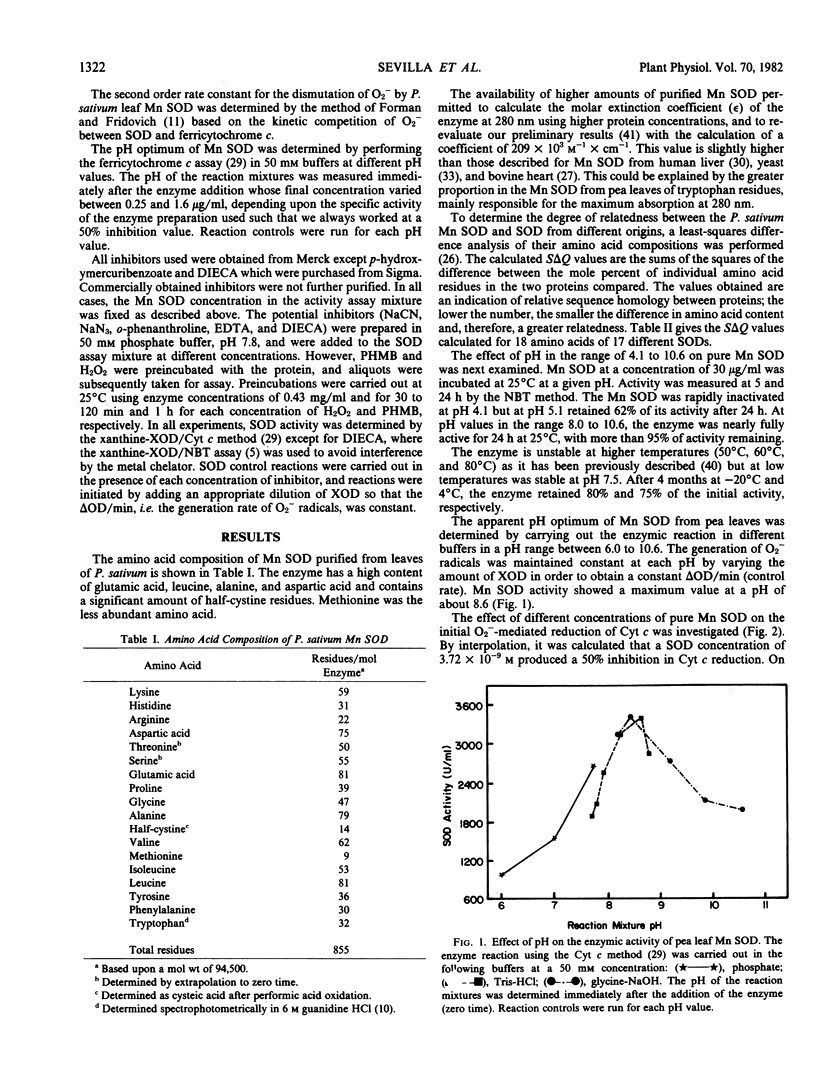
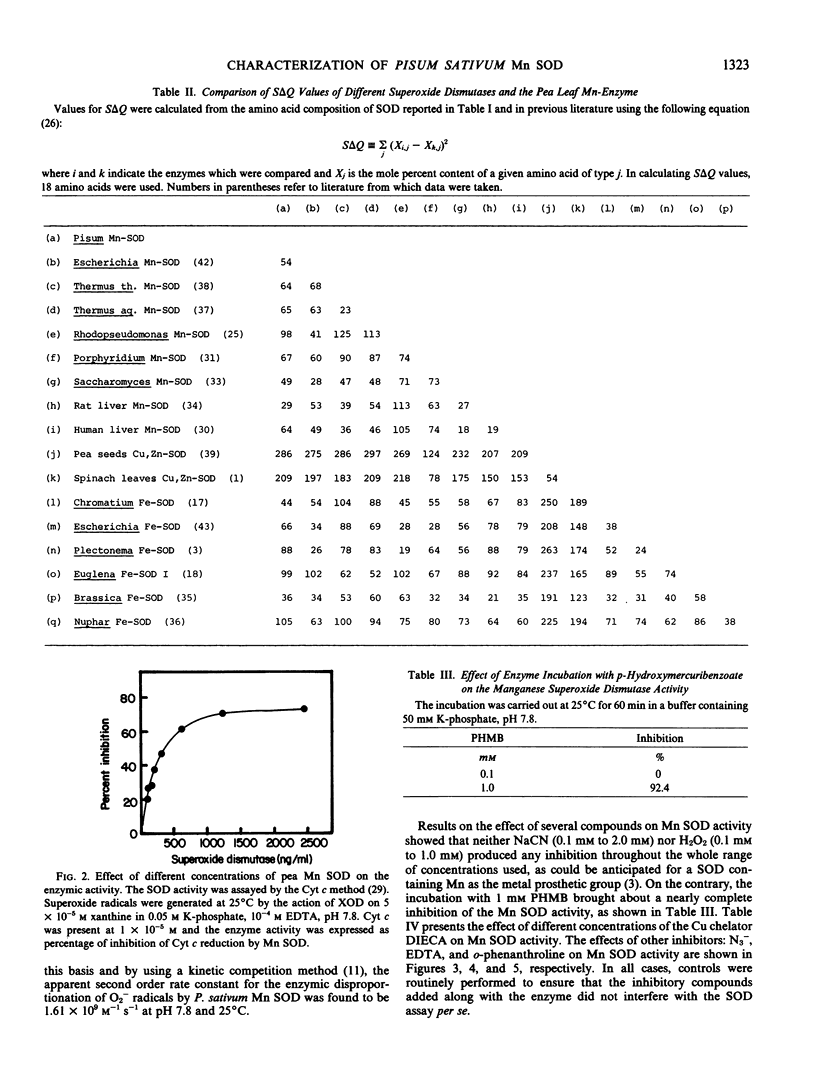
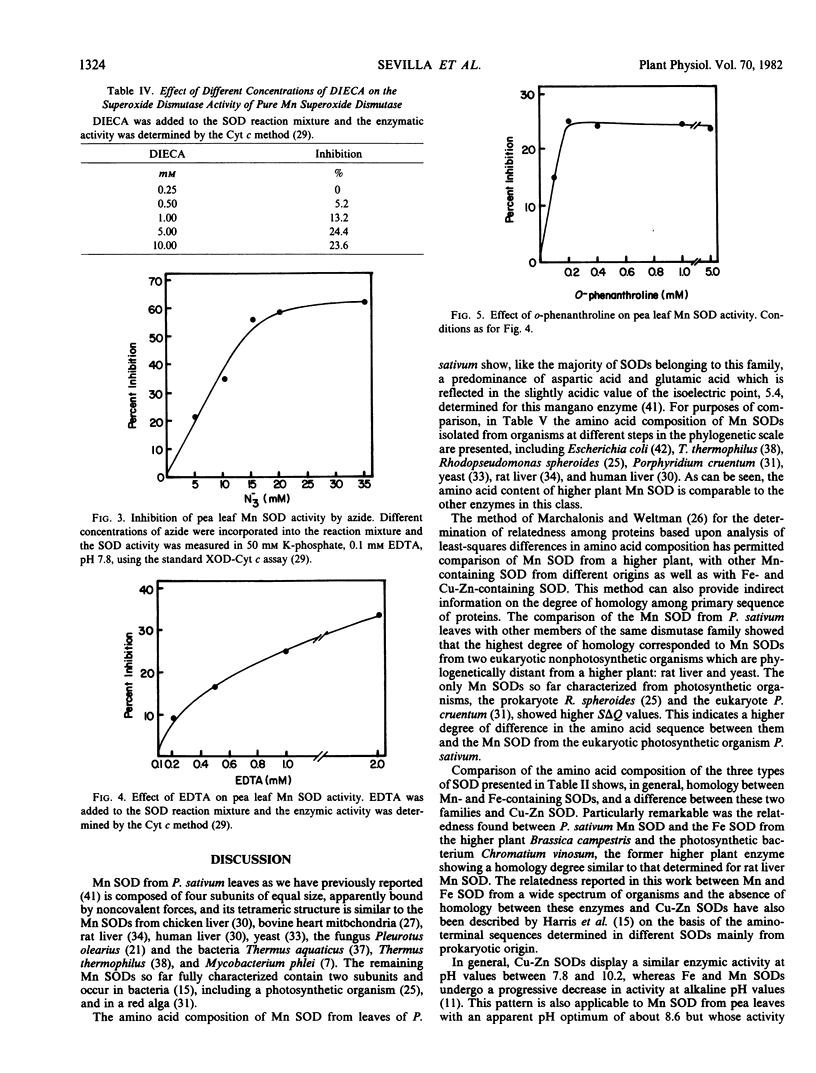
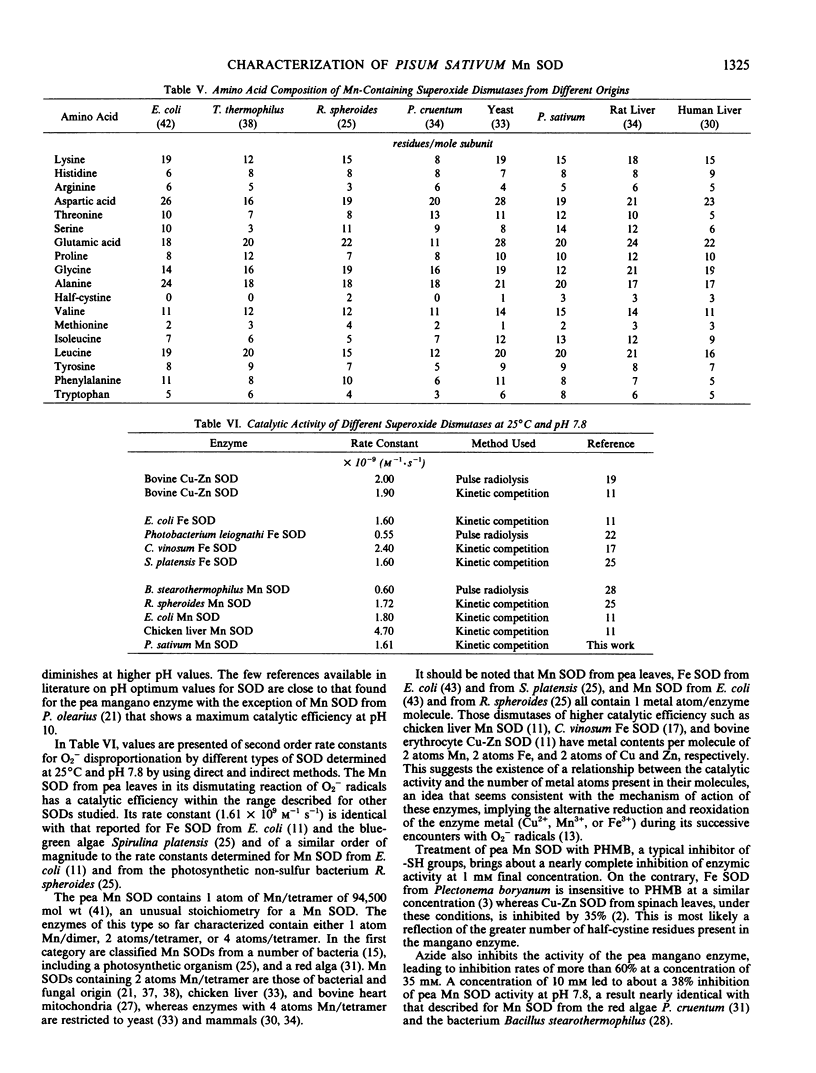
Abstract
A manganese-containing superoxide dismutase (EC 1.15.1.1) was fully characterized from leaves of the higher plant Pisum sativum L., var. Lincoln. The amino acid composition determined for the enzyme was compared with that of a wide spectrum of superoxide dismutases and found to have a highest degree of homology with the mitochondrial manganese superoxide dismutases from rat liver and yeast. The enzyme showed an apparent pH optimum of 8.6 and at 25°C had a maximum stability at alkaline pH values. By kinetic competition experiments, the rate constant for the disproportionation of superoxide radicals by pea leaf manganese superoxide dismutase was found to be 1.61 × 109 molar−1·second−1 at pH 7.8 and 25°C. The enzyme was not sensitive to NaCN or to H2O2, but was inhibited by N3−. The sulfhydryl reagent p-hydroxymercuribenzoate at 1 mm concentration produced a nearly complete inhibition of the manganese superoxide dismutase activity. The metal chelators o-phenanthroline, EDTA, and diethyldithiocarbamate all inhibited activity slightly in decreasing order of intensity. A comparative study between this higher plant manganese superoxide dismutase and other dismutases from different origins is presented.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asada K., Urano M., Takahashi M. Subcellular location of superoxide dismutase in spinach leaves and preparation and properties of crystalline spinach superoxide dismutase. Eur J Biochem. 1973 Jul 2;36(1):257–266. doi: 10.1111/j.1432-1033.1973.tb02908.x. [DOI] [PubMed] [Google Scholar]
- Asada K., Yoshikawa K., Takahashi M., Maeda Y., Enmanji K. Superoxide dismutases from a blue-green alga, Plectonema boryanum. J Biol Chem. 1975 Apr 25;250(8):2801–2807. [PubMed] [Google Scholar]
- Baum J. A., Scandalios J. G. Isolation and characterization of the cytosolic and mitochondrial superoxide dismutases of maize. Arch Biochem Biophys. 1981 Feb;206(2):249–264. doi: 10.1016/0003-9861(81)90089-8. [DOI] [PubMed] [Google Scholar]
- Beauchamp C. O., Fridovich I. Isozymes of superoxide dismutase from wheat germ. Biochim Biophys Acta. 1973 Jul 12;317(1):50–64. doi: 10.1016/0005-2795(73)90198-0. [DOI] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Chikata Y., Kusunose E., Ichihara K., Kusunose M. Purification of superoxide dismutases from Mycobacterium phlei. Osaka City Med J. 1975;21(2):127–136. [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- Forman H. J., Fridovich I. Superoxide dismutase: a comparison of rate constants. Arch Biochem Biophys. 1973 Sep;158(1):396–400. doi: 10.1016/0003-9861(73)90636-x. [DOI] [PubMed] [Google Scholar]
- Fridovich I. The biology of oxygen radicals. Science. 1978 Sep 8;201(4359):875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- Harris J. I., Auffret A. D., Northrop F. D., Walker J. E. Structural comparisons of superoxide dismutases. Eur J Biochem. 1980 May;106(1):297–303. doi: 10.1111/j.1432-1033.1980.tb06023.x. [DOI] [PubMed] [Google Scholar]
- Kanematsu S., Asada K. Ferric and manganic superoxide dismutases in Euglena gracilis. Arch Biochem Biophys. 1979 Jul;195(2):535–545. doi: 10.1016/0003-9861(79)90380-1. [DOI] [PubMed] [Google Scholar]
- Kanematsu S., Asada K. Superoxide dismutase from an anaerobic photosynthetic bacterium, Chromatium vinosum. Arch Biochem Biophys. 1978 Jan 30;185(2):473–482. doi: 10.1016/0003-9861(78)90191-1. [DOI] [PubMed] [Google Scholar]
- Klug D., Rabani J., Fridovich I. A direct demonstration of the catalytic action of superoxide dismutase through the use of pulse radiolysis. J Biol Chem. 1972 Aug 10;247(15):4839–4842. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lavelle F., McAdam M. E., Fielden E. M., Roberts P. B. A pulse-radiolysis study of the catalytic mechanism of the iron-containing superoxide dismutase from Photobacterium leiognathi. Biochem J. 1977 Jan 1;161(1):3–11. doi: 10.1042/bj1610003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavelle F., Michelson A. M. Purification et étude des deux superoxyde dismutases du champignon Pleurotus olearius. Biochimie. 1975;57(3):375–381. doi: 10.1016/s0300-9084(75)80314-2. [DOI] [PubMed] [Google Scholar]
- Lumsden J., Cammack R., Hall D. O. Purification and physicochemical properties of superoxide dismutase from two photosynthetic microorganisms. Biochim Biophys Acta. 1976 Jul 8;438(2):380–392. doi: 10.1016/0005-2744(76)90255-2. [DOI] [PubMed] [Google Scholar]
- Lumsden J., Hall D. O. Soluble & membrane-bound superoxide dismutases in a blue-green algae (Spirulina)and spinach. Biochem Biophys Res Commun. 1974 May 7;58(1):35–41. doi: 10.1016/0006-291x(74)90887-0. [DOI] [PubMed] [Google Scholar]
- Marklund S. Purification and characterization of a manganese containing superoxide dismutase from bovine heart mitochondria. Int J Biochem. 1978;9(5):299–306. doi: 10.1016/0020-711x(78)90101-5. [DOI] [PubMed] [Google Scholar]
- McAdam M. E., Levelle F., Fox R. A., Fielden E. M. A pulse-radiolysis study of the manganese-containing superoxide dismutase from Bacillus stearothermophilus. Biochem J. 1977 Jul 1;165(1):81–87. doi: 10.1042/bj1650081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Misra H. P., Fridovich I. Purification and properties of superoxide dismutase from a red alga, Porphyridium cruentum. J Biol Chem. 1977 Sep 25;252(18):6421–6423. [PubMed] [Google Scholar]
- Ravindranath S. D., Fridovich I. Isolation and characterization of a manganese-containing superoxide dismutase from yeast. J Biol Chem. 1975 Aug 10;250(15):6107–6112. [PubMed] [Google Scholar]
- Salin M. L., Bridges S. M. Isolation and Characterization of an Iron-Containing Superoxide Dismutase From Water Lily, Nuphar luteum. Plant Physiol. 1982 Jan;69(1):161–165. doi: 10.1104/pp.69.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin M. L., Bridges S. M. Isolation and characterization of an iron-containing superoxide dismutase from a eucaryote, Brassica campestris. Arch Biochem Biophys. 1980 May;201(2):369–374. doi: 10.1016/0003-9861(80)90524-x. [DOI] [PubMed] [Google Scholar]
- Salin M. L., Day E. D., Jr, Crapo J. D. Isolation and characterization of a manganese-containing superoxide dismutase from rat liver. Arch Biochem Biophys. 1978 Apr 15;187(1):223–228. doi: 10.1016/0003-9861(78)90027-9. [DOI] [PubMed] [Google Scholar]
- Sato S., Harris J. I. Superoxide dismutase from Thermus aquaticus. Isolation and characterisation of manganese and apo enzymes. Eur J Biochem. 1977 Mar 1;73(2):373–381. doi: 10.1111/j.1432-1033.1977.tb11328.x. [DOI] [PubMed] [Google Scholar]
- Sato S., Nakazawa K. Purification and properties of superoxide dismutase from Thermus thermophilus HB8. J Biochem. 1978 Apr;83(4):1165–1171. doi: 10.1093/oxfordjournals.jbchem.a132007. [DOI] [PubMed] [Google Scholar]
- Sawada Y., Oyama T., Yamazaki I. Preparation and physicochemical properties of green pea superoxide dismutase. Biochim Biophys Acta. 1972 May 12;268(2):305–312. doi: 10.1016/0005-2744(72)90325-7. [DOI] [PubMed] [Google Scholar]
- Steinman H. M. The amino acid sequence of mangano superoxide dismutase from Escherichia coli B. J Biol Chem. 1978 Dec 25;253(24):8708–8720. [PubMed] [Google Scholar]
- Yost F. J., Jr, Fridovich I. An iron-containing superoxide dismutase from Escherichia coli. J Biol Chem. 1973 Jul 25;248(14):4905–4908. [PubMed] [Google Scholar]


