Abstract
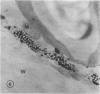
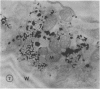
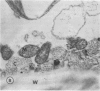
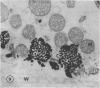
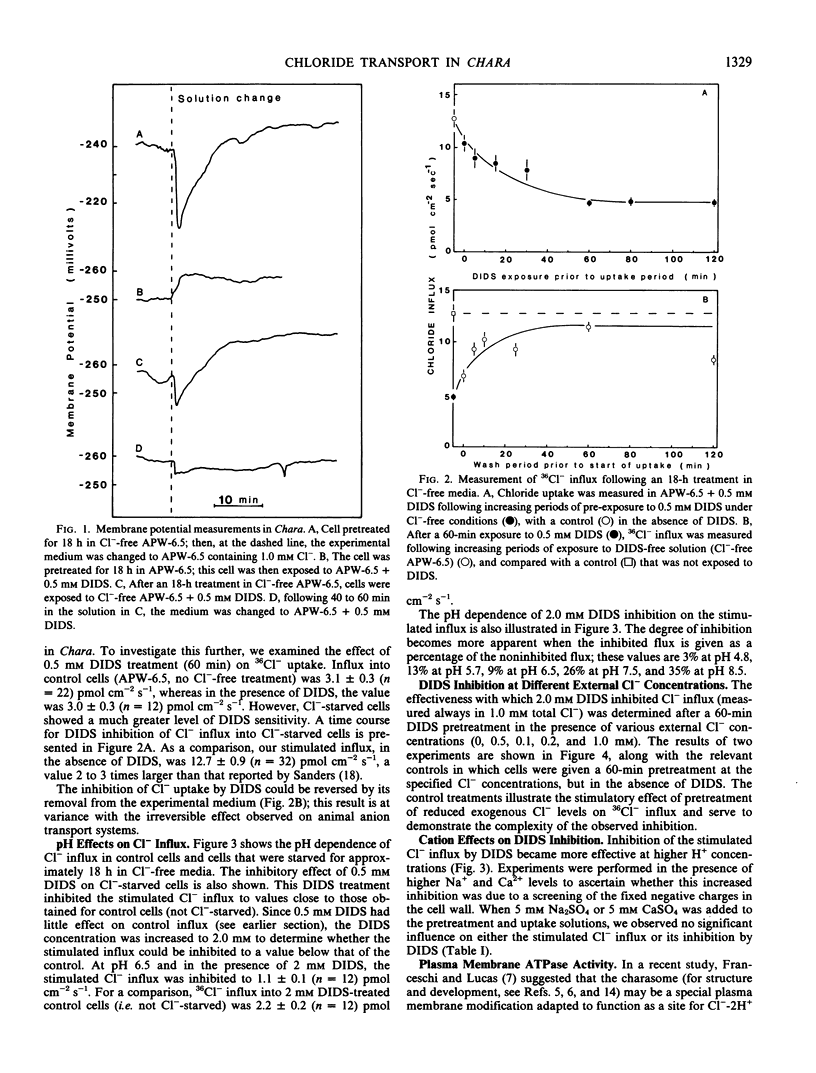
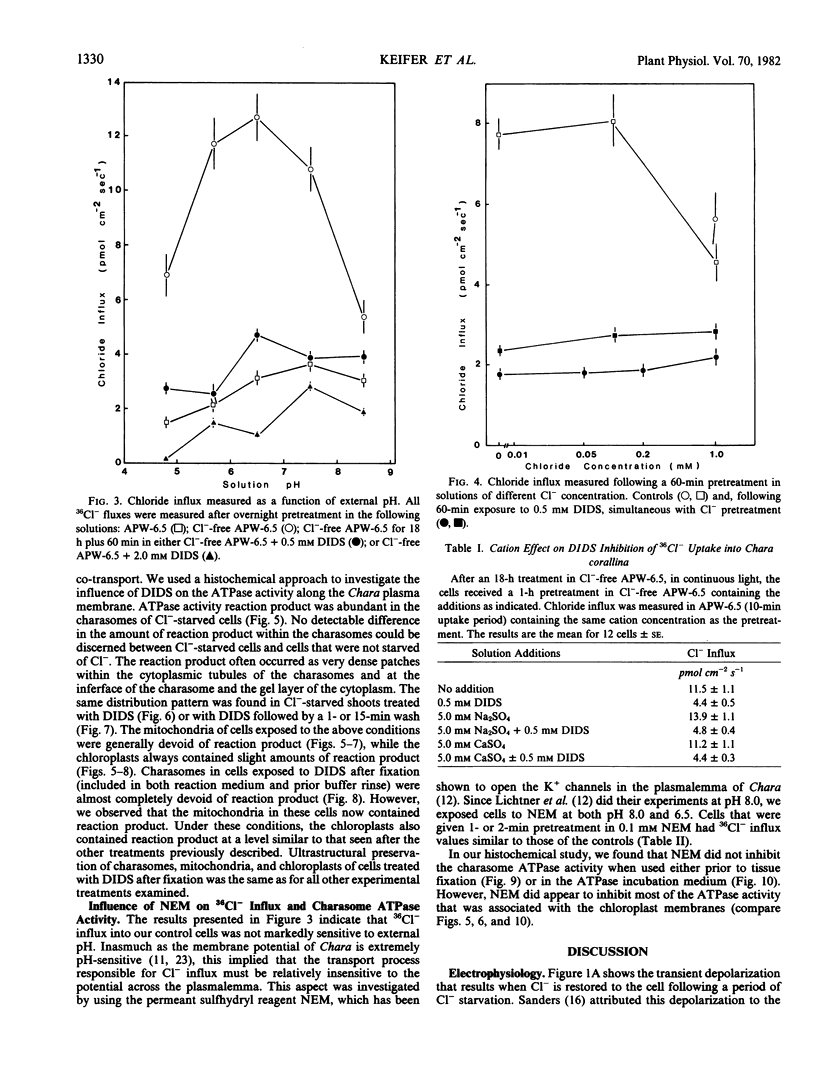
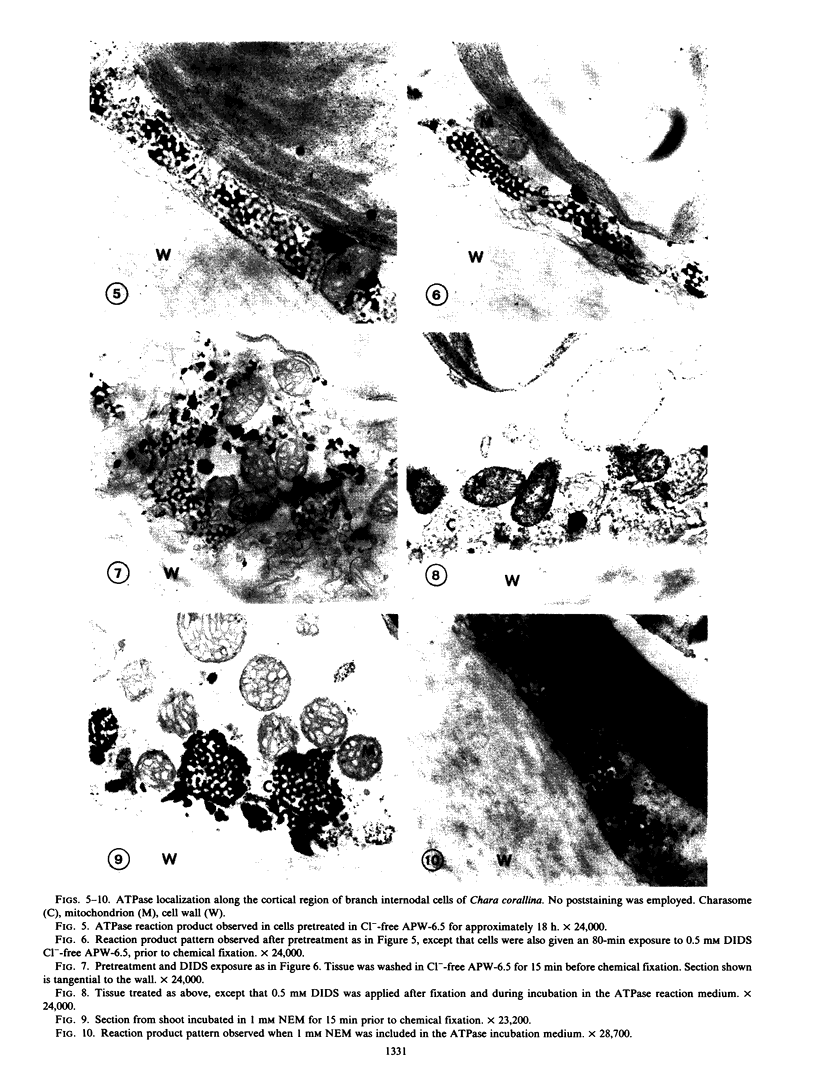
Chloride transport, presumably via a Cl−-2H+ co-transport system, was investigated in Chara corallina. At pH 6.5, the control influx (3.1 picomoles per centimeter2 per second) was stimulated 4-fold by an 18-hour Cl− starvation. The stimulated influx was inhibited to 4.7 picomoles per centimeter2 per second after a 60-minute pre-exposure to 0.5 millimolar 4,4′-diisothiocyano-2,2′-disulfonic acid stilbene (DIDS). This compares with a nonsignificant inhibition of the control under similar conditions. At 2 millimolar DIDS, both stimulated and control influx were inhibited to values of 1.1 and 2.2 picomoles per centimeter2 per second, respectively; in all cases, DIDS inhibition was reversible. Over the pH range 4.8 to 8.5, the control and DIDS-inhibited influx showed only slight pH sensitivity; in contrast, the stimulated flux was strongly pH dependent (pH 6.5 optimum). Inasmuch as changes in pH alter membrane potential, N-ethylmaleimide was used to depolarize the membrane; this had no effect on Cl− influx. A transient depolarization of the membrane (about 20 millivolts) was observed on restoration of Cl− to starved cells. The membrane also depolarized transiently when starved cells were exposed to 0.5 millimolar DIDS, but the depolarization associated with Cl− restoration was inhibited by a 40-minute pretreatment with DIDS. Exposure of control cells to DIDS caused only a small hyperpolarization (about 7 millivolts). DIDS may have blocked Cl− influx by inhibiting the putative plasmalemma H+-translocating ATPase. Histochemical studies on intact cells revealed no observable effect of DIDS on plasmalemma ATPase activity. However, DIDS application after fixation resulted in complete inhibition of ATPase activity.
The differential sensitivity of the stimulated and control flux to inhibition by DIDS may reflect an alteration of transport upon stimulation, but could also result from differences in pretreatment. The stimulated cells were pretreated with DIDS in the absence of Cl−, in contrast to the presence of Cl− during pretreatment of controls. The differential effect could result from competition between Cl− and DIDS for a common binding site. Our histochemical ATPase results indicate that Cl− transport and membrane ATPase are separate systems, and the latter is only inhibited by DIDS from the inside of the cell.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cabantchik Z. I., Knauf P. A., Rothstein A. The anion transport system of the red blood cell. The role of membrane protein evaluated by the use of 'probes'. Biochim Biophys Acta. 1978 Sep 29;515(3):239–302. doi: 10.1016/0304-4157(78)90016-3. [DOI] [PubMed] [Google Scholar]
- Cabantchik Z. I., Rothstein A. Membrane proteins related to anion permeability of human red blood cells. I. Localization of disulfonic stilbene binding sites in proteins involved in permeation. J Membr Biol. 1974;15(3):207–226. doi: 10.1007/BF01870088. [DOI] [PubMed] [Google Scholar]
- Keifer D. W., Lucas W. J. Potassium Channels in Chara corallina: CONTROL AND INTERACTION WITH THE ELECTROGENIC H PUMP. Plant Physiol. 1982 Apr;69(4):781–788. doi: 10.1104/pp.69.4.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keifer D. W., Spanswick R. M. Activity of the Electrogenic Pump in Chara corallina as Inferred from Measurements of the Membrane Potential, Conductance, and Potassium Permeability. Plant Physiol. 1978 Oct;62(4):653–661. doi: 10.1104/pp.62.4.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtner F. T., Lucas W. J., Spanswick R. M. Effect of Sulfhydryl Reagents on the Biophysical Properties of the Plasmalemma of Chara corallina. Plant Physiol. 1981 Oct;68(4):899–904. doi: 10.1104/pp.68.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley R. M. Isolation of Functionally Intact Rhodoplasts from Griffithsia monilis (Ceramiaceae, Rhodophyta). Plant Physiol. 1981 Jan;67(1):5–8. doi: 10.1104/pp.67.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W. Inhibition of anion transport in corn root protoplasts. Plant Physiol. 1981 Aug;68(2):435–438. doi: 10.1104/pp.68.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shami Y., Rothstein A., Knauf P. A. Identification of the Cl- transport site of human red blood cells by a kinetic analysis of the inhibitory effects of a chemical probe. Biochim Biophys Acta. 1978 Apr 4;508(2):357–363. doi: 10.1016/0005-2736(78)90337-1. [DOI] [PubMed] [Google Scholar]
- Spear D. G., Barr J. K., Barr C. E. Localization of hydrogen ion and chloride ion fluxes in Nitella. J Gen Physiol. 1969 Sep;54(3):397–414. doi: 10.1085/jgp.54.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]