Abstract
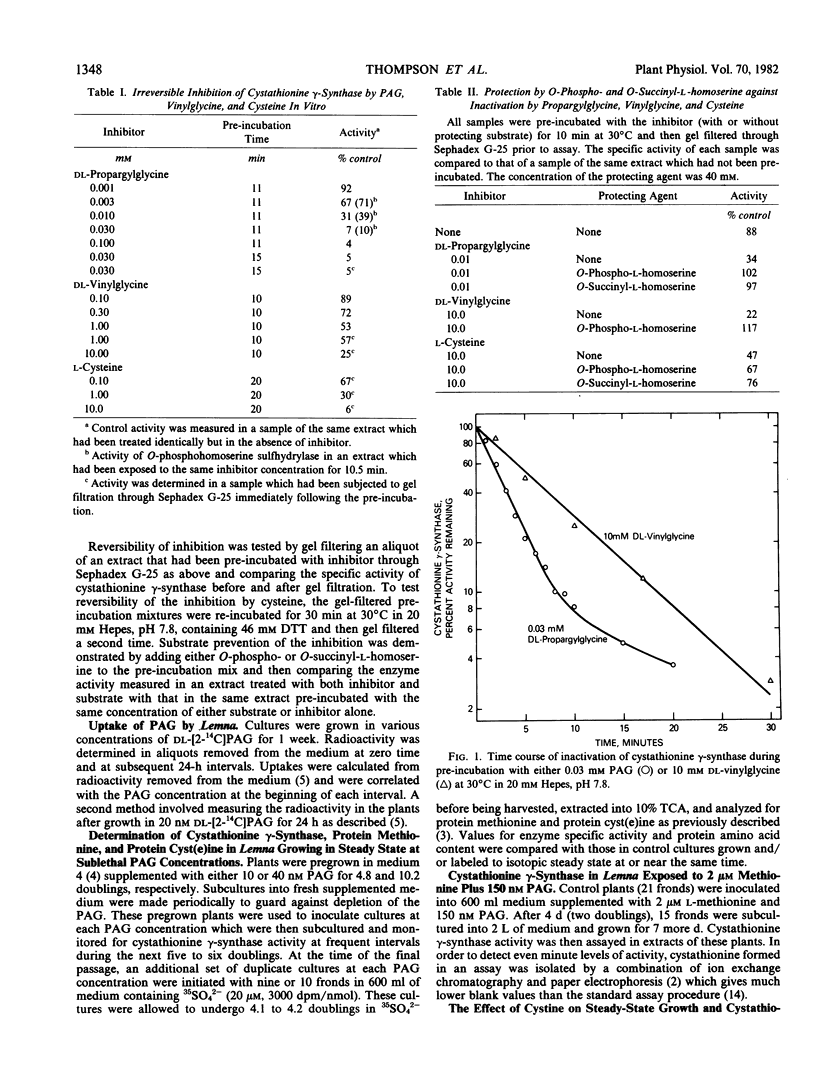
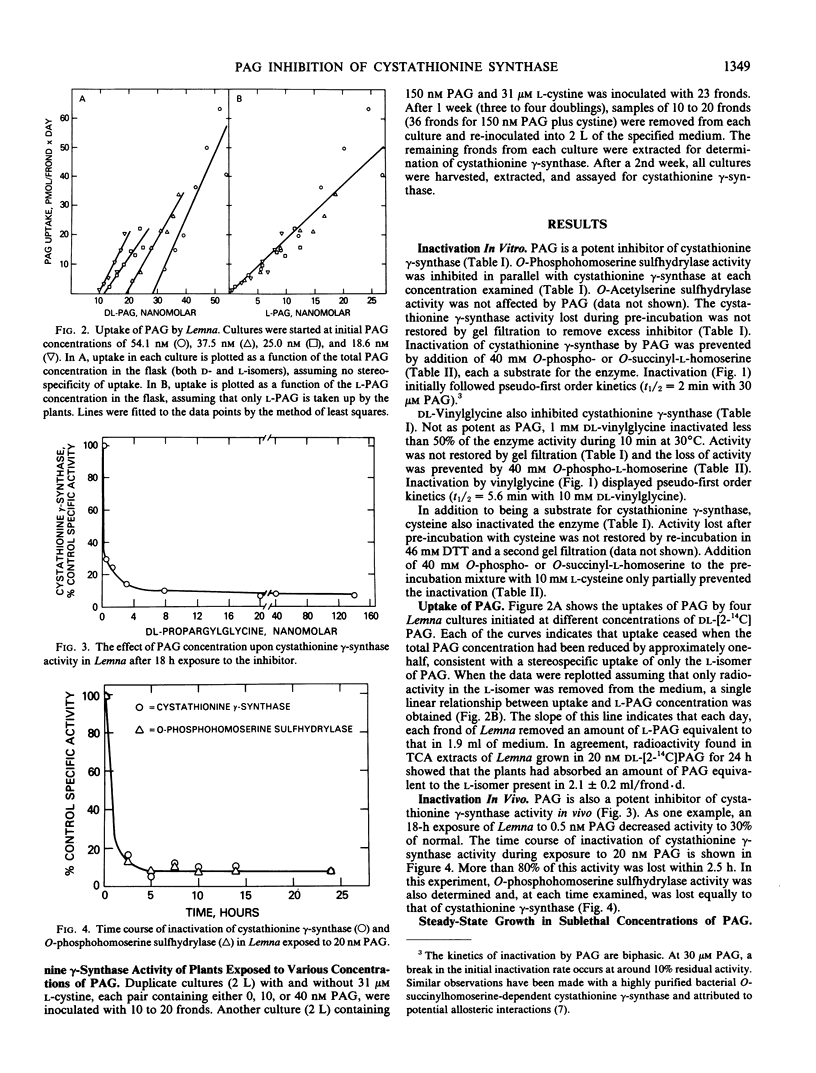
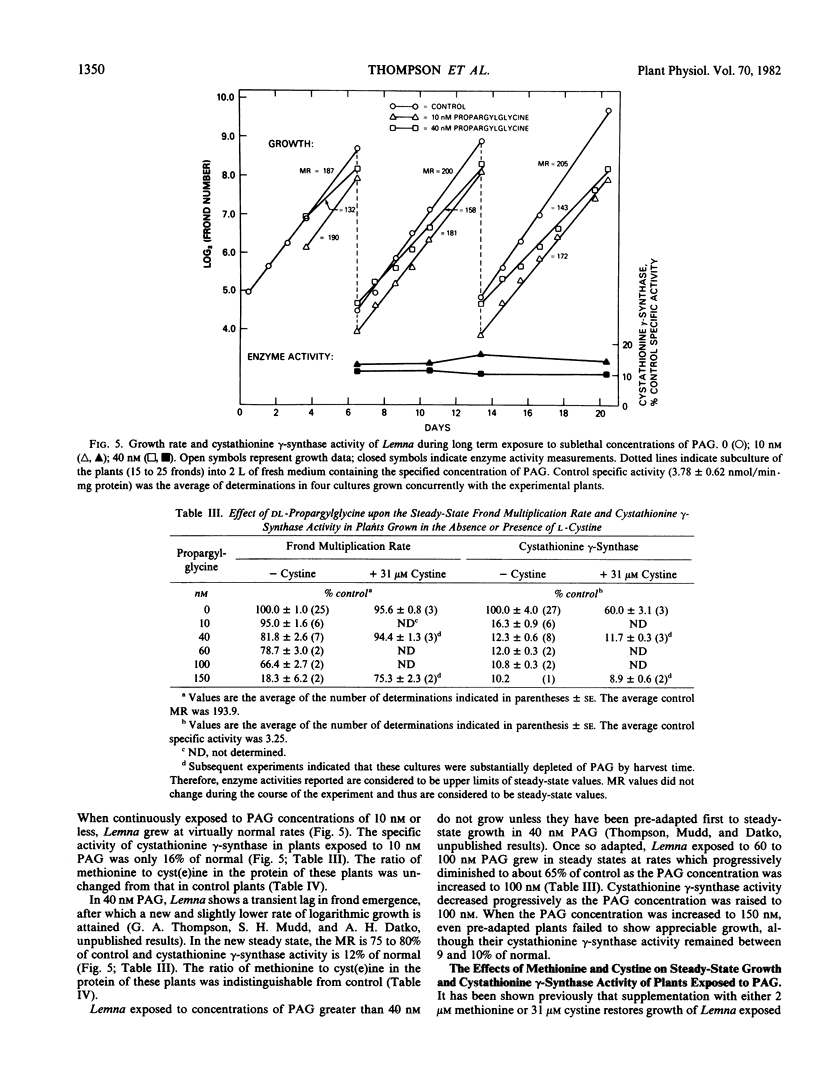
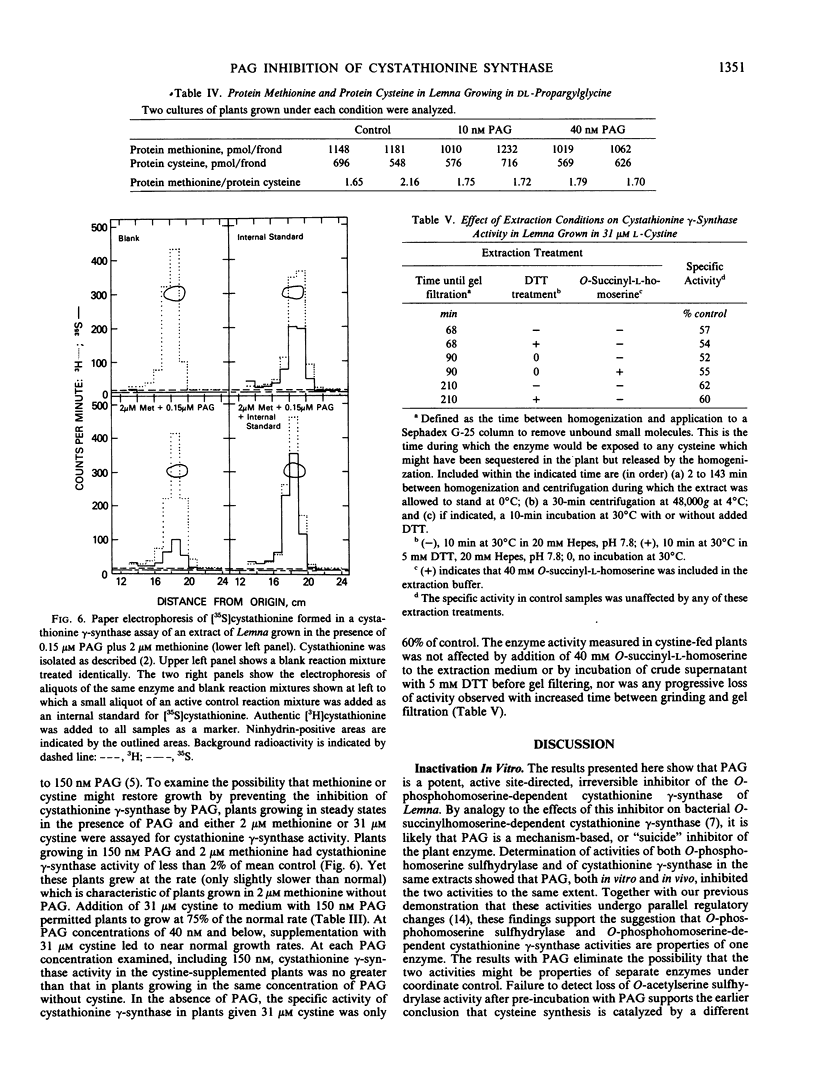
Propargylglycine, vinylglycine, and cysteine each cause irreversible inactivations of cystathionine γ-synthase (and, in parallel, of O-phosphohomoserine sulfhydrylase) activities in crude extracts of Lemna paucicostata. Inactivation by propargylglycine or vinylglycine is completely prevented by 40 millimolar O-phospho- or O-succinyl-l-homoserine; that by cysteine is only partially prevented. Propargylglycine (PAG), the most potent of these inhibitors, causes rapid and drastic inactivation of both activities in intact Lemna. Studies of plants growing in steady states in the presence of various concentrations (0-150 nanomolar) of PAG showed that 16% of control activity is necessary and sufficient to maintain normal rates of growth and methionine biosynthesis, and that 10% of control activity is essential for viability. Addition of either 2 micromolar methionine or 31 micromolar cystine to growth medium containing 150 nanomolar PAG permits growth at 75 to 100% of control rates when enzyme activity is less than 10% of control. Whereas methionine presumably rescues by directly providing the missing metabolite, cystine may rescue by enhancing substrate accumulation and thereby promoting flux through residual cystathionine γ-synthase. The results indicate that the down-regulation of cystathionine γ-synthase to 15% of control which occurs when plants are grown in 2 micromolar methionine (Thompson, Datko, Mudd, Giovanelli Plant Physiol 69: 1077-1083), by itself, is not sufficient to reduce the rate of methionine biosynthesis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Datko A. H., Giovanelli J., Mudd S. H. Homocysteine biosynthesis in green plants. O-Phosphorylhomoserine as the physiological substrate for cystathionine gamma-synthase. J Biol Chem. 1974 Feb 25;249(4):1139–1155. [PubMed] [Google Scholar]
- Datko A. H., Mudd S. H., Giovanelli J. Lemna paucicostata Hegelm. 6746: DEVELOPMENT OF STANDARDIZED GROWTH CONDITIONS SUITABLE FOR BIOCHEMICAL EXPERIMENTATION. Plant Physiol. 1980 May;65(5):906–912. doi: 10.1104/pp.65.5.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datko A. H., Mudd S. H., Giovanelli J., Macnicol P. K. Sulfur-containing Compounds in Lemna perpusilla 6746 Grown at a Range of Sulfate Concentrations. Plant Physiol. 1978 Oct;62(4):629–635. doi: 10.1104/pp.62.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datko A. H., Mudd S. H. Methionine biosynthesis in lemna: inhibitor studies. Plant Physiol. 1982 May;69(5):1070–1076. doi: 10.1104/pp.69.5.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston M., Jankowski D., Marcotte P., Tanaka H., Esaki N., Soda K., Walsh C. Suicide inactivation of bacterial cystathionine gamma-synthase and methionine gamma-lyase during processing of L-propargylglycine. Biochemistry. 1979 Oct 16;18(21):4690–4701. doi: 10.1021/bi00588a033. [DOI] [PubMed] [Google Scholar]
- Johnston M., Marcotte P., Donovan J., Walsh C. Mechanistic studies with vinylglycine and beta-haloaminobutyrates as substrates for cystathionine gamma-synthetase from Salmonella typhimurium. Biochemistry. 1979 May 1;18(9):1729–1738. doi: 10.1021/bi00576a015. [DOI] [PubMed] [Google Scholar]
- Madison J. T., Thompson J. F. Threonine synthetase from higher plants: stimulation by S-adenosylmethionine and inhibition by cysteine. Biochem Biophys Res Commun. 1976 Jul 26;71(2):684–691. doi: 10.1016/0006-291x(76)90842-1. [DOI] [PubMed] [Google Scholar]
- Penefsky H. S. Reversible binding of Pi by beef heart mitochondrial adenosine triphosphatase. J Biol Chem. 1977 May 10;252(9):2891–2899. [PubMed] [Google Scholar]
- Rando R. R. Mechanism-based irreversible enzyme inhibitors. Methods Enzymol. 1977;46:28–41. doi: 10.1016/s0076-6879(77)46007-5. [DOI] [PubMed] [Google Scholar]
- Rognes S. E., Lea P. J., Miflin B. J. S-adenosylmethionine--a novel regulator of aspartate kinase. Nature. 1980 Sep 25;287(5780):357–359. doi: 10.1038/287357a0. [DOI] [PubMed] [Google Scholar]
- Scannell J. P., Pruess D. L., Demny T. C., Weiss F., Williams T. Antimetabolites produced by microorganisms. II. L-2-amino-4-pentynoic acid. J Antibiot (Tokyo) 1971 Apr;24(4):239–244. doi: 10.7164/antibiotics.24.239. [DOI] [PubMed] [Google Scholar]
- Thompson G. A., Datko A. H., Mudd S. H., Giovanelli J. Methionine Biosynthesis in Lemna: STUDIES ON THE REGULATION OF CYSTATHIONINE gamma-SYNTHASE, O-PHOSPHOHOMOSERINE SULFHYDRYLASE, AND O-ACETYLSERINE SULFHYDRYLASE. Plant Physiol. 1982 May;69(5):1077–1083. doi: 10.1104/pp.69.5.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]


