Abstract
Background
This study aimed to assess the impact of duration of early mobilisation on survivors of critical illness. The hypothesis was that interventions lasting over 40 min, as per the German guideline, positively affect the functional status at ICU discharge.
Methods
Prospective single-centre cohort study conducted in two ICUs in Germany. In 684 critically ill patients surviving an ICU stay > 24 h, out-of-bed mobilisation of more than 40 min was evaluated.
Results
Daily mobilisation ≥ 40 min was identified as an independent predictor of an improved functional status upon ICU discharge. This effect on the primary outcome measure, change of Mobility-Barthel until ICU discharge, was observed in three different models for baseline patient characteristics (average treatment effect (ATE), all three models p < 0.001). When mobilisation parameters like level of mobilisation, were included in the analysis, the average treatment effect disappeared [ATE 1.0 (95% CI − 0.4 to 2.4), p = 0.16].
Conclusions
A mobilisation duration of more than 40 min positively impacts functional outcomes at ICU discharge. However, the maximum level achieved during ICU stay was the most crucial factor regarding adequate dosage, as higher duration did not show an additional benefit in patients with already high mobilisation levels.
Trial registration: Prospective Registry of Mobilization-, Routine- and Outcome Data of Intensive Care Patients (MOBDB), NCT03666286. Registered 11 September 2018—retrospectively registered,
Supplementary Information
The online version contains supplementary material available at 10.1186/s40560-023-00703-1.
Keywords: ICU, Mobilisation, Early mobilisation, Functional status, Physical therapy modalities
Introduction
Surviving critical illness involves not only recuperating from a potentially fatal condition, but also enduring persistent physical impairments and psychological challenges that may result in a diminished quality of life [1–4]. To reduce these side effects, maintaining the patient’s functional status during the intensive care unit (ICU) stay and preventing loss of independence is essential in modern intensive care medicine [2, 5]. Early mobilisation is a vital therapy approach to achieving this. A wide range of positive effects has been reported, such as reduced ICU and hospital length of stay (LOS), better short-term functional outcomes, and more delirium-free days [6–12]. The ability of early mobilisation to prevent the loss of muscle mass and maintain strength plays a significant role in combating intensive care unit-acquired weakness, one of the leading causes of functional decline in the critically ill [13–15]. However, important questions concerning the optimal dose of mobilisation, a complex interaction of mobilisation level, duration, frequency, and intensity remain unanswered [16]. Positive effects of early rehabilitation have been demonstrated for a higher level of mobilisation and early initiation of therapy within the first 72 h after ICU admission [7, 9, 17]. Still, the impact of frequency and duration of mobilisation on patient outcomes remains uncertain, and limited evidence is available [18–21]. In addition, the recently published TEAM trial has highlighted the potentially harmful effects of a high mobilisation dose and the ceiling effect that may accompany the increase in dosage [22]. A guideline on early mobilisation [23] recommending a daily dose of 40 min for critically ill patients may therefore be called into question as the referenced randomised controlled trials (RCT) and metanalyses are inconsistent and cannot be used to claim superiority of 40 min of daily mobilisation.
The aim of this study was to investigate the effects of a given duration of mobilisation on critically ill patients, considering patient characteristics and disease severity. More specifically, we investigated the impact of an average of more or less than 40 min of daily out-of-bed mobilisation on the outcome of survivors of critical illness.
Materials and methods
Study design, setting, and participants
This is an analysis of prospectively collected patient registry data (NCT03666286) from two interdisciplinary ICUs of the Department of Anesthesiology and Intensive Care Medicine, Klinikum Rechts der Isar, School of Medicine and Health, Technical University of Munich, Germany, between from April 2017 to April 2019. The data of critically ill patients were collected after obtaining written informed consent from them or their legal representative, in accordance with German law. The database has been approved by the Ethics Committee of the Faculty of Medicine, Technical University of Munich (Reference number 528/18, Ethics committee meeting of 22 December 2016). The inclusion criteria were age over 18 years and an expected ICU stay > 24 h, while the exclusion criterion was readmission to the intensive care unit.
Outcomes
The primary outcome measure was the change of functional status during ICU stay using the sum of the subdomains “Mobility” and “Transfer” of the Barthel Index [mobility-transfer-Barthel (MTB)] [24–26]. These subdomains ranged between 0 and 15 by steps of 5 and were summed up. A maximum sum score of 30 represents a fully independent person who can walk independently and transfer from bed to chair without assistance. An MTB of 0 indicates an entirely dependent patient in those domains. To identify changes in the functional status over time, we recorded the MTB at three time points: (1) pre-hospital, (2) at ICU discharge, and (3) at hospital discharge. Pre-hospital status was assessed retrospectively through interviews with the patient or their relatives, referring to the patient’s functional status 2 weeks prior to critical illness. Time points 2 and 3 were obtained by our study staff. The primary outcome, “Δ MTB ICU”, indicates the change between the pre-hospital assessment and ICU discharge and represents the loss of mobility during ICU stay [27]. Secondary outcome parameters included “Δ MTB hospital” (change between pre-hospital and hospital discharge), ICU LOS, hospital LOS, discharge to home, ICU, and hospital mortality.
Exposures
Patients received mobilisation therapy provided by experienced physiotherapists and ICU nurses, according to our hospital standards. To define the dose of the intervention, we recorded data regarding the initiation (to evaluate if early mobilisation applied (< 72 h) [6, 28]), frequency and duration of daily mobilisation, as well as the highest level reached in each session. The level of mobilisation was obtained by the Surgical Intensive Care Unit Optimal Mobilisation Score (SOMS), a validated tool that assesses the patient’s mobilisation capacity, ranging from 0 (no mobilisation) to 4 (ambulation) [29, 30]. The recorded duration of daily mobilisation included passive and active mobilisation and considered consecutive sessions (also with different levels of mobilisation) as one mobilisation unit. The average frequency per day is calculated by dividing the sum of all units of the patient by the total duration of the ICU stay.
Data collection
We collected baseline basic demographics, the reason for admission, and the respective department at ICU admission. Data upon admission included location before ICU admission, ICU admission category (sepsis, polytrauma, traumatic brain injury, non-traumatic brain injury, postoperative monitoring, cardiac failure, respiratory failure, and “other”), and diagnosis (e.g. sepsis or trauma) and several scores to characterise the cohort: baseline Glasgow Coma Scale (GCS), Clinical Frailty Scale (CFS) [26, 27], Charlson Comorbidity Index [28], Sequential Organ Failure Assessment Score (SOFA) [29] as well as standard laboratory and haemodynamic parameters. To record data on mobilisation practice, healthcare providers filled out a bedside form for each patient after each session. Our study staff performed a bedside quality analysis daily, and the data were prospectively maintained in an electronic database. By compiling these variables, we created a detailed profile of our cohort regarding their mobilisation ability. Patients without complete mobilisation records or who did not receive any out-of-bed mobilisation during their ICU stay (SOMS levels 0 and 1) were not included in the study. Furthermore, patients who passed away during their ICU stay were excluded due to the missing primary endpoint in the primary analysis.
This data collection profile included information on the patient's condition upon admission to the ICU and pre-morbid functional status measured by frailty, Mobility-Transfer-Barthel, and Charlson Comorbidity Index. We also recorded detailed information on the severity of illness using the SOFA, APACHE II, and Glasgow Coma Scale.
Statistics
We presented continuous variables as median [interquartile range (IQR)] and categorical variables in absolute numbers and percentages. Univariate analysis was conducted using Mann–Whitney U tests or Chi-square tests.
To measure the influence of the mean daily duration of mobilisation on the change in MTB from hospital admission to ICU discharge, the average treatment effect (ATE) [31] was calculated using linear regression models. First, an unadjusted ATE was calculated; in the second step, an adjusted ATE was calculated using a multivariate linear regression model. Parameters included in the models were: duration of daily mobilisation, patient characteristics (sex, BMI, age, ICU admission, invasive mechanical ventilation, frailty), ICU LOS, scores (GCS, APACHE II, SOFA, CCI), treating department and reason for ICU admission. We further performed analyses including the aforementioned covariates and adding mobilisation parameters to the model (mean mobilisation sessions per day, maximum SOMS level achieved). In the third step ATE with inverse probability weighting was calculated. Inverse probability weighting (IPW) [32] is a statistical method that involves adjusting for selection bias by assigning weights to the observed data based on the inverse of the probability of the observed sample being chosen. IPW was performed with the package WeightIt. [33] Here, different variants to perform the IPW can be analysed and compared (glm, gbm, energy, etc.). Of all the options, the energy [34] method provided the best results regarding balance, coefficient of variation, and adequate sample size. The balance was calculated using standardised mean differences (SMD) and proportion differences and shown using love plots. An SMD or difference in proportions of < 0.1 was considered balanced. All adjustment methods were repeated once with and once without mobilisation parameters. Model-based recursive partitioning [35] was used with a minimum bucket size of 10% of the study population to identify patient subgroups benefiting differently from mobilisation duration. Model-based recursive partitioning is a statistical method that constructs a tree by recursively splitting data into smaller, more homogeneous subgroups based on the average treatment effect within each subgroup. Here, the influence of the duration of mobilisation on the change in MTB until ICU discharge was set as an endpoint. For sensitivity analysis, all calculations were repeated for the full set of patients. For patients who died, we repeated the analysis imputing the missing endpoints. We used three different methods: MTB at ICU and hospital discharge were set to 0 (worst-case approach), MTB at ICU and hospital discharge were carried forward using the MTB at hospital admission, and a jump to reference imputation [36] using 2000 bootstrap samples (most stable method). A p < 0.05 was considered significant. All analyses were conducted using R version 4.3.0 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient and mobilisation characteristics
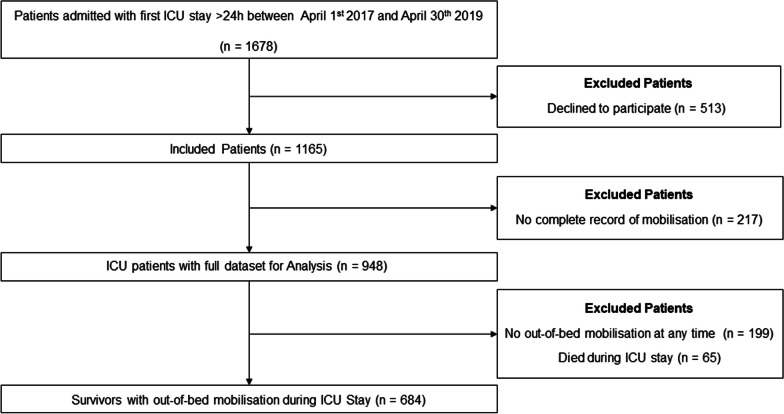
During a period of 2 years, 1165 critically ill patients were included. After excluding dead and in-bed mobilised patients, 684 were analysed (Fig. 1). The median age of our patients was 66 years, with the majority of patients being female (59.8%). Further baseline and demographic characteristics are presented in Table 1.
Fig. 1.
Strobe diagram
Table 1.
Patient characteristics
| All patients, n = 684 | Group of patients with | p-value | ||
|---|---|---|---|---|
| < 40 min per day, n = 412 | ≥ 40 min per day, n = 272 | |||
| Patient characteristics | ||||
| Age (years), median [IQR] | 66 [55–76] | 64 [54–73] | 70 [57–77] | 0.002 |
| Female, n (%) | 409 (59.8) | 234 (56.8) | 175 (64.3) | 0.049 |
| Body mass index (kg/m2), n (%) | 0.20 | |||
| Underweight | 40 (5.8) | 29 (7.0) | 11 (4.0) | |
| Normal | 289 (42.3) | 171 (41.5) | 118 (43.4) | |
| Overweight | 259 (37.9) | 149 (36.2) | 110 (40.4) | |
| Obese | 96 (14.0) | 63 (15.3) | 33 (12.1) | |
| MTB at hospital admission, median [IQR] | 30 [30–30] | 30 [30–30] | 30 [30–30] | 0.079 |
| Invasive mechanical ventilation, n (%) | 360 (52.6) | 247 (60.0) | 113 (41.5) | < 0.001 |
| ICU admission, n (%) | 0.74 | |||
| From home | 461 (67.4) | 280 (68.0) | 181 (66.5) | |
| From hospital | 212 (31.0) | 127 (30.8) | 85 (31.3) | |
| From nursing home | 8 (1.2) | 4 (1.0) | 4 (1.5) | |
| Unknown | 3 (0.4) | 1 (0.2) | 2 (0.7) | |
| Frailty, n (%) | 148 (21.6) | 78 (18.9) | 70 (25.7) | 0.034 |
| Scoring | ||||
| APACHE II, median [IQR] | 13 [10–17] | 14 [9–17] | 13 [10–17] | > 0.99 |
| SOFA, median [IQR] | 6 [4–8] | 6 [4–8] | 6 [3–8] | 0.039 |
| CCI, median [IQR] | 1 [0–3] | 1 [0–2] | 2 [0–3] | < 0.001 |
| GCS, median [IQR] | 14.5 [10–15] | 14 [8–15] | 15 [13–15] | < 0.001 |
| Department, n (%) | < 0.001 | |||
| Neurocritical | 281 (41.1) | 194 (47.1) | 87 (32.0) | |
| Surgical | 341 (49.9) | 191 (46.4) | 150 (55.1) | |
| Medical | 36 (5.3) | 18 (4.4) | 18 (6.6) | |
| Other | 26 (3.8) | 9 (2.2) | 17 (6.3) | |
| ICU admission reasons | ||||
| Sepsis, n (%) | 84 (12.3) | 44 (10.7) | 40 (14.7) | 0.12 |
| Polytrauma, n (%) | 27 (3.9) | 21 (5.1) | 6 (2.2) | 0.057 |
| Traumatic brain injury, n (%) | 80 (11.7) | 58 (14.1) | 22 (8.1) | 0.017 |
| Non-traumatic brain pathology, n (%) | 127 (18.6) | 94 (22.8) | 33 (12.1) | < 0.001 |
| Postoperative, n (%) | 169 (24.7) | 93 (22.6) | 76 (27.9) | 0.11 |
| Cardiac, n (%) | 34 (5.0) | 18 (4.4) | 16 (5.9) | 0.37 |
| Pulmonary, n (%) | 206 (30.1) | 101 (24.5) | 105 (38.6) | < 0.001 |
| Other, n (%) | 122 (17.8) | 73 (17.7) | 49 (18.0) | 0.92 |
| Mobilisation parameters | ||||
| Mean mobilisation sessions per day, median [IQR] | 0.20 [0.10–0.40] | 0.18 [0.09–0.33] | 0.25 [0.11–0.50] | 0.002 |
| Maximum SOMS level reached, n (%) | < 0.001 | |||
| 2 | 218 (31.9) | 190 (46.1) | 28 (10.3) | |
| 3 | 265 (38.7) | 148 (35.9) | 117 (43.0) | |
| 4 | 201 (29.4) | 74 (18.0) | 127 (46.7) | |
| Early mobilisation, n (%) | 447 (65.4) | 244 (59.2) | 203 (74.6) | < 0.001 |
| Hospital trajectory | ||||
| ICU length of stay (days), median [IQR] | 10 [4–22] | 9 [4–20] | 11 [5–26] | 0.037 |
| Hospital length of stay (days), median [IQR] | 29 [19–44] | 28 [19–41] | 31 [19–51] | 0.016 |
| Hospital mortality after ICU discharge, n (%) | 32 (4.7) | 21 (5.1) | 11 (4.0) | 0.52 |
Numbers are presented as n (%) or median [IQR]. “Frailty” is defined as Clinical Frailty Scale 5–9
ICU intensive care unit, IQR interquartile range, GCS Glasgow Coma Scale, APACHE Acute Physiology and Chronic Health Evaluation Score, SOFA Sepsis-Related Organ Failure Assessment Score, CCI Charlson Comorbidity index, MTB mobility-transfer-Barthel, SOMS Surgical ICU optimal mobilisation score
Primary and secondary endpoints
Daily mobilisation ≥ 40 min was identified as an independent predictor of an improved functional status upon ICU discharge. This effect on the primary outcome measure Δ MTB till ICU discharge was observed in the univariate [ATE 3.6 (95% CI 2.4–4.8), p < 0.001], in the adjusted multivariate model (without mobilisation parameters) [ATE 3.4 (95% CI 2.3–4.7), p < 0.001] and the IPW analysis (without mobilisation parameters) [ATE 3.1 (95% CI 1.9–4.4), p < 0.001] (Table 2). When mobilisation parameters were included in the analysis, the average treatment effect disappeared [multivariate analysis ATE 0.5 (95% CI − 0.7 to 1.7), p = 0.38], IPW analysis 0.3 [95% CI − 1.0 to 1.6], p = 0.67); see Additional file 1: Figs. S1 and S2 for the love plots and Additional file 1: Tables S1–3 for the full models. The effect of daily mobilisation on functional status upon hospital discharge provided the same results with significant improvement in the univariate [ATE 2.2 (95% CI 0.4–3.6), p = 0.016], in the adjusted multivariate model (without mobilisation parameters) [ATE 2.2 (95% CI 0.6–3.9), p = 0.008] and in the IPW analysis (without mobilisation parameters) [ATE 1.9 (95% CI 0.2–3.6), p = 0.03]. When mobilisation parameters were included in the analysis, the average treatment effect disappeared [multivariate analysis ATE − 0.7 (95% CI − 2.4 to 1.0), p = 0.39], IPW analysis − 1.1 [95% CI − 2.7 to 0.6], p = 0.22, Table 2 and Additional file 1: Table S4–6 for the full models on hospital discharge). The three imputation methods for deceased patients confirmed the primary analysis results (see Additional file 1: Tables S7).
Table 2.
Average treatment effects (ATE) of ≥ 40 min daily mobilisation on the primary and secondary endpoint
| Change in MTB until | ||||
|---|---|---|---|---|
| ICU discharge | Hospital discharge | |||
| ATE [95% CI] | p-value | ATE [95% CI] | p-value | |
| Univariate analysis | 3.6 [2.4–4.8] | < 0.001 | 2.2 [0.41–3.9] | 0.016 |
| Multivariate analysis | 3.4 [2.2–4.7] | < 0.001 | 2.2 [0.59–3.9] | 0.008 |
| Multivariate with mobilisation | 0.54 [− 0.66–1.7] | 0.38 | − 0.74 [− 2.4–0.96] | 0.39 |
| IPW | 3.1 [1.9–4.4] | < 0.001 | 1.9 [0.20–3.6] | 0.03 |
| IPW with mobilisation | 0.28 [− 1.0–1.6] | 0.67 | − 1.1 [− 2.7–0.62] | 0.22 |
Calculated using univariate, multivariate, and weighted linear regression models. Multivariate linear regression models were adjusted for all baseline patient characteristics once without mobilisation parameters and once with. Inverse probability weighting was performed in the same manner. MTB mobility-transfer-Barthel, ICU intensive care unit, IPW inverse probability weighting
Subgroup analyses
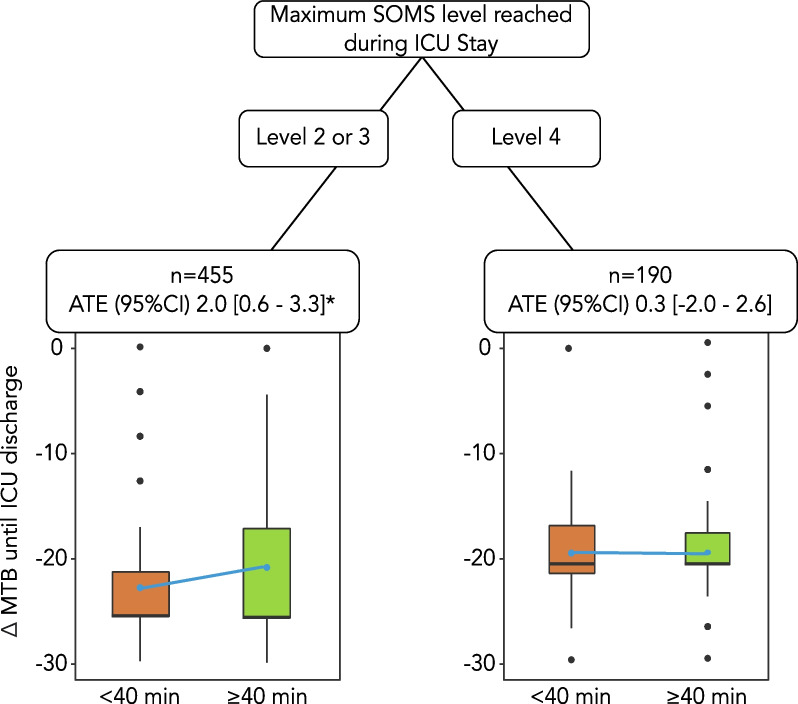
Model-based recursive partitioning was performed to characterise patient subgroups who benefit from mobilisation duration ≥ 40 min. The maximum SOMS level during the ICU stay was identified as the most crucial variable to positively affect the change in Mobility-Transfer-Barthel until ICU discharge (Fig. 2). Higher mobilisation levels during the ICU stay had a positive impact on the primary outcome, resulting in significantly less functional loss until ICU discharge if SOMS level 2 or 3 was reached [ATE 2.0 (95% CI 0.6–3.3), p = 0.001] (Fig. 2). If SOMS level 4 was reached, e.g. the patient was able to ambulate during ICU stay, there was no effect of the duration ≥ 40 min [ATE − 0.3 (95%CI − 2.0 to 2.6), p = 0.80] (Fig. 2).
Fig. 2.
Model-based recursive partitioning with all confounding variables for the influence of duration of mobilisation on ∆ MTB until ICU discharge. The minimum number of patients in each end node was set to 10% of the sample size. Blue points represent mean ∆ MTB until ICU discharge of each group. ATE were calculated using linear regression models. *= 0.001. ATE average treatment effects,MTB mobility-transfer-Barthel, ICU intensive care unit, SOMS Surgical ICU optimal mobilisation score
Discussion
In this analysis, we demonstrated that mobilisation for more than 40 min per day in an interdisciplinary critically ill cohort positively affected the change in mobility until discharge from the ICU. Our results suggest that a higher duration of mobilisation may help preserve the functionality of critically ill patients surviving the ICU stay. However, the maximum achieved mobilisation level was the most important of all mobilisation parameters influencing the outcome. Looking at the subgroups by mobilisation level, in patients with the highest mobilisation level (SOMS 4), the mobilisation duration of > 40 min was no longer statistically significant.
Three additional mobilisation parameters besides duration were included in our models: the time of onset (“early mobilisation”), the frequency per day, and the maximum level reached, while we did not include subjectively perceived intensity. Evidence on mobilisation duration alone and its optimum in critical care is limited. Only Schujmann et al. conducted a single-centre RCT in Brazil, where the intervention group received an average of 40 min of physiotherapy per day, leading to improved functional status and more independent patients on ICU discharge (96% vs 44%; p < 0.001) [36]. Our data showed a similar benefit of 40 min of mobilisation therapy on the functional status of the critically ill, confirming their findings in a general ICU cohort without a limitation to functionally independent patients.
The interaction between the different mobilisation components, however, remains complex. Watanabe et al. and Scheffenbichler et al. developed a score that considered both level and duration to compare low vs. high doses of mobilisation therapy. A high dose of mobilisation therapy was associated with a better functional outcome, reduced mortality, and a shorter ICU and hospital stay [19, 37]. However, the specific effect of duration cannot be determined from these studies.
Mazwi et al. employed the same score to analyse the effects of high vs. low doses of mobilisation on adverse discharge in stroke patients. Furthermore, they investigated the individual effects of duration and level of mobilisation on outcome. Longer mean mobilisation (> 41 min/day) correlated with lower odds of adverse discharge (OR: 0.11, 95%CI 0.05–0.23; p < 0.01) compared with shorter mean mobilisation (< 41 min/day). Adjustment for disease severity provided similar results. Patients who achieved the mobility level of ambulation were less likely to have a negative discharge than those who achieved a lower level (OR: 0.14, 95%CI 0.07–0.29; p < 0.01) [21]. Interestingly, their results indicate favourable outcomes for similar daily mobilisation duration as our data and highlight the importance of higher mobilisation levels. However, their primary outcome and patient cohort were distinct, focusing on a homogeneous group of stroke patients.
Our data suggested that level was an important component, especially if patients were able to achieve the capability of walking in the ICU. This is consistent with the findings of Paton et al. who demonstrated that higher levels of mobilisation, as measured by the IMS, resulted in improved long-term outcomes in both functional status and quality of life [20]. However, the impact of high mobilisation levels on outcomes appears to vary among subgroups of ICU patients. Fuest et al. confirmed that in severely frail patients, the maximum SOMS level achieved had the greatest influence on discharge to home, whereas in young trauma patients, a higher level was not associated with a superior chance of being discharged home [38]. Therefore, a uniform approach of mobilisation targeting higher levels of therapy does not appear to be useful in the heterogeneous group of critically ill patients. The recently published TEAM trial showed no significant benefit for longer and higher active mobilisation (+ 12.0 additional minutes per day) in long-term outcomes and had a higher incidence of adverse events during the intervention [39]. This confirmed that there is a ceiling effect of the dosage of mobilisation. Therefore the 40 min recommended in a guideline [23] may be too ambitious, and an individualised approach could be more meaningful.
There are several reasons that influence the length of mobilisation therapy: (1) patient-related, (2) provider-related and (3) organisational factors. Patient-related factors are probably the most important factor. The type and severity of the disease often limit the mobilisation that can be achieved. The intrinsic possibility and ability for out-of-bed mobilisation depends on the status prior to ICU admission and the current impact of the disease on it. To rule out this effect on the endpoints, a balanced group analysis as the used IPW is essential. Examples of provider-related factors are their workload, individual motivation or attitude towards mobilisation as well as their training [40]. Organisational factors include both the culture towards mobilisation (e.g. the existence of mobilisation teams or mobility champions) and the existence of standard operating procedures or local protocols [41, 42].
Generalisability and limitations
Although our study was based on single-centre data, a large number of patients and a diverse range of critically ill patients were strengthening factors of this prospective cohort study. Unlike other studies in this field, we did not exclude patients with a functional deficit prior to hospital admission or neurocritical patients. Nevertheless, our results should be externally validated, which must be considered as a limitation. Another important limitation was the exclusion of deceased patients and patients who could not be mobilised out-of-bed during the entire stay. This could introduce bias as patients in poor condition were excluded. This exclusion was justified because there was no primary endpoint for these patients, and thus, the intended analysis could not be performed. Second, the evaluation of mobilisation duration for patients who could not be mobilised at any time was not meaningful. Nevertheless, several sensitivity analyses confirmed the results of the primary analysis, which indicates a stable result. However, there were group differences in the severity of the disease between the patients we analysed for our study, which could affect the stability of the patient and potentially hinder mobilisation. This could have affected the duration of mobilisation and therefore introduced bias into our results. To address potential patient-related confounding, we performed inverse probability weighting, including disease severity scores, disease type, and baseline characteristics, department, and other aspects of mobilisation, which provided similar results. Nevertheless, residual confounding cannot be completely discounted. Another limitation of our study is that adverse events during the intervention were not evaluated. Since the publication of the TEAM trial, there could have been concerns that adverse events might increase if mobilisation lasted longer, here longer than 40 min. However, the adverse events in the TEAM trial did not show such an increase. Furthermore, the adverse events in the TEAM trial did not lead to significant differences in patient outcomes [39], which reduces their clinical relevance.
Conclusion
In conclusion, a mobilisation duration of more than 40 min in a group of survivors of critical illness had a positive effect on functional outcomes. Investigating the interaction of the different mobilisation dose components, the maximum mobilisation level achieved was the most important factor influencing the outcome. Moreover, in patients who were already able to ambulate, an increased duration of mobilisation did not result in any additional effect.
Supplementary Information
Additional file 1. Online supplementary Figures and Tables.
Acknowledgements
We would like to thank especially the team of nursing and physiotherapy in our ICUs for their engagement and their patience during the time of study implementation.
Author contributions
SJS and MB have designed the study and had the responsibility in the implementation. ML, KF and SJS were responsible for the data. KF wrote the first draft. BU was the study statistician. All authors reviewed and edited the manuscript and approved the submitted version.
Funding
Open Access funding enabled and organized by Projekt DEAL. Institutional funds only.
Data availability
The datasets used and analysed during the current study are available from the corresponding author on reasonable request and after signing a data sharing contract.
Declarations
Institutional review board statement
The database has been conducted according to the Declaration of Helsinki and approved by the Ethics Committee of the Faculty of Medicine, Technical University of Munich (528/18 from 22nd Dec 2016). The inclusion criteria were age over 18 years and an expected ICU stay > 24 h, while the exclusion criterion was readmission to the intensive care unit.
Consent for publication
The data of critically ill patients were collected after obtaining written informed consent from them or their legal representative, in accordance with German law.
Competing interests
SJS received grants and non-financial support from Reactive Robotics GmbH (Munich, Germany), ASP GmbH (Attendorn, Germany), STIMIT AG (Biel, Switzerland), ESICM (Geneva, Switzerland), grants, personal fees, and non-financial support from Fresenius Kabi Deutschland GmbH (Bad Homburg, Germany), grants from the Innovationsfond of The Federal Joint Committee (G-BA), personal fees from Springer Verlag GmbH (Vienna, Austria) for educational purposes and Advanz Pharma GmbH (Bielefeld, Germany), non-financial support from national and international societies (and their congress organisers) in the field of anesthesiology and intensive care medicine, outside the submitted work. Dr. Schaller holds stocks in small amounts from Alphabet Inc., Bayer AG, and Siemens AG; these holdings have not affected any decisions regarding his research or this study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hodgson CL, Udy AA, Bailey M, Barrett J, Bellomo R, Bucknall T, et al. The impact of disability in survivors of critical illness. Intensive Care Med. 2017;43(7):992–1001. doi: 10.1007/s00134-017-4830-0. [DOI] [PubMed] [Google Scholar]
- 2.Herridge MS, Tansey CM, Matte A, Tomlinson G, Diaz-Granados N, Cooper A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364(14):1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 3.Kamdar BB, Huang M, Dinglas VD, Colantuoni E, von Wachter TM, Hopkins RO, et al. Joblessness and lost earnings after acute respiratory distress syndrome in a 1-year national multicenter study. Am J Respir Crit Care Med. 2017;196(8):1012–1020. doi: 10.1164/rccm.201611-2327OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spies CD, Krampe H, Paul N, Denke C, Kiselev J, Piper SK, et al. Instruments to measure outcomes of post-intensive care syndrome in outpatient care settings—results of an expert consensus and feasibility field test. J Intensive Care Soc. 2021;22(2):159–174. doi: 10.1177/1751143720923597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rengel KF, Hayhurst CJ, Pandharipande PP, Hughes CG. Long-term cognitive and functional impairments after critical illness. Anesth Analg. 2019;128(4):772–780. doi: 10.1213/ANE.0000000000004066. [DOI] [PubMed] [Google Scholar]
- 6.Fuest K, Schaller SJ. Recent evidence on early mobilization in critical-ill patients. Curr Opin Anaesthesiol. 2018;31(2):144–150. doi: 10.1097/ACO.0000000000000568. [DOI] [PubMed] [Google Scholar]
- 7.Schweickert WD, Pohlman MC, Pohlman AS, Nigos C, Pawlik AJ, Esbrook CL, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373(9678):1874–1882. doi: 10.1016/S0140-6736(09)60658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tipping CJ, Harrold M, Holland A, Romero L, Nisbet T, Hodgson CL. The effects of active mobilisation and rehabilitation in ICU on mortality and function: a systematic review. Intensive Care Med. 2017;43(2):171–183. doi: 10.1007/s00134-016-4612-0. [DOI] [PubMed] [Google Scholar]
- 9.Schaller SJ, Anstey M, Blobner M, Edrich T, Grabitz SD, Gradwohl-Matis I, et al. Early, goal-directed mobilisation in the surgical intensive care unit: a randomised controlled trial. Lancet. 2016;388(10052):1377–1388. doi: 10.1016/S0140-6736(16)31637-3. [DOI] [PubMed] [Google Scholar]
- 10.Waldauf P, Jiroutková K, Krajčová A, Puthucheary Z, Duška F. Effects of rehabilitation interventions on clinical outcomes in critically ill patients: systematic review and meta-analysis of randomized controlled trials. Crit Care Med. 2020;48(7):1055–1065. doi: 10.1097/CCM.0000000000004382. [DOI] [PubMed] [Google Scholar]
- 11.Patel BK, Wolfe KS, Patel SB, Dugan KC, Esbrook CL, Pawlik AJ, et al. Effect of early mobilisation on long-term cognitive impairment in critical illness in the USA: a randomised controlled trial. Lancet Respir Med. 2023;11(6):563–572. doi: 10.1016/S2213-2600(22)00489-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schaller SJ, Scheffenbichler FT, Bose S, Mazwi N, Deng H, Krebs F, et al. Influence of the initial level of consciousness on early, goal-directed mobilization: a post hoc analysis. Intensive Care Med. 2019;45(2):201–210. doi: 10.1007/s00134-019-05528-x. [DOI] [PubMed] [Google Scholar]
- 13.Hermans G, Van den Berghe G. Clinical review: intensive care unit acquired weakness. Crit Care. 2015;19(1):274. doi: 10.1186/s13054-015-0993-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Aerde N, Meersseman P, Debaveye Y, Wilmer A, Gunst J, Casaer MP, et al. Five-year impact of ICU-acquired neuromuscular complications: a prospective, observational study. Intensive Care Med. 2020 doi: 10.1007/s00134-020-05927-5. [DOI] [PubMed] [Google Scholar]
- 15.Piva S, Fagoni N, Latronico N. Intensive care unit-acquired weakness: unanswered questions and targets for future research. F1000Res. 2019;8:508. doi: 10.12688/f1000research.17376.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayward KS, Churilov L, Dalton EJ, Brodtmann A, Campbell BCV, Copland D, et al. Advancing stroke recovery through improved articulation of nonpharmacological intervention dose. Stroke. 2021;52(2):761–769. doi: 10.1161/STROKEAHA.120.032496. [DOI] [PubMed] [Google Scholar]
- 17.Ding N, Zhang Z, Zhang C, Yao L, Yang L, Jiang B, et al. What is the optimum time for initiation of early mobilization in mechanically ventilated patients? A network meta-analysis. PLoS ONE. 2019;14(10):e0223151. doi: 10.1371/journal.pone.0223151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moss M. Early mobilization of critical care patients—still more to learn. N Engl J Med. 2022;387(19):1807–1808. doi: 10.1056/NEJMe2212360. [DOI] [PubMed] [Google Scholar]
- 19.Scheffenbichler FT, Teja B, Wongtangman K, Mazwi N, Waak K, Schaller SJ, et al. Effects of the level and duration of mobilization therapy in the surgical ICU on the loss of the ability to live independently: an international prospective cohort study. Crit Care Med. 2021;49(3):e247–e257. doi: 10.1097/CCM.0000000000004808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paton M, Lane R, Paul E, Cuthburtson GA, Hodgson CL. Mobilization during critical illness: a higher level of mobilization improves health status at 6 months, a secondary analysis of a prospective cohort study. Crit Care Med. 2021;49(9):e860–e869. doi: 10.1097/CCM.0000000000005058. [DOI] [PubMed] [Google Scholar]
- 21.Mazwi N, Lissak I, Wongtangman K, Platzbecker K, Albrecht L, Teja B, et al. Effects of mobility dose on discharge disposition in critically-ill stroke patients. PMR. 2023 doi: 10.1002/pmrj.13039. [DOI] [PubMed] [Google Scholar]
- 22.TEAM Study Investigators. The ACTG. Hodgson CL, Bailey M, Bellomo R, Brickell K, et al. Early active mobilization during mechanical ventilation in the ICU. N Engl J Med. 2022;387(19):1747–1758. doi: 10.1056/NEJMoa2209083. [DOI] [PubMed] [Google Scholar]
- 23.Bein T, Bischoff M, Brückner U, Gebhardt K, Henzler D, Hermes C, et al. S2e guideline: positioning and early mobilisation in prophylaxis or therapy of pulmonary disorders. Anaesthesist. 2015;64(1):1–26. doi: 10.1007/s00101-015-0071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahoney FI, Barthel DW. Functional evaluation: the Barthel index. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 25.Watanabe S, Kotani T, Taito S, Ota K, Ishii K, Ono M, et al. Determinants of gait independence after mechanical ventilation in the intensive care unit: a Japanese multicenter retrospective exploratory cohort study. J Intensive Care. 2019;7(1):53. doi: 10.1186/s40560-019-0404-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fuest KE, Lorenz M, Grunow JJ, Weiss B, Morgeli R, Finkenzeller S, et al. The functional trajectory in frail compared with non-frail critically ill patients during the hospital stay. Front Med. 2021;8:748812. doi: 10.3389/fmed.2021.748812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jesus FS, Paim Dde M, Brito Jde O, Barros Ide A, Nogueira TB, Martinez BP, et al. Mobility decline in patients hospitalized in an intensive care unit. Rev Bras Ter Intensiva. 2016;28(2):114–119. doi: 10.5935/0103-507X.20160025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hodgson CL, Berney S, Harrold M, Saxena M, Bellomo R. Clinical review: early patient mobilization in the ICU. Crit Care. 2013;17(1):207. doi: 10.1186/cc11820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piva S, Dora G, Minelli C, Michelini M, Turla F, Mazza S, et al. The surgical optimal mobility score predicts mortality and length of stay in an Italian population of medical, surgical, and neurologic intensive care unit patients. J Crit Care. 2015;30(6):1251–1257. doi: 10.1016/j.jcrc.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 30.Schaller SJ, Stäuble CG, Suemasa M, Heim M, Duarte IM, Mensch O, et al. The German validation study of the surgical intensive care unit optimal mobility score. J Crit Care. 2016;32:201–206. doi: 10.1016/j.jcrc.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 31.Holland PW. Statistics and causal inference. J Am Stat Assoc. 1986;81(396):945–960. doi: 10.1080/01621459.1986.10478354. [DOI] [Google Scholar]
- 32.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34(28):3661–3679. doi: 10.1002/sim.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greifer N, Greifer MN. Package ‘WeightIt’. CRAN. 2019.
- 34.Huling JD, Mak S. Energy balancing of covariate distributions. arXiv. 2020 doi: 10.48550/arXiv.2004.13962. [DOI] [Google Scholar]
- 35.Hothorn T, Hornik K, Zeileis A. Unbiased recursive partitioning: a conditional inference framework. J Comput Graph Stat. 2006;15(3):651–674. doi: 10.1198/106186006X133933. [DOI] [Google Scholar]
- 36.Schujmann DS, Teixeira Gomes T, Lunardi AC, Zoccoler Lamano M, Fragoso A, Pimentel M, et al. Impact of a progressive mobility program on the functional status, respiratory, and muscular systems of ICU patients: a randomized and controlled trial. Crit Care Med. 2020;48(4):491–497. doi: 10.1097/CCM.0000000000004181. [DOI] [PubMed] [Google Scholar]
- 37.Watanabe S, Morita Y, Suzuki S, Kochi K, Ohno M, Liu K, et al. Effects of the intensity and activity time of early rehabilitation on activities of daily living dependence in mechanically ventilated patients. Prog Rehabil Med. 2021;6:20210054. doi: 10.2490/prm.20210054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fuest KE, Ulm B, Daum N, Lindholz M, Lorenz M, Blobner K, et al. Clustering of critically ill patients using an individualized learning approach enables dose optimization of mobilization in the ICU. Crit Care. 2023;27(1):1. doi: 10.1186/s13054-022-04291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hodgson CL, Bailey M, Bellomo R, Brickell K, Broadley T, Buhr H, et al. Early active mobilization during mechanical ventilation in the ICU. N Engl J Med. 2022;387(19):1747–1758. doi: 10.1056/NEJMoa2209083. [DOI] [PubMed] [Google Scholar]
- 40.Hermes C, Nydahl P, Blobner M, Dubb R, Filipovic S, Kaltwasser A, et al. Assessment of mobilization capacity in 10 different ICU scenarios by different professions. PLoS ONE. 2020;15(10):e0239853. doi: 10.1371/journal.pone.0239853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dubb R, Nydahl P, Hermes C, Schwabbauer N, Toonstra A, Parker AM, et al. Barriers and strategies for early mobilization of patients in intensive care units. Ann Am Thorac Soc. 2016;13(5):724–730. doi: 10.1513/AnnalsATS.201509-586CME. [DOI] [PubMed] [Google Scholar]
- 42.Bruce R, Forry C. Integrating a mobility champion in the intensive care unit. Dimens Crit Care Nurs. 2018;37(4):201–209. doi: 10.1097/DCC.0000000000000306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Online supplementary Figures and Tables.
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request and after signing a data sharing contract.