. Beyond fundamental knowledge of human hearing, these programmes should yield a better understanding of how normal-hearing and hearing-impaired listeners monitor rural and city green and blue spaces and benefit from them, and whether rehabilitation devices (hearing aids and cochlear implants) restore natural soundscape perception and emotional responses back to normal. Importantly, they should also reveal whether and how humans hear the rapid changes in the environment brought about by human activity.
Keywords: natural soundscape, auditory ecology, human auditory system, hearing impairment, restorative environments, health, environmental awareness
Introduction
For about a century, substantial knowledge has been accumulated about how the human auditory system processes the human voice and speech in a variety of acoustic contexts (e.g., Pardo et al., 2021; Pisoni & Remez, 2005). However, little is known if any about how humans process natural soundscapes, that is – according to their proximal definition (see below) – the complex arrangements of biological and geophysical sounds shaped by sound propagation through non-anthropogenic habitats (Grinfeder et al., 2022; Pijanowski et al., 2011; see also Schulte-Fortkamp et al., 2023). This lack of information is quite surprising for three fundamental and practical reasons.
Firstly, the scientific study of natural soundscapes with an ecological perspective began several decades ago, triggered by the pioneering work of Schafer (1977), Truax (1978) and Jenkins (1985). This field, known as soundscape ecology (Pijanowski et al., 2011) or ecoacoustics (Farina & Gage, 2017; Sueur & Farina, 2015), has developed over the years the collection and analysis of massive, high-quality acoustic data from ecosystems through the use of autonomous recorders, standardized recording procedures, powerful signal-processing techniques, and machine-learning algorithms. This approach has helped researchers in this field to elaborate a rich theoretical framework based – amongst others – on key hypotheses detailed below such as acoustic adaptation (AAH; Marten & Marler, 1977; Morton, 1975; Wiley & Richards, 1978; see Ey & Fischer, 2009; Hardt & Benedict, 2020) and acoustic niche (ANH; Krause, 1987). Unfortunately, knowledge of the data, methods and concepts developed and used by these ecology disciplines remains relatively limited within the hearing science community. To this we can add the fact that research on soundscapes, conceived in an essentially perceptual and human-centered sense (ISO 12913-1; see also Fiebig, 2023) is developing strongly in the related and more applied field of environmental acoustics and urban sound planning (e.g., Engel et al., 2021; Erfanian et al., 2019; Genuit et al., 2023; Kang et al., 2016; Schulte-Fortkamp & Fiebig, 2023; for a comprehensive presentation, see Schulte-Fortkamp et al., 2023). However, environmental acoustics and hearing sciences evolve relatively independently.
Secondly, although half of the world population lives in rural environments (UN Habitat World Cities Report, 2022), most hearing research is currently motivated by the need to increase quality of life in urban environments. Moreover, recent demonstrations of the so-called “restorative effects” of nature exposure at both physiological and psychological levels for humans (Buxton et al., 2021; Hammoud et al., 2022; Ratcliffe, 2021) support the idea that substantial efforts are needed at local, national and international levels to offer to anyone (including city-dwellers with limited contact with nature) the possibility to regularly experience natural soundscapes through green/blue spaces, that is parks, woodlands, forests, rivers, lakes or beaches, among others, or protected nature reserves. Yet, hearing research generally focuses on enhancing communication in urban settings rather than environmental awareness.
Thirdly, from a more theoretical perspective, hearing, which is an ancestral function shared by other species equipped with auditory receptors, determines survival and reproduction through the ability to represent the immediate environment, orient, navigate and assess resources and dangers (Fay, 2009). In terms of evolution, this monitoring function would have preceded and led to acoustic communication. We can then reasonably assume that general auditory mechanisms distinct from those involved in speech processing and communication have been shaped by ancestral selective pressures related to the composition and regularities of natural soundscapes across terrestrial biomes during the evolution of tetrapods and, more specifically, humanity (Chen & Wiens, 2020). Following the pioneering work of Attias and Schreiner (1997) and Singh and Theunissen (2003), studies focusing on the statistics of natural acoustic scenes (McDermott & Simoncelli, 2011; McWalter & Dau, 2017; McWalter & McDermott, 2018; Traer & McDermott, 2016) have recently explored this monitoring function of the human auditory system that may involve acoustic cues and recruit neural mechanisms potentially distinct from those involved in speech perception in urban settings and cocktail party effects, but there is still much more research that needs to be done.
Our lack of knowledge about the auditory perception of natural soundscapes certainly has many origins and probably reflects the tendency of human hearing research to limit the exploration of auditory perception to simplistic stimuli (Schutz & Gillard, 2020). We can only speculate about this but in our view, three main biases may explain why this important topic has been ignored. For about a century, human hearing research seem to have been mostly driven by (i) the general trend towards urbanization (UN Habitat World Cities Report, 2022), (ii) the growing need to rehabilitate communication disorders due to increasing lifespan in developed countries (Roth et al., 2011), and (iii) the development of efficient radio telecommunication systems connecting humans around the planet. Indeed, auditory scientists mainly consider listening situations associated with urban environments, such as speech understanding at a “cocktail party,” in rooms and auditoria or in the presence of traffic or cafeteria noise (for reviews, see Pardo et al., 2021; Pisoni & Remez, 2005), recognition of isolated urban and mostly mechanical sounds (e.g., Gygi et al., 2004, Gygi & Shafiro, 2013; Shafiro, 2008; Shafiro et al., 2022), or identification of attributes (e.g., pleasantness, annoyance) of urban outdoor soundscapes (e.g., Axelsson et al., 2010; Irwin et al., 2011; Raimbault, 2006). These strong “communication and urban biases” have led auditory scientists to ignore other equally important situations associated with natural and rural environments such as orienting in the habitat, estimating day time or even seasonality by noting timely sound events, or assessing weather conditions by paying attention to wind, rain or stream sounds.
Sensory ecology aims at understanding how non-human species acquire, process and use information from their habitat through their sensory organs (Dusenbery, 1992; Stevens, 2013). Amongst others, the concepts of “sensory pollutant” (an external agent deteriorating the functions of a sensory system, and affecting consequently the short-term or long-term individual fitness) and “sensory danger zone” (areas where sensory pollutants alter animal activity) (Dominoni et al., 2020) introduced by sensory ecology have hardly been addressed from the perspective of human hearing, especially with the help of well-proven methods such as psychophysics, auditory neuroscience, experimental audiology and computational modelling. Recently, however, there have been a few attempts at better understanding the “relationship between the acoustic environments in which people live and their auditory needs in these environments” (Gatehouse et al., 1999). This auditory ecology, a term initially coined by Gatehouse et al. (1999), therefore refers to the listening environments in which humans live and work, the tasks to be undertaken in these environments and the importance of these tasks in daily life. The use of this concept – although raising increasing interest in hearing sciences – has been mostly restricted to the case of urban life (see for instance the consensus paper by Keidser et al. (2020, 2022) for a comprehensive critical review on ecological validity in hearing research). Urban habitats are relatively recent in humankind history and evolution, and natural soundscapes have preceded the apparition of Homo sapiens, some 300 000 years ago (Senter, 2008). Here we propose to apply the concept of auditory ecology coined by Gatehouse and col. to study a different and evolutionary-based question, namely how humans perceive ecological processes at work in natural habitats through their peripheral and central auditory system.
In view of the above, it is apparent that one should reconsider human hearing research in light of soundscape ecology, by adopting the tools, data, experimental paradigms, models and concepts of soundscape ecology and ecoacoustics. The present approach is new in that psychoacoustics, auditory neuroscience and audiology would strongly benefit from considering the use of acoustic databases recorded by ecologists and concepts derived from ecology when studying statistics of natural scenes, auditory mechanisms involved in auditory perception of biological and geophysical sound sources and auditory scene analysis by humans with normal hearing and sensorineural hearing loss. As a first step, we propose an extended definition of the field of “human auditory ecology” based on six general research directions, hereafter detailed. This extended definition focuses on the experimental and theoretical study of the capacity of the human auditory system to perceive ecological processes at work in non-anthropogenic environments. Table 1 summarizes these six directions and gives a general idea of how the present article is organised, from more fundamental questions (e.g., statistics of natural scenes, auditory mechanisms for life and water perception) to more applied ones (e.g., effects of hearing loss and rehabilitation strategies on biophony and geophony perception, awareness of environmental changes). Table 1 also indicates that all six directions present both fundamental and applied research questions and methods. However, directions I and II introduce and focus more on the methods of this research program, while directions V and VI describe only research questions of this program.
Table 1.
Six Research Directions in Human Auditory Ecology. HIREC Stands for Human-Induced Rapid Environmental Change.
| Research directions | Research questions | Methods | Applications/Translational aspects |
|---|---|---|---|
| I. High-quality, massive and ecologically valid natural-soundscape database |
|
|
|
| II. Experimental and computational paradigms |
|
|
|
| III. Specialized auditory mechanisms, emotional processing and domain specificity |
|
|
|
| IV. Universality and plasticity of auditory mechanisms involved in natural soundscape perception |
|
|
|
| V. Effects of hearing disorders and rehabilitation systems on natural-soundscape perception |
|
|
|
| VI. Impact of human activity on natural-soundscape perception |
|
|
|
Of all the questions asked in this article, the most obvious are: Direction I: Can we improve the search for statistics on natural auditory scenes by taking natural soundscapes into account? Direction II: What kind of auditory monitoring behaviors do we adopt for natural habitats? How does informational masking affect auditory perception of natural soundscapes? Do the principles of auditory scene analysis apply to natural soundscapes? What are the acoustic cues and auditory mechanisms distinguishing biophony from geophony? Do human beings perceive biodiversity by sound, and if so, what are the mechanisms engaged in biodiversity assessment? Direction III: Do emotional responses modulate the salience of the biological and geophysical components of natural soundscapes? Do restorative effects of natural soundscapes have evolutionary underpinnings? Direction IV: What roles do development and acquired expertise play in natural soundscape perception? Direction V: What are the consequences of cochlear damage and ageing on natural soundscape perception and to what extent do people with hearing disorders benefit from their rehabilitation device in terms of the perception of natural soundscapes? Direction VI: Do we perceive alterations in natural soundscapes resulting from human activity?
Six Directions for an Extended Research Program in Human Auditory Ecology
What Do We Mean by “Natural Soundscape”?
A natural soundscape refers to the case where the contribution of acoustic events resulting from human activity (so-called “anthropophony”) can be considered as negligible. As a consequence, natural soundscapes should be composed mainly of (i) biological sounds (“biophony,” i.e., animal vocalizations) and (ii) geophysical sounds (“geophony,” e.g., wind, rain, streams) both shaped by the sound propagation properties of the habitat under study (Krause, 1987). A recent perspective by Grinfeder et al. (2022) aimed at clarifying further the term “soundscape” the usage of which can be sometimes equivocal across scientific communities despite an ISO definition (ISO 12913-1) limiting this notion to a perceptual and human-centered construct. In an attempt to clarify and reconcile these different usages, Grinfeder et al. (2022) distinguished between distal, proximal and perceptual soundscapes. This distinction is illustrated in Figure 1.
Figure 1.
Soundscape functional block diagram. The “distal soundscape” is the spatial and temporal distribution of sounds in a predetermined area, in relation to sound propagation effects. Biotic (e.g., biodiversity), abiotic (e.g., streams, weather) and acoustic factors (e.g., reverberation) determine the distal soundscape. The “proximal soundscape” represents an ideal point of observation which includes external ambient sounds. The “perceptual soundscape” is the neural/psychological representation that the receiver builds through its sensory and cognitive apparatus (Adapted from Grinfeder et al., 2022).
Within this framework, studying soundscape perception in humans becomes a complex endeavour aiming to (i) unveil the relationship between the acoustic features of sound mixtures picked up at a given place and time by the peripheral auditory system of a human listener (the “proximal soundscapes”) and the characteristics of auditory percepts evoked by these proximal soundscapes (the “perceptual soundscapes”), and (ii) understand how human listeners use these percepts to infer important aspects of the “distal soundscape” (the actual distribution of sound sources within this habitat at different spatial and temporal scales) and take it into account to guide their behavior. In this respect, the “perceptual soundscape” corresponds to the ISO 12913-1 definition commonly used in environmental acoustics and urban planning; on the other hand, “proximal” and “distal soundscapes” – which use is more in line with current usage by the soundscape ecology and ecoacoustics community– correspond to purely acoustic phenomena.
Natural Soundscapes Are Highly Structured by Ecological Processes
Soundscape ecology has revealed that natural, proximal and distal soundscapes are structured spectro-temporal acoustic patterns, conveying meaningful information susceptible to help human or non-human listeners to build stable representations of their habitat, orient and navigate and assess resources (water, preys, shelter) and dangers (predators). This structuration results from the complex – but relatively well understood – causal chains described in Figure 1 (see Figure 6 in Grinfeder et al. (2022) for a more detailed description of these causal chains) and it can be illustrated in three ways. First, the biological component of natural soundscapes shows a strong and ubiquitous periodicity caused by the day and night cycle, with a chorus at dawn and at dusk in some locations, that is a double-peaked circadian pattern of biological activity (for a review, see Gil & Llusia, 2020). Second, annotations of large databases revealed that birds, insects and, to a lesser extent, amphibians participate the most to natural soundscapes across a wide variety of terrestrial biomes (Chen et al., 2021; Divyapriya & Pramod, 2019; Gasc, Anso et al., 2018; Gasc, Gottesman, 2018; Mullet et al., 2016; Phillips et al., 2018). Given that bird, insect and anuran sounds are quite different from mammal vocalizations with, among other, faster temporal modulations (Aubin & Bremond, 1983; Capranica et al., 1985; Catchpole & Slater, 2008; Fonseca, 2014; Gerhardt & Bee, 2007; Stein, 1968; Sueur & Aubin, 2003), this over-representation of birds, insects and amphibians imposes specific acoustic regularities (that is, unique statistical structures) to natural soundscapes, to which mammals, including human ancestors, have been exposed for at least several million years (Senter, 2008). Third, two influential hypotheses in soundscape ecology, namely the acoustic niche (ANH) and acoustic adaptation (AAH) hypotheses, posit that ecological feedback from natural habitats change animal vocalizations and their arrangements on an evolutionary time scale. According to the AAH (Morton, 1975), signals produced by vocalizing animals are shaped by environmental constraints such as the density and size of surrounding trees. If this hypothesis is correct, then the signals produced by species inhabiting the same ecosystem should show some acoustic similarity. According to the ANH (Krause, 1987), each vocalizing species occupies a particular acoustic space, or niche, mainly defined by the properties of the signals they produce. Such acoustic niches would be the result of a competition between species so that overlap is minimized and space partitioning maximized . Whereas the ANH should lead to acoustic divergence, the AAH should induce acoustic convergence. If true, these evolutionary impose acoustic similarities and dissimilarities between animal vocalizations that are highly specific to certain habitats, constraining strongly the biological composition of natural soundscapes across terrestrial biomes. This high level of structuration of natural soundscapes opens the path for ecologically valid tests of the general “correspondence principle” for auditory neuroscience based on the idea that regularities in the natural soundscape should be visible in the response properties of auditory neurons (Nelken et al., 1999), and the efficient-coding hypothesis (He et al., 2023; Lesica & Grothe, 2008; Lewicki, 2002; Park et al., 2021; Smith & Lewicki, 2006) postulating that sensory processing is optimized for natural stimuli.
Direction I. Of the Importance of Using High-Quality, Massive and Ecologically Valid Natural Soundscapes Databases
The rise of soundscape ecology and ecoacoustics has led to the collection of large acoustic databases (i.e., recordings of proximal soundscapes) built with high-quality equipment and standardized procedures (e.g., Sugai et al., 2019).
These databases cover a wide variety of terrestrial biomes (e.g., forests, savannahs, grasslands, meadows, chaparrals, tundras, deserts) affected to different degrees by human activity. These include open and closed habitats from tropical, sub-tropical, temperate and arctic biomes that are often recorded with a relatively high temporal resolution (in most cases, 1 min every 15 min) for many months and seasons, and sometimes for several years (e.g., Gage & Axel, 2013). Figure 2 shows two-dimensional amplitude-modulation (2D-AMi) spectra computed by a model of human auditory processing (Thoret et al., 2020; Varnet et al., 2017) for a corpus of five natural soundscapes recorded in distinct terrestrial biomes on different continents at dawn or early morning: a boreal forest, a tropical forest, a temperate forest, a desert, and a savannah. These 2D-AMi spectra were obtained by passing the recordings through two successive filterbanks (see Varnet et al., 2017, and Thoret et al., 2020, for more details) simulating the spectro-temporal analysis performed by the human auditory system. More precisely, all recordings were passed through a first bank of bandpass filters tuned in the audio-frequency domain that simulated peripheral (cochlear) filtering. Temporal envelopes were then extracted at the output of each simulated cochlear filter and passed through a second bank of bandpass filters simulating central modulation filtering by humans. More sophisticated models of human hearing have been developed and applied to a variety of listening situations including speech against various acoustic urban backgrounds and environmental sounds such as wind or rain (e.g., Biberger & Ewert, 2017; McDermott & Simoncelli, 2011; McWalter & Dau, 2017; Osses Vecchi et al., 2022). Nevertheless, the present model structure captures the main constraints affecting spectro-temporal processing by the human auditory system with the exception of temporal fine structure processing. Figure 3 shows the 2D-AMi spectra for a Kenyan savannah recorded at three moments of the day (dawn, dusk and night) to illustrate diel variations for a single terrestrial biome.
Figure 2.
Two-dimensional amplitude-modulation (2D-AMi) spectra computed by a model of human auditory system for a corpus of natural soundscapes. Five natural soundscapes recorded in distinct terrestrial biomes on different continents at dawn/early morning. From left to right, and top to bottom: a boreal forest (Location: Algonquin Park; GPS: 45°13′25.41″N 78°35′25.31″W; Altitude: 405 m; Time: 07:10 am; Date: 24.03.2007), a tropical forest (Location: Sumatra, Ketambe; GPS: 3°32′44.81″N 97°45′09.26″E; Altitude: 338 m; Time: 06:25 am; Date: 09.03.1991), a temperate forest (Location: Nutter point, Twin lakes, Cheboygan, Michigan, USA; GPS: Latitude 45.7052895°, Longitude −84.7278262°; Altitude: 210 m; Time: 07:30 am; Date: 11.04.2012), a desert (Location: Gray Ranch, Chihuahuan desert; GPS: 31°27′38.62″N 108°51′35.33″W; Altitude: 1572 m; Time: 05:58 am; Date: 27.04.1992) and a savannah (Location: Munguezi, Zimbabwe, Africa; GPS: 20°58′04.65″S/32°19′14.58″E; Altitude: 1468 m; Time: 04:22 am; Date: 30.09.1996). All sounds can be listened to from the recordings provided in the “Supplementary Material” section. Source: B. Krause, Wild Sanctuary. 2D-AMi spectra show the distribution of AM energy (as computed by the modulation index, AMi) plotted in dB (hue code) as a function of AM rate (in Hz, abscissa) and audio frequency (in Hz, ordinate) for each biome. Most of the AM energy is limited to relatively slow rates in the higher audio-frequency channels, reflecting mainly the contribution of biophony.
Figure 3.
2D-AMi spectra for a single terrestrial biome (an African savannah (Location; Kenya, Africa: GPS: 1°18′10.41″N/37°06′37.84″E; Date: 22.02.83) recorded at three moments of the day: dawn (05:15 am), dusk (06:20 pm) and night (08:00 pm). All sounds were 30-s long and were equated in long-term root-mean-square (rms) power. All sounds can be listened to from the recordings provided in the “Supplementary Material” section. Source: B. Krause, Wild Sanctuary. See Figure 2 for other details. Modulation spectra show large diurnal variations, reflecting mainly diel cycles in biological activity (i.e., dawn or dusk choruses produced by birds, insects and amphibians). Modulation spectra suggest that the dusk chorus should be perceived as more dramatic than the dawn one for that specific habitat.
The 2D-AMi spectra in Figures 2 and 3 clearly illustrate the differences across biomes and diel variations, suggesting that the central auditory system of humans should have access to sufficient sensory information to achieve accurate auditory discrimination of these biomes and their changes. This was recently demonstrated by Thoret et al. (2020) and Apoux et al. (2023) for temperate habitats using a machine-learning approach. Natural soundscapes databases are often associated with metadata that can be used to enhance signal analyses and interpretations. In addition, these databases have been analysed using various signal-processing methods, providing useful descriptions and classifications of spectral and temporal patterns and features associated with each biome, habitat, season, moment of the day, climatic conditions, ecological processes and human activity.
Extending the Study of Natural Scene Statistics
Importantly, the databases discussed in this paper do not show the biases of previous databases used to run brain-imaging and psychophysical experiments on biological-sound detection, animal and environmental sound-source categorization (e.g., Doehrmann et al., 2008; Engel et al., 2009; Lewis et al., 2005, 2009; Murray et al., 2006; Suied et al., 2010; Suied & Viaud-Delmon, 2009; Webster et al., 2017) or computational studies attempting to assess statistics of natural scenes and test efficient-coding principles (e.g., Attias & Schreiner, 1997; He et al., 2023; Lesica & Grothe, 2008; Lewicki, 2002; Park et al., 2021; Singh & Theunissen, 2003). In particular, many of these previous relatively small datasets suffered from an over representation of mammals and more specifically pets and domestic animals, while lacking more representative biotic sounds such as those produced by birds, insects and amphibians. Hearing sciences should capitalize on such material by collaborating actively with ecoacousticians in order to characterize better the statistics of natural auditory scenes and set up powerful experimental and/or computational paradigms with higher ecological validity exploring fundamental and potentially ancestral auditory mechanisms in humans.
Reconstructing Natural Soundscapes to Provide a “Ground Truth”
Knowledge gained from soundscape ecology (e.g., AAH, ANH) and the analysis of these natural soundscapes could then be used to generate artificial proximal soundscapes where biophony, geophony and also habitat acoustics could be manipulated systematically with high experimental control. Indeed, natural scenes may be “reconstructed” based on the acoustic and metadata data provided by ecoacousticians (e.g., bird vocalizations, insect stridulations, rain, wind and stream sounds, transfer function and reverberation time of the habitat) and knowledge about ecological processes at work (e.g., animal communication behavior, interactions between geophysical and biological processes). This would offer the possibility to control rigorously the number of individuals and species associated with a given wild habitat, their behavior in relationship with moment of the day, season and geophysical processes (e.g., dawn chorus, etc.), providing therefore a “ground truth” in behavioral and neuroscientific experiments (Grinfeder et al., 2022, 2023).
To date, the acoustic databases recorded by ecoacousticians do not provide the spatial information needed to assess the role of binaural auditory processing in the perception of natural soundscapes and recording quality could be improved. Efforts should be made to record natural soundscapes in wild habitats using devices that produce the human interaural time and intensity differences and, if possible, the human head-related transfer function (Genuit et al., 2023) as spatial cues may affect listening behavior and soundscape perception (Tarlao et al., 2022).
Direction II. Experimental and Computational Paradigms for Studying Natural Soundscape Perception
Identifying Monitoring Auditory Behaviors
The use of these novel databases calls for the design of appropriate experimental paradigms. In particular, it highlights the need for using psychophysical tasks targeting the repertoire of natural auditory behaviors in humans (Kingstone et al., 2008; Krakauer et al., 2017; Miller et al., 2022), that is behaviors involved in environmental monitoring (Keidser et al., 2020) by contrast with communication behaviors. The objective is to study the human capacity to process natural soundscape information in ecologically valid situations (see for instance: Holleman et al., 2020; Keidser et al., 2020; Lewkowicz, 2001; Neuhoff, 2004; Schmuckler, 2001; Sonkusare et al., 2019), that is for stimuli and tasks representative of those experienced in everyday life and relevant to the psychological process being investigated. However, a necessary first step would require the identification of the repertoire of natural behaviors for humans via ethnographic studies aiming at characterizing “ordinary listening behaviors” in rural or wild settings (e.g., Feld and Brenneis, 2004). Assessing the strength of wind, discharge of running waters, orienting at dusk or night without artificial light or more simply appreciating manifestations of life (bird, insects, …) in the surrounding environment may be important behaviors for people living in such places. This endeavour belongs to cognitive ethology (Kingstone et al., 2008), geography, anthropology and even sociology. Unfortunately, such ethnographic studies are clearly lacking. Some cognitive psychology studies suggest however that sensory processing and attention may differ between rural and urban elderly people (Hirst et al., 2022). These studies argue that, consistent with work conducted in environmental acoustics (e.g., De Coensel & Botteldooren, 2006), rural environments are less complex than urban ones and situations typical of urban daily life such as road crossing require global processing and divided attention more than focused attention (Cassarino & Setti, 2016). More work is clearly warranted to characterize differences between urban and rural and even wild settings, not only in terms of soundscape features (e.g., De Coensel et al., 2003) but also in terms of listening behaviors.
Here, it is important to note that the situations under study are dynamic environments where human listeners are engaged observers who actively modify their behavior based on feedback from the environment (see for instance Turchet et al., 2015, for an elegant demonstration of the role of interactive auditory feedback such as footstep sounds in modulating walking upon surface materials such as snow). To characterize properly the above-mentioned “ordinary listening behaviors,” it is therefore essential to consider the behavioral relevance of all soundscape features (biophony, geophony, propagation effects) for human listeners, in line with the ecological perspective conceptualized by Gibson (1966) in vision sciences, and more recently by Gaver (1993) and Neuhoff (2004) for hearing sciences. Indeed human listeners interact with dynamic environments with the focus on perceiving the causal properties of environmental sounds such as action types and source properties (e.g., materials) rather than the sounds the source produces (Heller et al., 2023; Houix et al., 2012; Lakatos et al., 1997; Lemaitre & Heller, 2012, 2013; Lutfi & Oh, 1997; Lutfi & Stoelinga, 2010; McAdams et al., 2010; Warren & Verbrugge, 1984). A systematic description of the different ways humans listen would also be useful to account for the variety of “ordinary listening behaviors” associated with natural soundscapes. For example, Truax (2001) distinguished between “listening-in-search,” “listening-in-readiness,” and “background listening.” Gaver (1993) distinguished between “everyday” and “musical” listening, with Helmholtz's “analytic” versus “synthetic” listening possibly being a special case of the former. It could be that different acoustic cues – global or local – are involved with different modes of listening. Generally speaking, these different listening modes certainly relate to the way in which auditory attention is focused on the different objects in the scene. For instance, a birdwatcher listening for a specific bird may be attending to the sounds of a temperate forest differently than a casual hiker out for a leisurely walk, a camper looking for a place to set up a tent, or someone who is presented with a recording of this forest and ask to tell if it is different from that of a grassland (e.g., Apoux et al., 2023), even if all of them are experiencing exactly the same acoustic scenes, that is the same proximal soundscape.
Experimental Paradigms for Laboratory Studies: Methodological Considerations
As for tasks, multiple-intervals, forced-choice discrimination procedures (e.g., the triangular/oddity method used by Apoux et al., 2023) may not correspond to ordinary listening situations, but they have the obvious advantage of limiting bias in decision making and are relatively easy to instruct (Hautus et al., 2021). Pilot experiments based on such paradigms showed that untrained human listeners can achieve high levels of consistency and relatively high levels of performance for habitat, moment of the day and season discrimination despite the large acoustic variability of natural soundscapes and limited life-long exposure to natural settings (Apoux et al., 2023). Recognition, identification, categorization and spatial tasks investigating navigation/orientation capacities may be more relevant to daily life and would ideally complement basic, forced-choice discrimination paradigms that may lack ecological validity. Recognition tasks (e.g., Cohen et al., 2009) should be deployed to study long-term memory of natural soundscapes and compare it to recognition memory of language, music and visual pictures. Identification tasks (e.g., Gygi et al., 2004; Shafiro, 2008, Shafiro et al., 2020) should allow us to establish the extent to which humans are able to associate a proximal soundscape with a type of habitat, season or a time of day. Being prone to criterion effects, free-sorting tasks (Strelnikov et al., 2018) may be used to study categorization processes for natural soundscapes but primarily as a pilot step. Change detection tasks (e.g., Cervantes Constantino et al., 2012; Petsas et al., 2016) where listeners would have to detect the presence of vital resources (e.g., water, preys) or danger (e.g., predators) embedded in congruent natural soundscape backgrounds may also probe the appropriate behaviors to be investigated. In addition, experimental paradigms that use relatively long stimuli (over seconds or minutes) where participants continuously perform a given task (e.g., indicating scenes that attract their attention as in Huang and Elhilali, 2017) would better replicate ordinary listening behaviors.
Applying Concepts of Energetic and Informational Masking to Natural Scenes
In such tasks, human performance and listening effort would be constrained by both energetic and informational masking effects. Informational masking effects have never been evaluated in this context and it is unclear whether uncertainty regarding the masker and similarity in characteristics of the target and masker – the two major components of informational masking (for reviews, see Kidd et al., 2008, and Lufti et al., 2013) – would play the same role in such situations than previously shown for speech perception tasks and urban settings. For instance, attentional resources may not be recruited in the same way for natural soundscapes, speech or urban backgrounds knowing that natural soundscapes are perceived as less distractive, and potentially enhance directed attention (Gould van Praag et al., 2017; see Ratcliffe, 2021, for a critical review). An information-theoretic approach (e.g., Stilp et al., 2018) may be used to explain informational masking effects with this perspective (Lufti et al., 2013) because entropy computed on soundscape databases already proved to be a useful predictor of biodiversity (Sueur et al., 2008; see Alcocer et al., 2022).
A New Perspective for the Study of Geophysical Sounds Perception
Geophony (wind, rain, stream sounds, etc.) is assumed to play an active role in ecological processes, including habitat selection (Doligez & Boulinier, 2008; Farina et al., 2021; Mullet et al., 2017), that is “the act of choosing the combination of available abiotic and biotic elements for the purpose of fulfilling the life history events of the organism” (Montgomery & Roloff, 2017). Water may have a special status in that respect, not only because of its acoustic characteristics (Geffen et al., 2011; Guyot et al., 2017; McDermott et al., 2009), but also because of its vital importance to living organisms as an essential nutrient. In that respect, water detection and discrimination tasks based on water sounds (e.g., streams of various discharge) embedded in natural soundscapes would allow for testing the efficiency of auditory mechanisms involved in the evaluation and monitoring of ecosystem resources by living organisms, a behavior likely to contribute to habitat preference and selection. Figure 4 shows the 2D-AMi spectra of a headwater forest stream in boreal Sweden (Klaus et al., 2019). The site was sampled several times to cover a large range of water temperature and discharge. AMi spectra show that the distribution of modulation energy shifts clearly towards lower audio-frequency channels and slower rates as discharge increases, suggesting that humans should be able to discriminate accurately changes in running waters.
Figure 4.
2D-AMi spectra for 2 recordings of a single headwater forest stream (Location: boreal Sweden (Övre Björntjärn: GPS: Latitude 64.126°, Longitude 18.776°; Date: 01 April–31 October 2012–2015. The bottom substrates of the stream were dominated by sand, gravel, cobbles and boulders. The site was sampled several times to cover a large range of water temperature (0–17 °C) and discharge (16.2–113.8 L. s−1)). All sounds can be listened to from the recordings provided in the “Supplementary Material” section. Source: Klaus et al. (2019). See Figure 2 for other details. Modulation spectra show large variations across the two recordings, reflecting mainly change in discharge (two discharge levels shown here: low level (left) and high level (right); cf. Klaus et al., 2019).
A New Perspective for the Study of Biological Sounds Perception and Auditory Scene Analysis: Life Detection and Biodiversity Assessment
In this perspective, studying the capacity of human listeners to categorize biological versus geophysical sounds using these large databases with high ecological validity would allow for the search of a “life detector” similar to the one speculated for the visual modality (Troje & Westhoff, 2006) following the discovery of biological motion (Johansson, 1973), but for the hearing modality. In any case, it appears necessary to revisit the statistics of biological sounds. For instance, the pioneering work of Singh and Theunissen (2003) and Lewis et al. (2009) (see Theunissen and Elie, 2014) suggests that high spectral modulations (harmonicity) and slow temporal modulations distinguish systematically biological from geophysical sounds.
Most of these previous studies used isolated sounds but biological sounds are rarely heard in isolation in real-world natural settings. Within natural soundscapes, biophony is mixed up with geophony to form specific and potentially unique combinations associated to a given habitat and biome. Further, the biological and geophysical constituents of natural soundscapes are shaped by the specific way sounds propagate in the habitat (for a review, see Grinfeder et al., 2022). Because of sound scattering caused by vegetation, closed environments such as deciduous or coniferous forests tend to attenuate low and high audio-frequency components, creating “sound windows,” and reverberation alters strongly the transmission of fast modulations. In comparison, open environments such as grasslands, savannahs, tundras or deserts show little reverberation but alter strongly the transmission of slow modulations because of atmospheric turbulence (Michelsen & Larsen, 1983; Richards & Wiley, 1980; see Forrest, 1994). For that reason, the importance of certain acoustic features for living sound-source categorisation may have been overestimated in previous studies. The work by Mouterde et al. (2014) illustrates perfectly this idea by showing how the harmonic structure and fast temporal modulations of zebra-finch vocalizations are dramatically attenuated by their propagation over about 250 m in their natural environment. The difference between open (e.g., desert, savannahs) and closed environments (boreal, tropical and temperate forests) is shown in Figure 2. Again, the perceptually inspired mid-level representations computed by a model of human auditory system distinguish relatively well open and closed settings, suggesting that humans should be able to discriminate between the two broad categories of natural habitats and take advantage of their distinctive features to navigate (for instance, at night and in the absence of artificial light). These differences should be taken into account when searching for universal and robust features able to distinguish biophony from geophony.
Further, being able to discriminate levels of abundance and species richness in natural soundscapes is most likely a crucial capacity allowing organisms – including humans – to assess the potential diversity of resources and thus the quality of a given habitat. Our capacity to estimate census number for bird populations (e.g., aerial counts of birds) through the visual modality (e.g., Dervieux et al., 1980; Erwin, 1982) may be taken as a first indication for the existence of sensory mechanisms engaged in biodiversity assessment. A couple of studies conducted in urban green spaces and hiking trails suggests that humans are indeed able to discriminate changes in biodiversity through the auditory modality. Unfortunately, this suggestion is only based on subjective reports collected via questionnaires (Ferraro et al., 2020; Fuller et al., 2007). The capacity to discriminate changes in biodiversity through the auditory modality may contribute to specific (and ancestral) behaviors such as habitat selection. However, it remains to be demonstrated if such a capacity reflects cross-modal non-symbolic numerosity (e.g., Feigenson et al., 2004) or dedicated auditory mechanisms. Here, it is important to note that our current understanding of the mechanisms engaged in auditory scene analysis has been gained mainly from artificial stimuli such as pure tones, clicks or noises and mixtures of speech or musical sounds (e.g., Bregman, 1990; Carlyon, 2014; Darwin, 1997; Moore & Gockel, 2002, 2012). It is thus unclear whether these general principles guiding auditory scene analysis would apply to natural scenes composed mainly of bird and insect sounds combined with wind, rain or stream textures and shaped by specific habitat acoustics. In that respect, the relative contribution of within- versus between-species acoustic disparities to the analysis of natural scenes and streaming processes remains unexplored. Pilot experiments investigating these abilities reveal that human listeners are able to discriminate variations in abundance and species richness for mixtures of bird vocalizations (McWalter & Lorenzi, 2022, 2023). More work is required to assess the extent to which humans can discriminate mixtures of vocalizations from different species in the presence or absence of geophony and whether this capacity depends on grouping/segregation auditory mechanisms identified so far (Mlynarski & McDermott, 2019). Work with speech material indicates that the “size” of the auditory scenes of multiple talkers is small, meaning that humans cannot distinguish more than about four-five speech sources comprising a scene (Kawashima & Sato, 2015; Yost et al., 2019; Zhong & Yost, 2017). Extending this investigation to scenes mainly composed of birds, amphibians and insects will test whether this perceptual limit applies to natural soundscapes.
Relevance of Computational Approaches
Because of the large size of the databases collected by ecoacousticians, it is now possible to train machine-learning algorithms to assess the best performance for the behavioral tasks under study, and subsequently evaluate how much and what information is lost, missed or ignored by (real) human observers in these tasks because of internal noise, limited attentional and memory capacities and/or suboptimal decision strategies. As an example, testing architectures where machine-learning algorithms are driven by the output of peripheral or mid-level auditory processing models (e.g., Apoux et al., 2023; Thoret et al., 2020) should prove useful in establishing the importance of low-level sensory cues and the contribution of more central mechanisms in natural soundscape perception. This model-driven approach offers a unique opportunity to map in greater depth the information-processing architecture of the human auditory system in the case of natural sounds and test for the existence of central mechanisms selectively tuned for biological sound-source detection and biodiversity assessment. This approach already proved to be successful for speech and music perception (e.g., Kell et al., 2018; Koumura et al., 2019, 2023; Saddler et al., 2021).
Direction III. Specialized Auditory Mechanisms, Emotional Processing and Domain Specificity
Is there anything special with the auditory perception of natural soundscapes in humans? So far, the empirical evidence is limited because previous studies assessed independently the perception of the biological and geophysical components of natural soundscapes (biophony and geophony, respectively), and therefore suffer from the various caveats listed above.
Detailed Versus Global Auditory Processing of Natural Soundscapes
Psychoacoustical studies conducted with human participants have shown very rapid auditory detection and categorization of biological sounds, compared to artificial stimuli (pure tones) or sounds produced by musical instruments (Isnard et al., 2019; Suied et al., 2010; Suied et al., 2014; Suied & Viaud-Delmon, 2009). Importantly, these studies also demonstrated specific and mandatory processing of animal vocalizations, with human listeners being unable to ignore an animal vocalization even when it was irrelevant to the task (Isnard et al., 2019).
Electrophysiological and brain-imaging studies identified specific neural structures and networks in the human brain involved in categorical perception of vocalizations (bilateral, anterior and middle superior temporal gyrus) distinct from those involved in the perception of animal-action sounds (bilateral posterior insulae) or geophysical sounds (bilateral dorsal-occipital and medial-parietal cortex) (Altmann et al., 2007; Doehrmann et al., 2008; Engel et al., 2009; Lewis et al., 2005; Murray et al., 2006; Webster et al., 2017; see Brefczynski-Lewis & Lewis, 2017).
Overall, these behavioral and neurophysiological data suggest that specialized auditory mechanisms are selectively involved in the perceptual representation of biophony and geophony. However, there is a clear lack of information on how biophony, geophony and habitat acoustics are integrated by the human auditory system to form a single and coherent percept of the close environment surrounding the listener that may eventually be stored in long-term episodic and semantic autobiographical memory and retrieved through auditory imagery (e.g., Agus et al., 2010; Cohen et al., 2009; Demany & Semal, 2008; Tekcan et al., 2015). Such an integration process may depend on “listening mode” (see above), and may not occur when the human observer is searching for specific sounds within the scene or interacting dynamically with it, as in navigating the scene to achieve a specific goal. According to that view, this integration can only occur when the observer listens to the proximal soundscape as a “background” (i.e., when auditory attention is “diffuse”).
Sensory Versus Emotional Processing of Natural Soundscapes
The effects of natural soundscapes on the autonomous nervous system have been repeatedly demonstrated using physiological measures (e.g., electrocardiography; see below), with listening to natural soundscapes facilitating recovery from everyday cognitive fatigue and reducing physiological and psychological stress (Gould van Praag et al., 2017; for critical reviews, see Ratcliffe, 2021, and Erfanian et al., 2019). These observations are closely linked to an ongoing debate on the evolutionary origins of this emotional response and possible preferences for certain natural landscapes associated with the history of the human species (Ratcliffe, 2021). This fundamental question deserves to be studied in collaboration with evolutionary biologists and psychologists. Certain points raise the complex relationship between auditory and emotional processing.
For instance, it is unclear whether the response of the autonomous nervous system is triggered by natural soundscapes as a whole, biophony alone (e.g., birds: Buxton et al., 2021; Hammoud et al., 2022; biodiversity: Ferraro et al., 2020; Fuller et al., 2007) or geophony alone (e.g., water: Buxton et al., 2021). It is therefore conceivable that the restorative effects produced by natural soundscapes may only be triggered when listening to the soundscape holistically, that is when auditory attention is not focused on the specific object. Experiments manipulating the observers’ listening modes and their physiological effects on the autonomous nervous system would make it possible to test this hypothesis. Recent work suggests that human listeners may be able to estimate global properties of natural soundscapes, a view that contrasts with the idea that auditory perception of complex scenes relies on the segregation of the multiple sound sources they comprise (McMullin et al., 2023). Taken together, these elements raise the important question of whether the observer's listening mode determines both the integration of the elements of the scene into a coherent percept (“the perceptual soundscape”), the ability to estimate the global properties of the scene and the observer's emotional reaction.
To our knowledge, only two brain-imaging studies based on functional magnetic resonance imaging (fMRI) explored the neural correlates of soundscape perception in humans. The first study conducted by Irwin et al. (2011) focused on urban soundscapes rather than natural soundscapes, although the stimulus database included green spaces (parks), birdsongs, wind and thunder. Interestingly, this study showed that the brain response to urban soundscapes engages two distinct neural pathways involved in sound analysis and emotional processing: brain regions in the ascending auditory system (inferior colliculus, medial geniculate body, and auditory cortex) produced a significant response to the acoustic characteristics of the soundscapes but not to the rated pleasantness of the same sounds; in contrast, brain regions in the paralimbic system (amygdala and posterior insula) produced a significant response to the emotional valence of the soundscapes, but not by acoustic input. The second study compared artificial to natural soundscapes (Gould van Praag et al., 2017). Consistent with the notion that natural soundscapes evoke strong emotional responses, this study showed that activation of the middle insula of the left hemisphere is significantly increased when listening to natural soundscapes (re: artificial soundscapes). In addition, a decrease in heart-rate variability was observed when listening to natural soundscapes (re: artificial soundscapes). This change in heart-rate variability is considered as a biomarker of stress reduction reflecting a shift in autonomic balance toward parasympathetic (“rest-digest”) activation with a concomitant reduction in sympathetic (“fight-flight”) activation within the cardiovascular system. Overall, these findings suggest that auditory perception of natural soundscapes triggers automatically positive emotional responses via the autonomous nervous system that may in turn modulate soundscape analysis. In line with this, vision research demonstrated that emotion facilitates and potentiates the perceptual benefits of attention (Phelps et al., 2006). The interaction between emotional and low-level auditory mechanisms should therefore be taken into account when studying auditory perception of natural soundscapes. For instance, emotional responses may modulate informational masking effects depending on whether the background is an urban soundscape showing strong mechanical (and thus, aversive) acoustic components and negative arousal (e.g., Irwin et al., 2011) or a natural, biodiverse soundscape triggering a positive arousal (Ferraro et al., 2020; Fuller et al., 2007; Gould van Praag et al., 2017). In this context, it might be interesting to test whether emotional response modulates salience of the biological and/or geophysical components of natural soundscapes (Kayser et al., 2005; Kaya & Elhilali, 2014; Huang & Elhilali, 2017; Kothinti et al., 2021; see De Coensel & Botteldooren (2010) and Filipan et al. (2019) for computational modelling of saliency-based attention to urban soundscapes).
Domain Specificity
A good starting point would be to compare systematically the modulation statistics of natural soundscapes, rural soundscapes, urban soundscapes and speech using the framework developed by Singh and Theunissen (2003) and more importantly, the sensory cues conveyed by these sounds. This is reminiscent of previous work conducted in the visual modality that attempted to characterize the low-level visual features responsible for the perception of “naturalness” (e.g., Berman et al., 2014). Figure 5 shows the mid-level representations computed at the output of our model of human auditory system (Thoret et al., 2020; Varnet et al., 2017) for a corpus of sentences recorded in two languages showing distinct rhythmic organizations (English and French). These perceptually inspired representations illustrate how speech sounds only partly overlap with the main components of natural soundscapes in the human auditory space. This suggest that auditory cues distinct from those involved in human communication may be engaged in the monitoring of natural soundscapes.
Figure 5.
2D-AMi spectra for a corpus of sentences recorded in two different languages showing distinct rhythmic organizations from a single female speaker: English (left) – a stress-based language – and French (right) – a syllable-based language (adapted from Varnet et al., 2017; speech database from Mehler et al., 2004; Ramus et al., 1999; with permission from F. Ramus). All sounds can be listened to from the recordings provided in the “Supplementary Material” section. See Figure 2 for other details. Speech sounds show high AM energy in mid audio-frequency channels (around 1 kHz) at relatively slow rates (<10 Hz).
Figure 6 shows 2D-AMi spectra computed for urban soundscapes (a construction site in New York, USA, and a street with fast traffic in Marseille, France). The perceptually inspired representations are quite different from those associated with natural soundscapes (see Figure 2 for comparison). For the construction site, modulation energy caused by mechanical sounds (here, the repetitive impulsive sound of a jackhammer) is found at mid-high audio-frequency channels and at both slow and fast temporal-modulation channels. For the street, modulation energy caused by fast-moving cars is found at all audio-frequency channels and mostly at fast temporal channels (see Botteldooren et al., 2006, De Coensel et al.,2003, and De Coensel and Botteldooren, 2007, for a systematic characterization of the spectro-temporal modulation cues conveyed by urban versus rural soundscapes). Obviously, urban settings contain more “man-made” sounds (anthrophony) and higher levels of acoustic noise (European Environment Agency, 2020), while rural, green and blue spaces, national parks and wild areas contain more open spaces and biological sounds associated with animal activity (biophony). The present simulations suggest that natural and urban areas generate quite dramatic acoustic and sensory contrasts, that should pose different sensory and cognitive demands to humans (for instance, see Cassarino & Setti, 2015, 2016). These preliminary observations should also incite hearing scientists to assess simultaneously perceptual capacities and emotional responses for humans using combined behavioral and physiological or brain-imaging techniques to study how these two relate to each other.
Figure 6.
2D-AMi spectra for two urban soundscapes. Left: construction site mid-town (duration: 30 s), NY, USA (04.2007); right: street with fast traffic (duration: 10 s), Marseille, France (1999), at midday. NY soundscape: modulation energy in the mid and high audio-frequency channels at relatively fast rates (>10 Hz) corresponds to the repetitive sound of a jackhammer. Marseille soundscape: modulation energy at fast rates over the entire audio-frequency range corresponds to sound of cars (estimated speed: 70 km/h). All sounds can be listened to from the recordings provided in the “Supplementary Material” section. Sources: (NY, USA) B. Krause, Wild Sanctuary; (Marseille, France) S. Meunier, LMA, CNRS.
Direction IV. Universality and Plasticity of Auditory Mechanisms Involved in Natural Soundscape Perception
Relevance of Comparative Approaches
A research programme dedicated to human auditory ecology should obviously include a comparative approach to assess the universality of auditory mechanisms engaged in natural soundscape perception and the role of learning and plasticity. The effects of natural environments on the production, transmission and perception of non-human animal vocalizations have been extensively explored over the last decades (e.g., Ey & Fischer, 2009), and a wealth of studies have investigated sound detection, localization and recognition in natural backgrounds for a variety of non-human species (for reviews, see for instance Manley & Fuchs, 2011). However, this is often conducted in the context of vocal communication and noisy/cocktail-party like situations (e.g., Wiley, 2015). To the best of our knowledge, these studies have not yet explored how these different non-human species discriminate between habitats and their variations. Obviously, differences in micro-habitats are expected to alter strongly the magnitude and perceptual weight of certain acoustic cues such as those produced by ground effects in the case of insects or amphibians. Moreover, absolute sensitivity (differences in listening bandwidth) and suprathreshold auditory capacities (e.g., differences in (audio)frequency selectivity, temporal resolution and temporal modulation selectivity) should alter strongly the perceptual weight of spectral and temporal cues across mammals (including humans), birds, reptiles, amphibians and insects (for reviews, see Dooling et al., 2000; Fay & Popper, 1994, 1999; Hoy et al., 1998; Köppl, 2014).
Discussing these differences is beyond the scope of this perspective article. However, to illustrate the influence of auditory abilities, Figure 7 shows the 2D-AMi spectra computed in response to the five natural soundscapes used in Figure 2 using our auditory model where audible bandwidth, audio-frequency selectivity and temporal resolution were grossly adjusted to Zebra Finch characteristics. Zebra Finch live in arid, open environments that may be compared to the desert and savannah used in here (Mouterde et al., 2014). Visual inspection of these 2D-AMi spectra suggests that all five habitats should be easily distinguished by a Zebra Finch. Still, some important differences between modulation spectra can be found when comparing Figures 2 and 7, suggesting – not surprisingly – that humans and Zebra Finch may not perceive the characteristics of each auditory scene in a similar manner. Of course, differences in needs, plasticity and cognitive capacities are also expected to modulate strongly the importance of the same cues across species. A comparison with humans using the same natural soundscapes and tasks would allow the universality of the auditory mechanisms involved in the perception of natural soundscapes to be studied. These comparative studies could, for example, focus on the ability to discriminate habitats and their circadian and seasonal changes; the ability to perceive presence, abundance, and diversity of living beings; or the ability to perceive and locate the presence of water in the habitat. Such an endeavour would require setting up tight collaborations between neuroethologists, ecoacousticians and psychoacousticians. Importantly, this endeavour would also require to abandon the anthropocentric definition of soundscapes (ISO 12913-1) (International Organization for Standardization; International Organization for Standardization; Mitchell et al., 2023).
Figure 7.
2D-AMi spectra computed by a gross model of Zebra Finch auditory system for a corpus of five natural soundscapes recorded in distinct terrestrial biomes on different continents at dawn/early morning. From left to right, and top to bottom: a boreal forest, a tropical forest, a temperate forest, a desert and a savannah. Model parameters: (F. Theunissen, personal communication). Audible bandwidth was limited to the 0.5–9 kHz range (Amin et al., 2007). To facilitate comparison with humans 2D-AMi spectra (Figure 2), the output of filters was set to zero for all components outside the audible range. Frequency selectivity was estimated as being twice poorer than for humans (Henry & Lucas, 2010): the width of peripheral audio filters, estimated using the ERB (equivalent rectangular bandwidth) scale, was enlarged by a factor 2 compared to humans (Moore, 2007). As for humans, a modulation filterbank with a quality factor Q of 1 (i.e., 1-oct wide modulation filters) was used to model temporal-envelope processing; however, the highest best modulation rate of modulation filters was set to 100 Hz - compared to 150 Hz for humans (Amin et al., 2010; Woolley et al., 2005). See Figure 2 for legend details and for a comparison with human 2D-AMi spectra.
Relevance of Learning Paradigms, Developmental Studies and Cross-Cultural Approaches
It is also reasonable to assume that knowledge, culture, exposure and expertise influence our ability to perceive natural soundscapes. In this respect, hunter-fisher-gatherers living in a wild environment should perform better in ecologically valid tasks that require monitoring natural soundscapes. Consistent with this idea, Guastavino (2003) showed that expertise influences listening mode : experts tend to adopt a more analytical listening strategy prioritizing precision whereas non experts attend to soundscapes in a more holistic way. Cross-cultural approaches have proven successful in the case of music perception (e.g., McPherson et al., 2020). In addition, a recent study showed that the environment in which we grow up (i.e., an urban or rural one) shapes our ability to navigate (Coutrot et al., 2022). This finding calls for further research exploring the perception of natural soundscapes by comparing the abilities of people living in urban, rural and wilderness environments, but also of experts (e.g., birdwatchers) versus naive listeners. These results should be compared with those of previous studies showing an influence of listener's expertise on the categorization of urban sounds (e.g., Lemaitre et al., 2010). Developmental studies comparing auditory perception of environmental sounds across infants, children and adults are also warranted (e.g., Martínez-Castilla et al., 2015). As suggested in a recent study conducted on naive urban listeners, 10 h of training are not sufficient to affect natural soundscape discrimination capacities (Apoux et al., 2023). However, this study used feedback that may have limited the influence of individual differences in listening strategies, helping them to find quickly the optimal decision rule. Psychoacoustical experiments investigating inter-individual differences and learning effects are also warranted to understand better the contribution of plasticity and decisional factors in natural soundscape perception. Whatever the approach (comparative, developmental, etc.), it seems important to consider the way in which the observer – human or non-human, newborn, child or adult – interacts with specific sound sources and their properties, and listening mode.
Direction V. Effects of Hearing Disorders and Rehabilitation Systems on Natural Soundscape Perception
Acknowlegment and Study of Restorative Effects for Hearing-Impaired People
Some studies suggested that species richness (i.e., biodiversity) and water (stream sounds), two important factors shaping biophony and geophony, respectively, contribute to the amount of well-being and so-called “restorative effects” associated with the auditory experience of natural soundscapes for humans (Buxton et al., 2021; Ferraro et al., 2020; Fuller et al., 2007). Thus, for hearing-impaired persons visiting natural places such as green or blue spaces in cities and for those living in rural environments, quality of life and health should not only depend on efficient communication, but also on accurate perception of natural soundscapes and the appropriate emotional response associated with this perceptual experience. This is particularly important as the ageing population in developed countries increases and tends to develop hearing difficulties of sensory and cognitive origin (Roth et al., 2011). As for urban environments, improving contact with nature in cities through access to green spaces with biodiverse habitats has been suggested to contribute to the quality of life for city dwellers (Gunnarsson et al., 2017). However, hearing-impaired persons may not fully benefit from these green spaces if their auditory experience is degraded, as suggested by a study based on a questionnaire indicating that people with hearing problems (i.e., tinnitus, sensorineural hearing loss) show less restorative effects than normal-hearing people when visiting urban parks (Payne, 2008a, 2008b). This hypothesis is partially supported by recent work showing that valence and arousal ratings of pleasant, neutral, and unpleasant nonverbal sounds (e.g., animal vocalizations or environmental sounds) by hearing-impaired listeners are less extreme (i.e., less pleasant and less unpleasant) than ratings by normal-hearing listeners (Tawdrous et al., 2022).
Assessment of Natural Soundscape Perception via Hearing Aids and Cochlear Implants
Hearing aids are expected to rehabilitate the deficits associated with sensorineural hearing loss via multiband amplification and compression. Surveys indicate that satisfaction with hearing aids is strongly related to the listening environment (Kochkin, 2011). Modern devices now incorporate adaptive environment classification algorithms (Hayes, 2021). However, these algorithms have been mostly designed and tested for urban settings and large differences appear across products for complex environments (Yellamsetty et al., 2020). It is therefore unclear whether such algorithms would enhance natural soundscape perception and whether amplification and compression would improve or disrupt soundscape perception (Johnson, 2022) given their limited success for speech (Armstrong et al., 2022). The same issues should be considered for cochlear implants (Shafiro et al., 2022). Recent results indicate, for example, that cochlear implantees do not react in the same way as normal-hearing listeners to affective sounds entering their auditory peri-personal space. Moreover, only normal-hearing listeners tend to perceive closer sounds as more arousing than distant sounds, even though both normal-hearing and hearing-impaired listeners perceive sound valence similarly (Bahadori et al., 2021). Altogether, these elements call for an in-depth exploration of the effects of hearing loss and the possibility to restore accurate perceptual capacities and appropriate emotional responses via hearing aids and cochlear implants.
In a pilot study, Miller-Viacava et al. (2023) assessed the ability to discriminate natural soundscapes recorded in a temperate terrestrial biome for normal-hearing and hearing-impaired participants with bilateral, mild-to-severe sensorineural hearing loss. Discrimination of the habitat, season and period of the day was measured using an oddity (forced choice) paradigm. The scores of hearing-impaired participants were poorer than normal. On average, they were relatively well accounted for by the scores of normal-hearing participants tested with stimuli spectrally shaped to match the frequency-dependent reduction in audibility of hearing-impaired participants. However, hearing-impaired scores were not significantly correlated with audiometric thresholds and age. These results suggest that the ability of hearing-impaired people to discriminate natural soundscapes is severely disrupted. These deficits are only partly accounted for by reduced audibility. Supra-threshold auditory deficits (such as reduced frequency selectivity, loss of fast-acting amplitude compression and abnormal temporal-fine structure coding) and individual listening strategies may also explain differences between normal-hearing and hearing-impaired persons.
Figure 8 shows the effects of reduced frequency selectivity caused by the loss of outer hair cells in the cochlea on the mid-level auditory representations of natural soundscapes. The simulations aiming to reproduce the loss in spectral resolution of humans with moderately-severe sensorineural hearing loss were conducted for all five biomes used in Figure 2. Broadened cochlear filters alter substantially these auditory representations by smearing important spectro-temporal features likely to be used to perceive soundscapes. Still, visual inspection of these preliminary simulations reveals that reduced frequency selectivity spares gross spectro-temporal differences between natural soundscapes, indicating that hearing-impaired listeners may retain some capacity to discriminate between habitats, although fine details of each auditory scene may be lost. This is consistent with the outcome of a recent psychoacoustical study conducted by Scheuregger et al. (2021) showing that the time-averaged statistic representations of sound textures (e.g., insects chirping, rain, water) provide listeners with cues which are robust to the effects of sensorineural hearing loss.
Figure 8.
Effects of reduced frequency selectivity caused by the loss of outer hair cells in the cochlea on the mid-level auditory representation of the five natural soundscapes used in Figure 2: From left to right, and top to bottom: a boreal forest, a tropical forest, a temperate forest, a desert and a savannah. Source: B. Krause, Wild Sanctuary. 2D-AMi spectra are computed for the A) normal-hearing and B) hearing-impaired model of human auditory system. Moderately-severe sensorineural hearing loss is simulated by enlarging the width of cochlear filters, estimated using the ERB (equivalent rectangular bandwidth) scale by a factor 4 (Moore, 2007). See Varnet et al. (2017) and Thoret et al. (2020) for model details.
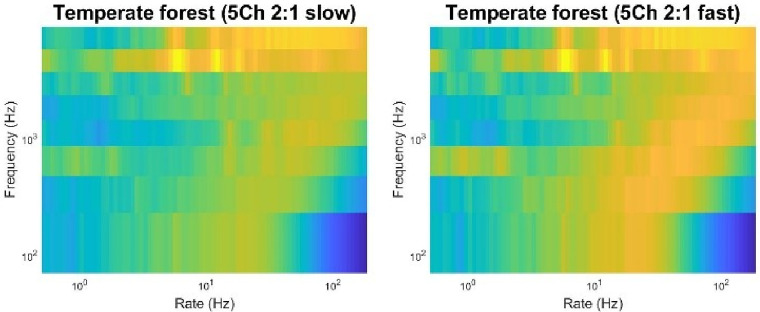
More work is therefore warranted to characterize further and explain the effects of sensorineural hearing loss on perception and emotional response to natural soundscapes and urban green spaces. More work is also needed to assess the extent to which alterations in soundscape perception and emotional responses can be restored back to normal via hearing aids or cochlear implants that were initially designed for the rehabilitation of communication in urban settings. Figure 9 shows the effects of slow and fast wide-dynamic range multi-band compression on the 2D-AMi spectra calculated at the output of our model of impaired auditory system, in response to the natural soundscape recorded in a temperate forest at dawn. Compression alters aspects of these perceptual representations, and more so with fast than with slow compression, suggesting that hearing-aid processing may degrade perception of natural soundscapes to some extent.
Figure 9.
Effects of multiband compression on the mid-level auditory representation of a natural soundscape (a temperate forest) used in Figure 2. Simulations are conducted for a simulated 5-channel hearing aid (Moore et al., 2011) with either slow (left panel; attack time constant: 250 ms; release time constant: 1250 ms) or fast (bottom panel; attack time constant: 15 ms; release time constant: 50 ms) compression speed. Compression ratio is set to 2:1. The audiometric thresholds of the audiogram N4 – a moderately severe hearing loss (Bisgaard et al., 2010) – are entered into the NAL-NL2 prescription. Sounds are then processed by the same model of impaired auditory system as in Figure 8. Compression has detrimental effects on the perceptual representation of the natural soundscape, these effects being larger for fast compared to slow compression.
Direction VI. Impact of Human Activity on Natural Soundscape Perception
Auditory Awareness of Effects of Human Activity
In her seminal book, Rachel Carson (1962) triggered widespread environmental awareness in society by pointing out the capacity of our auditory system to detect dramatic changes in biodiversity caused by human activity in our close environment (in this case, massive extinction of insects and birds caused by chemical pollutants). This certainly highlights an important function of our auditory system as a warning system that deserves further study (e.g., Pereira et al., 2012). However, this warning function is most likely based on more ancestral and general mechanisms used to monitor our close acoustic environment such as those described in the preceding sections of this article. It is now a well-accepted fact that natural environments are rapidly changing as a result of human activity, profoundly altering animal behavior and fitness (e.g., Hale & Swearer, 2016). From the present perspective, this “human-induced rapid environmental change” (HIREC) has numerous impacts including changes in sound propagation characteristics, and drastic modifications in acoustic patterns associated with biological activity as well as meteorological and climatic conditions (Sueur et al., 2019).
Figure 10 shows the change in 2D-AMi spectra in a natural setting recorded in California (USA) over a single year. The changes resulted from selective logging (a deforestation practice consisting in removing a single tree about every 20 m). The differences between 2D-AMi spectra allow us to anticipate the strong auditory changes associated with this technique supposedly harmless to ecosystems and hardly detectable via the visual modality. These simulations point to the potentially dramatic sensory effects of direct and indirect HIREC.
Figure 10.
Top and bottom panels, respectively: photographs and 2D-AMi spectra of two soundscapes recorded in a sub-alpine meadow surrounded by ponderosa pine, juniper and lodge pole pine trees, with a clear mountain stream bisecting the habitat) over a 1 single year (Location: Lincoln Meadow, Yuba Pass, California, USA; GPS: 39°35′29.26″N/120°30′02.53″W; Altitude: 2100 m; Weather: Clear/partly cloudy (27°C), before (Date: 06/88) and after (Date: 06/89) selective logging at dawn. A tree-cutting protocol called selective logging (i.e., taking out a tree every 20 m) was expected to have no negative impact on the environment. All sounds can be listened to from the recordings provided in the “Supplementary Material” section. Source: B. Krause, Wild Sanctuary. In the 1989 recording, modulation energy has dropped substantially in high audio-frequency channels and slow temporal-modulation channels, reflecting large changes in biophony. This is not visible on the two photographs taken at the same place and time.
For all the reasons cited above, humans as any species equipped with an auditory system should be able to perceive HIREC, provided that crucial cues affected by HIREC are preserved in long-term auditory memory (however, see Cohen et al., 2009). So-called “sensory danger zones” caused by sensory pollutants such as anthropogenic sounds (e.g., traffic noise) are expanding. They not only alter animal behavior, reproduction and survival in large scales through auditory masking, distraction and misleading (Dominoni et al., 2020) – that is, energetic and informational masking according to the current psychoacoustical paradigm – but also human health and cognitive performance, as suggested by recent studies on “restorative effects” associated with exposure to natural soundscapes (Buxton et al., 2021; Lercher & Dzhambov, 2023; Ratcliffe, 2021). Perception of biodiversity (Ferraro et al., 2020), bird vocalizations (Hammoud et al., 2022) and water (Buxton et al., 2021) seem to play a particularly important role in this respect. Further work is warranted to better understand how the human auditory system perceives and adapts to these changes, and to which extent these changes alter sensory capacities and emotional reactions, and more broadly health. This aspect of the research program in human auditory ecology should be conducted in close collaboration with geographers who have already started exploring human perception of HIREC (e.g., Sourdril et al., 2017)
Human Auditory Ecology and Urban Design
A better understanding of how the human auditory system perceives and adapts to HIREC has many implications including recommendations for the design of green and blue spaces in sustainable cities and management of national parks (e.g., Kang et al., 2016), and development of hearing aids more suited to soundscape processing. Improving contact with nature in cities through access to green spaces (parks, forests, etc.), with biodiverse habitats and blue spaces (rivers, lakes, coastal water) has been suggested to contribute to quality of life for city dwellers, although the evidence is limited because of the insufficient number of studies and heterogeneity of exposure assessment (for critical reviews, see Gascon et al., 2015, and Erfanian et al., 2019). The dramatic auditory contrast between urban soundscapes recorded in two neighbour areas of the same city – a park and an adjacent street with high traffic – is illustrated in Figure 11, corroborating the possibility to attenuate drastically the perception of anthropophony and enhance the perception of biodiversity in urban green spaces.
Figure 11.
2D-AMi spectra for two urban soundscapes recorded in neighbouring places on the same day (duration: 30 s). Left: green space (Jardin de l'insectarium; 15/06/2020 at 06:00 am). Right: street with high traffic adjacent to the green space (Boulevard de l’Hôpital; 15/06/2020 at 03:10 am). Both recordings were made in Paris, France. For the green space, modulation energy in the high audio-frequency channels and slow temporal channels reflects the contribution of biophony (here, bird vocalizations). For the street, modulation energy caused by cars is found at all audio-frequency channels but mostly at faster temporal channels. All sounds can be listened to from the recordings provided in the “Supplementary Material” section. Source: S Haupert & J Sueur, Museum National d’Histoire Naturelle, CNRS.
As an example, a large-cohort study based on smartphone momentary assessment showed time-lasting mental-health benefits of listening and seeing birdlife, both in healthy people and people diagnosed with depression (Hammoud et al., 2022). This study provides support for the introduction of protection policies encouraging biodiverse habitats in urban and rural areas, or alternatively encourages more drastic changes in lifestyle and the way people inhabit their territory. It also provides support for mental healthcare policy prescribing nature-based activities (e.g., regular visits to habitats with a high degree of birdlife, such as parks). However, these efforts – which could be conceived as part of the ecosystem services of cities whose importance can only increase with climate change – could be undermined if the information conveyed by these green and blue spaces is not perceived or distorted because the ageing population in developed countries tends to develop hearing difficulties of sensory and cognitive origin (Roth et al., 2011). The success of this venture will depend on the ability to bridge the gap between audiology, sensory ecology and environmental acoustics.
Conclusions
Human auditory ecology – the scientific study of the complex and dynamic interactions between humans and their acoustic environment – should not be limited to the case of urban settings and communication, but should be extended to perception of wild or rural soundscapes and ecological processes at work in non-anthropogenic habitats. General knowledge gained in soundscape ecology and ecoacoustics should be taken into account by hearing scientists. Massive high-quality acoustic databases recorded by ecoacousticians combined with appropriate experimental paradigms targeting relevant monitoring behaviors should help setting up investigations of human perception with higher ecological validity. This would challenge our current knowledge of basic auditory mechanisms and auditory scene analysis and unveil monitoring capacities distinct from those engaged in communication. Their universality, specificity and the possibility to enhance them through life-long exposure, plasticity, training and cultural transmission are open to question. Moreover, natural soundscapes trigger specific emotional responses in humans that are already well documented and deserve to be studied in depth. Computational modelling, as illustrated throughout this paper, should effectively guide this endeavour. The extension of this research program to audiology should also improve our understanding of the perceptual consequences of cochlear and central damage and prompt useful evolutions in hearing aid processing. This also offers new perspectives to improve the quality of life – and more broadly health – of people living in rural settings or people visiting regularly green or blue spaces within cities or national parks. Human auditory ecology may also suggest ways to be better prepared to address transition to sustainable urban and rural environments.
Supplemental Material
Supplemental material, sj-wav-1-tia-10.1177_23312165231212032 for Human Auditory Ecology: Extending Hearing Research to the Perception of Natural Soundscapes by Humans in Rapidly Changing Environments by Christian Lorenzi, Frédéric Apoux, Elie Grinfeder, Bernie Krause, Nicole Miller-Viacava and Jérôme Sueur in Trends in Hearing
Supplemental material, sj-wav-2-tia-10.1177_23312165231212032 for Human Auditory Ecology: Extending Hearing Research to the Perception of Natural Soundscapes by Humans in Rapidly Changing Environments by Christian Lorenzi, Frédéric Apoux, Elie Grinfeder, Bernie Krause, Nicole Miller-Viacava and Jérôme Sueur in Trends in Hearing
Supplemental material, sj-wav-3-tia-10.1177_23312165231212032 for Human Auditory Ecology: Extending Hearing Research to the Perception of Natural Soundscapes by Humans in Rapidly Changing Environments by Christian Lorenzi, Frédéric Apoux, Elie Grinfeder, Bernie Krause, Nicole Miller-Viacava and Jérôme Sueur in Trends in Hearing
Supplemental material, sj-wav-4-tia-10.1177_23312165231212032 for Human Auditory Ecology: Extending Hearing Research to the Perception of Natural Soundscapes by Humans in Rapidly Changing Environments by Christian Lorenzi, Frédéric Apoux, Elie Grinfeder, Bernie Krause, Nicole Miller-Viacava and Jérôme Sueur in Trends in Hearing
Supplemental material, sj-wav-5-tia-10.1177_23312165231212032 for Human Auditory Ecology: Extending Hearing Research to the Perception of Natural Soundscapes by Humans in Rapidly Changing Environments by Christian Lorenzi, Frédéric Apoux, Elie Grinfeder, Bernie Krause, Nicole Miller-Viacava and Jérôme Sueur in Trends in Hearing
Supplemental material, sj-wav-6-tia-10.1177_23312165231212032 for Human Auditory Ecology: Extending Hearing Research to the Perception of Natural Soundscapes by Humans in Rapidly Changing Environments by Christian Lorenzi, Frédéric Apoux, Elie Grinfeder, Bernie Krause, Nicole Miller-Viacava and Jérôme Sueur in Trends in Hearing
Supplemental material, sj-wav-7-tia-10.1177_23312165231212032 for Human Auditory Ecology: Extending Hearing Research to the Perception of Natural Soundscapes by Humans in Rapidly Changing Environments by Christian Lorenzi, Frédéric Apoux, Elie Grinfeder, Bernie Krause, Nicole Miller-Viacava and Jérôme Sueur in Trends in Hearing
Supplemental material, sj-wav-8-tia-10.1177_23312165231212032 for Human Auditory Ecology: Extending Hearing Research to the Perception of Natural Soundscapes by Humans in Rapidly Changing Environments by Christian Lorenzi, Frédéric Apoux, Elie Grinfeder, Bernie Krause, Nicole Miller-Viacava and Jérôme Sueur in Trends in Hearing
Supplemental material, sj-wav-9-tia-10.1177_23312165231212032 for Human Auditory Ecology: Extending Hearing Research to the Perception of Natural Soundscapes by Humans in Rapidly Changing Environments by Christian Lorenzi, Frédéric Apoux, Elie Grinfeder, Bernie Krause, Nicole Miller-Viacava and Jérôme Sueur in Trends in Hearing
Supplemental material, sj-wav-10-tia-10.1177_23312165231212032 for Human Auditory Ecology: Extending Hearing Research to the Perception of Natural Soundscapes by Humans in Rapidly Changing Environments by Christian Lorenzi, Frédéric Apoux, Elie Grinfeder, Bernie Krause, Nicole Miller-Viacava and Jérôme Sueur in Trends in Hearing
Supplemental material, sj-wav-11-tia-10.1177_23312165231212032 for Human Auditory Ecology: Extending Hearing Research to the Perception of Natural Soundscapes by Humans in Rapidly Changing Environments by Christian Lorenzi, Frédéric Apoux, Elie Grinfeder, Bernie Krause, Nicole Miller-Viacava and Jérôme Sueur in Trends in Hearing
Supplemental material, sj-wav-12-tia-10.1177_23312165231212032 for Human Auditory Ecology: Extending Hearing Research to the Perception of Natural Soundscapes by Humans in Rapidly Changing Environments by Christian Lorenzi, Frédéric Apoux, Elie Grinfeder, Bernie Krause, Nicole Miller-Viacava and Jérôme Sueur in Trends in Hearing
Supplemental material, sj-wav-13-tia-10.1177_23312165231212032 for Human Auditory Ecology: Extending Hearing Research to the Perception of Natural Soundscapes by Humans in Rapidly Changing Environments by Christian Lorenzi, Frédéric Apoux, Elie Grinfeder, Bernie Krause, Nicole Miller-Viacava and Jérôme Sueur in Trends in Hearing
Supplemental material, sj-wav-14-tia-10.1177_23312165231212032 for Human Auditory Ecology: Extending Hearing Research to the Perception of Natural Soundscapes by Humans in Rapidly Changing Environments by Christian Lorenzi, Frédéric Apoux, Elie Grinfeder, Bernie Krause, Nicole Miller-Viacava and Jérôme Sueur in Trends in Hearing
Supplemental material, sj-wav-15-tia-10.1177_23312165231212032 for Human Auditory Ecology: Extending Hearing Research to the Perception of Natural Soundscapes by Humans in Rapidly Changing Environments by Christian Lorenzi, Frédéric Apoux, Elie Grinfeder, Bernie Krause, Nicole Miller-Viacava and Jérôme Sueur in Trends in Hearing
Supplemental material, sj-wav-16-tia-10.1177_23312165231212032 for Human Auditory Ecology: Extending Hearing Research to the Perception of Natural Soundscapes by Humans in Rapidly Changing Environments by Christian Lorenzi, Frédéric Apoux, Elie Grinfeder, Bernie Krause, Nicole Miller-Viacava and Jérôme Sueur in Trends in Hearing
Supplemental material, sj-wav-17-tia-10.1177_23312165231212032 for Human Auditory Ecology: Extending Hearing Research to the Perception of Natural Soundscapes by Humans in Rapidly Changing Environments by Christian Lorenzi, Frédéric Apoux, Elie Grinfeder, Bernie Krause, Nicole Miller-Viacava and Jérôme Sueur in Trends in Hearing
Supplemental material, sj-wav-18-tia-10.1177_23312165231212032 for Human Auditory Ecology: Extending Hearing Research to the Perception of Natural Soundscapes by Humans in Rapidly Changing Environments by Christian Lorenzi, Frédéric Apoux, Elie Grinfeder, Bernie Krause, Nicole Miller-Viacava and Jérôme Sueur in Trends in Hearing
Acknowledgments
Natural and urban soundscape recordings were made available by the Bernie Krause Natural Sound Archive (BKNSA) currently licensed exclusively to Wild Sanctuary, Inc., except for urban recordings in Paris and Marseille (France) that were made available by Jérôme Sueur and Sylvain Haupert (Museum National d’Histoire Naturelle) and Sabine Meunier (LMA, CNRS). The running water and speech database (French & English sentences) were made available by Marcus Klaus (Klaus et al., 2019) and Franck Ramus (Ramus, 2023; Ramus et al., 1999), respectively. The authors wish to thank Andrew Oxenham and two reviewers for very valuable comments and suggestions that helped us improving substantially this perspective paper. The authors are particularly grateful to the constructive and enlighting comments and suggestions of Valeriy Shafiro.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by ANR-17-EURE-0017, ANR-20-CE28-0011, ANR-20-CE28-0011 (Hearbiodiv), and chair CFM data ENS-PSL.
ORCID iDs: Christian Lorenzi https://orcid.org/0000-0001-7240-1653
Frédéric Apoux https://orcid.org/0000-0002-9300-6537
Supplementary Material: Supplementary material (audio recordings) is available at DOI: 10.5281/zenodo.10069144.
References
- Agus T. R., Thorpe S. J., Pressnitzer D. (2010). Rapid formation of robust auditory memories: Insights from noise. Neuron, 66, 610–618. 10.1016/j.neuron.2010.04.014 [DOI] [PubMed] [Google Scholar]
- Alcocer I., Lima H., Sugai L. S. M., Llusia D. (2022). Acoustic indices as proxies for biodiversity: A meta-analysis. Biological Reviews, 97, 2209–2236. 10.1111/brv.12890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmann C. F., Doehrmann O., Kaiser J. (2007). Selectivity for animal vocalizations in the human auditory cortex. Cerebral Cortex, 17, 2601–2608. 10.1093/cercor/bhl167 [DOI] [PubMed] [Google Scholar]
- Amin N., Doupe A., Theunissen F. E. (2007). Development of selectivity for natural sounds in the songbird auditory forebrain. Journal of Neurophysiology, 97, 3517–3531. 10.1152/jn.01066.2006 [DOI] [PubMed] [Google Scholar]
- Amin N., Gill P., Theunissen F. (2010). Role of the Zebra Finch auditory thalamus in generating complex representations for natural sounds. Journal of Neurophysiology, 104, 784–798. 10.1152/jn.00128.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apoux F., Miller-Viacava N., Ferriere R., Dai H., Krause B., Sueur J., Lorenzi C. (2023). Auditory discrimination of natural soundscapes. The Journal of the Acoustical Society of America, 153, 2706–2753. 10.1121/10.0017972 [DOI] [PubMed] [Google Scholar]
- Armstrong A., Lam C. C., Sabesan S., Lesica N. A. (2022). Compression and amplification algorithms in hearing aids impair the selectivity of neural responses to speech. Nature Biomedical Engineering, 6, 717–730. 10.1038/s41551-021-00707-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attias H., Schreiner C. E. (1997). Temporal low-order statistics of natural sounds. Advances in Neural Information Processing Systems, 9, 27–33. [Google Scholar]
- Aubin T., Bremond J.-C. (1983). The process of species-specific song recognition in the Skylark Alauda arvensis. An experimental study by means of synthesis. Zeitschrift für Tierpsychologieol, 61, 141–152. 10.1111/j.1439-0310.1983.tb01334.x [DOI] [Google Scholar]
- Axelsson O., Nilsson M. E., Berglund B. (2010). A principal components model of soundscape perception. The Journal of the Acoustical Society of America, 128, 2836–2846. 10.1121/1.3493436 [DOI] [PubMed] [Google Scholar]
- Bahadori, M., Barumerli, R., Geronazzo, M., Cesari, P. (2021). Action planning and affective states within the auditory peripersonal space in normal hearing and cochlearimplanted listeners. Neuropsychologia, 155, 107790. 10.1016/j.neuropsychologia.2021.107790 [DOI] [PubMed] [Google Scholar]
- Berman M. G., Hout M. C., Kardan O., Hunter M. R., Yourganov G., et al. (2014). The perception of naturalness correlates with low-level visual features of environmental scenes. PLoS One, 9(12), e114572. 10.1371/journal.pone.0114572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biberger T., Ewert S. D. (2017). The role of short-time intensity and envelope power for speech intelligibility and psychoacoustic masking. The Journal of the Acoustical Society of America, 142, 1098–1111. 10.1121/1.4999059 [DOI] [PubMed] [Google Scholar]
- Bisgaard N., Vlaming M. S. M. G., Dahlquist M. (2010). Standard audiograms for the IEC 60118-15 measurement procedure. Trends in Amplification, 14, 113–120. 10.1177/1084713810379609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botteldooren D., De Coensel B., De Muer T. (2006). The temporal structure of urban soundscapes. Journal of Sound and Vibration, 292, 105–123. 10.1016/j.jsv.2005.07.026 [DOI] [Google Scholar]
- Brefczynski-Lewis J. A., Lewis J. W. (2017). Auditory object perception: A neurobiological model and prospective review. Neuropsychologia, 105, 223–242. 10.1016/j.neuropsychologia.2017.04.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bregman A. S. (1990). Auditory scene analysis: The perceptual organization of sound. Bradford Books, MIT Press. ISBN: 9780262521956. [Google Scholar]
- Buxton R. T., Pearson A. L., Allou C., Fristrup K., Wittemyer G. (2021). A synthesis of health benefits of natural sounds and their distribution in national parks. Proceedings of the National Academy of Sciences of the United States of America, 118, 10.1073/pnas.2013097118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capranica R. R., Rose G. J., Brenowitz E. A. (1985). Time resolution in the auditory systems of Anurans. In Michelsen A. (Ed.), Time resolution in auditory systems (pp. 58–73). Springer. Proceedings in Life Sciences. 10.1007/978-3-642-70622-6_4 [DOI] [Google Scholar]
- Carlyon R. P. (2014). How the brain separates sounds. Trends in Cognitive Sciences, 8, 465–471. 10.1016/j.tics.2004.08.008 [DOI] [PubMed] [Google Scholar]
- Carson R. (1962). Silent spring. Houghton Mifflin. [Google Scholar]
- Cassarino M., Setti A. (2015). Environment as ‘brain training’: A review of geographical and physical environmental influences on cognitive ageing. Ageing Research Reviews, 23, 167–182. 10.1016/j.arr.2015.06.003 [DOI] [PubMed] [Google Scholar]
- Cassarino M., Setti A. (2016). Complexity as key to designing cognitive-friendly environments for older people. Frontiers in Psychology, 7, 1329. 10.3389/fpsyg.2016.01329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catchpole C. K., Slater P. J. B. (2008). Bird song: Biological themes and variations (2nd ed.). Cambridge University Press. 10.1017/CBO9780511754791 [DOI] [Google Scholar]
- Cervantes Constantino F., Pinggera L., Paranamana S., Kashino M., Chait M. (2012). Detection of appearing and disappearing objects in complex acoustic scenes. PLoS One, 7(9), e46167. 10.1371/journal.pone.0046167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Luo Y., Mammides C., Cao K., Zhu S., Goodale E. (2021). The relationship between acoustic indices, elevation, and vegetation, in a forest plot network of southern China. Ecological Indicators, 129, 107942. 10.1016/j.ecolind.2021.107942 [DOI] [Google Scholar]
- Chen Z., Wiens J. J. (2020). The origins of acoustic communication in vertebrates. Nature Communications, 11, 369. 10.1038/s41467-020-14356-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. A., Horowitz T. S., Wolfe J. M. (2009). Auditory recognition memory is inferior to visual recognition memory. Proceedings of the National Academy of Science USA, 106(14), 6008–6010. 10.1073/pnas.0811884106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutrot A., Manley E., Goodroe S., Gahnstrom C., Filomena G., Yesiltepe D., Dalton R. C., Wiener J. M., Hölscher C., Hornberger M., Spiers H. J. (2022). Entropy of city street networks linked to future spatial navigation ability. Nature, 604, 104–110. 10.1038/s41586-022-04486-7 [DOI] [PubMed] [Google Scholar]
- Darwin C. J. (1997). Auditory grouping. Trends in Cognitive Sciences, 1, 327–333. 10.1016/S1364-6613(97)01097-8 [DOI] [PubMed] [Google Scholar]
- De Coensel B., Botteldooren D. (2006). The quiet rural soundscape and how to characterize it. Acta Acustica United with Acustica, 92, 887–897. [Google Scholar]
- De Coensel B., Botteldooren D. (2007). The rhythm of the urban soundscape. Noise & Vibration Worldwide, 38, 11–17. 10.1260/095745607782689827 [DOI] [Google Scholar]
- De Coensel B., Botteldooren D. (2010). A model of saliency-based auditory attention to environmental sound [Paper presentation]. 20th International Congress on Acoustics (ICA-2010), Sydney, pp. 1–8. [Google Scholar]
- De Coensel B., Botteldooren D., Muer T. (2003). 1/f Noise in rural and urban soundscapes. Acta Acustica United with Acustica, 89, 287–295. [Google Scholar]
- Demany L., Semal C. (2008). The role of memory in auditory perception. In Yost W. A., Popper A. N., Fay R. R. (Eds.), Auditory perception of sound sources (Vol. 29). Springer. Springer Handbook of Auditory Research. 10.1007/978-0-387-71305-2_4 [DOI] [Google Scholar]
- Dervieux A., Lebreton J. D., Tamisier A. (1980). Technique et fiabilité des dénombrements aériens de canards et de foulques hivernant en Camargue. Revue D'Écologie (La Terre et La Vie), 34, 69–99. 10.3406/revec.1980.4056 [DOI] [Google Scholar]
- Divyapriya C., Pramod P. (2019). Ornithophony in the soundscape of Anaikatty Hills, Coimbatore, Tamil Nadu, India. Journal of Threatened Taxa, 11, 14471–14483. 10.11609/jott.4948.11.12.14471-14483 [DOI] [Google Scholar]
- Doehrmann O., Naumer M. J., Volz S., Kaiser J., Altmann C. F. (2008). Probing category selectivity for environmental sounds in the human auditory brain. Neuropsychologia, 46, 2776–2786. 10.1016/j.neuropsychologia.2008.05.011 [DOI] [PubMed] [Google Scholar]
- Doligez B., Boulinier T. (2008). Habitat selection and habitat suitability preferences. In Jørgensen S. E., Fath B. D. (Eds.), Behavioral ecology, encyclopedia of ecology 3 (pp. 1810–1830). Elsevier. [Google Scholar]
- Dominoni D. M., Halfwerk W., Baird E., Buxton R. T., Fernandez-Juricic E., Fristrup K. M., McKenna M. F., Mennitt D. J., Perkin E. K., Seymoure B. M., Stoner D. C., Tennessen J. B., Toth C. A., Tyrrell L. P., Wilson A., Francis C. D., Carter N. H., Barber J. R. (2020). Why conservation biology can benefit from sensory ecology. Nature Ecology & Evolution, 4, 502–511. 10.1038/s41559-020-1135-4 [DOI] [PubMed] [Google Scholar]
- Dooling R. J., Fay R. R., Popper A. N. (2000). Comparative hearing: Birds and reptiles. Springer Handbook of Auditory Research. 10.1007/978-1-4612-1182-2_1 [DOI] [Google Scholar]
- Dusenbery D. (1992). Sensory ecology. W. H. Freeman. [Google Scholar]
- Engel L. R., Frum C., Puce A., Walker N. A., Lewis J. W. (2009). Different categories of living and non-living sound-sources activate distinct cortical networks. Neuroimage, 47, 1778–1791. 10.1016/j.neuroimage.2009.05.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel M. S., Fiebig A., Pfaffenbach C., Fels J. (2021). A review of the use of psychoacoustic indicators on soundscape studies. Current Pollution Reports, 7, 359–378. 10.1007/s40726-021-00197-1 [DOI] [Google Scholar]
- Erfanian M., Mitchell A. J., Kang J., Aletta F. (2019). The psychophysiological implications of soundscape: A systematic review of empirical literature and a research agenda. International Journal of Environmental Research and Public Health, 16, 3533. 10.3390/ijerph16193533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwin R. M. (1982). Observer variability in estimating numbers: An experiment. Journal of Field Ornithology, 53, 159–167. [Google Scholar]
- European Environment Agency. (2020). Potential quiet areas in Europe based upon the quietness suitability index (QSI). European Environment Agency, 2020. https://www.eea.europa.eu/data-and-maps/figures/quietness-suitability-index-qsi-1
- Ey E., Fischer J. (2009). The acoustic adaptation hypothesis- A review of the evidence from birds, anurans, and mammals. Bioacoustics, 19, 21–48. 10.1080/09524622.2009.9753613 [DOI] [Google Scholar]
- Farina A., Gage S. H. (2017). Ecoacoustics: The ecological role of sounds. John Wiley & Sons. ISBN: 978-1-119-23069-4. [Google Scholar]
- Farina A., Mullet T. C., Bazabayeva T. A., Tazhibayeva T., Bulatova D., Li P. (2021). Perspectives on the ecological role of geophysical sounds. Frontiers in Ecology and Evolution, 9, 748398. 10.3389/fevo.2021.748398 [DOI] [Google Scholar]
- Fay R. (2009). Soundscapes and the sense of hearing of fishes. Integrative Zoology, 4, 26–32. 10.1111/j.1749-4877.2008.00132.x [DOI] [PubMed] [Google Scholar]
- Fay R. R., Popper A. N. (1994). Comparative hearing: Mammals. Springer Handbook of Auditory Research. 10.1007/978-1-4612-2700-7_1 [DOI] [Google Scholar]
- Fay R. R., Popper A. N. (1999). Comparative hearing: Fish and amphibians. Springer Handbook of Auditory Research. 10.1007/978-1-4612-0533-3_1 [DOI] [Google Scholar]
- Feigenson L., Dehaene S., Spelke E. (2004). Core systems of number. Trends in Cognitive Sciences, 8, 307–314. 10.1016/j.tics.2004.05.002 [DOI] [PubMed] [Google Scholar]
- Feld S., Brenneis D. (2004). Doing anthropology in sound. American Ethnologist, 31, 461–474. 10.1525/ae.2004.31.4.461 [DOI] [Google Scholar]
- Ferraro D. M., Miller Z. D., Ferguson L. A., Taff B. D., Barber J. R., Newman P., Francis C. D. (2020). The phantom chorus: Birdsong boosts human well-being in protected areas. Proceedings of the Royal Society B: Biological Sciences, 287, 20201811. 10.1098/rspb.2020.1811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebig A. (2023). Soundscape: A construct of human experience. In Schulte-Fortkamp B., Fiebig A., Sisneros J. A., Popper A. N., Fay R. R. (Eds.), Soundscapes : Humans and their acoustic environment (pp. 23–48). Springer Nature Switzerland AG. Springer Handbook of Auditory Research 76. 10.1007/978-3-031-22779-0_6 [DOI] [Google Scholar]
- Filipan K., De Coensel B., Aumond P., Can A., Lavandier C., Botteldooren D. (2019). Auditory sensory saliency as a better predictor of change than sound amplitude in pleasantness assessment of reproduced urban soundscapes. Building and Environment, 148, 730–741. 10.1016/j.buildenv.2018.10.054 [DOI] [Google Scholar]
- Fonseca P. J. (2014). Cicada acoustic communication. In Hedwig B. (Ed.), Insect hearing and acoustic communication. Animal signals and communication (Vol. 1). Springer. 10.1007/978-3-642-40462-7_7 [DOI] [Google Scholar]
- Forrest T. G. (1994). From sender to receiver: Propagation and environmental effects on acoustic signals. American Zoologist, 34, 644–654. 10.1093/icb/34.6.644 [DOI] [Google Scholar]
- Fuller R. A., Irvine K. N., Devine-Wright P., Warren P. H., Gaston K. J. (2007). Psychological benefits of greenspace increase with biodiversity. Biology Letters, 3, 390–394. 10.1098/rsbl.2007.0149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage S., Axel A. (2013). Visualization of temporal change in soundscape power of a Michigan lake habitat over a 4-year period. Ecological Informatics, 21, 100–109. 10.1016/j.ecoinf.2013.11.004 [DOI] [Google Scholar]
- Gasc A., Anso J., Sueur J., Jourdan H., Desutter-Grandcolas L. (2018). Cricket calling communities as an indicator of the invasive ant Wasmannia auropunctata in an insular biodiversity hotspot. Biological Invasions, 20, 1099–1111. 10.1007/s10530-017-1612-0 [DOI] [Google Scholar]
- Gasc G., Gottesman B., Francomano D., Jung J., Durham M., Mateljak J., Pijanowski B. C. (2018). Soundscapes reveal disturbance impacts: Biophonic response to wildfire in the Sonoran Desert Sky Islands. Landscape Ecology, 33, 1399–1415. 10.1007/s10980-018-0675-3 [DOI] [Google Scholar]
- Gascon M., Triguero-Mas M., Martínez D., Dadvand P., Forns J., Plasència A., Nieuwenhuijsen M. J. (2015). Mental health benefits of long-term exposure to residential green and blue spaces: A systematic review. International Journal of Environmental Research and Public Health, 12, 4354–4379. 10.3390/ijerph120404354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatehouse S., Elberling C., Naylor G. (1999). Aspects of auditory ecology and psychoacoustic function as determinants of benefits from and candidature for non-linear processing in hearing aids. In Rasmussen A. N., Osterhammel P. A., Anderson T., Poulsen T. (Eds.), Auditory models and non-linear hearing instruments (18th Danavox Symposium) (pp. 221–233). Holmens Trykkeri. [Google Scholar]
- Gaver W. W. (1993). What in the world do we hear?: An ecological approach to auditory event perception. Ecological Psychology, 5, 1–29. 10.1207/s15326969eco0501_1 [DOI] [Google Scholar]
- Geffen M. N., Gervain J., Werker J. F., Magnasco M. O. (2011). Auditory perception of self-similarity in water sounds. Frontiers in Integrative Neuroscience, 5. 10.3389/fnint.2011.00015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genuit K., Schulte-Fortkamp B., Fiebig A. (2023). Psychoacoustics in soundscape research. In Schulte-Fortkamp B., Fiebig A., Sisneros J. A., Popper A. N., Fay R. R. (Eds.), Soundscapes: Humans and their acoustic environment (pp. 157–184). Springer Nature Switzerland AG. Springer Handbook of Auditory Research 76. 10.1007/978-3-031-22779-0_6 [DOI] [Google Scholar]
- Gerhardt H. C., Bee M. A. (2007). Recognition and localization of acoustic signals. In Narins P. M., Feng A. S., Fay R. R., Popper A. N. (Eds.), Hearing and sound communication in amphibians (Vol. 28, pp. 113–146). Springer. Springer Handbook of Auditory Research. 10.1007/978-0-387-47796-1_5 [DOI] [Google Scholar]
- Gibson J. J. (1966). The senses considered as perceptual systems (335 pages). Houghton Mifflin. ISBN: 0313239614. [Google Scholar]
- Gil D., Llusia D. (2020). The bird dawn chorus revisited. In Mathevon N., Aubin T. (Eds.), Coding strategies in vertebrate acoustic communication (pp. 45–90). Springer International Publishing. 10.1007/978-3-030-39200-0_3 [DOI] [Google Scholar]
- Gould van Praag C. D., Garfinkel S. N., Sparasci O., Mees A., Philippides A. O., Ware M., Ottaviani Cristina, Critchley H. D. (2017). Mind-wandering and alterations to default mode network connectivity when listening to naturalistic versus artificial sounds. Scientific Reports, 7, 45273. 10.1038/srep45273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinfeder E., Lorenzi C., Haupert S., Sueur J. (2022). What do we mean by “soundscape”? A functional description. Frontiers in Ecology and Evolution, 10, 894232. 10.3389/fevo.2022.894232 [DOI] [Google Scholar]
- Grinfeder E., Lorenzi C., Haupert S., Sueur J. (2023). Evascape, a new tool to reconstruct terrestrial soundscapes for ecoacoustic and psychoacoustic research [Paper presentation]. XXVIIIth International Bioacoustics Congress (IBAC), 27 October–1 November 2023, Hokkaido, Japan. [Google Scholar]
- Guastavino C. E. (2003). Etude sémantique et acoustique de la perception des basses fréquences dans l’environnement sonore urbain [PhD dissertation]. [Google Scholar]
- Gunnarsson B., Kez I., Hedblom M., Ode Sang Å. (2017). Effects of biodiversity and environment-related attitude on perception of urban green space. Urban Ecosystems, 20, 37–49. 10.1007/s11252-016-0581-x [DOI] [Google Scholar]
- Guyot P., Houix O., Misdariis N., Susini P., Pinquier J., André-Obrecht R. (2017). Identification of categories of liquid sounds. The Journal of the Acoustical Society of America, 142, 878–889. 10.1121/1.4996124 [DOI] [PubMed] [Google Scholar]
- Gygi B., Kidd G., Watson C. (2004). Spectral-temporal factors in the identification of environmental sounds. The Journal of the Acoustical Society of America, 115, 1252–1265. 10.1121/1.1635840 [DOI] [PubMed] [Google Scholar]
- Gygi B., Shafiro V. (2013). Auditory and cognitive effects of aging on perception of environmental sounds in natural auditory scenes. Journal of Speech Language and Hearing Research, 56, 1595–1612. 10.1044/1092-4388(2013/12-0283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale R., Swearer S. E. (2016). Ecological traps: Current evidence and future directions. Proceedings of the Royal Society B: Biological Sciences, 283, 20152647. 10.1098/rspb.2015.2647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammoud R., Tognin S., Burgess L., Bergou N., Smythe M., Gibbons J., Davidson N., Afifi A., Bakolis I., Mechelli A. (2022). Smartphone-based ecological momentary assessment reveals mental health benefits of birdlife. Scientific Reports, 12, 17589. 10.1038/s41598-022-20207-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardt B., Benedict L. (2020). Can you hear me now? A review of signal transmission and experimental evidence for the acoustic adaptation hypothesis. Bioacoustics, 30, 716–742. 10.1080/09524622.2020.1858448 [DOI] [Google Scholar]
- Hautus M., Macmillan N., Creelman C. (2021). Detection theory: A user's guide. Routledge. 10.4324/9781003203636 [DOI] [Google Scholar]
- Hayes D. (2021). Environmental classification in hearing aids. Seminars in Hearing, 42, 186–205. 10.1055/s-0041-1735175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F., Stevenson I. H., Escabi M. A. (2023). Two stages of bandwidth scaling drives efficient neural coding of natural sounds. PLOS Computational Biology, 19(2), e1010862. 10.1371/journal.pcbi.1010862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller L. M., Oszczapinska U., Smith J. M. (2023). The causal properties of sounds predict their recognition and misidentification. The Journal of the Acoustical Society of America, 153(3_supplement), A363. 10.1121/10.0019172 [DOI] [Google Scholar]
- Henry K. S., Lucas J. R. (2010). Auditory sensitivity and the frequency selectivity of auditory filters in the Carolina chickadee, Poecile carolinensis. Animal Behaviour, 80, 497–507. 10.1016/j.anbehav.2010.06.012 [DOI] [Google Scholar]
- Hirst R. J., Cassarino M., Kenny R. A., Newell F. N., Setti A. (2022). Urban and rural environments differentially shape multisensory perception in ageing. Aging, Neuropsychology, and Cognition, 29, 197–212. 10.1080/13825585.2020.1859084 [DOI] [PubMed] [Google Scholar]
- Holleman G. A., Hooge I. T. C., Kemner C., Hessels R. S. (2020). The ‘real-world approach’ and its problems: A critique of the term ecological validity. Frontiers in Psychology, 11, 721. 10.3389/fpsyg.2020.00721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houix O., Lemaitre G., Misdariis N., Susini P., Urdapilleta I. (2012). A perceptive categorization of sounds produced the interaction of solid objects. Journal of the Acoustical Society of America, 18, 52–80. 10.1121/1.3384757 [DOI] [Google Scholar]
- Hoy R. R., Popper A. N., Fay R. R. (1998). Comparative hearing: Insects. Springer Handbook of Auditory Research. ISBN: 978-1-4612-6828-4. [Google Scholar]
- Huang N., Elhilali M. (2017). Auditory salience using natural soundscapes. The Journal of the Acoustical Society of America, 141, 2163–2176. 10.1121/1.4979055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Organization for Standardization. ISO/TS 12913-2: 2014. Acoustics-Soundscape- Part 1: Definition and conceptual framework . Geneva, Switzerland. Retrieved from https://www.iso.org/obp/ui/#iso:std:iso:12913:-1:ed-1:v1:en
- Irwin A., Hall D. A., Peters A., Plack C. J. (2011). Listening to urban soundscapes: Physiological validity of percept.ual dimensions. Psychophysiology, 48, 258–268. 10.1111/j.1469-8986.2010.01051.x [DOI] [PubMed] [Google Scholar]
- Isnard V., Chastres V., Viaud-Delmon I., Suied C. (2019). The time course of auditory recognition measured with rapid sequences of short natural sounds. Scientific Reports, 9, 8005. 10.1038/s41598-019-43126-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins J. J. (1985). Acoustic information for objects, places and events. In Warren W. H., Shaw R. E. (Eds.), Persistence and change (pp. 115–138). Erlbaum. [Google Scholar]
- Johansson G. (1973). Visual perception of biological motion and a model for its analysis. Perception & Psychophysics, 14, 201–211. 10.3758/BF03212378 [DOI] [Google Scholar]
- Johnson E. M. (2022). Improving speech intelligibility without sacrificing environmental sound recognition [PhD dissertation]. Ohio State University. [Google Scholar]
- Kang J., Aletta F., Gjestland T. T., Brown L. A., Botteldooren D., Schulte-Fortkamp B., Lercher P., van Kamp I., Genuit K., Fiebig A., Bento Coelho J. L., Maffei L., Lavia L. (2016). Ten questions on the soundscapes of the built environment. Building and Environment, 108, 284–294. 10.1016/j.buildenv.2016.08.011 [DOI] [Google Scholar]
- Kawashima T., Sato T. (2015). Perceptual limits in a simulated “Cocktail party”. Attention, Perception, & Psychophysics, 77, 2108–2120. 10.3758/s13414-015-0910-9 [DOI] [PubMed] [Google Scholar]
- Kaya E. M., Elhilali M. (2014). Investigating bottom-up auditory attention. Frontiers in Human Neuroscience, 8, 1–12. 10.3389/fnhum.2014.00327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser C., Petkov C. I., Lippert M., Logothetis N. K. (2005). Mechanisms for allocating auditory attention: An auditory saliency map. Current Biology, 15, 1943–1947. 10.1016/j.cub.2005.09.040 [DOI] [PubMed] [Google Scholar]
- Keidser G., Naylor G., Brungart D. G., Caduff A., Campos J., Carlile S., Carpenter M. G., Grimm G., Hohmann V., Holube I., Launer S., Lunner T., Mehra R., Rapport F., Slaney M., Smeds K. (2020). The quest for ecological validity in hearing science: What it is, why it matters, and how to advance it. Ear & Hearing, 41, 5S–19S. 10.1097/AUD.0000000000001241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keidser G., Naylor G., Brungart D. G., Caduff A., Campos J., Carlile S., Carpenter M. G., Grimm G., Hohmann V., Holube I., Launer S., Lunner T., Mehra R., Rapport F., Slaney M., Smeds K. (2022). Comment on the point of view “ecological validity, external validity and mundane realism in hearing science”. Ear & Hearing, 43, 1601–1602. 10.1097/AUD.0000000000001241 [DOI] [PubMed] [Google Scholar]
- Kell A. J. E., Yamins D. L. K., Shook E. N., Norman-Haignere S. V., McDermott J. H. (2018). A task-optimized neural network replicates human auditory behavior, predicts brain responses, and reveals a cortical processing hierarchy. Neuron, 98, 630–644.e16. 10.1016/j.neuron.2018.03.044 [DOI] [PubMed] [Google Scholar]
- Kidd G., Jr, Mason C. R., Richards V. M., Gallun F. J., Durlach N. I. (2008). Informational masking. In Yost W. A., Popper A. N., Fay R. R. (Eds). Auditory perception of sound sources (pp. 143–190). Springer. 10.1007/978-0-387-71305-2_6 [DOI] [Google Scholar]
- Kingstone A., Smilek D., Eastwood J. D. (2008). Cognitive ethology: A new approach for studying human cognition. British Journal of Psychology, 99, 317–340. 10.1348/000712607X251243 [DOI] [PubMed] [Google Scholar]
- Klaus M., Geibrink E., Hotchkiss E. R., Karlsson J. (2019). Listening to air–water gas exchange in running waters. Limnology and Oceanography: Methods, 17, 395–414. 10.1002/lom3.10321 [DOI] [Google Scholar]
- Kochkin S. (2011). Marketrak VIII patients report improved quality of life with hearing aid usage. The Hearing Journal, 64, 25–26. 10.1097/01.HJ.0000399150.30374.45 [DOI] [Google Scholar]
- Kothinti S. R., Huang N., Elhilali M. (2021). Auditory salience using natural scenes: An online study. The Journal of the Acoustical Society of America, 150, 2952–2966. 10.1121/10.0006750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koumura T., Terashima H., Furukawa S. (2019). Cascaded tuning to amplitude modulation for natural sound recognition. The Journal of Neuroscience, 39, 5517–5533. 10.1523/JNEUROSCI.2914-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koumura T., Terashima H., Furukawa S. (2023). Human-like modulation sensitivity emerging through optimization to natural sound recognition. The Journal of Neuroscience, 43, 3876–3894. https://doi.org/0.1523/JNEUROSCI.2002-22.2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köppl C. (2014). Insights from comparative hearing research. Springer Handbook of Auditory Research. 10.1007/978-1-4614-9077-7 [DOI] [Google Scholar]
- Krakauer J. W., Ghazanfar A. A., Gomez-Marin A., MacIver M. A., Poeppel D. (2017). Neuroscience needs behavior: Correcting a reductionist bias. Neuron, 93, 480–490. 10.1016/j.neuron.2016.12.041 [DOI] [PubMed] [Google Scholar]
- Krause B. (1987). Bioacoustics, habitat ambience in ecological balance. Whole Earth Review, 57, 14–18. ISBN: 0749-5056. [Google Scholar]
- Lakatos S., McAdams S., Causse R. (1997). The representation of auditory source characteristics: Simple geometric forms. Perception & Psychophysics, 59, 1180–1190. 10.3758/BF03214206 [DOI] [PubMed] [Google Scholar]
- Lemaitre G., Heller L. M. (2012). Auditory perception of material is fragile while action is strikingly robust. The Journal of the Acoustical Society of America, 131, 1337–1348. 10.1121/1.3675946 [DOI] [PubMed] [Google Scholar]
- Lemaitre G., Heller L. M. (2013). Evidence for a basic level in a taxonomy of everyday action sounds. Experimental Brain Research, 226, 253–264. 10.1007/s00221-013-3430-7 [DOI] [PubMed] [Google Scholar]
- Lemaitre G., Houix O., Misdariis N., Susini P. (2010). Listener expertise and sound identification influence the categorization of environmental sounds. Journal of Experimental Psychology: Applied, 16, 16–32. 10.1037/a0018762 [DOI] [PubMed] [Google Scholar]
- Lercher P., Dzhambov A. M. (2023). Soundscape and health. In Schulte-Fortkamp B., Fiebig A., Sisneros J. A., Popper A. N., Fay R. R. (Eds.), Soundscapes: Humans and their acoustic environment (pp. 243–276). Springer Nature Switzerland AG. Springer Handbook of Auditory Research 76. 10.1007/978-3-031-22779-0_6 [DOI] [Google Scholar]
- Lesica N. A., Grothe B. (2008). Efficient temporal processing of naturalistic sounds. PLoS One, 3, e1655. 10.1371/journal.pone.0001655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewicki M. S. (2002). Efficient coding of natural sounds. Nature Neuroscience, 5, 356–363. 10.1038/nn831 [DOI] [PubMed] [Google Scholar]
- Lewis J. W., Brefczynski J. A., Phinney R. E., Janik J. J., De Yoe E. A. (2005). Distinct cortical pathways for processing tool versus animal sounds. Journal of Neuroscience, 25, 5148–5158. 10.1523/JNEUROSCI.0419-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. W., Talkington W. J., Walker N. A., Spirou G. A., Jajosky A., Frum C., Brefczynski-Lewis J. A. (2009). Human cortical organization for processing vocalizations indicates representation of harmonic structure as a signal attribute. The Journal of Neuroscience, 29, 2283–2296. 10.1523/JNEUROSCI.4145-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewkowicz D. J. (2001). The concept of ecological validity: What are its limitations and is it bad to be invalid? Infancy, 2, 437–450. 10.1207/S15327078IN0204_03 [DOI] [PubMed] [Google Scholar]
- Lufti R. A., Gilbertson L., Heo I., Chang A. C., Stamas J. (2013). The information-divergence hypothesis of informational masking. The Journal of the Acoustical Society of America, 134, 2160–2170. 10.1121/1.4817875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutfi R. A., Oh E. L. (1997). Auditory discrimination of material changes in a struck-clamped bar. The Journal of the Acoustical Society of America, 102, 3647–3656. 10.1121/1.420151 [DOI] [PubMed] [Google Scholar]
- Lutfi R. A., Stoelinga C. N. J. (2010). Sensory constraints on auditory identification of the material and geometric properties of struck bars. The Journal of the Acoustical Society of America, 127, 350–360. 10.1121/1.3263606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley G. A., Fuchs P. A. (2011). Recent advances in comparative hearing. Hearing Research, 273, 1–6. 10.1016/j.heares.2011.01.005 [DOI] [PubMed] [Google Scholar]
- Marten K., Marler P. M. (1977). Sound transmission and its significance for animal vocalization. I. Temperate habitats. Behavioral Ecology and Sociobiology, 2, 271–290. 10.1007/BF00299740 [DOI] [Google Scholar]
- Martínez-Castilla P., García-Nogales MÁ, Campos R., Rodríguez M. (2015). Environmental sound recognition by timbre in children with Williams syndrome. Child Neuropsychology, 21(1), 90–105. 10.1080/09297049.2013.876492 [DOI] [PubMed] [Google Scholar]
- McAdams S., Roussarie V., Chaigne A., Giordano B. L. (2010). The psychomechanics of simulated sound sources: Material properties of impacted thin plates. The Journal of the Acoustical Society of America, 128, 1401–1413. 10.1121/1.3466867 [DOI] [PubMed] [Google Scholar]
- McDermott J. H., Oxenham A. J., Simoncelli E. P. (2009). Sound texture synthesis via filter statistics. Proceedings of IEEE Workshop on Applications of Signal Processing to Audio and Acoustics, New Paltz, NY, USA, pp. 297–300. 10.1109/ASPAA.2009.5346467. [DOI] [Google Scholar]
- McDermott J. H., Simoncelli E. P. (2011). Sound texture perception via statistics of the auditory periphery: Evidence from sound synthesis. Neuron, 71, 926–940. 10.1016/j.neuron.2011.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMullin M. A., Huggins N. C., Gygi B., Snyder J. S. (2023, February). Dimensionality of natural auditory scene perception: A factor analysis study [Paper presentation]. 46th MidWinter Meeting of the Association for Research in Otolaryngology (ARO), 11–15 February, Orlando, FL, USA. [Google Scholar]
- McPherson M. J., Sophia D. E., Durango A., Ossandon T., Valdés J., Undurraga E. A., Jacoby N., Godoy R. A., McDermott J. H. (2020). Perceptual fusion of musical notes by native Amazonians suggests universal representations of musical intervals. Nature Communications, 11, 2786. 10.1038/s41467-020-16448-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWalter R., Dau T. (2017). Cascaded amplitude modulations in sound texture perception. Frontiers in Neuroscience, 11, 485. 10.3389/fnins.2017.00485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWalter R., Lorenzi C. (2022, June). Listening to biodiversity [Paper presentation]. XIXth International Symposium on Hearing, 19–24 June, Lyon, France. sciencesconf.org:ish2022:383523. [Google Scholar]
- McWalter R., Lorenzi C. (2023, February). Grouping and segregating sound sources in natural environments [Paper presentation]. 46th MidWinter Meeting of the Association for Research in Otolaryngology (ARO), 11–15 February, Orlando, Florida, USA. [Google Scholar]
- McWalter R., McDermott J. H. (2018). Adaptive and selective time averaging of auditory scenes. Current Biology, 28, 1405–1418.e10. 10.1016/j.cub.2018.03.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehler J., Sebastian-Galls N., Nespor M. (2004). Biological foundations of language: Language acquisition, cues for parameter setting and the bilingual infant. In Gazzaniga M. S. (Ed.), The new cognitive neuroscience (pp. 825–836). MIT Press. ISBN-10: 0262071959. [Google Scholar]
- Michelsen A., Larsen O. N. (1983). Strategies for acoustic communication in complex environments. In Huber F., Mark H. (Eds), Neuroethology and behavioral physiology (pp. 321–331). Springer-Verlag. 10.1007/978-3-642-69271-0_23 [DOI] [Google Scholar]
- Miller C. T., Gire D., Hoke K., Huk A. C., Kelley D., Leopold D. A., Smear M. C., Theunissen F., Yartsev M., Niell C. M. (2022). Natural behavior is the language of the brain. Current Biology, 32, R482–R493. 10.1016/j.cub.2022.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller-Viacava N., Lazard D., Delmas T., Krause B., Apoux F., Lorenzi C. (2023, February). Effects of sensorineural hearing loss on auditory discrimination of natural soundscapes [Paper presentation]. 46th MidWinter Meeting of the Association for Research in Otolaryngology (ARO), 11–15 February, Orlando, Florida, USA. [Google Scholar]
- Mitchell A., Aletta F., Oberman T., Kang J. (2023, September). How do we define soundscape? [Paper presentation]. Forum Acusticum 2023, 10th Convention of the European Acoustics Association, 11–15 September, Turin, Italy. [Google Scholar]
- Młynarski W., McDermott J. H. (2019). Ecological origins of perceptual grouping principles in the auditory system. Proceedings of the National Academy of Sciences, 116, 25355–25364. 10.1073/pnas.1903887116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery R. A., Roloff G. J. (2017). Habitat selection. In Reference module in life sciences. Elsevier. ISBN: 978-0-12-809633-8. [Google Scholar]
- Moore B. C. J. (2007). Cochlear hearing loss: Physiological, psychological and technical issues (p. 332). Wiley. ISBN-10: 047051633X. [Google Scholar]
- Moore B. C. J., Gockel H. (2002). Factors influencing sequential stream segregation. Acta Acustica United with Acustica, 88, 320–333. [Google Scholar]
- Moore B. C. J., Gockel H. (2012). Properties of auditory stream formation. Philosophical Transactions of the Royal Society B: Biological Sciences, 367, 919–931. 10.1098/rstb.2011.0355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, B. C. J., Füllgrabe, C., & Stone, M. A. (2011). Determination of preferred parameters for multichannel compression using individually fitted simulated hearing AIDS and paired comparisons. Ear and Hearing, 32, 556–568. 10.1097/AUD.0b013e31820b5f4c [DOI] [PubMed] [Google Scholar]
- Morton E. S. (1975). Ecological sources of selection on avian sounds. The American Naturalist, 109, 17–34. 10.1086/282971 [DOI] [Google Scholar]
- Mouterde S. C., Theunissen F. E., Elie J. E., Vignal C., Mathevon N. (2014). Acoustic communication and sound degradation: How do the individual signatures of male and female Zebra Finch calls transmit over distance? PLoS One, 9, e102842. 10.1371/journal.pone.0102842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullet T. C., Farina A., Gage S. H. (2017). The acoustic habitat hypothesis: An ecoacoustics perspective on species habitat selection. Biosemiotics, 10, 319–336. 10.1007/s12304-017-9288-5 [DOI] [Google Scholar]
- Mullet T. C., Gage S. H., Morton J. M., Huettmann F. (2016). Temporal and spatial variation of a winter soundscape in south-central Alaska. Landscape Ecology, 31, 1117–1137. 10.1007/s10980-015-0323-0 [DOI] [Google Scholar]
- Murray M. M., Camen C., Gonzalez Andino S. L., Bovet P., Clarke S. (2006). Rapid brain discrimination of sounds of objects. The Journal of Neuroscience, 26, 1293–1302. 10.1523/JNEUROSCI.4511-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelken I., Rotman Y., Bar Yosef O. (1999). Responses of auditory-cortex neurons to structural features of natural sounds. Nature, 397, 154–157. 10.1038/16456 [DOI] [PubMed] [Google Scholar]
- Neuhoff J. G. (2004). Ecological psychoacoustics: Introduction and history. In Neuhoff J. G. (Ed.), Ecological psychoacoustics (pp. 1–13). Elsevier Academic Press. [Google Scholar]
- Osses Vecchi A., Varnet L., Carney L. H., Dau T., Bruce I. C., Verhulst S., Majdak P. (2022). A comparative study of eight human auditory models of monaural processing. Acta Acustica, 6, 17. 10.1051/aacus/2022008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo J. S., Nygaard L. C., Remez R. E., Pisoni D. B. (2021). The handbook of speech perception (2nd ed., pp. 1–755). Wiley. 10.1002/9781119184096 [DOI] [Google Scholar]
- Park S., Salles A., Allen K., Moss C. F., Elhilali M. (2021). Natural statistics as inference principles of auditory tuning in biological and artificial midbrain networks. eNeuro, 8(3), ENEURO.0525-20.2021. 10.1523/ENEURO.0525-20.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne S. R. (2008a). Are perceived soundscapes within urban parks restorative? Proceedings of Acoustics, 08, 5519–5524. [Google Scholar]
- Payne S. R. (2008b). Are perceived soundscapes within urban parks restorative? The Journal of the Acoustical Society of America, 123(5 Supplement), 3809. 10.1121/1.2935525 [DOI] [Google Scholar]
- Pereira A. G., Cruz A., Lima S. Q., Moita M. A. (2012). Silence resulting from the cessation of movement signals danger. Current Biology, 22, R627–R628. 10.1016/j.cub.2012.06.015 [DOI] [PubMed] [Google Scholar]
- Petsas T., Harrison J., Kashino M., Furukawa S., Chait M. (2016). The effect of distraction on change detection in crowded acoustic scenes. Hearing Research, 341, 179–189. 10.1016/j.heares.2016.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps E. A., Ling S., Carrasco M. (2006). Emotion facilitates perception and potentiates the perceptual benefits of attention. Psychological Science, 17, 292–299. 10.1111/j.1467-9280.2006.01701.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips Y. F., Towsey M., Roe P. (2018). Revealing the ecological content of long-duration audio-recordings of the environment through clustering and visualisation. PLoS One, 13, e0193345. 10.1371/journal.pone.0193345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijanowski B. C., Villanueva-Rivera L. J., Dumyahn S. L., Farina A., Krause B. L., Napoletano B. M., Gage S. H., Pieretti N. (2011). Soundscape ecology: The science of sound in the landscape. Bioscience, 61, 203–216. 10.1525/bio.2011.61.3.6 [DOI] [Google Scholar]
- Pisoni D. B., Remez R. E. (Eds.). (2005). The handbook of speech perception. Blackwell. ISBN 0-631-22927-2. [Google Scholar]
- Raimbault M. (2006). Qualitative judgments of urban soundscapes: Questioning questionnaires and semantic scales. Acta Acustica United with Acustica, 92, 929–937. [Google Scholar]
- Ramus F. (2023, January 4). Correlates of linguistic rhythm in the speech signal - Dataset. 10.17605/OSF.IO/RPVFJ [DOI] [PubMed] [Google Scholar]
- Ramus F., Nespor M., Mehler J. (1999). Correlates of linguistic rhythm in the speech signal. Cognition, 73, 265–292. 10.1016/S0010-0277(00)00101-3 [DOI] [PubMed] [Google Scholar]
- Ratcliffe E. (2021). Sound and soundscape in restorative natural environments: A narrative literature review. Frontiers in Psychology, 12, 570563. 10.3389/fpsyg.2021.570563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards D. G., Wiley R. H. (1980). Reverberation and amplitude fluctuations in the propagation of sound in a forest: Implications for animal communication. American Naturalist, 115, 381–399. 10.1086/283568 [DOI] [Google Scholar]
- Roth T. N., Hanebuth D., Probst R. (2011). Prevalence of age-related hearing loss: A review. European Archives in Otorhinolaryngology, 268, 1101–1107. 10.1007/s00405-011-1597-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saddler M. R., Gonzalez R., McDermott J. H. (2021). Deep neural network models reveal interplay of peripheral coding and stimulus statistics in pitch perception. Nature Communications, 12, 7278. 10.1038/s41467-021-27366-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer R. M. (1977). The soundscape: Our sonic environment and the tuning of the world (301 pages). Alfred Knopf. ISBN: 0394409663. [Google Scholar]
- Scheuregger O., Hjortkjær J., Dau T. (2021). Identification and discrimination of sound textures in hearing-impaired and older listeners. Trends in Hearing, 25, 23312165211065608. 10.1177/23312165211065608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmuckler M. A. (2001). What is ecological validity? A dimensional analysis. Infancy, 2, 419–436. 10.1207/S15327078IN0204_02 [DOI] [PubMed] [Google Scholar]
- Schulte-Fortkamp B., Fiebig A. (2023). Soundscape: The development of a new discipline. In Schulte-Fortkamp B., Fiebig A., Sisneros J. A., Popper A. N., Fay R. R. (Eds.), Soundscapes: Humans and their acoustic environment (pp. 1–21). Springer Nature Switzerland AG. Springer Handbook of Auditory Research 76. 10.1007/978-3-031-22779-0_6 [DOI] [Google Scholar]
- Schulte-Fortkamp B., Fiebig A., Sisneros J. A., Popper A. N., Fay R. R. (2023). Soundscapes: Humans and their acoustic environment (330 pages). Springer Nature Switzerland AG, Springer Handbook of Auditory Research 76. 10.1007/978-3-031-22779-0 [DOI] [Google Scholar]
- Schutz M., Gillard J. (2020). On the generalization of tones: A detailed exploration of non-speech auditory perception stimuli. Scientific Reports, 10, 9520. 10.1038/s41598-020-63132-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senter P. (2008). Voices of the past: A review of Paleozoic and Mesozoic animal sounds. Historical Biology, 20, 255–287. 10.1080/08912960903033327 [DOI] [Google Scholar]
- Shafiro V. (2008). Identification of environmental sounds with varying spectral resolution. Ear & Hearing, 29, 401–420. 10.1097/AUD.0b013e31816a0cf1 [DOI] [PubMed] [Google Scholar]
- Shafiro V., Hebb M., Walker C., Oh J., Hsiao Y., Brown K., Sheft S., Li Y., Vasil K., Moberly A. C. (2020). Development of the basic auditory skills evaluation battery for online testing of cochlear implant listeners. American Journal of Audiology, 29, 577–590. 10.1044/2020_AJA-19-00083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafiro V., Luzum N., Moberly A. C., Harris M. S. (2022). Perception of environmental sounds in cochlear implant users: A systematic review. Frontiers in Neuroscience, 15, 788899. 10.3389/fnins.2021.788899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N. C., Theunissen F. E. (2003). Modulation spectra of natural sounds and ethological theories of auditory processing. The Journal of the Acoustical Society of America, 114, 3394–3411. 10.1121/1.1624067 [DOI] [PubMed] [Google Scholar]
- Smith E. C., Lewicki M. S. (2006). Efficient auditory coding. Nature, 439, 978–982. 10.1038/nature04485 [DOI] [PubMed] [Google Scholar]
- Sonkusare S., Breakspear M., Guo C. (2019). Naturalistic stimuli in neuroscience: Critically acclaimed. Trends in Cognitive Sciences, 23, 699–714. 10.1016/j.tics.2019.05.004 [DOI] [PubMed] [Google Scholar]
- Sourdril A., Welch-Devine M., Andrieu E., Bélaïdi N. (2017). Do April showers bring May flowers? Knowledge and perceptions of local biodiversity influencing understanding of global environmental change. A presentation of the PIAF project. Natures Sciences Sociétés, 25, 56–62. 10.1051/nss/2017009 [DOI] [Google Scholar]
- Stein R. S. (1968). Modulation in bird sounds. The Auk, 85, 229–243. 10.2307/4083583 [DOI] [Google Scholar]
- Stevens M. (2013). Sensory ecology, behaviour, and evolution. Oxford University Press. [Google Scholar]
- Stilp C. E., Kiefte M., Kluender K. R. (2018). Discovering acoustic structure of novel sounds. The Journal of the Acoustical Society of America, 143, 2460–2473. 10.1121/1.5031018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strelnikov K., Collett E., Gaillard P., Truy E., Déguine O., Marx M., Barone P. (2018). Categorisation of natural sounds at different stages of auditory recovery in cochlear implant adult deaf patients. Hearing Research, 367, 182–194. 10.1016/j.heares.2018.06.006 [DOI] [PubMed] [Google Scholar]
- Sueur J., Aubin T. (2003). Specificity of cicada calling songs in the genus Tibicina (Hemiptera: Cicadidae). Systematic Entomology, 28, 481–492. 10.1046/j.1365-3113.2003.00222.x [DOI] [Google Scholar]
- Sueur J., Farina A. (2015). Ecoacoustics: The ecological investigation and interpretation of environmental sound. Biosemiotics, 8, 493–502. 10.1007/s12304-015-9248-x [DOI] [Google Scholar]
- Sueur J., Krause B., Farina A. (2019). Climate change is breaking Earth’s beat. Trends in Ecology & Evolution, 34, 971–973. 10.1016/j.tree.2019.07.014 [DOI] [PubMed] [Google Scholar]
- Sueur J., Pavoine S., Hamerlynck O., Duvail S. (2008). Rapid acoustic survey for biodiversity appraisal. PLoS One, 3, e4065. https://doi.org/10.1371/https://doi.org/ journal.pone.0004065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugai L., Desjonquères C., Silva T., Llusia D. (2019). A roadmap for survey designs in terrestrial acoustic monitoring. Remote Sensing in Ecology and Conservation, 6, 220–235. 10.1002/rse2.131 [DOI] [Google Scholar]
- Suied C., Agus T. R., Thorpe S. J., Mesgarani N., Pressnitzer D. (2014). Auditory gist: Recognition of very short sounds from timbre cues. The Journal of the Acoustical Society of America, 135, 1380–1391. 10.1121/1.4863659 [DOI] [PubMed] [Google Scholar]
- Suied C., Susini P., McAdams S., Patterson R. D. (2010). Why are natural sounds detected faster than pips? The Journal of the Acoustical Society of America, 127, EL105–EL110. 10.1121/1.3310196 [DOI] [PubMed] [Google Scholar]
- Suied C., Viaud-Delmon I. (2009). Auditory-visual object recognition time suggests specific processing for animal sounds. PLoS One, 4(4), e5256. 10.1371/journal.pone.0005256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarlao C., Steele D., Guastavino C. (2022). Assessing the ecological validity of soundscape reproduction in different laboratory settings. PLoS One, 17(6), e0270401. 10.1371/journal.pone.0270401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawdrous M. M., D’Onofrio K. L., Gifford R., Picou E. M. (2022). Emotional responses to non-speech sounds for hearing-aid and bimodal cochlear-implant listeners. Trends in Hearing, 26, 233121652210830. 10.1177/23312165221083091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekcan Aİ, Yılmaz E., Kaya Kızıloz B., Karadoller D. Z., Mutafoğlu M., Aktan Erciyes A. (2015). Retrieval and phenomenology of autobiographical memories in blind individuals. Memory (Hove, England), 23, 329–339. 10.1080/09658211.2014.886702 [DOI] [PubMed] [Google Scholar]
- Theunissen F. E., Elie J. E. (2014). Neural processing of natural sounds. Nature Reviews Neuroscience, 15, 355–366. 10.1038/nrn3731 [DOI] [PubMed] [Google Scholar]
- Thoret E., Varnet L., Boubenec Y., Ferriere R., Le Tourneau F.-M., Krause B., Lorenzi C. (2020). Characterizing amplitude and frequency modulation cues in natural soundscapes: A pilot study in four habitats of a biosphere reserve. The Journal of the Acoustical Society of America, 147, 3260–3274. 10.1121/10.0001174 [DOI] [PubMed] [Google Scholar]
- Traer J., McDermott J. H. (2016). Statistics of natural reverberation enable perceptual separation of sound and space. Proceedings of the National Academy of Sciences USA, 113(48), E7856–E7865. 10.1073/pnas.1612524113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troje N. F., Westhoff C. (2006). The inversion effect in biological motion perception: Evidence for a ‘‘life detector’’? Current Biology, 16, 821–824. 10.1016/j.cub.2006.03.022 [DOI] [PubMed] [Google Scholar]
- Truax B. (1978). The world soundscape project's handbook for acoustic ecology (171 pages). Music of the environment series, 5. A.R.C. Publications. ISBN 0-88985-011-9. [Google Scholar]
- Truax B. (2001). Acoustic communication (2nd ed.). Ablex Publishing. [Google Scholar]
- Turchet L., Camponogara I., Cesari P. (2015). Interactive footstep sounds modulate the perceptual-motor aftereffect of treadmill walking. Experimental Brain Research, 233, 205–214. 10.1007/s00221-014-4104-9 [DOI] [PubMed] [Google Scholar]
- UN habitat World cities report. (2022). Envisaging the future of cities . United Nations Human Settlements Programme (UN-Habitat). Retrieved from https://unhabitat.org/world-cities-report-2022-envisaging-the-future-of-cities. ISBN: 978-92-1-132894-3
- Varnet L., Ortiz-Barajas M. C., Guevara Erra R., Gervain J., Lorenzi C. (2017). A cross-linguistic study of speech modulation spectra. The Journal of the Acoustical Society of America, 142, 1976–1989. 10.1121/1.5006179 [DOI] [PubMed] [Google Scholar]
- Warren W. H., Verbrugge R. R. (1984). Auditory perception of breaking and bouncing events: A case study in ecological acoustics. Journal of Experimental Psychology: Human Perception and Performance, 10, 704–712. 10.1037/0096-1523.10.5.704 [DOI] [PubMed] [Google Scholar]
- Webster P. J., Skipper-Kallal L. M., Frum C. A., Still H. N., Ward B. D., Lewis J. W. (2017). Divergent human cortical regions for processing distinct acoustic-semantic categories of natural sounds: Animal action sounds vs. vocalizations. Frontiers in Neuroscience, 10, 579. 10.3389/fnins.2016.00579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley R. H. (2015). Noise matters: The evolution of communication. 10.4159/9780674287044. [DOI]
- Wiley R. H., Richards D. G. (1978). Physical constraints on acoustic communication in the atmosphere: Implications for the evolution of animal vocalizations. Behavioral Ecology and Sociobiology, 3, 69–94. 10.1007/BF00300047 [DOI] [Google Scholar]
- Woolley S., Fremouw T., Hsu A., Theunissen F. (2005). Tuning for spectro-temporal modulations as a mechanism for auditory discrimination of natural sounds. Nature Neuroscience, 8, 1371–1379. 10.1038/nn1536 [DOI] [PubMed] [Google Scholar]
- Yellamsetty A., Ozmeral E. J., Budinsky R. A., Eddins D. A. (2020). A comparison of environment classification among premium hearing instruments. Trends in Hearing, 25, 1–14. 10.1177/2331216520980968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yost W. A., Pastore M. T., Pulling K. R. (2019). The relative size of auditory scenes of multiple talkers. The Journal of the Acoustical Society of America, 146, EL219–EL224. 10.1121/1.5125007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong X., Yost W. A. (2017). How many images are in an auditory scene? The Journal of the Acoustical Society of America, 141(4), 2882–2892. 10.1121/1.4981118 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-wav-1-tia-10.1177_23312165231212032 for Human Auditory Ecology: Extending Hearing Research to the Perception of Natural Soundscapes by Humans in Rapidly Changing Environments by Christian Lorenzi, Frédéric Apoux, Elie Grinfeder, Bernie Krause, Nicole Miller-Viacava and Jérôme Sueur in Trends in Hearing
Supplemental material, sj-wav-2-tia-10.1177_23312165231212032 for Human Auditory Ecology: Extending Hearing Research to the Perception of Natural Soundscapes by Humans in Rapidly Changing Environments by Christian Lorenzi, Frédéric Apoux, Elie Grinfeder, Bernie Krause, Nicole Miller-Viacava and Jérôme Sueur in Trends in Hearing
Supplemental material, sj-wav-3-tia-10.1177_23312165231212032 for Human Auditory Ecology: Extending Hearing Research to the Perception of Natural Soundscapes by Humans in Rapidly Changing Environments by Christian Lorenzi, Frédéric Apoux, Elie Grinfeder, Bernie Krause, Nicole Miller-Viacava and Jérôme Sueur in Trends in Hearing
Supplemental material, sj-wav-4-tia-10.1177_23312165231212032 for Human Auditory Ecology: Extending Hearing Research to the Perception of Natural Soundscapes by Humans in Rapidly Changing Environments by Christian Lorenzi, Frédéric Apoux, Elie Grinfeder, Bernie Krause, Nicole Miller-Viacava and Jérôme Sueur in Trends in Hearing
Supplemental material, sj-wav-5-tia-10.1177_23312165231212032 for Human Auditory Ecology: Extending Hearing Research to the Perception of Natural Soundscapes by Humans in Rapidly Changing Environments by Christian Lorenzi, Frédéric Apoux, Elie Grinfeder, Bernie Krause, Nicole Miller-Viacava and Jérôme Sueur in Trends in Hearing
Supplemental material, sj-wav-6-tia-10.1177_23312165231212032 for Human Auditory Ecology: Extending Hearing Research to the Perception of Natural Soundscapes by Humans in Rapidly Changing Environments by Christian Lorenzi, Frédéric Apoux, Elie Grinfeder, Bernie Krause, Nicole Miller-Viacava and Jérôme Sueur in Trends in Hearing
Supplemental material, sj-wav-7-tia-10.1177_23312165231212032 for Human Auditory Ecology: Extending Hearing Research to the Perception of Natural Soundscapes by Humans in Rapidly Changing Environments by Christian Lorenzi, Frédéric Apoux, Elie Grinfeder, Bernie Krause, Nicole Miller-Viacava and Jérôme Sueur in Trends in Hearing
Supplemental material, sj-wav-8-tia-10.1177_23312165231212032 for Human Auditory Ecology: Extending Hearing Research to the Perception of Natural Soundscapes by Humans in Rapidly Changing Environments by Christian Lorenzi, Frédéric Apoux, Elie Grinfeder, Bernie Krause, Nicole Miller-Viacava and Jérôme Sueur in Trends in Hearing
Supplemental material, sj-wav-9-tia-10.1177_23312165231212032 for Human Auditory Ecology: Extending Hearing Research to the Perception of Natural Soundscapes by Humans in Rapidly Changing Environments by Christian Lorenzi, Frédéric Apoux, Elie Grinfeder, Bernie Krause, Nicole Miller-Viacava and Jérôme Sueur in Trends in Hearing
Supplemental material, sj-wav-10-tia-10.1177_23312165231212032 for Human Auditory Ecology: Extending Hearing Research to the Perception of Natural Soundscapes by Humans in Rapidly Changing Environments by Christian Lorenzi, Frédéric Apoux, Elie Grinfeder, Bernie Krause, Nicole Miller-Viacava and Jérôme Sueur in Trends in Hearing
Supplemental material, sj-wav-11-tia-10.1177_23312165231212032 for Human Auditory Ecology: Extending Hearing Research to the Perception of Natural Soundscapes by Humans in Rapidly Changing Environments by Christian Lorenzi, Frédéric Apoux, Elie Grinfeder, Bernie Krause, Nicole Miller-Viacava and Jérôme Sueur in Trends in Hearing
Supplemental material, sj-wav-12-tia-10.1177_23312165231212032 for Human Auditory Ecology: Extending Hearing Research to the Perception of Natural Soundscapes by Humans in Rapidly Changing Environments by Christian Lorenzi, Frédéric Apoux, Elie Grinfeder, Bernie Krause, Nicole Miller-Viacava and Jérôme Sueur in Trends in Hearing
Supplemental material, sj-wav-13-tia-10.1177_23312165231212032 for Human Auditory Ecology: Extending Hearing Research to the Perception of Natural Soundscapes by Humans in Rapidly Changing Environments by Christian Lorenzi, Frédéric Apoux, Elie Grinfeder, Bernie Krause, Nicole Miller-Viacava and Jérôme Sueur in Trends in Hearing
Supplemental material, sj-wav-14-tia-10.1177_23312165231212032 for Human Auditory Ecology: Extending Hearing Research to the Perception of Natural Soundscapes by Humans in Rapidly Changing Environments by Christian Lorenzi, Frédéric Apoux, Elie Grinfeder, Bernie Krause, Nicole Miller-Viacava and Jérôme Sueur in Trends in Hearing
Supplemental material, sj-wav-15-tia-10.1177_23312165231212032 for Human Auditory Ecology: Extending Hearing Research to the Perception of Natural Soundscapes by Humans in Rapidly Changing Environments by Christian Lorenzi, Frédéric Apoux, Elie Grinfeder, Bernie Krause, Nicole Miller-Viacava and Jérôme Sueur in Trends in Hearing
Supplemental material, sj-wav-16-tia-10.1177_23312165231212032 for Human Auditory Ecology: Extending Hearing Research to the Perception of Natural Soundscapes by Humans in Rapidly Changing Environments by Christian Lorenzi, Frédéric Apoux, Elie Grinfeder, Bernie Krause, Nicole Miller-Viacava and Jérôme Sueur in Trends in Hearing
Supplemental material, sj-wav-17-tia-10.1177_23312165231212032 for Human Auditory Ecology: Extending Hearing Research to the Perception of Natural Soundscapes by Humans in Rapidly Changing Environments by Christian Lorenzi, Frédéric Apoux, Elie Grinfeder, Bernie Krause, Nicole Miller-Viacava and Jérôme Sueur in Trends in Hearing
Supplemental material, sj-wav-18-tia-10.1177_23312165231212032 for Human Auditory Ecology: Extending Hearing Research to the Perception of Natural Soundscapes by Humans in Rapidly Changing Environments by Christian Lorenzi, Frédéric Apoux, Elie Grinfeder, Bernie Krause, Nicole Miller-Viacava and Jérôme Sueur in Trends in Hearing