Abstract
Demethylation inhibitor (DMI)-resistant strains of the plant pathogenic fungus Penicillium digitatum were shown to be simultaneously resistant to cycloheximide, 4-nitroquinoline-N-oxide (4NQO), and acriflavine. A PMR1 (Penicillium multidrug resistance) gene encoding an ATP-binding cassette (ABC) transporter (P-glycoprotein) was cloned from a genomic DNA library of a DMI-resistant strain (LC2) of Penicillium digitatum by heterologous hybridization with a DNA fragment containing an ABC-encoding region from Botrytis cinerea. Sequence analysis revealed significant amino acid homology to the primary structures of PMR1 (protein encoded by the PMR1 gene) and ABC transporters of Saccharomyces cerevisiae (PDR5 and SNQ2), Schizosaccharomyces pombe (HBA2), Candida albicans (CDR1), and Aspergillus nidulans (AtrA and AtrB). Disruption of the PMR1 gene of P. digitatum DMI-resistant strain LC2 demonstrated that PMR1 was an important determinant of resistance to DMIs. The effective concentrations inhibiting radial growth by 50% (EC50s) and the MICs of fenarimol and bitertanol for the PMR1 disruptants (Δpmr1 mutants) were equivalent to those for DMI-sensitive strains. Northern blot analysis indicated that severalfold more PMR1 transcript accumulated in the DMI-resistant strains compared with those in DMI-sensitive strains in the absence of fungicide. In both DMI-resistant and -sensitive strains, transcription of PMR1 was strongly enhanced within 10 min after treatment with the DMI fungicide triflumizole. These results suggested that the toxicant efflux system comprised of PMR1 participates directly in the DMI resistance of the fungus.
Sterol demethylation inhibitors (DMIs) have a common site of action within the fungal sterol biosynthesis pathway, which is the demethylation of sterols at position 14 by sterol 14-α-demethylase (p450-14DM). This group of fungicides is widely used to control a broad spectrum of plant pathogenic fungi belonging to the classes Ascomycetes, Basidiomycetes, and Deuteromycetes. Based on genetic studies with Aspergillus nidulans (41) and Erysiphe graminis f. sp. hordei (19), DMI resistance in fungi is controlled not by a single gene but by more complex genes. For this reason, the possibility of occurrence of resistant fungal strains to DMIs has been considered to be relatively low. However, many DMI-resistant field strains have developed recently in various plant pathogenic fungi (18).
In theory, DMI resistance can be based on a target site mutation of the p450-14DM. Indeed, recent studies demonstrated that alteration of p450-14DM activity (21, 40) or overexpression of p450-14DM (39) was involved in resistance to DMIs. More recently, several amino acid alterations in p450-14DM were shown to be associated with DMI resistance (7, 27).
Another mechanism of DMI resistance may depend on a decreased accumulation of fungicides in the mycelium due to enhanced energy-dependent toxicant efflux, as has been shown for A. nidulans (11–13), Botrytis cinerea (37), and Penicillium italicum (9, 10). In Candida albicans, resistance to azole fungicides was based on an energy-dependent efflux mechanism operated by the ATP-binding cassette (ABC) transporter CDR1 (29, 34). ABC transporters (P-glycoproteins) are known to be responsible for multidrug resistance in prokaryotic and eukaryotic organisms (2, 15, 16, 31, 35). In the most recent study, atrA and atrB genes encoding ABC transporters were cloned from a filamentous fungus, A. nidulans, and it was demonstrated that an atrB transgene rendered Saccharomyces cerevisiae resistant to azole fungicide (6). These data prove that the ABC transporter may play a significant role in fungicide sensitivity and resistance (8).
In our previous study, the energy-dependent efflux operated by PDR5 was exhibited to be a main determinant of DMI resistance in S. cerevisiae (28a). The MICs of DMIs (triflumizole and bitertanol) for Δpdr5 mutants (PDR5 disruptants) were 20 times lower than that for the parental yeast strain.
In this study, we investigated the relationship between DMI resistance and multidrug resistance in the field isolates of the citrus green mold Penicillium digitatum by cloning and characterizing a gene (designated PMR1) encoding an ABC transporter for DMI efflux.
MATERIALS AND METHODS
Fungi, bacteria, and vector DNA.
DMI-resistant strains LC2, M1, and I1 of the citrus green mold P. digitatum were isolated from the surfaces of imported lemons. DMI-sensitive strains DF1 and U1 were isolated from imported lemons and oranges, respectively. DMI-sensitive strain PD5 was isolated from domestic satsuma mandarin (Citrus unshiu Marc.). Escherichia coli DH5α was used for plasmid propagation. Strain XL1-Blue MRA(P2) was used for phage DNA manipulation. Plasmid vector pUC19 and pBluescriptII SK+ (Stratagene) were used for subcloning and sequencing. Phage vector λDASH II (Stratagene) was used for genomic library construction.
Toxicant sensitivity assay.
Toxicants tested included four DMIs (triflumizole, fenarimol, bitertanol, and pyrifenox), one antibiotic (cycloheximide), and two mutagens (acriflavine and 4-nitroquinoline-N-oxide [4NQO]). After incubation for 2 days at 25°C on potato dextrose agar (PDA; Eiken), the inoculum disks (4 mm in diameter) of P. digitatum were taken from the margins of actively growing cultures. The inoculum was placed on PDA (Difco) containing each toxicant described above. Fungal sensitivities to toxicants were determined by measuring the effective concentration inhibiting radial growth by 50% (EC50) or the MIC after 3 days of incubation at 25°C in the dark. Experiments were performed in duplicate.
Cloning and sequencing.
Basic methods for DNA manipulations were as described elsewhere (32). Total genomic DNA was isolated from P. digitatum LC2 by the following modification of a method described elsewhere (43). About 1 g of fresh mycelia was ground to a fine powder under liquid nitrogen. The powder was suspended in 5 ml of extraction buffer (0.7 M NaCl, 50 mM Na2EDTA, 50 mM Tris-HCl [pH 8.0], 1% sodium dodecyl sulfate [SDS]), and then the suspension was incubated for 30 min at 50°C. DNA was extracted several times from the suspension with phenol-chloroform-isoamyl alcohol (24:24:1), twice with chloroform-isoamyl alcohol (24:1), and precipitated with 2-propanol. High-molecular-weight DNA was spooled out with a pipette tip, washed in 70% ethanol, vacuum dried, and resuspended finally in TE buffer (10 mM Tris-HCl, 1 mM Na2EDTA [pH 8.0]). This genomic DNA was partially digested with MboI to generate the maximum yield of DNA fragments in the size range of 10 to 20 kb. The 10- to 20-kb fragments were size fractionated by agarose gel electrophoresis as described elsewhere (1). These DNA fragments were ligated into the BamHI site of λDASH II and packaged with Gigapack II Plus Packaging extracts (Stratagene). This genomic library was screened by plaque hybridization with the 2.1-kb EcoRI fragment of BMR1 of B. cinerea (see Results). After the plaque screening, DNA fragments from positive clones were subcloned into pBluescriptII SK(+) and sequenced by the dideoxy chain termination method (33). The sequence data were assembled and analyzed with GENETYX software (Software Development Co., Ltd.). One of the positive clones containing a complete open reading frame (ORF) similar to the ABC transporter gene was designated pPD602 and used for the following experiments. The gene was designated PMR1 (Penicillium multidrug resistance).
PMR1 gene disruption.
On the basis of the nucleotide and amino acid sequences of PMR1 in pPD602, an EcoRV fragment containing the translation initiation codon as well as approximately three-fourths of the PMR1 (protein encoded by PMR1 gene)-coding region was subcloned into the EcoRV site of pBluescriptII SK+. An internal 3.0-kb AatI-SmaI fragment was replaced by a 4.0-kb BglII-XbaI fragment containing the hygromycin B resistance gene cassette (Hygr) excised from pAN7-1 (30) (see Fig. 2A). The resultant plasmid, which was designated pUNE10, was linearized with EcoRV and was used for the transformation of P. digitatum LC2. Preparation and transformation of the fungal protoplasts were carried out by methods described previously (20). The transformants were selected on PDA containing hygromycin B at a concentration of 100 μg/ml. A total of 199 hygromycin B-resistant transformants were isolated. After single-spore isolation, sensitivity to 10 μg of triflumizole or 0.5 μg of cycloheximide per ml was examined.
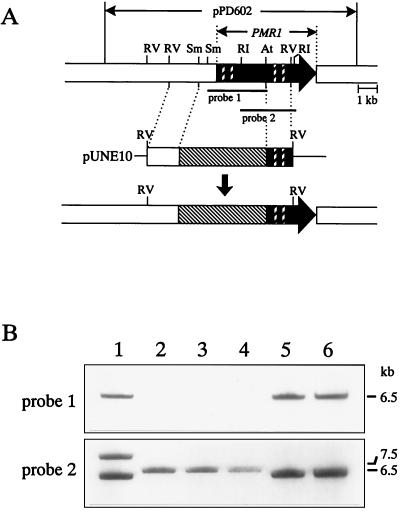
FIG. 2.
Disruption of PMR1. (A) Restriction map of pPD602 containing PMR1 and schematic diagram of disruption of PMR1. Black arrow, ORF of PMR1; open boxes, flanking regions of PMR1; bold striped boxes, Walker motifs; black lines, probes for Southern and Northern blot analysis; filled boxes, ORF. Disruption vector pUNE10 contains the hygromycin B resistance gene cassette (Hygr) (thin striped box) flanked by the 5′ upstream region and the 3′ end of the PMR1 gene. Double homologous recombination between the PMR1 locus and the disruption vector pUNE10 results in gene replacement. Restriction enzymes are as follows: At, ArtI; RI, EcoRI; RV, EcoRV; and Sm, SmaI. (B) Southern blot analysis of Δpmr1 mutants. The genomic DNAs extracted from ectopic transformant TF01 (lane 1); Δpmr1 mutants DIS07, DIS33, and DIS96 (lanes 2 to 4); parental strain LC2 (lane 5); and DMI-sensitive strain PD5 were digested with EcoRV and separated in a 0.8% agarose gel. The blot was probed with probe 1 (2.5-kb SmaI-ArtI fragment) and was reprobed with probe 2 (2.3-kb EcoRI fragment).
Gene disruption of PMR1 was confirmed by Southern hybridization analysis. Five micrograms of genomic DNA from the triflumizole-sensitive transformants as well as the ectopic transformants and wild-type strains was digested with EcoRV, size separated in a 0.8% agarose gel, and transferred to a GeneScreen Plus membrane (DuPont NEN) by vacuum blotting with VacuGene (Bio-Rad). Hybridization with the 2.5-kb AatI-SmaI fragment containing the 5′ end of the PMR1 gene (probe 1) (see Fig. 2A) and the 2.3-kb EcoRI fragment containing the central region of the PMR1 gene (probe 2) (see Fig. 2A) was performed with the enhanced chemiluminescence (ECL) system (Amersham) under the conditions recommended by the manufacturer. Finally, three PMR1 disruptants (Δpmr1 mutants) were designated DIS07, DIS33, and DIS96.
RNA isolation and Northern blot analysis.
Conidia of DMI-sensitive strains PD5, DF1, and U1; DMI-resistant strains LC2, M1, and I1; and the Δpmr1 mutant DIS33 were incubated in potato dextrose broth (Difco) for 1 day at 25°C with shaking (80 strokes/min). To investigate whether transcription of PMR1 was induced by a toxicant, mycelia of PD5 and LC2 were treated with 50 μg of triflumizole per ml for 10 min or 1 h. Fungal total RNA was extracted by acid guanidinium thiocyanate-phenol-chloroform extraction as previously described (25), except that the fresh mycelia were ground into fine powders under liquid nitrogen. Approximately 50 μg of total RNA was electrophoresed on a 1% agarose gel containing formaldehyde (32), transferred onto GeneScreen Plus membrane by VacuGene, and hybridized with probe 2 by using the ECL system.
Nucleotide sequence accession number.
The sequence reported here is available under GenBank accession no. AB010442.
RESULTS
Multidrug resistance of DMI-resistant strains of P. digitatum.
Toxicant sensitivity assaying of the DMI-resistant strains of P. digitatum indicated that all of the strains tested (LC2, M1, and I1) were highly resistant to cycloheximide, 4NQO, and acriflavine compared with the DMI-sensitive strains (PD5, DF1, and U1). The EC50s of these toxicants for the resistant strains were approximately two to seven times higher than those for the sensitive strains (Table 1). These results showed that the DMI resistance in P. digitatum is highly correlated with multidrug resistance.
TABLE 1.
Multidrug resistance in P. digitatum
| Toxicant | Result for the following strains (μg/ml)a:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PD5
|
DF1
|
U1
|
LC2
|
M1
|
I1
|
|||||||
| EC50 | MIC | EC50 | MIC | EC50 | MIC | EC50 | MIC | EC50 | MIC | EC50 | MIC | |
| Triflumizole | 0.08 | 1 | 0.04 | 1 | 0.01 | 1 | 2.06 | >100 | 1.28 | >100 | 2.79 | >100 |
| Fenarimol | 0.28 | 5 | 0.32 | 5 | 0.29 | 5 | 2.29 | >100 | 2.67 | >100 | 5.26 | >100 |
| Bitertanol | 0.22 | 5 | 0.28 | 5 | 0.31 | 5 | 3.19 | >100 | 2.72 | >100 | 9.59 | >100 |
| Pyrifenox | 0.37 | 10 | 0.64 | 10 | 0.75 | 10 | >50 | >100 | >50 | >100 | >50 | >100 |
| Cycloheximide | 0.07 | 1 | 0.04 | 1 | 0.09 | 1 | 0.16 | 2 | 0.28 | 2 | 0.18 | 2 |
| 4NQO | 0.36 | 5 | 0.45 | 5 | 0.24 | 5 | 1.70 | 10 | 1.79 | 10 | 1.38 | 10 |
| Acriflavin | 0.77 | 5 | 1.10 | 5 | 0.44 | 5 | 1.58 | 50 | 1.74 | 50 | 1.16 | 50 |
DMI-sensitive strains (PD5, DF1, and U1) and DMI-resistant strains (LC2, I1 and M1) were used for analysis of the multidrug resistance.
Cloning and characterization of PMR1.
Recently, we cloned a gene (BMR1) encoding the ABC transporter from the plant pathogenic fungus B. cinerea by heterologous hybridization with the PDR5 gene from S. cerevisiae (28a). A 2.1-kb EcoRI fragment of BMR1, which contains an ABC-coding region, was used as the probe for screening of the homologous clones from the genomic library of P. digitatum LC2. Three positive clones (insert sizes, 14.0, 15.0, and 16.5 kb) were obtained. These three clones were shown to contain the same PMR1 gene.
A total of 5,260 nucleotides of sequence, from 382 bp upstream from the translation initiation codon to 429 bp downstream of the putative stop codon of the PMR1 gene, was determined. An ORF of 4,446 nucleotides encoding a protein of 1,482 amino acids (166 kDa) was predicted. No introns were found in this region, which was confirmed by cDNA sequencing (data not shown).
A homology search indicated that the PMR1 ORF is significantly similar to several ABC transporters. Alignments of amino acid sequences showed 48.8% identity with CDR1 of C. albicans (29), 47.7% identity with PDR5 (STS1/YDR1) of S. cerevisiae (3, 4, 17), 46.5% identity with AtrA of A. nidulans (6), 39.4% identity with HBA2 of Schizosaccharomyces pombe (38), and 38.8% identity with SNQ2 of S. cerevisiae (36).
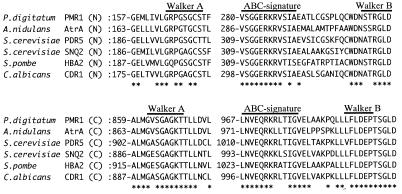
Hydropathy analysis according to a method described elsewhere (26) suggested that the predicted structure of PMR1 is typical of an ABC transporter and is characterized by two membrane-anchored hydrophobic moieties with six transmembrane stretches (residues 492 to 511, 518 to 541, 575 to 594, 601 to 620, 630 to 650, and 743 to 759 and residues 1162 to 1185, 1198 to 1216, 1247 to 1265, 1283 to 1301, 1317 to 1335, and 1449 to 1467, respectively), alternating with two intracellular ATP-binding hydrophilic moieties. Walker A motifs (GxSGxGKST) (42) were located at residues 164 to 172 and 862 to 870, and Walker B motifs (LxxDEP/AxxxLD) (42) were located at residues 306 to 311 and 988 to 993 (Fig. 1B); both of these were located in the hydrophilic domains corresponding to similar positions in the yeast ABC transporters.
FIG. 1.
Multiple alignment of the ATP-binding cassette of P. digitatum PMR1 and representative fungus and yeast homologues. The ABC sequence of PMR1 was aligned with those of AtrA from A. nidulans (6), PDR5 from S. cerevisiae (3), SNQ2 from S. cerevisiae (36), HBA2 from S. pombe (38), and CDR1 from C. albicans (29) in the NH2-terminal (N) and COOH-terminal (C) domains. Bar above sequences indicate the Walker A and B and the ABC signature motifs. The amino acid residue number for each protein is indicated at the beginning of each sequence. Asterisks indicate identical residues.
Disruption of PMR1.
From a total of 199 hygromycin B-resistant transformants, three DMI-sensitive transformants (1.5% of the total transformants) were obtained after single-spore isolation followed by a DMI sensitivity assay. Southern blot analysis confirmed that the PMR1 genes of the DMI-sensitive transformants (DIS07, DIS33, and DIS96) were disrupted, since all of them showed no hybridization with probe 1 (Fig. 2B). Moreover, when the same blot was reprobed with probe 2, the ectopic transformant and the wild-type strains showed an expected hybridization signal at 6.5 kb (Fig. 2B), whereas the three Δpmr1 mutants showed a hybridization signal at 7.5 kb, which was the length expected for a gene replacement (Fig. 2B).
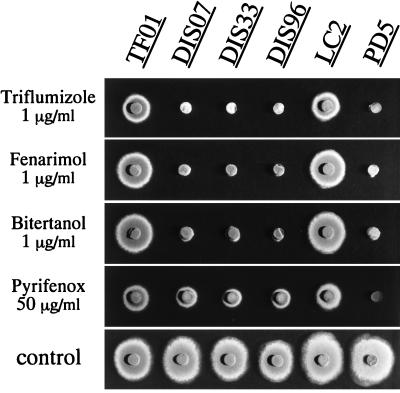
These results indicated that PMR1 is closely connected with the DMI resistance of P. digitatum. The Δpmr1 mutants were unable to grow on PDA plates containing the following toxicants at concentrations that allowed the growth of parental strain LC2: 1 μg of fenarimol, 1 μg of bitertanol, and 1 μg of triflumizole per ml (Fig. 3). On the other hand, the effect of PMR1 disruption on pyrifenox sensitivity was not as significant (Fig. 3; Table 2). The EC50s and MICs of cycloheximide, 4NQO, and acriflavine for the Δpmr1 mutants were almost the same as those for LC2 (data not shown). The Δpmr1 mutants were viable, grew normally, and caused the same symptoms on the inoculated citrus fruits as the parental strain, indicating that PMR1 is not an essential gene for normal growth and pathogenicity.
FIG. 3.
DMI sensitivity of the Δpmr1 mutants. TF01 (ectopic transformant with pUNE10); Δpmr1 mutants DIS07, DIS33, and DIS96; DMI-resistant strain LC2 (parental strain of the transformants); and DMI-sensitive strain PD5 were cultured for 3 days at 25°C on PDA plates containing triflumizole (1 μg/ml), bitertanol (1 μg/ml), fenarimol (1 μg/ml), or pyrifenox (50 μg/ml).
TABLE 2.
Effect of PMR1 disruption on DMI sensitivity in P. digitatum
| Toxicant | Result with the following strains (μg/ml)a:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PD5
|
DIS07
|
DIS33
|
DIS96
|
LC2
|
||||||
| EC50 | MIC | EC50 | MIC | EC50 | MIC | EC50 | MIC | EC50 | MIC | |
| Triflumizole | 0.08 | 1 | 0.44 | 10 | 0.37 | 10 | 0.48 | 10 | 2.06 | >100 |
| Fenarimol | 0.28 | 5 | 0.33 | 5 | 0.28 | 5 | 0.37 | 5 | 2.29 | >100 |
| Bitertanol | 0.22 | 5 | 0.20 | 5 | 0.17 | 5 | 0.24 | 5 | 3.19 | >100 |
| Pyrifenox | 0.37 | 5 | 34.4 | >100 | 26.8 | >100 | 19.2 | >100 | >50 | >100 |
DMI-sensitive strain PD5; Δpmr1 mutants DIS07, DIS33, and DIS96; and the parental strain LC2 (DMI-resistant strain) were used for analysis of the effect of the Δpmr1 mutation.
Northern blot analysis.
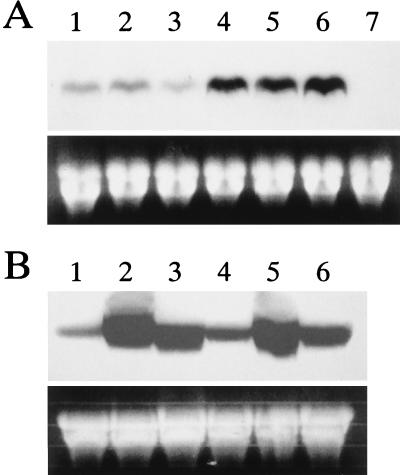
The steady-state levels of PMR1 mRNA in DMI-sensitive and -resistant strains of P. digitatum in the absence of the toxicant are shown in Fig. 4A. PMR1 transcripts were detectable in all of the sensitive strains tested (Fig. 4A). In the resistant strains, the levels of PMR1 expression were several times higher than those in the sensitive strains (Fig. 4A). No transcript was detectable in the Δpmr1 mutant DIS33 (Fig. 4A).
FIG. 4.
Northern blot analysis of PMR1 expression. (A) Expression of PMR1 in P. digitatum in the absence of triflumizole. Conidia from each strain were grown in the absence of triflumizole in PDB at 25°C for 1 day. Total RNA was prepared as described in Materials and Methods. Approximately 50 μg of total RNA from each strain (lanes: 1, sensitive strain PD5; 2, sensitive strain DF1; 3, sensitive strain U1; 4, resistant strain LC2; 5, resistant strain M1; 6, resistant strain I1; and 7, Δpmr1 mutant DIS33) was loaded into each lane. Ethidium bromide-stained rRNA bands are shown for comparison of the quantities of loaded RNAs. (B) Time course of expression of PMR1 in the presence of triflumizole. Approximately 50 μg of total RNA from DMI-sensitive strain PD5 (lanes 1 to 3) and DMI-resistant strain LC2 (lanes 4 to 6) after treatment with triflumizole (50 μg/ml) was loaded into each lane. Lanes: 1 and 4, 0 min of treatment; 2 and 5, 10 min of treatment; 3 and 6, 60 min of treatment.
When 50 μg of triflumizole per ml was supplemented in PDB, transcription of PMR1 in both DMI-sensitive and -resistant strains was strongly enhanced within 10 min after triflumizole treatment (Fig. 4B), and afterwards the accumulation of mRNA was decreased in 60 min. No significant difference in the levels of mRNA accumulation between both strains was observed in the presence of triflumizole (Fig. 4B).
DISCUSSION
We describe the cloning and characterization of the PMR1 gene encoding an ABC transporter of P. digitatum, the causal agent of citrus green mold. This is the first report to indicate the presence of an ABC transporter and its role in fungicide resistance in field isolates of a plant pathogenic fungus.
The major molecular determinants mediating multidrug resistance are known to be P-glycoproteins, which reduce the cytoplasmic concentration of toxic drugs by driving them out of the cells (2, 15, 16, 31, 35). In our previous study, we cloned a gene involved in the resistance to DMIs from S. cerevisiae and identified it with the pleiotropic drug-resistant gene PDR5 (28a). For this reason, it is reasonable to suppose that the resistance to some pesticides such as DMIs in filamentous fungi can be mediated by multidrug efflux transporters. At the present time, such a transporter-mediated resistance mechanism to cytotoxic compounds is thought to be universal and indispensable for almost all organisms, from bacteria to humans. The DMI-resistant strains of P. digitatum examined in this study showed resistance to cycloheximide, 4NQO, and acriflavine (Table 1). Therefore, it is likely that multidrug efflux transporters could perform an important role in resistance to these toxicants in P. digitatum. However, although the EC50s and the MICs of fenarimol and bitertanol for Δpmr1 mutants were equivalent to those for the DMI-sensitive strains (Table 2; Fig. 3), the sensitivities of the Δpmr1 mutants to pyrifenox (Table 2; Fig. 3), cycloheximide, 4NQO, and acriflavine (data not shown) did not change significantly. In S. cerevisiae, a number of ABC transporters which confer multidrug resistance have been identified. Therefore, in P. digitatum, it seemed reasonable to think that there are likewise several homologues of PMR1 which confer resistance to pyrifenox, cycloheximide, 4NQO, and acryflavine. The present study has at least shown that the ABC transporter PMR1 is the main determinant of resistance to fenarimol and bitertanol in P. digitatum and that the resistance level might depend on the efflux capacity of PMR1 but not on the alteration of the target enzyme p450-14DM, as reported previously (7, 21, 27, 39, 40).
A common mechanism underlying multidrug resistance is overexpression of P-glycoprotein. The PMR1 transcript was shown to be expressed at a higher level without toxicant in DMI-resistant strains compared with DMI-sensitive strains (Fig. 4). A similar result was reported for A. nidulans, in which isogenic imazalil-resistant mutants carrying imaB have a higher basal level of transcription of atrA than the wild-type strain (6). However, since all strains used in the present study were field isolates of different origins, more experiments, such as the transformation of sensitive strains with expression vector including PMR1, are necessary to confirm that higher levels of gene expression confer a stronger multidrug-resistant phenotype. Transcription of PMR1 in all strains was enhanced within 10 min after the addition of triflumizole. This result is similar to the transcriptional induction of PDR5 and SNQ2 in S. cerevisiae (17) and atrA and atrB in A. nidulans (6) by the toxicants.
In A. nidulans (11–13), B. cinerea (37), and P. italicum (9, 10), the uptake of DMIs into the sensitive strains was initially fast, and the accumulation of the toxicant increased to the maximum 10 min after addition of the toxicant, followed by a decline. In contrast, the initial phase of rapid fenarimol uptake was completely lacking in those resistant strains. These results and our present data suggest that the efflux system in resistant cells is constitutively active and becomes immediately operative upon contact with fungicides. Accordingly, the high fungicide concentration sufficient for saturation of the target sites in the sensitive strains might not be achieved in resistant cells. In the case of yeast multidrug resistance, some transcriptional regulatory factors affecting the transcript level of PDR5 were identified (3, 5, 14). Recently, the target sequences of these regulators (PDR1 and PDR3), which belong to the C6 zinc cluster family, were identified in the promoter regions of PDR5 (22, 23) and SNQ2 (28). We are now analyzing the trans-acting and cis-acting factors involved in the regulation of PMR1 transcription. However, taking account of the rather small difference in the basal PMR1 transcription level between resistant and sensitive strains, this aspect seems insufficient to elucidate the complete mechanisms of resistance. It was suggested that an amino acid conversion in a human P-glycoprotein MDR1 confers multidrug resistance (24). It is not evident at present whether any mutation in PMR1 also confers multidrug resistance in P. digitatum.
Further analysis of the regulation of PMR1 expression is necessary to clarify the mechanism of DMI resistance by PMR1.
Finally, we presume that the ABC transporter-mediated toxicant efflux mechanism might play an important role in fungicide resistance among almost all of the plant pathogenic fungi, since we detected the PMR1 homologues in several species of plant pathogenic fungi belonging to the classes Oomycetes, Ascomycetes, Basidiomycetes, and Deuteromycetes (unpublished data).
ACKNOWLEDGMENTS
We gratefully acknowledge J. E. Hamer and M. Urban (Purdue University) for critical reading of the manuscript, valuable advice, and correction of English; and H. Hamamoto (The University of Tokyo) for useful suggestions.
This study was supported by the program for promotion of basic research activities for innovative biosciences of the Bio-oriented Technology Research Advancement Institution (BRAIN).
REFERENCES
- 1.Ausbel F M, Brentand R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: Green Publishing Associates and Wiley-Interscience; 1987. [Google Scholar]
- 2.Balzi E, Goffeau A. Yeast multidrug resistance: the PDR network. J Bioenerg Biomembr. 1995;27:71–76. doi: 10.1007/BF02110333. [DOI] [PubMed] [Google Scholar]
- 3.Balzi E, Wang M, Leterme S, Van Dyck L, Goffeau A. PDR5, a novel yeast multidrug resistance conferring transporter controlled by the transcription regulator PDR1. J Biol Chem. 1994;269:2206–2214. [PubMed] [Google Scholar]
- 4.Bissinger P H, Kuchler K. Molecular cloning and expression of the Saccharomyces cerevisiae STS1 gene product. A yeast ABC transporter conferring mycotoxin resistance. J Biol Chem. 1994;269:4180–4186. [PubMed] [Google Scholar]
- 5.Delaveau T, Delahodde A, Carvajal E, Subik J, Jacq C. PDR3, a new yeast regulatory gene, is homologous to PDR1 and controls and multidrug resistance phenomenon. Mol Gen Genet. 1994;244:501–511. doi: 10.1007/BF00583901. [DOI] [PubMed] [Google Scholar]
- 6.Del Sorbo G, Andrade A C, Van Nistelrooy J G, Van Kan J A, Balzi E, De Waard M A. Multidrug resistance in Aspergillus nidulans involves novel ATP-binding cassette transporters. Mol Gen Genet. 1997;254:417–426. doi: 10.1007/s004380050434. [DOI] [PubMed] [Google Scholar]
- 7.Delye C, Laigret F, Corio-Costet M F. A mutation in the 14 alpha-demethylase gene of Uncinula necator that correlates with resistance to a sterol biosynthesis inhibitor. Appl Environ Microbiol. 1997;63:2966–2970. doi: 10.1128/aem.63.8.2966-2970.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Waard M A. Significance of ABC transporters in fungicide sensitivity and resistance. Pectic Sci. 1997;51:271–275. [Google Scholar]
- 9.De Waard M A, Van Nistelrooy J G. Accumulation of SBI fungicide in wild-type and fenarimol-resistant isolates of Penicillium italicum. Pestic Sci. 1988;22:371–382. [Google Scholar]
- 10.De Waard M A, Van Nistelrooy J G. Differential accumulation of fenarimol by a wild type isolate and fenarimol-resistant isolates of Penicillium italicum. Neth J Plant Pathol. 1984;90:143–153. [Google Scholar]
- 11.De Waard M A, Van Nistelrooy J G. An energy-dependent efflux mechanism for fenarimol in a wild type strain and fenarimol-resistant mutants of Aspergillus nidulans. Pestic Biochem Physiol. 1980;13:255–266. [Google Scholar]
- 12.De Waard M A, Van Nistelrooy J G. Induction of fenarimol-efflux activity in Aspergillus nidulans by fungicides inhibiting sterol biosynthesis. J Gen Microbiol. 1981;126:483–489. doi: 10.1099/00221287-126-2-483. [DOI] [PubMed] [Google Scholar]
- 13.De Waard M A, Van Nistelrooy J G. Inhibitors of energy-dependent efflux of fungicide fenarimol by Aspergillus nidulans. Exp Mycol. 1987;11:1–10. [Google Scholar]
- 14.Dexter D, Moye-Rowley W S, Wu A L, Golin J. Mutations in the yeast PDR3, PDR4, PDR7 and PDR9 pleiotropic (multiple) drug resistance loci affect the transcript level of an ATP binding cassette transporter encoding gene, PDR5. Genetics. 1994;136:505–515. doi: 10.1093/genetics/136.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gottesman M M, Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- 16.Higgins C F. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- 17.Hirata D, Yano K, Miyahara K, Miyakawa T. Saccharomyces cerevisiae YDR1, which encodes a member of the ATP-binding cassette (ABC) superfamily, is required for multidrug resistance. Curr Genet. 1994;26:285–294. doi: 10.1007/BF00310491. [DOI] [PubMed] [Google Scholar]
- 18.Hollomon D W. Resistance to azole fungicides in the field. Biochem Soc Trans. 1993;21:1047–1051. doi: 10.1042/bst0211047. [DOI] [PubMed] [Google Scholar]
- 19.Hollomon D W, Butters J, Clark J. Proceedings of the British Crop Protection Conference—Pests and Diseases. Vol. 2. Croydon, United Kingdom: British Crop Protection Council Publications; 1984. Genetic control of triadimenol resistance in barley powdery mildew; pp. 477–482. [Google Scholar]
- 20.Ito Y, Johnson R, Scott B. Integrative transformation of the mycotoxin-producing fungus, Penicillium paxilli. Curr Genet. 1994;25:508–513. doi: 10.1007/BF00351670. [DOI] [PubMed] [Google Scholar]
- 21.Joseph-Horne T, Hollomon D, Loeffler R S, Kelly S L. Altered P450 activity associated with direct selection for fungal azole resistance. FEBS Lett. 1995;374:174–178. doi: 10.1016/0014-5793(95)01102-k. [DOI] [PubMed] [Google Scholar]
- 22.Katzmann D J, Burnett P E, Golin J, Mahe Y, Moye-Rowley W S. Transcriptional control of the yeast PDR5 gene by the PDR3 gene product. Mol Cell Biol. 1994;14:4653–4661. doi: 10.1128/mcb.14.7.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katzmann D J, Hallstrom T C, Mahe Y, Moye-Rowley W S. Multiple Pdr1p/Pdr3p binding sites are essential for normal expression of the ATP binding cassette transporter protein-encoding gene PDR5. J Biol Chem. 1996;271:23049–23054. doi: 10.1074/jbc.271.38.23049. [DOI] [PubMed] [Google Scholar]
- 24.Kioka N, Tsubota J, Kakehi Y, Komano T, Gottesman M M, Pastan I, Ueda K. P-glycoprotein gene (MDR1) cDNA from human adrenal: normal P-glycoprotein carries Gly185 with an altered pattern of multidrug resistance. Biochem Biophys Res Commun. 1989;162:224–231. doi: 10.1016/0006-291x(89)91985-2. [DOI] [PubMed] [Google Scholar]
- 25.Kormanec J, Farkasovsky M. Isolation of total RNA from yeast and bacteria and detection of rRNA in Northern blots. BioTechniques. 1994;17:838–842. [PubMed] [Google Scholar]
- 26.Kyte J, Doolittle R F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 27.Loffler J, Kelly S L, Hebart H, Schumacher U, Lass-Florl C, Einsele H. Molecular analysis of cyp51 from fluconazole-resistant Candida albicans strains. FEMS Microbiol Lett. 1997;151:263–268. doi: 10.1111/j.1574-6968.1997.tb12580.x. [DOI] [PubMed] [Google Scholar]
- 28.Mahe Y, Parle-McDermott A, Nourani A, Delahodde A, Lamprecht A, Kuchler K. The ATP-binding cassette multidrug transporter Snq2 of Saccharomyces cerevisiae: a novel target for the transcription factors Pdr1 and Pdr3. Mol Microbiol. 1996;20:109–117. doi: 10.1111/j.1365-2958.1996.tb02493.x. [DOI] [PubMed] [Google Scholar]
- 28a.Nakaune, R., et al. Unpublished data.
- 29.Prasad R, De Wergifosse P, Goffeau A, Balzi E. Molecular cloning and characterization of a novel gene of Candida albicans, CDR1, conferring multiple resistance to drugs and antifungals. Curr Genet. 1995;27:320–329. doi: 10.1007/BF00352101. [DOI] [PubMed] [Google Scholar]
- 30.Punt P J, Oliver R P, Dingemanse M A, Pouwels P H, van den Hondel C A. Transformation of Aspergillus based on the hygromycin B resistance marker from Escherichia coli. Gene. 1987;56:117–124. doi: 10.1016/0378-1119(87)90164-8. [DOI] [PubMed] [Google Scholar]
- 31.Roepe P D. The role of the MDR protein in altered drug translocation across tumor cell membranes. Biochim Biophys Acta. 1995;1241:385–405. doi: 10.1016/0304-4157(95)00013-5. [DOI] [PubMed] [Google Scholar]
- 32.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 33.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanglard D, Kuchler K, Ischer F, Pagani J L, Monod M, Bille J. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob Agents Chemother. 1995;39:2378–2386. doi: 10.1128/aac.39.11.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schinkel A H, Borst P. Multidrug resistance mediated by P-glycoproteins. Semin Cancer Biol. 1991;2:213–226. [PubMed] [Google Scholar]
- 36.Servos J, Haase E, Brendel M. Gene SNQ2 of Saccharomyces cerevisiae, which confers resistance to 4-nitroquinoline-N-oxide and other chemicals, encodes a 169 kDa protein homologous to ATP-dependent permeases. Mol Gen Genet. 1993;236:214–218. doi: 10.1007/BF00277115. [DOI] [PubMed] [Google Scholar]
- 37.Stehmann C, de Waard M A. Accumulation of tebuconazole by isolates of Botrytis cinerea differing in sensitivity to sterol demethylation inhibiting fungicides. Pectic Sci. 1995;45:311–318. [Google Scholar]
- 38.Turi T G, Rose J K. Characterization of a novel Schizosaccharomyces pombe multidrug resistance transporter conferring brefeldin A resistance. Biochem Biophys Res Commun. 1995;213:410–418. doi: 10.1006/bbrc.1995.2147. [DOI] [PubMed] [Google Scholar]
- 39.Van den Brink H J, Van Nistelrooy H J, de Waard M A, Van den Hondel C A, Van Gorcom R F. Increased resistance to 14 alpha-demethylase inhibitors (DMIs) in Aspergillus niger by coexpression of the Penicillium italicum eburicol 14 alpha-demethylase (cyp51) and the A. niger cytochrome P450 reductase (cprA) genes. J Biotechnol. 1996;49:13–18. doi: 10.1016/0168-1656(96)01403-4. [DOI] [PubMed] [Google Scholar]
- 40.Van Nistelrooy J G, Van den Brink J M, Van Kan J A, Van Gorcom R F, de Waard M A. Isolation and molecular characterization of the gene encoding eburicol 14 alpha-demethylase (CYP51) from Penicillium italicum. Mol Gen Genet. 1996;250:725–733. doi: 10.1007/BF02172984. [DOI] [PubMed] [Google Scholar]
- 41.Van Tuyl J M. Genetis of fungal resistance to systemic fungicides. Meded Landbouwhogesch Wagening. 1977;77:1–136. [Google Scholar]
- 42.Walker J E, Saraste M, Runswick M J, Gay N J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1:945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoder O C. Cochliobolus heterostrophus, cause of Southern corn leaf blight. Adv Plant Pathol. 1988;6:93–112. [Google Scholar]