ABSTRACT
A large group of keratin genes (n=54 in the human genome) code for intermediate filament (IF)-forming proteins and show differential regulation in epithelial cells and tissues. Keratin expression can be highly informative about the type of epithelial tissue, differentiation status of constituent cells and biological context (e.g. normal versus diseased settings). The foundational principles underlying the use of keratin expression to gain insight about epithelial cells and tissues primarily originated in pioneering studies conducted in the 1980s. The recent emergence of single cell transcriptomics provides an opportunity to revisit these principles and gain new insight into epithelial biology. Re-analysis of single-cell RNAseq data collected from human and mouse skin has confirmed long-held views regarding the quantitative importance and pairwise regulation of specific keratin genes in keratinocytes of surface epithelia. Furthermore, such analyses confirm and extend the notion that changes in keratin gene expression occur gradually as progenitor keratinocytes commit to and undergo differentiation, and challenge the prevailing assumption that specific keratin combinations reflect a mitotic versus a post-mitotic differentiating state. Our findings provide a blueprint for similar analyses in other tissues, and warrant a more nuanced approach in the use of keratin genes as biomarkers in epithelia.
Keywords: Keratin, Intermediate filament, Transcriptomics, Single-cell biology, Proliferation, Differentiation, Homeostasis
Summary: An analysis of publicly available sc-RNAseq data from human and mouse epidermis to determine foundational principles and emerging paradigms regarding the significance of keratin gene expression.
INTRODUCTION
Keratin genes and proteins serve as unparalleled markers towards the identity and status of epithelial cells throughout the body. This reality has been known and exploited for over 40 years (Moll et al., 1982; Fuchs et al., 1985; O'Guin et al., 1990; Fuchs, 1995; Moll et al., 2008). Whole-genome sequencing efforts in the past two decades have expanded our knowledge of the extent of keratin gene diversity and their utility as epithelial markers (Hesse et al., 2004; Schweizer et al., 2006). In humans, 28 type I and 26 type II intermediate filament (IF) genes code for keratin proteins of sizes ranging between 40 and 70 kDa (Schweizer et al., 2006). The dual nature of keratin IF genes (Fuchs et al., 1981) reflects a strict requirement for heteropolymerization of keratin proteins into 10 nm IFs (Coulombe and Fuchs, 1990; Hatzfeld and Weber, 1990; Steinert, 1990). Accordingly, epithelial cells must coordinate the expression of (at least) one type I and (at least) one type II gene to assemble an IF network in their cytoplasm (Sun et al., 1983; Fuchs et al., 1985; Fuchs, 1995; Kim and Coulombe, 2007). Pioneering studies, largely conducted in the 1980s, established that many type I and II keratin genes are tightly regulated in a pairwise fashion (Sun et al., 1983; Fuchs et al., 1985; Cooper and Sun, 1986; Eichner et al., 1986). Furthermore, these pioneering studies revealed the specificity of keratin genes and proteins for the type of epithelial cells, their differentiation status, and whether they are engaged in an adaptative or disease process (Sun et al., 1983; Fuchs et al., 1985; O'Guin et al., 1990; Fuchs, 1995; Moll et al., 2008). Relative to internal ‘simple’ epithelia that occur in the gut, liver, kidney and pancreas (Omary, 2017), surface epithelia, such as those lining up the skin, oral mucosa and cornea, exhibit a greater complexity in keratin expression (Fuchs and Green, 1980; Fuchs, 1995; Moll et al., 2008). The defining features of the keratin gene family, including their transcriptional regulation and the primary structure of their protein products, are highly conserved in terrestrial mammals (Ehrlich et al., 2019), emphasizing their usefulness and significance as epithelial biomarkers. Accordingly, the signature elements that typify keratin genes and proteins continue to be utilized by researchers and clinicians to interpret epithelial phenotypes when studying disease samples and relevant experimental models (Moll et al., 2008; Rashmi et al., 2009; Karantza, 2011; Sharma et al., 2019).
RESULTS
Revisiting the significance of keratin expression through single-cell genomics
The increasing accessibility of single-cell RNAseq (scRNAseq) datasets provides a powerful opportunity to revisit the fundamental principles that have been assumed and relied on when using keratin expression to monitor epithelial cell identity or status. In this text, using the epidermis of both human and mouse as examples, we took advantage of available scRNAseq data and paid special attention to three popular notions regarding keratin regulation in surface epithelia; first, that keratins are among the most highly expressed genes in keratinocytes; second, that specific pairings of type I and II keratin genes indeed show tight co-regulation in progenitor and differentiating keratinocytes; and third, that specific cell states and localization within epidermis are sharply reflected in keratin expression.
Human trunk skin features a low density of epithelial appendages (hair, glands) along with a relatively thin, low complexity epidermis (Fig. 1A), facilitating an assessment of these principles. We re-analyzed a dataset from human trunk skin reported in Cheng et al. (Cheng et al., 2018; European Genome-phenome Archive accession no. EGAS00001002927) because it features a robust representation of living keratinocytes from across the epidermal sheet (Fig. S1A). A total of 24,979 keratinocytes from this study were included in our analyses (see Materials and Methods section). Given the strategic importance of transgenic mouse models to study epithelial biology in health and disease, we also analyzed a dataset from mouse ear epidermis produced by Lukowski et al. (Lukowski et al., 2018; ArrayExpress accession no. E-MTAB-6429) (Fig. S3A). A total of 4761 keratinocytes from the Lukowski study were included in our analyses (see Materials and Methods section).
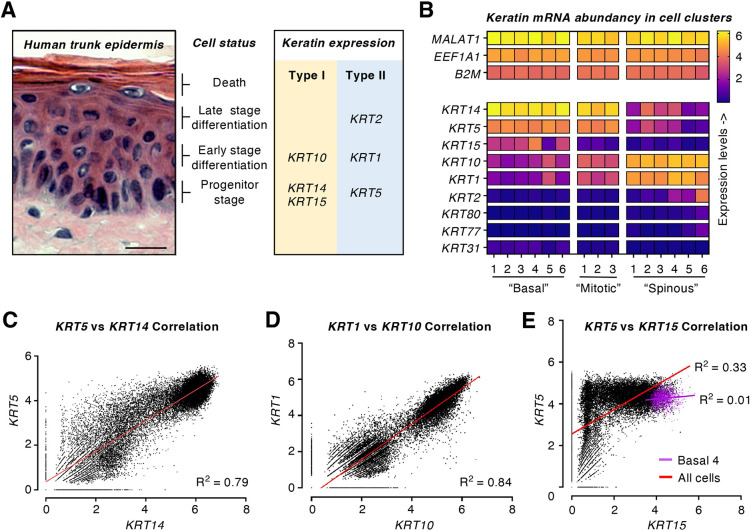
Fig. 1.
Abundant and pairwise expression of keratin genes in human trunk skin. (A) Cryosection of healthy human trunk skin, stained with hematoxylin-eosin (archival material obtained with institutional IRB approval). The status of keratinocytes and their corresponding keratin expression are indicated on the right. Scale bar: 20 μm. (B) Heatmap of keratin gene expression in the 15 Seurat clusters of keratinocytes in healthy human trunk skin, analyzed by scRNAseq (Basal1-6, Mitotic1-3 and Spinous1-6). The genes shown at the top (MALAT1, EEF1A1 and B2M) represent genes that are highly expressed across all clusters. (C–E) Correlations between specific type I and type II keratin genes across the entire array of epidermal keratinocytes (n=24,979 cell). Red lines represent linear correlations, and r2 values are reported. (C) KRT5 versus KRT14. (D) KRT1 versus KRT10. (E) KRT5 versus KRT15. In E, the magenta line conveys the linear correlation for the Basal4 cluster, specifically. Data set published by Cheng et al. (2018) and reanalyzed using a standard Seurat pipeline (see Materials and Methods section). Data set publicly available at EGAS00001002927.
Abundance of keratin gene expression in normal human interfollicular epidermis
Keratinocytes of conventional interfollicular epidermis primarily express six keratin genes. These are the type II IF KRT5 and the type I IFs KRT14 and KRT15, characteristic of progenitor cells located in the basal layer (Fuchs and Green, 1980; Nelson and Sun, 1983; Lloyd et al., 1995), the type II KRT1 and type I KRT10, characteristic of early stage differentiating cells (Fuchs and Green, 1980; Woodcock-Mitchell et al., 1982), and the type II IF KRT2, characteristic of late stage differentiating cells (Collin et al., 1992a,b) (Fig. 1A). The number of reads for a given gene, when normalized to library size, directly relates to abundance of mRNA transcripts in that given cell in a scRNAseq data set. MALAT1 (Wu et al., 2015), EEF1A1 (Abbas et al., 2015) and B2M (Li et al., 2016) are examples of genes for which the number of reads is consistently at the top across most cell clusters in Cheng et al. (2018) (Fig. 1B), thus providing a reference for highly expressed genes in human epidermis.
In relatively undifferentiated keratinocytes (e.g. ‘Basal1-6’ clusters; Fig. S1A), KRT5 and especially KRT14 are among the highest abundance mRNAs in human trunk skin epidermis (Fig. 1B; Table S1). Relative to KRT5 and KRT14, the KRT15 mRNA is more restricted in its expression domain and expression levels (Fig. 1B). KRT15 is one of the ten most expressed genes in the ‘Basal4’ cluster (Table S1), which is populated by cells exhibiting a strong basal lamina gene signature (POSTN, DST and COL17A1), high levels of KRT14 mRNA (Tables S1 and S2), and that are predominantly in the G1 phase of the cell cycle (Fig. S1D,E). Across all relevant clusters, the average number of reads per cell for the type I KRT14, either alone or combined with KRT15, are markedly higher than those for KRT5, the sole type II keratin gene expressed in undifferentiated keratinocytes of interfollicular epidermis (Table S2, Fig. S2A). These observations also apply to mitotic keratinocytes (‘Mitotic1-3’ clusters; see Fig. 1B, Tables S1 and S2). Since the size of mature mRNAs is similar for type I (<2.0 kb) and type II keratins (<2.5 kb) (Fuchs et al., 1981; Roop et al., 1983), size-related biases likely do not contribute to differences in transcript abundance.
In keratinocytes undergoing differentiation (e.g. ‘Spinous1-6’ clusters; Fig. S1A), KRT1 and KRT10 represent highly abundant transcripts (Fig. 1B; Table S1). In this instance, the number of reads for KRT1 and KRT10 transcripts is closer to a 1:1 ratio (Fig. S2A, Table S2). The KRT2 mRNA is considerably more restricted in both its expression domain and levels relative to each of KRT1 and KRT10 (Fig. 1B; Table S1). KRT2 is one of the ten most expressed genes in only one cluster (Spinous6), which is populated by cells that express late differentiation markers (Table S1) and that are in G1 phase of the cell cycle (Fig. S1D,E).
These scRNAseq analyses confirm the notion that the six keratin genes known to be expressed at substantial levels in keratinocytes of the epidermis, namely, the type II IF KRT1, KRT2, and KRT5 and the type I IF KRT10, KRT14, and KRT15 genes, are among the most highly expressed genes in cells making up this epithelium.
Pairwise regulation of keratin genes in human trunk epidermis
The Cheng et al. dataset (Cheng et al., 2018) also provides a straightforward opportunity to re-examine pairwise regulation of type I and II keratin genes in healthy human epidermis. Along with the KRT8–KRT18 pair emblematic of simple epithelia (Omary, 2017), KRT5–KRT14 and KRT1–KRT10 pairs are understood to be among the tightest keratin pairings in all of epithelial biology. Expression of keratin pairs at a single-cell level was plotted across all epidermal keratinocytes, totaling 24,979 cells, and linear correlations in keratin expression across cells and cell clusters in human trunk skin were assessed. Very high Pearson correlation coefficients occur between KRT5 and KRT14 mRNA reads (r2=0.79; P<0.0001; Fig. 1C) and KRT1 and KRT10 mRNA reads (r2=0.84; P<0.0001; Fig. 1D). We proceeded to test whether similar conclusions apply when considering relevant individual clusters (Table S3). A reduction in statistical robustness is expected for such analyses due to smaller cell numbers in single clusters along with assessing correlations in restricted ranges such as defined clusters (Bland and Altman, 2011). Regardless, KRT1 and KRT10 are highly correlated in the ‘Spinous4’ cluster (n=1007 cells; r2=0.82; P<0.0001), which features the highest number of reads for both genes. Similarly, KRT5 and KRT14 show a lesser though significant correlation in, for example, the ‘Basal1’ cluster (n=5037 cells; r2=0.35; P<0.0001), which shows the highest levels of KRT14 mRNA reads (Table S3). The degree to which KRT5 and KRT14, and KRT1 and KRT10, are each coordinately regulated is truly remarkable when factoring in the notion that type I and type II keratin genes are clustered on separate chromosomes in the human genome and that of other mammals (Powell et al., 1986; Lessin et al., 1988; Romano et al., 1988). As of yet, there is no mechanistic basis for the co-regulation of type I and II keratin genes in either general or specific terms.
Keratin gene-specific correlations are far less compelling for the more restricted KRT15 (type I; progenitor keratinocytes) and KRT2 (type II; differentiating keratinocytes) genes. Across the entire array of keratinocytes in human trunk skin, KRT15 and KRT5 (type II) exhibit a markedly lower Pearson correlation coefficient (r2=0.33) than KRT5–KRT14 (Fig. 1E). Surprisingly, KRT15 exhibits a higher Pearson correlation coefficient with type I KRT14 (r2=0.48) (Table S3). Importantly, high expression of two keratins does not necessarily imply linear correlation at a single-cell level. For instance, linear regression analysis indicates no correlation between KRT15 and KRT5 (r2=0.01) in the cell cluster featuring the highest expression of the two genes (‘Basal4’; Fig. 1E, Table S3). For KRT2, the correlation observed with type I KRT10 also appears to be relatively weak, given r2=0.14 for trunk skin cells as a whole. In this case, the two genes show a stronger correlation in the cluster that expresses KRT2 at the highest level (‘Spinous6’, r2=0.56; Fig. S2B, Table S3). From these findings, we infer that there are keratin genes, for example, KRT15, for which pairwise regulation does not represent a compelling attribute at the mRNA transcript level, at least in human trunk skin. It will prove interesting to test whether this notion applies to other epithelia in which KRT15 transcript levels are higher relative to KRT14 and/or other type I keratins.
Emerging concept in keratinocyte biology – hybrid expression of progenitor- and differentiation-related keratins
The transition that occurs between progenitor and differentiating keratinocytes in interfollicular epidermis is considered by many to be sharp and tightly coupled to the transition between KRT5–KRT14 and KRT1–KRT10 expression. Onset of KRT1 and KRT10 expression in healthy interfollicular epidermis is widely believed to occur when progenitor keratinocytes in the basal compartment become post-mitotic and enter the suprabasal compartment. However, this notion does not accurately reflect foundational studies on keratin expression published in the 1980s (Fuchs and Green, 1980; Schweizer et al., 1984; Regnier et al., 1986; Roop, 1987). Such views on the respective significance of KRT5–KRT14 and KRT1–KRT10 expression originate, in part, from the spatial staining patterns afforded by several (but not all) antibodies to these keratin proteins in immunostained tissue sections under standard imaging conditions (e.g. ‘suprabasal’ K14 is often equated with hyperproliferation in the literature). Indeed, combining experimental evidence with technical advances in sequencing, a growing body of literature reports on keratinocytes with hybrid features including the expression of progenitor- and differentiation-related keratins. Initial observations of these populations were established in 2020 in both newborn mice (Lin et al., 2020) and neonatal human foreskin (Wang et al., 2020) where scRNAseq data identified hybrid interfollicular epidermal transition cells. Although both studies utilized experimental methods to validate these findings in vivo [RNA-FISH in Lin et al. (2020) and immunohistochemistry in Wang et al. (2020)], they differed in the observed proportion of transitional cells in the dataset (with 20% observed in newborn mouse skin versus 4% in human neonatal foreskin). Although these discrepancies could reflect differences on a tissue or organismal level (which our analysis supports), we note that the exact proportion of these hybrid cells among all keratinocytes could be biased due to methodological differences in cell dissociation, impacting the keratinocyte populations sequenced and the heterogeneity reflected (Kim et al., 2020). Additional studies have since utilized immunohistochemistry to observe these transitional cells, and identify them as basal cells committed for differentiation (Krt14+ Krt10+), yet capable of cycling (Aragona et al., 2020). Utilizing a pulse-chase BrdU approach Aragona et al. observed that 36% of cycling cells in mouse dorsal skin were positive for both K14 and K10, suggesting that such transient cells might still actively cycle under homeostatic conditions (Aragona et al., 2020). Most recently, this paradigm was further explored under live imaging in vivo utilizing lineage tracing and a Krt10 expression reporter (Cockburn et al., 2021). Further supporting the existence of a cycling, transient population, the authors were able to follow basal cell commitment to differentiation under live imaging, noting the induction of Krt10, and observing proliferation of these hybrid, differentiation-committed cells (Cockburn et al., 2021).
With these observations in mind, we wanted to identify whether an approach focused on keratin regulation supports these emerging findings, as well as identify whether such a population might be conserved in the human trunk epidermis. We set out to examine these issues anew through a multi-pronged approach that included (1) relating KRT14 and KRT10 expression in the 24,979 single epidermal keratinocytes from the Cheng et al. (Cheng et al., 2018) human data set and (2) analyzing this relationship from the perspective of genes that are reliably associated with the G2/M phase of the cell cycle and localization to the basal layer of the epidermis.
Relating KRT10 to KRT14 in a scatter plot (Fig. 2A) reveals that large numbers of epidermal keratinocytes partition to a KRT14high/KRT10low subgroup (53% of the pool), consistent with a progenitor character, or to a KRT14low/KRT10high subgroup (37% of the pool), consistent with a differentiating character. The observation that the KRT14high/KRT10low group represents a larger group of cells in the scRNAseq data is not surprising and could be due to methodological ease of obtaining single cells from the basal rather than the suprabasal differentiating compartment of epidermis (Kim et al., 2020; Burja et al., 2022). However, we note that the dataset contains a large fraction of cells expressing late-stage differentiation markers (such as KRT2; Fig. 1B), and that 37% of the keratinocyte pool represents 9230 cells, indicative of the robustness of the dataset in capturing subpopulations of keratinocytes at varying stages of differentiation. With this caveat alleviated to a significant degree, we find that, surprisingly, a sizable fraction of keratinocytes express appreciable levels of both KRT14 and KRT10 transcripts (9.5% of the pool). That such KRT14high/KRT10high keratinocytes exhibit a hybrid character is further substantiated by their content in mRNAs associated with both a progenitor state (e.g. POSTN, DST and COL17A) and a differentiated state [e.g. DMKN, DSP, KRTDAP and SBSN]. In contrast, no cells with hybrid characteristics were identified when KRT15 and KRT2 levels were related in such a scatter plot (Fig. S2C).
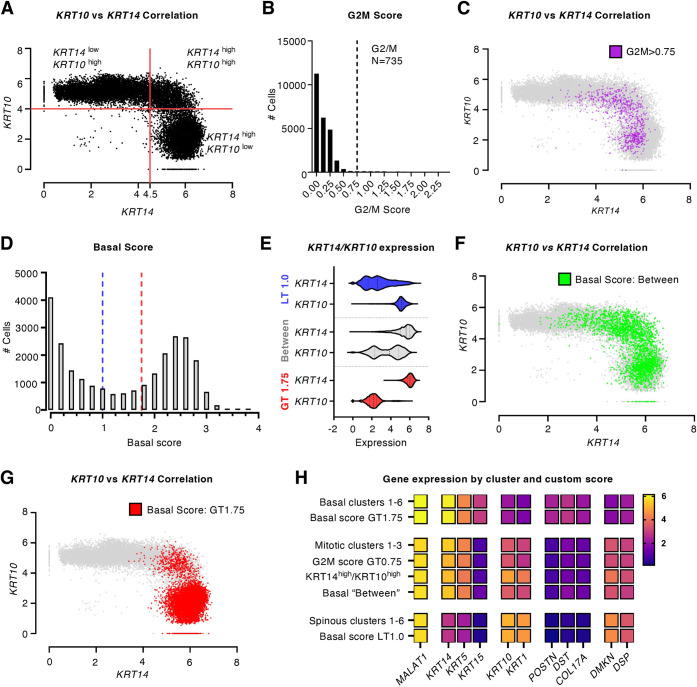
Fig. 2.
scRNAseq data identifies a KRT10high/KRT14high hybrid keratinocyte population. (A) Correlation of KRT10 and KRT14 in human trunk epidermis (n=24,979 cells). Quadrants were defined by abundancy of cells expressing high KRT14 (>4.5) or high KRT10 (>4.0). (B) Histogram reporting on the composite G2/M score across the same cell population. Dashed line represents the threshold for G2/M cells (>0.75, ∼3% of cells). (C) Mapping of G2/M cells with a high (>0.75) G2/M score (pink) on the KRT10-KRT14 scatter plot shown in A. (D) Histogram reporting on the composite ‘Basal Score’ across the population of epidermal keratinocytes. Blue and red dashed lines partition the cells into low (less than 1.0 or LT1.0), high (greater than 1.75 or GT1.75) and ‘Between’ basal score groups. (E) Violin plots of KRT14 and KRT10 expression in cells with LT1.0, ‘Between’, and GT1.75 basal scores. Dashed lines are boundaries between the ‘LT1.0’, ‘Between’ and ‘GT1.75’ subgroups. (F–G). Mapping of cells with a (F) ‘between’ and a (G) GT1.75 basal score on the KRT10-KRT14 scatter plot shown in A. (H) Heatmap representation of expression levels for genes associated with basal and spinous differentiation state in Seurat clusters and custom scores. Data set published by Cheng et al. (2018) and reanalyzed using a standard Seurat pipeline (see Materials and Methods section). Data set publicly available at EGAS00001002927.
Among the myriad of questions that arise about the KRT14high/KRT10high keratinocytes with hybrid characteristics, two that stand out relate to their mitotic competency and location within the trunk epidermis. To examine the mitotic state of keratinocytes exhibiting hybrid features, we devised a combined G2/M score based on average expression of five genes that are highly specific to this phase of the cell cycle – MKI67 (Booth et al., 2014), TOP2A (Earnshaw et al., 1985), CDC20 (Kramer et al., 2000), BUB3 (Kalitsis et al., 2000) and CCNB2 (Nigg, 2001). The rationale for using these five genes in combination is to stabilize the variation one would expect from data points for a single gene. Sorting all epidermal keratinocytes from human trunk skin according to this combined G2/M score yielded a left-skewed, strongly asymmetric distribution, as expected (Fig. 2B). To identify keratinocytes that are indisputably mitotic, we applied a high cut-off filter, set at 0.75, yielding 735 out of 24,979 keratinocytes, or 2.94% of the total cell population (Fig. 2B). Cells with such a high G2/M score are almost entirely comprised within the ‘Mitotic2’ cluster in the original Uniform Manifold Approximation and Projection (UMAP) data (Table S4). Moreover, KRT14 and KRT5 are among the highest expressed genes in this subgroup (Fig. 2H). Remarkably, however, so are KRT10 and KRT1, along with other genes previously associated with keratinocyte differentiation including DMKN (Bazzi et al., 2007) and DSP (Lechler and Fuchs, 2007) (Fig. 2H). Mapping keratinocytes showing a high composite G2/M score [greater than 0.75 (GT 0.75)] onto the KRT10/KRT14 scatter plot reveals that cells expressing mitotic genes occur in both the KRT14high/KRT10low and KRT14high/KRT10high quadrants (Fig. 2C). These findings suggest the existence of a keratinocyte population that have initiated differentiation, as reflected through expression of KRT10, KRT1 and several additional markers (DMKN, PSP, KRTDAP and SBSN), yet can maintain a mitotic signature in human trunk skin epidermis.
We next sought to assess the location of keratinocytes with hybrid features in human trunk skin epidermis. A combined basal layer score incorporating the expression levels for three genes encoding proteins specifically involved in anchoring keratinocytes to the basal lamina, DST (Sawamura et al., 1991), POSTN (Nishiyama et al., 2011), and COL17A1 (McGrath et al., 1995), was devised. Individually, DST, POSTN and COL17A1 show similar distributions across cells in this data set (Fig. S2D). Sorting all keratinocytes (n=24,979) using the combined basal score yields a classic bimodal distribution, reflecting in part the expected downregulation of basal lamina associated genes within the suprabasal compartment (Fig. 2D). Keratinocytes were next partitioned into three subsets based on the basal score (Fig. 2D). When considering either ‘top marker genes’ or ‘highest-expressed genes’, keratinocytes exhibiting a high basal score (GT 1.75; n=12,123 cells) are characterized by a strong progenitor signature (e.g. Fig. 2E,H) consistent with a location within the basal layer of epidermis. On the other hand, keratinocytes with a low basal score [less than (LT) 1.0; n=10,402 cells] show a strong differentiation signature when using the same criteria (Fig. 2E,H), consistent with a location away from the basal compartment. With such expectations met, it proves interesting to analyze the subset of keratinocytes showing an intermediate basal score (‘between’; n=2454 cells) in this analysis (Fig. 2D). Cells with a intermediate basal score express both KRT14 and KRT10 at appreciable levels (Fig. 2E) and populate a broad fraction of keratinocytes as sorted in the KRT10/KRT14 scatter plot analysis (Fig. 2F). Remarkably, a sizable fraction (34%) of keratinocytes with an intermediate (‘between’) basal score map to the KRT14high/ KRT10high subgroup in the KRT10/KRT14 scatter plot (Fig. 2F). This enrichment (34% observed versus 18% expected, χ2=2216, P<0.0001) is striking since KRT14high/ KRT10high hybrid cells populate considerably smaller fractions of both high and low basal scoring groups (e.g. 3.6% of cells within the GT 1.75 subset versus 10% expected, and 10.2% of cells in LT 1.0 versus 10% expected). Conversely, 19% of keratinocytes exhibiting a high (GT 1.75) basal score map to the KRT14high/KRT10high subgroup in the KRT10/KRT14 scatter plot (Fig. 2G).
Taken together, the evidence stemming from analyses of scRNAseq data collected from human trunk skin suggests the existence of a sizable subpopulation of keratinocytes that: (1) reside in the basal layer; (2) express intermediate levels of KRT5–KRT14, KRT1–KRT10 and additional genes reflecting a differentiating status (Fig. 2H); and (3) can undergo mitosis as reflected by relatively high levels of G2/M gene transcripts. From this, we conclude that changes in keratin gene expression occur quite gradually as keratinocytes of the epidermis transit from a progenitor to a differentiating state (see Stoler et al., 1988), and also that expression of the differentiation-related KRT1 and KRT10 can occur in keratinocytes undergoing mitosis in the epidermis. The outcome of this keratin expression-focused computational analysis thus confirms and extends the efforts of others combining computational and experimental approaches (Aragona et al., 2020; Lin et al., 2020; Wang et al., 2020; Cockburn et al., 2021).
Although our computational analysis focuses on the transcriptional landscape of keratinocytes, it remains important to bridge the gap and reexamine the relationship between mRNA and protein levels when studying this emerging concept in keratinocyte biology. Work by our lab and others have attempted to address these questions in the past, measuring cellular protein levels of K5, K14 and K15 in sorted basal keratinocytes (Sun and Green, 1978; Feng et al., 2013). K5 and K14, in particular, are highly expressed at both the mRNA and protein levels in basal keratinocytes. Follow-up studies at the protein level are especially important considering evidence for a role of post-translational modifications in keratinocyte differentiation (Guo et al., 2020) and will further establish our definitions of low/high expression of genes/protein and their transitions across keratinocytes differentiation and cycling.
Key attributes of keratin gene regulation are conserved in mouse ear skin epidermis
Transgenic mouse models have made an enormous contribution to our understanding of gene function and disease mechanisms in human skin epithelia, prompting us to next assess whether the principles emanating from our analysis of human trunk skin scRNAseq data also apply to mouse skin. To do so, we re-analyzed the scRNAseq data set from Lukowski et al. (2018) reporting on intact mouse ear skin (see Materials and Methods section and Fig. S3A), which arguably shares many attributes with human trunk skin. Focusing on the six cell clusters totaling 4761 cells that comprise epidermal keratinocytes, we confirmed that Krt5 and especially Krt14 reads occur at high levels relative to known abundant transcripts (e.g. Eef1a1) in clusters enriched in progenitor keratinocytes (‘Basal1,2’; Fig. 3A). Likewise, Krt1 and especially Krt10 reads occur at high levels relative to abundant genes in clusters enriched in differentiating keratinocytes (‘Spinous1-3’; Fig. 3A). Moreover, Krt5 and Krt14 genes are highly correlated across the entire array of 4761 keratinocytes in mouse ear (r2=0.74; Fig. 3B), whereas, again, the degree to which Krt15 is correlated to Krt5 is lower (r2=0.53; data not shown). In this setting, Krt10 transcripts are far better correlated to those of Krt2 (r2=0.35; Fig. 3C) compared to Krt1 (r2=0.13; data not shown), a novel and interesting finding that merits follow-up studies. Relating Krt14 to Krt10 expression through a scatter plot analysis of all keratinocytes from mouse ear epidermis gives rise to a distribution which, again, includes Krt14high/Krt10high hybrid keratinocytes in addition to the expected Krt14high/Krt10low and Krt14low/Krt10high keratinocyte subsets (Fig. 3D). We also analyzed this cell population through the G2/M composite score, exactly as done for human skin (see Fig. 2B). Filtering the resulting distribution using a high G2/M score (>0.6) yielded 266, or 5.5%, keratinocytes (Fig. 3E) that largely binned to the mitotic cluster group (81%; Table S4). In contrast to the human trunk dataset, relatively few keratinocytes with a high G2/M score map to the hybrid keratinocytes in mouse ear skin, given that only 1.4% of Krt14high/Krt10high cells exceed the composite G2/M score threshold (Fig. 3F; by comparison, 8.3% of the high score G2/M cells map to this hybrid group in human trunk skin). Instead, 91% of all high G2/M scoring cells reside in the Krt14high/Krt10low group (Fig. 3F). Whether these differences are due to the lower statistical power and/or missing keratinocyte subpopulations in the mouse skin study (Lukowski et al., 2018), or are related to the reality of distinct body location (human trunk versus mouse ear), or reflect deeper and more significant biological and/or evolutionary differences between human and mouse skin, remains to be explored.
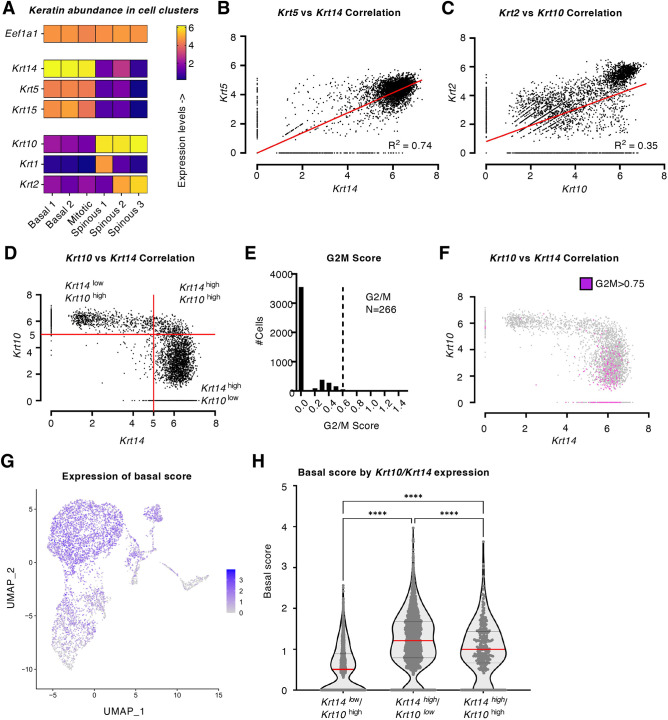
Fig. 3.
Keratin expression in mouse ear skin. (A) Heatmap of keratin gene expression in the six Seurat clusters of keratinocytes (n=4761 cells) in healthy mouse ear skin, analyzed by scRNAseq (Basal1-2; Mitotic and Spinous1-3). Eef1a1 provides a reference for a highly expressed gene across all clusters. (B,C) Correlations between specific type I and type II keratin genes across the entire array of epidermal keratinocytes in mouse ear skin (n=4979 cells). Red lines represent linear correlations, and r2 values are reported. (B) Krt5 versus Krt14. (C) Krt2 versus Krt10. (D) Correlation of Krt10 and Krt14 in mouse ear epidermis. Quadrants were defined by abundancy of cells expressing high Krt14 (>5.0) or high Krt10 (>5.0). (E) Histogram reporting on the composite G2/M score across the same cell population. Dashed line represent threshold for G2/M cells (>0.6, ∼5.5% of cells). (F) Mapping of G2/M cells with a high (>0.6) G2/M score (pink) on the Krt10-Krt14 scatter plot shown in D. (G) Mapping of the basal score for individual cells on the original UMAP from the mouse ear skin data set (see Fig. S3). (H) Violin plot representation of basal score in cells that express low and/or high combinations of Krt14 and Krt10 (see D). The red lines represent the median and dashed lines the quartile positions. ****P<0.0001 (adjusted) as tested by one-way ANOVA with Tukey's multiple comparisons test. Data set published by Lukowski et al. (2018) and reanalyzed using a standard Seurat pipeline (see Materials and Methods section). Data set publicly available at E-MTAB-6429.
Utilizing basal lamina-anchoring genes (Postn, Dst and Col17A1; see Fig. S4A), as we did when analyzing the human keratinocyte dataset, we compiled an average basal score for each cell and examined the distribution of scores for epidermal keratinocytes across mouse ear skin. The resulting distribution (Fig. S4B) did not follow a classic bimodal shape as it did for human trunk skin epidermis, again possibly owing to the underrepresentation of differentiating keratinocytes in the data set. Still, plotting the basal score on the original UMAP revealed that keratinocytes with a high basal score (GT 1.8) are contained within the ‘Basal 1-2’ and ‘Mitotic’ cell clusters (93% of cells; Fig. 3G,H). We then analyzed the distribution of the basal score in the setting of a scatter analysis relating Krt14 and Krt10 expression. Krt14high/Krt10low cells are defined by an average basal score (1.23±0.01; mean±s.e.m.) that is significantly higher than that of Krt14low/Krt10high cells (0.53±0.01, P<0.0001). Importantly, and consistent with our analysis of human trunk skin data, Krt14high/Krt10high hybrid keratinocytes in mouse skin epidermis show an intermediate score (1.0±0.03; Fig. 3H), suggesting that these cells maintain a similar location and progenitor-like gene expression. Although more analyses are needed, such observations confirm that the key principles governing keratin gene expression in the interfolliclar epidermis of human trunk skin (Figs 1,2) are conserved in mouse skin (Fig. 3).
DISCUSSION
The power of scRNAseq analyses has exposed a significant molecular heterogeneity within the classically defined ‘progenitor‘ and ‘differentiating‘ compartments in human and mouse interfollicular epidermis (Joost et al., 2016; Cheng et al., 2018; Lukowski et al., 2018; Aragona et al., 2020; Dekoninck et al., 2020; Haensel et al., 2020; Lin et al., 2020; Wang et al., 2020; Cockburn et al., 2021). Whether this heterogeneity reflects a continuum of cellular states in the setting of a single developmental or differentiation pathway, distinct classes of progenitor stem cells and/or co-existence of distinct differentiation pathways within epidermis is a fundamentally important issue that must await further experimentation and consideration (see Lin et al., 2020; Wang et al., 2020; Cockburn et al., 2021). Here, we considered specific aspects of keratin expression in interfolliclar epidermis from a perspective of scRNAseq analyses, and offer the following three conclusions. First, keratin-encoding genes are among the most abundantly expressed genes in epidermal keratinocytes. Second, the type I KRT14 and type II KRT5 and the type I KRT10 and type II KRT1 genes are each tightly co-regulated at the mRNA transcript level in epidermal keratinocytes, independently of the cellular state, thereby justifying a designation of pairwise regulation for them. By contrast, two additional keratin genes expressed at significant levels in the epidermis, the type II KRT2 in late stage differentiating keratinocytes and the type I KRT15 expressed in undifferentiated keratinocytes in the basal layer, do not show tight co-regulation with a dedicated partner keratin gene of the complementary type, at least at the transcript level. Third, our analyses support recent observations of changes in keratin gene expression occurring while keratinocytes transit from a progenitor to a differentiating state in the interfollicular epidermis, and also that expression of the differentiation-related KRT1 and KRT10 genes occurs in keratinocytes that maintain a mitotic gene expression signature. Although onset of KRT1-KRT10 expression clearly conveys an engagement of keratinocytes towards differentiation, it does not necessarily reflect a post-mitotic state, consistent with early studies in the field (Schweizer et al., 1984; Regnier et al., 1986) and more recent studies also based on high-throughput single cell analyses (Lin et al., 2020; Cockburn et al., 2021). We predict that similar conclusions will be attained when such analyses will be conducted on other surface epithelia. Whether keratinocytes undergo a ‘gradual model’ of differentiation from KRT14+ to KRT10+, or a ‘stepwise model’, wherein KRT14+/KRT10+ represents a transition state in keratinocyte differentiation, is an open issue that remains to be solved. Regardless, a higher resolution understanding of keratinocyte differentiation will provide significant insights in our understanding of skin homeostasis and disease.
The analyses reported here help shed new light on several open issues of high significance in the fields of keratin- and keratinocyte-related biology. First, how is the transcription of specific keratin type I and II genes, located amidst large keratin gene clusters located on two different chromosomes (chromosome 12 and 17 in the human), so exquisitely coordinated as a function of the keratinocyte journey within the epidermis? The answer to this puzzle likely resides, at least in part, in the three-dimensional organizational of chromosome domains, including the type I and type II keratin gene clusters, and the epidermal differentiation complex, in mammalian genomes (Botchkarev, 2017). Second, how is a 1:1 molar ratio between type I and II keratin proteins achieved (Kim et al., 1984; Giudice and Fuchs, 1987) under circumstances where individual keratinocytes show an imbalance between type I keratin transcripts, which are often dominant, and type II keratin transcripts? There is evidence that excess keratin of one type is inherently unstable and rapidly degraded (Giudice and Fuchs, 1987; Kulesh et al., 1989), and also that keratin proteins are subject to ubiquitylation and proteasome-mediated degradation (Magin et al., 1998; Ku and Omary, 2000). Such post-transcriptional and post-translational layers of regulation are likely important since some keratins are seemingly not subject to a transcription-level match with a partner of the opposite type (e.g. KRT2 and KRT15 in epidermis). Related to this, the nature of the mechanisms that set individual keratin proteins to an optimal level in keratinocytes are also unknown. Again, here, pioneering studies in the field showed that type I and II keratins readily form heterooligomers in various types of epithelial cells (Hatzfeld and Franke, 1985), adding to the complexity of this issue. Finally, one wonders about the significance and role, if any, of the mixing of progenitor-type (e.g. K5, K14 and K15) and differentiation-type (e.g. K1 and K10) keratins in hybrid keratinocytes at the time of their commitment to differentiation. A recent study provided strong evidence that K14-dependent disulfide bonding regulates a YAP1/Hippo-driven mechanism that gates entry of keratinocytes into differentiation within epidermis, with the key biochemical determinants conserved in some type I keratins (e.g. K10 and K9) but not others (e.g. K15 and K19) (Guo et al., 2020). Recent advances in spatial mRNAseq (Marx, 2021) further provide opportunities to observe transcriptional changes of keratinocytes as they initiate and undergo differentiation. Considering the spatially hierarchical organization of cell states within the epidermis, this method is poised to inform, and possibly revolutionize, our understanding of emerging questions regarding keratinocyte differentiation in mammalian interfollicular epidermis. Thus, there are many properties and virtues awaiting to be discovered and further characterized in greater detail when it comes to keratin proteins, their regulation and roles in surface epithelia such as epidermis.
MATERIALS AND METHODS
Analysis of scRNAseq data from human trunk skin
A standard ‘LogNormalize’ (natural-log) Seurat pipeline (Hao et al., 2021) was applied for the re-analysis of a data set from Cheng et al. (2018) (European Genome-phenome Archive accession no. EGAS00001002927). Cells with defined characteristics (between 500 and 7500 unique features; less than 15% mitochondrial content) were retained, yielding a total of 27,452 cells with informative content. Principal component analysis was applied for dimensional reduction, with the top 30 principal components retained for UMAP visualization (Fig. S1A), construction of a shared nearest neighbor (SNN) graph, and subsequent clustering using the Louvain algorithm with a resolution setting of 0.5. This yielded 21 statistically distinct cell clusters (Fig. S1A).
A review of ‘top marker genes’ and ‘most expressed genes’ showed that 15 clusters corresponded to epidermal keratinocytes, two corresponded to appendageal keratinocytes, two corresponded to melanocytes, one consisted of Langerhans cells, and the last one was T cell related (data not shown). Analyzing the entire array of cells partitioned in this fashion for specific marker genes, such as KRT14 (Fig. S1B), KRT10 (Fig. S1C), KRT2 (data not shown) and genes reflecting the S and G2/M phase of the cell cycle (Fig. S1D,E) attests to a good balance between progenitor, actively dividing and differentiating keratinocytes in this dataset.
All analyzes of keratin expression were restricted to the 15 clusters comprising epidermal keratinocytes, totaling 24,979 cells. Specifically, the six ‘Basal’ keratinocyte clusters were defined by high abundance of KRT5 and KRT14, the six spinous keratinocyte clusters defined by high abundance of KRT1 and KRT10, and the three ‘Mitotic’ clusters were characterized as keratinocytes based on high abundance of KRT14. The six clusters excluded from the keratinocyte analysis (and their markers) were ‘Langerhans’ (high CD74), ‘T-cell-like’ (high CXCR4 and TRBC2), two ‘Appendageal’ (high GJB2, TM4SF1, CYP1B1 and KRT6A), and two ‘Melanocytes’ (high DCT and PMEL). Given that our analyses were focused on the expression of keratins and not the clusters themselves, the latter were not reprocessed after the removal of the six non-keratinocyte clusters. It is worth noting, however, that data renormalizing and re-clustering resulted in one fewer cluster where ‘Basal 1’ and ‘Basal 6’ were no longer independent, with all other cells largely assigned to the same original clusters (in this case, 12 principal components were used instead of 15 to account for the decrease in information after removing four cell types).
Analysis of scRNAseq data from mouse ear skin
The same approach was used to reanalyze a data set from Lukowski et al. (2018) (ArrayExpress accession no. E-MTAB-6429), yielding nine statistically distinct cell clusters (Fig. S3A). Adjustments made for lower cell counts and therefore lower dimensional data in this set included using the top 12 principal components and a resolution setting of 0.4 when clustering. Review of the ‘top marker genes’ and ‘most expressed genes’ showed that six clusters corresponded to epidermal keratinocytes (including a mitotic one), two corresponded to keratinocytes from epithelial appendages, and the last one corresponded to melanocytes (data not shown). Expression levels for Krt14, Krt10 and cell cycle genes are related in panels of Fig. S3B–E.
As with the human dataset, the two ‘Basal’ and one ‘Mitotic’ clusters were defined by high expression of Krt14, whereas the three ‘Spinous’ clusters were defined by high Krt1. The ‘Melanocytes’ and ‘Appendages’ clusters respectively showed high Dcn and high Defb6, Fst, Gstm5 and Gsta3. All analyzes of keratin expression in mouse skin were restricted to the six clusters comprising epidermal keratinocytes, totaling 4,761 cells.
Composite scores and statistical analyses
Composite scores were generated using a simple average of log expression values for a set of markers. For the ‘G2/M score’ the gene markers used were MKI67, TOP2A, CDC20, BUB3 and CCNB2. For the ‘S phase’ score, the gene markers used were RPA2, UHRF1, ATAD2, RFC2 and RRM2. For the ‘Basal score’ the gene markers used were DST, POSTN and COL17A1. The Wilcoxon rank sum test was used for all top marker detection and differential expression analyses with tested genes limited to those detected at a minimum fraction of 0.25 in at least one of the clusters being compared to ignore genes less frequently expressed.
Supplementary Material
Acknowledgements
The authors are very grateful to Dr Raymond Cho (UCSF, USA) and Dr Joseph Powell (University of Queensland, Australia) for making their scRNAseq data sets publicly available, and to members of the Coulombe laboratory for support.
Footnotes
Funding
These efforts were supported in part by the National Institutes of Health (grant R01 AR079418 to P.A.C. and grant T32 CA009676 to C.J.R.). E.C. received support from the Center for Plasticity and Organ Design (CPOD, T32 5T32HD00750) at the University of Michigan and from the National Psoriasis Foundation (#960805). Deposited in PMC for release after 12 months.
References
- Abbas, W., Kumar, A. and Herbein, G. (2015). The eEF1A proteins: at the crossroads of oncogenesis, apoptosis, and viral infections. Front. Oncol. 5, 75. 10.3389/fonc.2015.00075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona, M., Sifrim, A., Malfait, M., Song, Y., Van Herck, J., Dekoninck, S., Gargouri, S., Lapouge, G., Swedlund, B., Dubois, C.et al. (2020). Mechanisms of stretch-mediated skin expansion at single-cell resolution. Nature 584, 268-273. 10.1038/s41586-020-2555-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzi, H., Fantauzzo, K. A., Richardson, G. D., Jahoda, C. A. B. and Christiano, A. M. (2007). Transcriptional profiling of developing mouse epidermis reveals novel patterns of coordinated gene expression. Dev. Dyn. 236, 961-970. 10.1002/dvdy.21099 [DOI] [PubMed] [Google Scholar]
- Bland, J. M. and Altman, D. G. (2011). Correlation in restricted ranges of data. BMJ 342, d556. 10.1136/bmj.d556 [DOI] [PubMed] [Google Scholar]
- Booth, D. G., Takagi, M., Sanchez-Pulido, L., Petfalski, E., Vargiu, G., Samejima, K., Imamoto, N., Ponting, C. P., Tollervey, D., Earnshaw, W. C.et al. (2014). Ki-67 is a PP1-interacting protein that organises the mitotic chromosome periphery. eLife 3, e01641. 10.7554/eLife.01641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botchkarev, V. A. (2017). The molecular revolution in cutaneous biology: chromosomal territories, higher-order chromatin remodeling, and the control of gene expression in keratinocytes. J. Invest. Dermatol. 137, e93-e99. 10.1016/j.jid.2016.04.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burja, B., Paul, D., Tastanova, A., Edalat, S. G., Gerber, R., Houtman, M., Elhai, M., Bürki, K., Staeger, R., Restivo, G.et al. (2022). An optimized tissue dissociation protocol for single-cell RNA sequencing analysis of fresh and cultured human skin biopsies. Front. Cell Dev. Biol. 10, 872688. 10.3389/fcell.2022.872688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, J. B., Sedgewick, A. J., Finnegan, A. I., Harirchian, P., Lee, J., Kwon, S., Fassett, M. S., Golovato, J., Gray, M., Ghadially, R.et al. (2018). Transcriptional programming of normal and inflamed human epidermis at single-cell resolution. Cell Rep. 25, 871-883. 10.1016/j.celrep.2018.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockburn, K., Annusver, K., Ganesan, S., Mesa, K., Kawaguchi, K., Kasper, M. and Greco, V. (2021). Gradual differentiation uncoupled from cell cycle exit generates heterogeneity in the epidermal stem cell layer. bioRxiv. 10.1101/2021.01.07.425777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin, C., Moll, R., Kubicka, S., Ouhayoun, J.-P. and Franke, W. W. (1992a). Characterization of human cytokeratin 2, an epidermal cytoskeletal protein synthesized late during differentiation. Exp. Cell Res. 202, 132-141. 10.1016/0014-4827(92)90412-2 [DOI] [PubMed] [Google Scholar]
- Collin, C., Ouhayoun, J.-P., Grund, C. and Franke, W. W. (1992b). Suprabasal marker proteins distinguishing keratinizing squamous epithelia: cytokeratin 2 polypeptides of oral masticatory epithelium and epidermis are different. Differentiation 51, 137-148. 10.1111/j.1432-0436.1992.tb00690.x [DOI] [PubMed] [Google Scholar]
- Cooper, D. and Sun, T. T. (1986). Monoclonal antibody analysis of bovine epithelial keratins. Specific pairs as defined by coexpression. J. Biol. Chem. 261, 4646-4654. 10.1016/S0021-9258(17)38550-2 [DOI] [PubMed] [Google Scholar]
- Coulombe, P. A. and Fuchs, E. (1990). Elucidating the early stages of keratin filament assembly. J. Cell Biol. 111, 153-169. 10.1083/jcb.111.1.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekoninck, S., Hannezo, E., Sifrim, A., Miroshnikova, Y. A., Aragona, M., Malfait, M., Gargouri, S., de Neunheuser, C., Dubois, C., Voet, T.et al. (2020). Defining the design principles of skin epidermis postnatal growth. Cell 181, 604-620.e622. 10.1016/j.cell.2020.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw, W. C., Halligan, B., Cooke, C. A., Heck, M. M. and Liu, L. F. (1985). Topoisomerase II is a structural component of mitotic chromosome scaffolds. J. Cell Biol. 100, 1706-1715. 10.1083/jcb.100.5.1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich, F., Fischer, H., Langbein, L., Praetzel-Wunder, S., Ebner, B., Figlak, K., Weissenbacher, A., Sipos, W., Tschachler, E. and Eckhart, L. (2019). Differential evolution of the epidermal keratin cytoskeleton in terrestrial and aquatic mammals. Mol. Biol. Evol. 36, 328-340. 10.1093/molbev/msy214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichner, R., Sun, T. T. and Aebi, U. (1986). The role of keratin subfamilies and keratin pairs in the formation of human epidermal intermediate filaments. J. Cell Biol. 102, 1767-1777. 10.1083/jcb.102.5.1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, X., Zhang, H., Margolick, J. B. and Coulombe, P. A. (2013). Keratin intracellular concentration revisited: implications for keratin function in surface epithelia. J. Invest. Dermatol. 133, 850-853. 10.1038/jid.2012.397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs, E. (1995). Keratins and the skin. Annu. Rev. Cell Dev. Biol. 11, 123-154. 10.1146/annurev.cb.11.110195.001011 [DOI] [PubMed] [Google Scholar]
- Fuchs, E. and Green, H. (1980). Changes in keratin gene expression during terminal differentiation of the keratinocyte. Cell 19, 1033-1042. 10.1016/0092-8674(80)90094-X [DOI] [PubMed] [Google Scholar]
- Fuchs, E. V., Coppock, S. M., Green, H. and Cleveland, D. W. (1981). Two distinct classes of keratin genes and their evolutionary significance. Cell 27, 75-84. 10.1016/0092-8674(81)90362-7 [DOI] [PubMed] [Google Scholar]
- Fuchs, E., Hanukoglu, I., Marchuk, D., Grace, M. P. and Kim, K. H. (1985). The nature and significance of differential keratin gene expression. [Review]. Ann. N. Y. Acad. Sci. 455, 436-450. 10.1111/j.1749-6632.1985.tb50427.x [DOI] [PubMed] [Google Scholar]
- Giudice, G. J. and Fuchs, E. (1987). The transfection of epidermal keratin genes into fibroblasts and simple epithelial cells: evidence for inducing a type I keratin by a type II gene. Cell 48, 453-463. 10.1016/0092-8674(87)90196-6 [DOI] [PubMed] [Google Scholar]
- Guo, Y., Redmond, C. J., Leacock, K. A., Brovkina, M. V., Ji, S., Jaskula-Ranga, V. and Coulombe, P. A. (2020). Keratin 14-dependent disulfides regulate epidermal homeostasis and barrier function via 14-3-3σ and YAP1. eLife 9, e53165. 10.7554/eLife.53165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haensel, D., Jin, S., Sun, P., Cinco, R., Dragan, M., Nguyen, Q., Cang, Z., Gong, Y., Vu, R., MacLean, A. L.et al. (2020). Defining epidermal basal cell states during skin homeostasis and wound healing using single-cell transcriptomics. Cell Rep. 30, 3932-3947.e6. 10.1016/j.celrep.2020.02.091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao, Y., Hao, S., Andersen-Nissen, E., Mauck, W. M., III, Zheng, S., Butler, A., Lee, M. J., Wilk, A. J., Darby, C., Zager, M.et al. (2021). Integrated analysis of multimodal single-cell data. Cell 184, 3573-3587.e29. 10.1016/j.cell.2021.04.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzfeld, M. and Franke, W. W. (1985). Pair formation and promiscuity of cytokeratins: formation in vitro of heterotypic complexes and intermediate-sized filaments by homologous and heterologous recombinations of purified polypeptides. J. Cell Biol. 101, 1826-1841. 10.1083/jcb.101.5.1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzfeld, M. and Weber, K. (1990). The coiled coil of in vitro assembled keratin filaments is a heterodimer of type I and II keratins: use of site-specific mutagenesis and recombinant protein expression. J. Cell Biol. 110, 1199-1210. 10.1083/jcb.110.4.1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse, M., Zimek, A., Weber, K. and Magin, T. M. (2004). Comprehensive analysis of keratin gene clusters in humans and rodents. Eur. J. Cell Biol. 83, 19-26. 10.1078/0171-9335-00354 [DOI] [PubMed] [Google Scholar]
- Joost, S., Zeisel, A., Jacob, T., Sun, X., La Manno, G., Lönnerberg, P., Linnarsson, S. and Kasper, M. (2016). Single-cell transcriptomics reveals that differentiation and spatial signatures shape epidermal and hair follicle heterogeneity. Cell Syst. 3, 221-237.e9. 10.1016/j.cels.2016.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalitsis, P., Earle, E., Fowler, K. J. and Choo, K. H. A. (2000). Bub3 gene disruption in mice reveals essential mitotic spindle checkpoint function during early embryogenesis. Genes Dev. 14, 2277-2282. 10.1101/gad.827500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karantza, V. (2011). Keratins in health and cancer: more than mere epithelial cell markers. Oncogene 30, 127-138. 10.1038/onc.2010.456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S. and Coulombe, P. A. (2007). Intermediate filament scaffolds fulfill mechanical, organizational, and signaling functions in the cytoplasm. Genes Dev. 21, 1581-1597. 10.1101/gad.1552107 [DOI] [PubMed] [Google Scholar]
- Kim, K. H., Marchuk, D. and Fuchs, E. (1984). Expression of unusually large keratins during terminal differentiation: balance of type I and type II keratins is not disrupted. J. Cell Biol. 99, 1872-1877. 10.1083/jcb.99.5.1872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, D., Chung, K. B. and Kim, T.-G. (2020). Application of single-cell RNA sequencing on human skin: Technical evolution and challenges. J. Dermatol. Sci. 99, 74-81. 10.1016/j.jdermsci.2020.06.002 [DOI] [PubMed] [Google Scholar]
- Kramer, E. R., Scheuringer, N., Podtelejnikov, A. V., Mann, M. and Peters, J.-M. (2000). Mitotic regulation of the APC activator proteins CDC20 and CDH1. Mol. Biol. Cell 11, 1555-1569. 10.1091/mbc.11.5.1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku, N.-O. and Omary, M. B. (2000). Keratins turn over by ubiquitination in a phosphorylation-modulated fashion. J. Cell Biol. 149, 547-552. 10.1083/jcb.149.3.547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulesh, D. A., Cecena, G., Darmon, Y. M., Vasseur, M. and Oshima, R. G. (1989). Posttranslational regulation of keratins: degradation of mouse and human keratins 18 and 8. Mol. Cell. Biol. 9, 1553-1565. 10.1128/MCB.9.4.1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechler, T. and Fuchs, E. (2007). Desmoplakin: an unexpected regulator of microtubule organization in the epidermis. J. Cell Biol. 176, 147-154. 10.1083/jcb.200609109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessin, S. R., Huebner, K., Isobe, M., Croce, C. M. and Steinert, P. M. (1988). Chromosomal mapping of human keratin genes: evidence of non-linkage. J. Invest. Dermatol. 91, 572-578. 10.1111/1523-1747.ep12477087 [DOI] [PubMed] [Google Scholar]
- Li, L., Dong, M. and Wang, X.-G. (2016). The implication and significance of beta 2 microglobulin: a conservative multifunctional regulator. Chin. Med. J. (Engl) 129, 448-455. 10.4103/0366-6999.176084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, Z., Jin, S., Chen, J., Li, Z., Lin, Z., Tang, L., Nie, Q. and Andersen, B. (2020). Murine interfollicular epidermal differentiation is gradualistic with GRHL3 controlling progression from stem to transition cell states. Nat. Commun. 11, 5434. 10.1038/s41467-020-19234-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd, C., Yu, Q. C., Cheng, J., Turksen, K., Degenstein, L., Hutton, E. and Fuchs, E. (1995). The basal keratin network of stratified squamous epithelia: defining K15 function in the absence of K14. J. Cell Biol. 129, 1329-1344. 10.1083/jcb.129.5.1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukowski, S. W., Tuong, Z. K., Noske, K., Senabouth, A., Nguyen, Q. H., Andersen, S. B., Soyer, H. P., Frazer, I. H. and Powell, J. E. (2018). Detection of HPV E7 transcription at single-cell resolution in epidermis. J. Invest. Dermatol. 138, 2558-2567. 10.1016/j.jid.2018.06.169 [DOI] [PubMed] [Google Scholar]
- Magin, T. M., Schröder, R., Leitgeb, S., Wanninger, F., Zatloukal, K., Grund, C. and Melton, D. W. (1998). Lessons from keratin 18 knock-out mice: Formation of novel keratin filaments, secondary loss of keratin 7 and accumulation of liver-specific keratin 8-positive aggregates. J. Cell Biol. 140, 1441-1451. 10.1083/jcb.140.6.1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx, V. (2021). Method of the Year: spatially resolved transcriptomics. Nat. Methods 18, 9-14. 10.1038/s41592-020-01033-y [DOI] [PubMed] [Google Scholar]
- McGrath, J. A., Gatalica, B., Christiano, A. M., Si, K., Owaribe, K., McMillan, J. R., Eady, R. A. J. and Uitto, J. (1995). Mutations in the 180-kD bullous pemphigoid antigen (BPAG2), a hemidesmosomal transmembrane collagen (COL17A1), in generalized atrophic benign epidermolysis bullosa. Nat. Genet. 11, 83-86. 10.1038/ng0995-83 [DOI] [PubMed] [Google Scholar]
- Moll, R., Franke, W. W., Schiller, D. L., Geiger, B. and Krepler, R. (1982). The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. [Review]. Cell 31, 11-24. 10.1016/0092-8674(82)90400-7 [DOI] [PubMed] [Google Scholar]
- Moll, R., Divo, M. and Langbein, L. (2008). The human keratins: biology and pathology. Histochem. Cell Biol. 129, 705-733. 10.1007/s00418-008-0435-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson, W. G. and Sun, T. T. (1983). The 50- and 58-kdalton keratin classes as molecular markers for stratified squamous epithelia: cell culture studies. J. Cell Biol. 97, 244-251. 10.1083/jcb.97.1.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg, E. A. (2001). Mitotic kinases as regulators of cell division and its checkpoints. Nat. Rev. Mol. Cell Biol. 2, 21-32. 10.1038/35048096 [DOI] [PubMed] [Google Scholar]
- Nishiyama, T., Kii, I., Kashima, T. G., Kikuchi, Y., Ohazama, A., Shimazaki, M., Fukayama, M. and Kudo, A. (2011). Delayed re-epithelialization in periostin-deficient mice during cutaneous wound healing. PLoS ONE 6, e18410. 10.1371/journal.pone.0018410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Guin, W. M., Schermer, A., Lynch, M. and Sun, T.-T. (1990). Differentiation-specific expression of keratin pairs. In Cellular and Molecular Biology of Intermediate Filaments (ed. Goldman R. D. and Steinert P. M.), pp. 301-334. Plenum Publishing Corp. [Google Scholar]
- Omary, M. B. (2017). Intermediate filament proteins of digestive organs: physiology and pathophysiology. Am. J. Physiol. Gastrointest. Liver Physiol. 312, G628-G634. 10.1152/ajpgi.00455.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell, B. C., Cam, G. R., Fietz, M. J. and Rogers, G. E. (1986). Clustered arrangement of keratin intermediate filament genes. Proc. Natl. Acad. Sci. USA 83, 5048-5052. 10.1073/pnas.83.14.5048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashmi, R., Rao, K. S. J. and Basavaraj, K. H. (2009). A comprehensive review of biomarkers in psoriasis. Clin. Exp. Dermatol. 34, 658-663. 10.1111/j.1365-2230.2009.03410.x [DOI] [PubMed] [Google Scholar]
- Regnier, M., Vaigot, P., Darmon, M. and Prunieras, M. (1986). Onset of epidermal differentiation in rapidly proliferating basal keratinocytes. J. Invest. Dermatol. 87, 472-476. 10.1111/1523-1747.ep12455517 [DOI] [PubMed] [Google Scholar]
- Romano, V., Bosco, P., Rocchi, M., Costa, G., Leube, R. E., Franke, W. W. and Romeo, G. (1988). Chromosomal assignments of human type I and type II cytokeratin genes to different chromosomes. Cytogenet. Cell Genet. 48, 158-151. 10.1159/000132612 [DOI] [PubMed] [Google Scholar]
- Roop, D. R. (1987). Regulation of keratin gene expression during differentiation of epidermal and vaginal epithelial cells. Curr. Top. Dev. Biol. 22, 195-207. 10.1016/S0070-2153(08)60104-0 [DOI] [PubMed] [Google Scholar]
- Roop, D. R., Hawley-Nelson, P., Cheng, C. K. and Yuspa, S. H. (1983). Keratin gene expression in mouse epidermis and cultured epidermal cells. Proc. Natl. Acad. Sci. USA 80, 716-720. 10.1073/pnas.80.3.716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawamura, D., Li, K., Chu, M. L. and Uitto, J. (1991). Human bullous pemphigoid antigen (BPAG1). Amino acid sequences deduced from cloned cDNAs predict biologically important peptide segments and protein domains. J. Biol. Chem. 266, 17784-17790. 10.1016/S0021-9258(18)55195-4 [DOI] [PubMed] [Google Scholar]
- Schweizer, J., Kinjo, M., Fürstenberger, G. and Winter, H. (1984). Sequential expression of mRNA-encoded keratin sets in neonatal mouse epidermis: basal cells with properties of terminally differentiating cells. Cell 37, 159-170. 10.1016/0092-8674(84)90311-8 [DOI] [PubMed] [Google Scholar]
- Schweizer, J., Bowden, P. E., Coulombe, P. A., Langbein, L., Lane, E. B., Magin, T. M., Maltais, L., Omary, M. B., Parry, D. A. D., Rogers, M. A.et al. (2006). New consensus nomenclature for mammalian keratins. J. Cell Biol. 174, 169-174. 10.1083/jcb.200603161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, P., Alsharif, S., Fallatah, A. and Chung, B. M. (2019). Intermediate filaments as effectors of cancer development and metastasis: a focus on Keratins, Vimentin, and Nestin. Cells 8, 497. 10.3390/cells8050497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert, P. M. (1990). The two-chain coiled-coil molecule of native epidermal keratin intermediate filaments is a type I-type II heterodimer. J. Biol. Chem. 265, 8766-8774. 10.1016/S0021-9258(19)38954-9 [DOI] [PubMed] [Google Scholar]
- Stoler, A., Kopan, R., Duvic, M. and Fuchs, E. (1988). Use of monospecific antisera and cRNA probes to localize the major changes in keratin expression during normal and abnormal epidermal differentiation. J. Cell Biol. 107, 427-446. 10.1083/jcb.107.2.427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, T. T. and Green, H. (1978). Keratin filaments of cultured human epidermal cells. Formation of intermolecular disulfide bonds during terminal differentiation. J. Biol. Chem. 253, 2053-2060. 10.1016/S0021-9258(19)62353-7 [DOI] [PubMed] [Google Scholar]
- Sun, T.-T., Eichner, R., Nelson, W. G., Tseng, C. G. S., Weiss, R. A., Jarvinen, M. and Woodcock-Mitchell, J. (1983). Keratin classes: molecular markers for different types of epithelial differentiation. J. Invest. Dermatol. 81, 109s-115s. 10.1111/1523-1747.ep12540831 [DOI] [PubMed] [Google Scholar]
- Wang, S., Drummond, M. L., Guerrero-Juarez, C. F., Tarapore, E., MacLean, A. L., Stabell, A. R., Wu, S. C., Gutierrez, G., That, B. T., Benavente, C. A.et al. (2020). Single cell transcriptomics of human epidermis identifies basal stem cell transition states. Nat. Commun. 11, 4239. 10.1038/s41467-020-18075-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock-Mitchell, J., Eichner, R., Nelson, W. G. and Sun, T. T. (1982). Immunolocalization of keratin polypeptides in human epidermis using monoclonal antibodies. J. Cell Biol. 95, 580-588. 10.1083/jcb.95.2.580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Y., Huang, C., Meng, X. and Li, J. (2015). Long noncoding RNA MALAT1: insights into its biogenesis and implications in human disease. Curr. Pharm. Des. 21, 5017-5028. 10.2174/1381612821666150724115625 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.