ABSTRACT
Transcription is an essential process of DNA metabolism, yet it makes DNA more susceptible to DNA damage. THSC/TREX-2 is a conserved eukaryotic protein complex with a key role in mRNP biogenesis and maturation that prevents genome instability. One source of such instability is linked to transcription, as shown in yeast and human cells, but the underlying mechanism and whether this link is universal is still unclear. To obtain further insight into the putative role of the THSC/TREX-2 complex in genome integrity, we have used Caenorhabditis elegans mutants of the thp-1 and dss-1 components of THSC/TREX-2. These mutants show similar defective meiosis, DNA damage accumulation and activation of the DNA damage checkpoint. However, they differ from each other regarding replication defects, as determined by measuring dUTP incorporation in the germline. Interestingly, this specific thp-1 mutant phenotype can be partially rescued by overexpression of RNase H. Furthermore, both mutants show a mild increase in phosphorylation of histone H3 at Ser10 (H3S10P), a mark previously shown to be linked to DNA–RNA hybrid-mediated genome instability. These data support the view that both THSC/TREX-2 factors prevent transcription-associated DNA damage derived from DNA–RNA hybrid accumulation by separate means.
Keywords: Caenorhabditis elegans, THSC/TREX-2 complex, Replication, DNA–RNA hybrids, Genome instability
Summary: We used C. elegans THP-1 and DSS-1 mutants to study the role of the THSC/TREX-2 complex in genome integrity. Both factors prevent transcription-associated DNA damage derived from DNA–RNA hybrid accumulation.
INTRODUCTION
Defects in the coupling of transcription with mRNA processing and export results in DNA damage and genome instability (Gaillard et al., 2013; Gómez-González and Aguilera, 2019). Understanding the link between mRNA biogenesis and genome instability has benefited strongly from studies of the THO complex, which is required for transcription elongation, mRNA export and genome integrity (Luna et al., 2012). Mutations in THO complex components give rise not only to transcription defects but also to genetic instability exhibited as hyper-recombination and DNA damage accumulation. These phenotypes are partially explained by the accumulation of R-loops in THO mutants in yeast, worms and human cells. An R-loop is a three-stranded structure formed during transcription by hybridization of the nascent RNA with the transcribed DNA strand, displacing the non-transcribed strand as single-stranded DNA (ssDNA) (Huertas and Aguilera, 2003; Castellano-Pozo et al., 2012a; Domínguez-Sánchez et al., 2011b). Although R-loops form naturally as key intermediates in specific cellular processes, such as E. coli plasmid replication, mitochondrial DNA replication or immunoglobulin (Ig) class switching (Aguilera and García-Muse, 2012), unscheduled R-loops are an important player in genome instability. Increasing evidence from species ranging from yeast to mammals has revealed that mRNA processing defects cause genetic instability in an R-loop-dependent manner, since the removal of these DNA–RNA hybrids partially suppresses the genetic instability phenotypes (Crossley et al., 2019). Previously, we have used the simple metazoan Caenorhabditis elegans to uncover a new role for the THO complex in development and meiosis and demonstrated that the defects in meiosis in THO mutants are partially due to replication impairment derived from R-loop accumulation (Castellano-Pozo et al., 2012a,b). Moreover, we have reported a link between R-loops and phosphorylation of histone H3 at Ser10 (H3S10P), a marker of chromatin condensation, and dimethylation of histone H3 at Lys9 (H3K9me2), a marker of heterochromatin, which suggests a role for RNA in chromatin compaction or condensation (Castellano-Pozo et al., 2013; García-Pichardo et al., 2017). Interestingly, the H3K9me2 modification has also been linked to R-loops occurring at transcription termination regions of some genes as well as in some rare fragile sites (Skourti-Stathaki et al., 2014; Groh et al., 2014). This connection between chromatin structure and R-loop accumulation adds new elements to understand how R-loops modulate genome dynamics.
The THSC/TREX-2 complex is conserved from yeast to humans and is involved in mRNP biogenesis (García-Oliver et al., 2012; Rondón et al., 2010). It interacts with the nuclear pore complex (NPC) and in yeast is constituted by the stable association of the multi-domain protein Sac3 (GANP, also known as MCM3AP, in mammals) with the Thp1 (PCID2 in mammals), Sus1 (ENY2 in mammals), Cdc31 (CEN2, encoded by CETN2 in mammals) and Sem1 (DSS1, also known as SEM1, in mammals) subunits (Fischer et al., 2002; Gallardo et al., 2003; Lei et al., 2003), among which Cdc31 and Sem1 have also been characterized as a centrin and a 19S proteosome subunit, respectively (Faza et al., 2009; Fischer et al., 2004; Wilmes et al., 2008). In addition to these functions, the mammalian proteins PCID2 and DSS1 have been linked to BRCA2 (breast cancer susceptibility gene 2 product), a component of the homologous recombination machinery (Bhatia et al., 2014; Marston et al., 1999; Yang et al., 2002). Similarly to the defects in THO mutants, it is believed that the genetic instability and transcriptional defects observed in yeast THSC/TREX-2 mutants (Gallardo et al., 2003; Santos-Pereira et al., 2014) are due to R-loop accumulation, because co-transcriptional cleavage of the nascent RNA reduces both defects to some extent (González-Aguilera et al., 2008). However, our laboratory has shown that depletion of PCID2, despite increasing genomic instability, does not lead to detectable accumulation of R-loops (Bhatia et al., 2014). To shed light on this conundrum we have used the nematode C. elegans to characterize two putative members of the C. elegans THSC/TREX-2 complex. We have observed that lack of the C. elegans THP-1 component of THSC/TREX-2 leads to sterility, DNA damage accumulation and replication defects at the germline. Importantly, microinjection of RNase H partially restores Cy3–dUTP incorporation in the nematode germline, consistent with R-loops interfering with replication progression. Interestingly, there is only a mild increase in the H3S10P chromatin mark in the THSC/TREX-2 mutants analyzed, strengthening the idea that R-loops interfere with replication in different ways. Our data provides evidence that THSC/TREX-2 contributes to the maintenance of genome integrity in part by preventing R-loop accumulation. Hence this study highlights that cells have developed alternative ways to avert DNA–RNA hybrids and minimize their incidence as a source of DNA damage and genome instability.
RESULTS
C. elegans THSC/TREX-2 complex is essential for fertility
To analyze the role of the THSC/TREX-2 complex in C. elegans, we first performed a search for THSC/TREX-2 component orthologs based on sequence comparisons (García-Oliver et al., 2012). The putative components of the C. elegans THSC/TREX-2 complex are summarized in Table S1. In yeast, Thp1, Sac3, Sus1 and Cdc31 are stable associated subunits of the THSC/TREX-2 complex (Gallardo et al., 2003), whereas Sem1, which has been shown to be an integrating component of THSC/TREX-2 (Wilmes et al., 2008; Faza et al., 2009), is a subunit of the 19S proteasome that interacts with other factors. Such interactions have also been observed for the mammalian Sem1 ortholog DSS1, which interacts with BRCA2 (Marston et al., 1999; Yang et al., 2002). To investigate the similar or different effects of these two THSC/TREX-2 complex members, we decided to work with the following two candidates for further analysis: the C27F2.10 gene (from now on referred to as thp-1), a homolog of yeast THP1 and human PCID2; and dss-1, a homolog of yeast SEM1 and human DSS1. The C. elegans THP-1 protein displays 45% and 27% amino acid sequence identity with human PCID2 and yeast Thp1, respectively (Fischer et al., 2002; Jani et al., 2012) (Table S1). Similar to human PCID2 (Umlauf et al., 2013), we found that THP-1 is a nuclear protein that accumulates at the nuclear periphery, possibly at NPCs (Fig. S1A,B). The C. elegans DSS-1 protein displays 49% and 40% amino acid sequence identity with human DSS1 and yeast Sem1, respectively (Pispa et al., 2008) (Table S1).
We investigated the two THSC/TREX-2 C. elegans orthologs by characterizing two deletion mutants, thp-1(tm3507) and dss-1(tm370), which are predicted to encode non-functional truncated proteins. The thp-1 gene spans 1.9 kb, including nine exons, and is predicted to encode an mRNA that produces a 413-amino-acid protein (Fig. S1C). The thp-1(tm3507) allele carries a deletion of 255 bp, which can be detected by single-worm PCR (Fig. S1D), and is predicted to encode a non-functional truncated protein of 64 amino acids, corresponding to the first three exons and part of the fourth exon. The dss-1 gene has previously been characterized as crucial for embryogenesis, larval growth and oogenesis (Pispa et al., 2008). Lethality assays revealed that the thp-1(tm3507) allele conferred complete sterility, since the homozygous mutant strain laid no eggs (Table 1), whereas wild-type animals [N2(wt)] and heterozygous thp-1(tm3507) mutants showed the expected lethal percentages of 0.05% and 62.86%, respectively. The lethality observed in the heterozygous mutant was due to the nature of the balancer system used (Edgley et al., 2006). The data demonstrate that lack of the THSC/TREX-2 component THP-1 leads to sterility, similar to the phenotype previously described for the dss-1 mutant (Pispa et al., 2008). Therefore, C. elegans thp-1 is an essential gene, as is dss-1; however, homozygous mutants produced by self-fertilization of heterozygous hermaphrodite parents were rescued beyond L4 stage by maternal contribution of thp-1 and dss-1 mRNA, allowing the analysis of mutants defective in either gene within adult tissues.
Table 1.
C. elegans thp-1 is an essential gene
To determine whether the sterility phenotype was caused by a defect during meiosis, we analyzed thp-1(tm3507) and dss-1(tm370) germlines. Each germline is spatially polarized in a distal-to-proximal manner with respect to mitotic proliferation and progression through meiotic prophase I. In the distal region, nuclei proliferate by mitotic divisions, acting as stem cells until they reach the transition zone where meiosis starts (Garcia-Muse and Boulton, 2007). 4,6-Diamidino-2-phenylindole (DAPI) staining of thp-1(tm3507) and dss-1(tm370) germlines revealed mitotic regions that were slightly reduced compared to that of N2(wt) regarding the number of mitotic nuclei (Fig. 1).
Fig. 1.
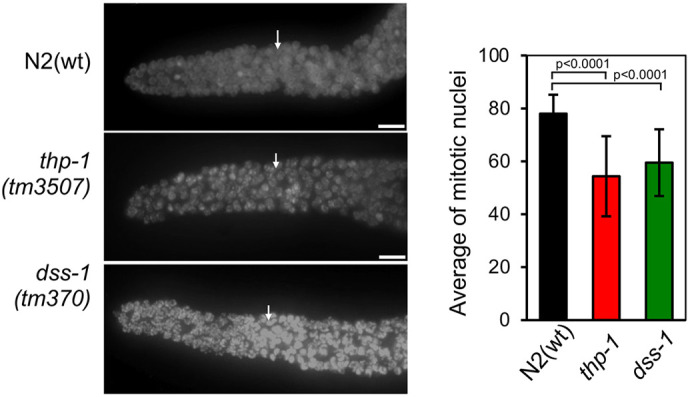
Characterization of mitosis in THSC/TREX-2 C. elegans mutants. Left: representative images of the mitotic region of fixed germlines counterstained with DAPI from adult hermaphrodites of the indicated genotypes. The arrow indicates the initiation of the transition zone. Right: quantification of mitotic nuclei of N2(wt), thp-1 mutant and dss-1 mutant strains (n=8, n=15 and n=10, respectively). Error bars indicate the s.d. P-values were calculated using a two-tailed unpaired Student's t-test. Scale bars: 10 µm.
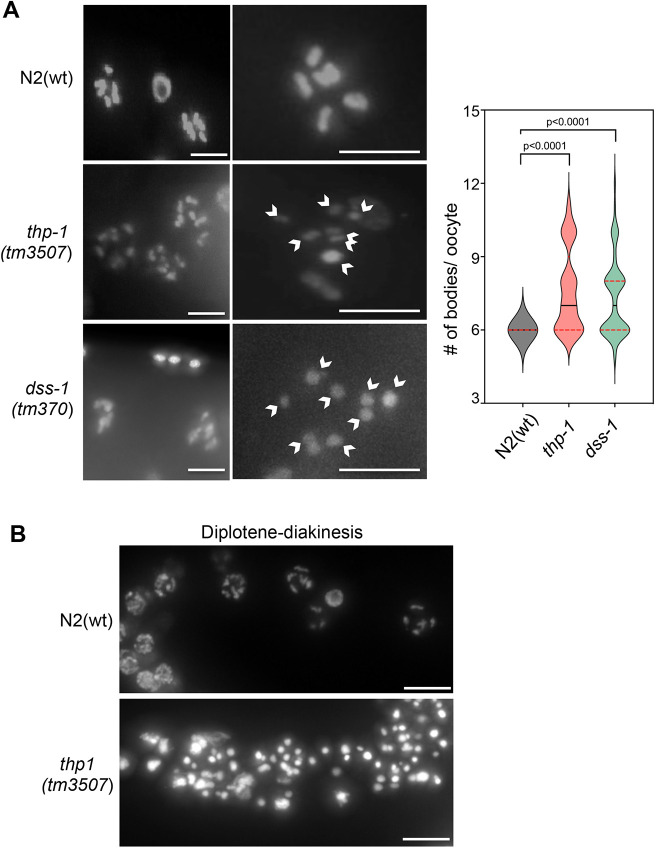
At the proximal region of the germline, wild-type oocytes always have nuclei with six discernible DAPI-stained bivalent chromosomes, indicative of successful meiosis I. In C. elegans any variation in this number is considered an aneuploidy due to defective meiosis. Indeed, as can be seen in Fig. 2A, N2(wt) oocytes showed six bivalent chromosomes (6±0.0, mean±s.d.; n=51), whereas in the case of the thp-1 mutant, oocytes with six normally condensing chromosomes as well as oocytes with more than six bivalent chromosomes were observed (7.6±1.67; n=45). This is an observation shared with the dss-1 mutant, where oocytes also showed a deviation from the expected number of six condensed chromosomes (7.32±1.59; n=65) (Fig. 2A), a phenotype that has previously been shown to increase in severity with the age of the animal (Pispa et al., 2008). This implies that both thp-1 and dss-1 mutants have abnormal diakinesis. In 24 h post-L4 animals, we were not able to see any difference in the number of oocytes between the mutants and N2(wt) (4 oocytes); however, older thp-1 animals showed alterations in the organization of the proximal region, making it difficult to differentiate diplotene and diakinesis (Fig. 2B).
Fig. 2.
Diakinesis is abnormal in THSC/TREX-2 C. elegans mutants. (A) Left: representative images of the diakinesis germline region of fixed germlines counterstained with DAPI from wild-type N2, thp-1 mutant and dss-1 mutant adult hermaphrodites. Middle: magnified images showing single oocytes. Arrowheads indicate DNA bodies. Right: quantification of DAPI-stained bodies per oocyte from N2(wt), thp-1 mutant and dss-1 mutant strains (n=51, n=45 and n=65, respectively). Violin plots show the distribution of values, with the mean (solid line) and s.d. (dotted line) indicated. P-values were calculated using a two-tailed unpaired Student's t-test. Scale bars: 5 µm. (B) Representative images of diplotene and diakinesis region of fixed germlines from 36 h post-L4 hermaphrodites of N2(wt) and thp-1(tm3507) strains. Images are representative of 10 animals. Scale bars: 10 µm.
DNA damage and checkpoint activation in thp-1 and dss-1 mutants
Next, we analyzed whether the diakinesis defect could be a consequence of early meiotic defects. One essential feature of successful meiosis is meiotic recombination, which is required for the generation of genetic variation and for proper chromosome segregation. Meiotic recombination is initiated by SPO-11-dependent double-strand break (DSB) formation (Dernburg et al., 1998) and requires RAD-51, a member of the RecA strand-exchange protein family, to catalyze the invasion of DNA single-strand overhangs into a recipient double-strand DNA to initiate formation of D loops and the later steps of meiotic recombination (West, 2003). First, to examine DSBs during meiosis in thp-1 and dss-1 mutants, we analyzed RAD-51 foci (Alpi et al., 2003). In N2(wt) worms, the levels of RAD-51 foci increased upon entrance to the transition zone (zone 2), peaked at mid-pachytene stage (zone 4) and disappeared by the end of pachytene (zone 5; Fig. 3). Upon entrance into meiosis (zone 3), thp-1 and dss-1 mutants accumulated RAD-51 foci earlier than N2(wt) worms (Fig. 3; Fig. S2), suggesting that the DSBs observed could originate from problems during mitotic replication or pre-meiotic replication at the entrance of the transition zone. Also, in these mutants we observed that RAD-51 foci remained until the end of the germline, implying that DSBs do not repair with the same efficiency as in N2(wt) animals (Fig. 3; Fig. S2). With the aim of checking whether the increase in DSBs observed in the thp-1 mutant was due to a defect in their repair, we performed a DSB repair analysis. Animals at 24 h post-L4 stage were treated with 75 Gy (which generates mainly DSBs) and left for 36–48 h before RAD-51 foci accumulation was analyzed. At mitotic and transition zone regions of N2(wt) animals, the levels of RAD-51 were similar to those in the non-treated control worms, whereas the irradiated thp-1 animals showed a decrease in the percentage of nuclei without RAD-51 foci with respect to that of the non-treated control (Fig. S3). This was also observed in the meiotic regions, although the N2(wt) had not completely recovered at 36 h, the thp-1 mutant showed a more dramatic accumulation of DSBs when comparing both irradiated and untreated animals (Fig. S3). These results suggest that DSB repair is compromised in the thp-1 mutants.
Fig. 3.
C. elegans thp-1 and dss-1 mutants show increased levels of RAD-51 foci. (A) Representative images of complete fixed germlines from adult hermaphrodites of the indicated genotypes immunostained using anti-RAD-51 antibodies (αRAD-51; green in +DAPI images) and counterstained with DAPI (blue). Scale bars: 10 µm. (B) Representative images of the mitotic and the pachytene regions of fixed germlines from adult hermaphrodites of the indicated genotypes immunostained with anti-RAD-51 antibodies and counterstained with DAPI. Scale bars: 5 µm. (C) Quantification of percentage of germline nuclei with RAD-51 foci. Zone 1 (mitosis), zone 2 (transition zone), zones 3–5 (pachytene) and zone 6 (diplotene) of N2(wt), thp-1 mutant and dss-1 mutant strains are shown (n=18, n=20 and n=20, respectively). Error bars indicate the s.d. Statistical analysis is shown in Fig. S3.
To determine whether the increase in DSBs in thp-1 and dss-1 mutants was a readout of DNA damage caused by replication problems, we performed immunofluorescence using antibodies against the single-strand binding protein RPA-1, which is involved in replication and recombination, and against the DNA damage checkpoint protein ATL-1, which is recruited to sites of DNA damage, where it activates the DNA damage checkpoint (Garcia-Muse and Boulton, 2005; Stergiou et al., 2011). Unlike N2(wt) worms, thp-1 and dss-1 mutants exhibited regions of ssDNA, as indicated by the presence of RPA-1 and ATL-1 foci in mitotic and meiotic nuclei (Fig. 4; Fig. S4). This is consistent with the interpretation that DNA damage occurs (throughout the germline) in thp-1 and dss-1 mutants, and subsequently activates the DNA damage checkpoint. This phenotype is reminiscent of that observed in thoc-2 mutants (Castellano-Pozo et al., 2012a) and mutants with defects during S phase, such as atl-1 and clk-2, in which endogenous DNA breaks arise from replication defects (Ahmed et al., 2001; Garcia-Muse and Boulton, 2005).
Fig. 4.
THSC/TREX-2 mutants show DNA damage accumulation and checkpoint activation. (A) Representative images of fixed germlines from adult hermaphrodites of the indicated genotypes immunostained using anti-ATL-1 (αATL-1) and anti-RPA-1 (αRPA-1) antibodies and counterstained with DAPI (blue). Images are representative of three experiments. Scale bars: 10 µm. (B) Representative images of fixed germlines from adult N2(wt) and thp-1(tm3507) hermaphrodites carrying rpa-1::yfp, immunostained for RPA-1::YFP and counterstained with DAPI (blue). Scale bars: 5 µm. (C) Quantification of the percentage of germline nuclei with RPA-1::YFP foci. Zones 3–6 represent equal parts of the pachytene region of animals as described in B (n=3 each). Error bars indicate the s.d. Statistical analysis is shown in Fig. S4.
DNA replication impairment in thp-1 mutants
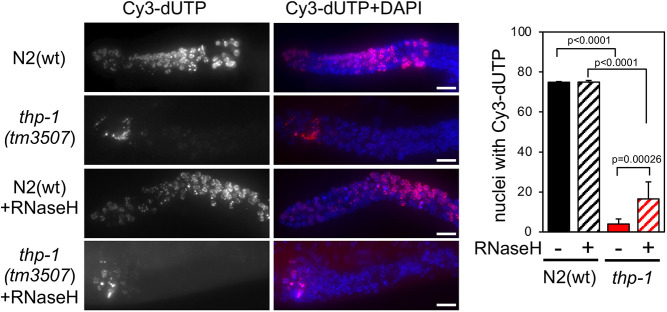
To examine whether the absence of THP-1 and DSS-1 proteins of the THSC/TREX-2 complex hinders replication, we assessed incorporation of deoxyribonucleotides into germline DNA after microinjection of Cy3–dUTP into the germline of young adult hermaphrodites (Castellano-Pozo et al., 2012a; Jaramillo-Lambert et al., 2007). In N2(wt) and dss-1 worms, all germlines showed Cy3–dUTP incorporation in all mitotic nuclei (Fig. 5; Fig. S5). However, the number of nuclei that incorporated Cy3–dUTP was significantly reduced in thp-1 mutants (Fig. 5). This result demonstrates that mitotic replication is impaired in thp-1 mutants but not in dss-1 mutants.
Fig. 5.
Mitotic replication is impaired in C. elegans thp-1 germlines, and this is partially alleviated by RNase H microinjection. Left: representative images of fixed germlines from adult hermaphrodites of the indicated genotype 2.5 h after microinjection with Cy3–dUTP (red in Cy3–dUTP+DAPI images) with or without RNase H. Germlines were counterstained with DAPI (blue). Right: graph shows the average percentage of mitotic nuclei with Cy3–dUTP incorporation for N2(wt) and thp-1 mutant animals that were either untreated or treated with RNaseH [N2(wt) untreated, n=19; N2(wt) treated, n=15; thp-1 untreated, n=25; thp-1 treated. n=15]. Error bars indicate the s.d. P-values were calculated using a two-tailed unpaired Student's t-test. Scale bars: 10 µm.
In yeast and C. elegans, other mRNA biogenesis mutants, such as mutants from the THO complex, also show replication defects. These defects have been linked to R-loop accumulation (Castellano-Pozo et al., 2012a; Huertas and Aguilera, 2003; Wellinger et al., 2006). To address directly whether this was also the case in the thp-1 mutant, we co-microinjected Escherichia coli RNase H1, which specifically digests the RNA moiety of DNA–RNA hybrids, into the worms in our in vivo replication assay (Castellano-Pozo et al., 2012a). Co-microinjection of RNase H1 did not affect incorporation of Cy3–dUTP in N2(wt) worms, whereas in thp-1 mutants RNase H1 treatment caused a clear increase in Cy3–dUTP labeled nuclei (Fig. 5). Although incomplete, a partial recovery of mitosis proficiency was possible upon RNase H1 microinjection. This result suggests that R-loops can form in thp-1 C. elegans mutants and could be responsible for the mitotic replication impairment that leads to the DNA damage observed.
The mechanism and factors responsible for genome instability mediated by R-loops are not yet well understood. We previously found that R-loops are linked to H3S10P, a mark of chromatin condensation, which led us to propose a model in which R-loops trigger the formation of condensed chromatin patches that would interfere with replication and/or transcription (Castellano-Pozo et al., 2012a). This conclusion was reinforced by the identification of yeast histone mutants unable to phosphorylate histone H3S10P (due to the lack of the residue or its mutation to alanine), which are able to accumulate high levels of R-loops without compromising genome integrity (García-Pichardo et al., 2017). To determine whether R-loop-mediated chromatin modifications could explain the genome instability of THSC/TREX-2 C. elegans mutants, we performed immunostaining against H3S10P. Normally, an N2(wt) germline shows H3S10P signal at 1–2 mitotic nuclei, correlating with chromosome condensation during mitosis, and at the compacted bivalents of the diakinesis region (Fig. 6; Fig. S6). We observed a slight increase in nuclei with H3S10P signal in both mutants, which was clearer in thp-1 animals (Fig. 6). In this mutant, some nuclei with H3S10P signal were observed beyond the germline mitotic region, reaching the transition zone and early pachytene regions (Fig. 6; Fig. S6). In addition, we observed H3S10P at later meiosis stages; in N2(wt) the compacted six bivalents showed a bright signal, as did those in the thp-1 and dss-1 mutants. We noticed that in 36 h post-L4 worms, when diakinesis is more disorganized in the thp-1 mutant, H3S10P signal could be seen at the late pachytene region (Fig. 2B; Fig. S6B). Thus, at least part of the observed replication impairment could be due to chromatin compaction triggered by R-loops, as has been shown in yeast and mammals (Castellano-Pozo et al., 2013).
Fig. 6.
THSC/TREX-2 mutants show mild H3S10P accumulation. Left: representative images of fixed germlines from adult hermaphrodites of the indicated genotypes immunostained with anti-H3S10P antibody (αH3S10P; red in +DAPI images) and counterstained with DAPI (blue). Right: graph shows the number of mitotic nuclei with H3S10P signal per gonad of the N2(wt), thp-1 mutant and dss-1 mutant strains (n=14, n=22 and n=11, respectively). Mean±s.d. are shown. P-values were calculated using a two-tailed unpaired Student's t-test. Scale bars: 10 µm.
DISCUSSION
Failures of DNA metabolic processes such as DNA replication and repair are a major source of DNA damage accumulation in cells (Aguilera and García-Muse, 2013). Importantly, it has been shown that DNA damage occurs at higher levels at regions with high transcriptional activity. Indeed, the loss of several RNA processing factors is associated with genome instability, which is explained, in part, by the formation of co-transcriptional DNA–RNA hybrids (Aguilera and García-Muse, 2012; García-Muse and Aguilera, 2019). To further understand this process in pluricellular organisms, we have analyzed mitosis and meiosis in C. elegans depleted of the THSC/TREX-2 mRNP biogenesis complex. Analysis of deletion mutants of two components of THSC/TREX-2 complex, thp-1(tm3507) and dss-1(tm370), revealed that this complex is required for fertility, since the mutants did not lay eggs. The absence of eggs was not due to compromised gonad development, since a two-arm gonad with developing germ cells was present in all mutant worms examined. Germline analysis of both THSC/TREX-2 mutants reveled defects in diakinesis, since oocyctes with an abnormal number of bivalents were observed. Nevertheless, the eggless phenotype cannot be explained by the diakinesis defects observed in the thp-1 and dss-1 mutants, since other mutants with defective diakinesis do form and lay eggs, and the lethality is observed at the embryonic stage. This is the case for mutants of brc-2, the C. elegans homolog of DNA repair gene BRCA2 (Martin et al., 2005; Pispa et al., 2008). BRCA2 interacts with DSS1 (Marston et al., 1999; Yang et al., 2002), and loss of either BRCA2 or DSS1 results in defects in homologous recombination (West, 2003). However, in brc-2 mutant oocytes, the chromosomes aggregate instead of condensing normally and eggs are produced, unlike in thp-1 and dss-1 mutants. One proposed explanation for the oogenesis defects in the dss-1 mutant is an aberrant regulation of the degradation of some essential protein required for oogenesis, on the basis that DSS1 is also a component of the proteasome (Krogan et al., 2004). Another possibility may be related to the relationship between DSS1 and BRCA2 (Bhatia et al., 2014). However, the fact that the mutant of the THSC/TREX-2 mRNA biogenesis complex component thp-1 shares the same eggless phenotype suggests a second possibility of an indirect effect caused by a defect in the mRNA biogenesis of specific meiotic genes. Nevertheless, our observations open a third possibility, which would need further investigation, in which R-loop accumulation also contributes to this eggless phenotype, as described previously for mutants of the THO complex (Castellano-Pozo et al., 2012a,b).
C. elegans thp-1 and dss-1 mutants show a clear increase in genome instability, as determined by accumulation of RPA-1 and RAD-51 foci and checkpoint activation. DNA damage accumulation, if not properly repaired, leads to genetic alterations such as mutations, gross chromosomal rearrangements, hyper-recombination and loss of heterozygosis, all of which are indicators of genome instability. Genome instability may result from failures of different DNA processes; however, failure in DNA replication is one of the most common causes (Aguilera and García-Muse, 2013). Replication impairment leads to ssDNA gaps and DSB accumulation. Most likely this DNA damage accumulation is the source of the genomic instability observed in thp-1 mutants. Indeed, loss of the THSC/TREX-2 complex in the worm leads to impaired replication, as observed in our in vivo replication assay. One cause of this defect could be the replication–transcription conflicts favored by the presence of DNA–RNA hybrids. In agreement with this, replication impairment can be partially suppressed by the addition of RNase H, which removes DNA–RNA hybrids. This observation supports that the defective replication due to the loss of a core component of the THSC/TREX-2 complex is in part caused by DNA–RNA hybrids, as is also the case for defects resulting from the absence of a functional THO complex in all organisms analyzed previously (Castellano-Pozo et al., 2012a; Huertas and Aguilera, 2003; Domínguez-Sánchez et al., 2011a). In contrast, loss of dss-1 does not hamper replication, suggesting that the genomic instability observed in this mutant is more likely to be related to BRC-2 activity (Pispa et al., 2008; Bhatia et al., 2014). In addition to causing the generation of DSBs, R-loops have recently been shown to accumulate at DSBs, especially those induced in transcriptionally active loci. However, the significance of this is still unclear, since data suggesting that R-loops can actively participate in DSB repair and data suggesting that they can interfere with DSB repair have both been reported (Aguilera and Gómez-González, 2017; Marnef and Legube, 2021). We have observed that thp-1 mutants show a delay in repair, which is in harmony with a detrimental effect of R-loops in DSB repair. On the other hand, it has been shown that spontaneous R-loops must be removed to avoid genomic instability and that repair factors such as BRCA2 play a role in this (Bhatia et al., 2014; D'Alessandro et al., 2018), and, as we mentioned above, this could underlie the differences observed between the thp-1 and dss-1 mutants. Therefore, our results show how two components of the C. elegans THSC/TREX-2 complex differently impact genome stability.
Co-transcriptional assembly of proteins on the nascent RNA is a strategy to prevent formation of DNA–RNA hybrids that is conserved from bacteria to eukaryotes (García-Muse and Aguilera, 2019; Aguilera and García-Muse, 2012). In bacteria, translation occurs co-transcriptionally, likely preventing the invasion of the RNA molecule during its synthesis (Gowrishankar and Harinarayanan, 2004). In yeast and metazoans, many RNA processing and splicing factors assemble within the RNA strand, therefore preventing the accumulation of DNA–RNA hybrids (Huertas and Aguilera, 2003; Li and Manley, 2005; Domínguez-Sánchez et al., 2011a). The number and complexity of RNA processing, splicing and export factors is increased in metazoans compared to those in yeast. This could be behind the fact that, although depletion of yeast THSC/TREX-2 complex leads to genome instability that is partially dependent on DNA–RNA hybrids (González-Aguilera et al., 2008), this effect is not that evident upon depletion of vertebrate PCID2, the ortholog of THP-1. Although PCID2 prevents genome instability, its depletion does not lead to a detectable accumulation of DNA–RNA hybrids (Bhatia et al., 2014); however we cannot exclude the possibility that this is due to limitations of the methodology used, or that accumulation of DNA–RNA hybrids could be evident upon depletion of other components of the THSC/TREX-2 complex, such as GANP (also known as MCM3AP). Our results in C. elegans correlate with those obtained previously in yeast, in which the THSC/TREX-2 complex helps prevent genome instability derived from DNA–RNA hybrid accumulation, opening the possibility that these THSC/TREX-2 functions might have evolved after the divergence of nematodes from the main metazoan lineage.
How the presence of DNA–RNA hybrids causes replication–transcription conflicts is yet not completely resolved. A higher impact of hybrids in convergent versus co-directional collisions has been described previously (Hamperl et al., 2017; García-Rubio et al., 2018; Lang et al., 2017). We have described previously that DNA–RNA hybrids can alter chromatin, increasing the presence of the condensation mark H3S10P and the heterochromatin mark H3K9me2 in nematode THO mutants (Castellano-Pozo et al., 2013). Similar observations have been obtained in other DNA–RNA hybrid-accumulating mutants from yeast to metazoans (Colak et al., 2014; Loomis et al., 2014; Skourti-Stathaki et al., 2014). The relevance of histone marks has been further confirmed by the identification of yeast histone mutations that facilitate DNA–RNA hybrids but do not cause instability, in agreement with the fact that they are not able to accumulate H3S10P (García-Pichardo et al., 2017). Importantly, such histone mutations suppress the genome instability phenotype of hpr1 and sen1 mutants because they prevent H3S10 phosphorylation (García-Pichardo et al., 2017). Unlike mutation of the THO complex, the loss of THSC/TREX-2 complex components in C. elegans causes a mild increase in the condensation mark H3S10P, whereas replication is impaired at similar levels in mutants of both complexes. This suggests that a putative premature condensation may not be the only way by which DNA–RNA hybrids can generate transcription-associated genome instability. In this sense, we have recently shown that hybrid stabilization by overexpression of the Yra1 RNA-binding protein also causes R-loop-mediated genome instability (Gavaldá et al., 2016; García-Rubio et al., 2018).
Lastly, there are important differences between the THO and THSC/TREX-2 complexes in the likely mechanism by which R-loop accumulation leads to genetic instability. During mRNA biogenesis, the two complexes act at different steps. The THO complex is recruited to chromatin and is required early during transcription elongation and mRNA export. Additional proteins would be recruited to the nascent mRNP via the THO complex to subsequently facilitate the recruitment of THSC/TREX-2 to bind the mRNP to the NPC. Importantly, it has been shown that physical proximity of transcribed chromatin to NPCs restrains the formation of pathological R-loops during transcription (García-Benítez et al., 2017). This observation agrees with the gene-gating hypothesis, which proposes that localization of transcribed DNA at the NPC facilitates the formation of an export-competent mRNP (Blobel, 1985). While this mechanism is well characterized in yeast, it is less defined in mammalian cells, for which further studies of cells depleted of different THSC/TREX-2- factors are required. In this regard, C. elegans has been shown to have developmental and stress-induced gene gating (Rohner et al., 2013), and we provide evidence that the THSC/TREX-2 complex accumulates at the nuclear periphery. This leads to the hypothesis that R-loop-dependent phenotypes observed in the THSC/TREX-2 mutants could originate from defects in the association with the NPC, in line with data provided for Mlp1 and Mlp2 yeast mutants (Garcia-Benitez et al., 2017).
MATERIALS AND METHODS
Strains and maintenance
Standard methods were used for the maintenance and manipulation of C. elegans strains (Brenner, 1974). The wild-type Bristol N2 and JK2739 nematode strains were provided by the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources. dss-1(tm370) was kindly provided by Dr Jäntti (VTT Technical Research Centre of Finland Ltd.; Pispa et al., 2008) and thp-1(tm3507) was generated and kindly provided by Dr Mitani (Tokyo Women's Medical University, National Biosource Project). The C. elegans thp-1(tm3507) deletion was backcrossed six times with wild-type Bristol N2 and then balanced with JK2739. The presence of thp-1(tm13507) and dss-1(tm350) deletion alleles was determined by nested PCR (primers are listed in Table S2). The RPA-1::YFP(ops263) has been described previously (Stergiou et al., 2011).
Embryonic lethality was scored by comparing the number of eggs that hatch to produce viable progeny versus the total number of eggs laid. Briefly, L4 hermaphrodites grown at 20°C were individually plated. The animals were transferred to new plates once every 24 h until the egg laying stopped. Laid eggs and hatched larvae were counted every day. The total number of single hermaphrodites for each stain is indicated in Table 1. Homozygous hermaphrodites used were thp-1(tm3507) mutants derived from thp-1(tm3507) heterozygous parents.
For DNA repair analysis after irradiation, 24 h post-L4 animals were exposed to 75 Gy of γ-ray from a BioBeam8000 (Gamma-service Medical GmbH). After 36–48 h, post-irradiation worms were processed for gonad analysis by immunofluorescence. As a control, non-irradiated plates were maintained in parallel.
To express GFP::THP-1, we inserted a full-length thp-1 PCR product amplified from N2 genomic DNA into plasmid pBN16, which contains a heat-inducible hsp-16.41 promoter and the 3′ UTR of unc-54 (Ródenas et al., 2012). The resulting plasmid, designated pBN467, was used for Mos-mediated single copy integration into ttTi5605 on chrII of EG4322 (Frøkjær-Jensen et al., 2008) to generate strain BN967, which next was crossed to BN903, which has mKate2 inserted into the 5′-end of the mel-28 coding sequence, resulting in strain BN974. Tagging of MEL-28, which localizes to nuclear pore complexes, with mKate2 was performed similarly to the protocol for insertion of GFP into the mel-28 locus (Gómez-Saldivar et al., 2016).
Immunostaining
Immunofluorescence stainings for Figs 2 and 5 were performed as described previously (Martin et al., 2005). Gonads from 24 h post-L4 adults were dissected in PBS with levamisole (10 mM concentration, Sigma) on poly-lysine-coated slides, fixed for 20 min in 4% paraformaldehyde and replaced for 10 min in TBSBTx [TBS containing 0.5% BSA (TBSB) and 0.4% Triton X-100]. The slides were washed twice for 10 min and once for 30 min with TBSB. Slides were then incubated overnight at 4°C with the primary antibodies (Table S3). The next day, gonads were washed three times in TBSB, each for 30 min at room temperature, and then incubated for 2 h with the secondary antibody in TBSB (Table S3). Gonads were washed three times for 30 min in TBSB and mounted with 10 μl Vectashield (with 1 μg/ml DAPI; Vector Laboratories and Sigma) per sample for further analysis.
Immunofluorescence stainings for Figs S3 and S5 were performed as described previously (Castellano-Pozo et al., 2020) with slight modifications. Gonads from 24 h post-L4 adults (with the exception of Fig. S3, for which 72 h post-L4 gonads were analyzed) were dissected in TBSTw (TBS containing 1% Tween 20) on poly-lysine-coated slides, fixed for 5 min in 1% paraformaldehyde, frozen in liquid nitrogen and permeabilized for 10 min in methanol at −20°C. The slides were washed with TBSTw three times for 5 min and blocked for 30 min with TBSTwB (TBSTw containing 0.7% BSA). The slides were incubated overnight at 4°C with primary antibodies (Table S3). The next day, gonads were washed three times in TBSTw, each for 10 min, and were then incubated for 2 h with the secondary antibody in TBSB (Table S3). Gonads were washed once in TBSTw containing DAPI (1 μg/ml) for 10 min and then washed four times for 10 min each in TBSTw before being mounted with 10 μl Vectashield per sample for further analysis.
In situ detection of germline DNA synthesis
Direct incorporation of Cy3–dUTP (Amersham Bioscences) into germline nuclei was performed as described previously (Castellano-Pozo et al., 2012a). The injection mix consisted of 50 μM Cy3–dUTP (Amersham Bioscences, Piscataway, NJ) in PBS, pH 7.2. After ∼2.5 h of exposure to the Cy3–dUTP, gonads were dissected, fixed and stained with DAPI. The total number of cells that incorporated Cy3–dUTP was determined for each dissected germline.
Fluorescence microscopy
Images from Figs 1A, 2A, 3A, 4 and 5 were acquired using a Leica DM6000B inverted microscope with 40× HCXPL-APO/1.25 OIL or 63× HCXPL-APO/1.40 OIL objective, and images were captured using Leica LAS-AF computer software. Regions of interest were projected into one dimension using Adobe Photoshop CS4 software. Images from all other figures were acquired using a Leica DM6000 inverted microscope with 100× HCXPL-APO/1.40 OIL objective, and images were captured using Leica LAS-AF computer software. Regions of interest were projected into one dimension using Adobe Photoshop CS4 software.
Quantification of Fig. 2 was performed by counting 10 nuclei of each region from animals along independent experiments (n is indicated in the figure legend). Quantification of Fig. 3C and Fig. S4 was performed as described previously (Castellano-Pozo et al., 2020). In brief, all nuclei from animals were counted. Data are presented as the percentage of nuclei in the different categories based on the number of foci/nuclei.
Confocal images of GFP::THP-1 and mKate2::MEL-28 were acquired on a Nikon A1R microscope equipped with a Plan Apo VC 60×/1.4 objective (Nikon, Tokyo, Japan) using a pinhole of 1.2 airy unit. Nematodes were grown at 25°C to achieve mild induction of GFP::THP-1 expression. Quantification of fluorescence signal was performed using Fiji (https://fiji.sc/), drawing a line 5 pixels wide and 5 µm long from the nuclear center outwards. Background values were obtained from nematodes without expression of fluorescent proteins.
Statistical analysis
Statistical significance was determined with a two-tailed unpaired Student's t-test or one-way ANOVA with Dunnett's post-hoc test using PRISM software (Graphpad Software Inc.).
Supplementary Material
Acknowledgements
We thank Shohei Mitani at the National Bioresource Project and Dr Jäntti for kindly providing thp-1(tm3507) and dss-1(tm370). Some strains were provided by the Caenorhabditis Genetics Center (CGC), which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). We thank A. Gartner for reagents, and C. Ayuso and U. Galindo for technical assistance.
Footnotes
Author contributions
Conceptualization: T.G.-M, A.A.; Methodology: A.Z., L.P.C., N.F.-F., M.G.-R., P.A., T.G.-M.; Validation: A.Z.; Formal analysis: A.Z., L.P.C., T.G.-M.; Investigation: A.Z., T.G.-M.; Resources: P.A., T.G.-M.; Writing - original draft: T.G.-M.; Writing - review & editing: T.G.-M., A.A.; Supervision: T.G.-M., A.A.; Funding acquisition: A.A.
Funding
This work was supported by grants from the Spanish Ministerio de Economía y Competitividad (BFU2016-75058-P), the European Research Council (ERC; Advanced Investigator Grant, ERC2014 AdG669898 TARLOOP) and the European Union (European Regional Development Fund).
Peer review history
The peer review history is available online at https://journals.biologists.com/jcs/article-lookup/doi/10.1242/jcs.258435.
References
- Aguilera, A. and García-Muse, T. (2012). R loops: from transcription byproducts to threats to genome stability. Mol. Cell 46, 115-124. 10.1016/j.molcel.2012.04.009 [DOI] [PubMed] [Google Scholar]
- Aguilera, A. and García-Muse, T. (2013). Causes of genome instability. Annu. Rev. Genet. 47, 1-32. 10.1146/annurev-genet-111212-133232 [DOI] [PubMed] [Google Scholar]
- Aguilera, A. and Gómez-González, B. (2017). DNA-RNA hybrids: the risks of DNA breakage during transcription. Nat. Struct. Mol. Biol. 24, 439-443. 10.1038/nsmb.3395 [DOI] [PubMed] [Google Scholar]
- Ahmed, S., Alpi, A., Hengartner, M. O. and Gartner, A. (2001). C. elegans RAD-5/CLK-2 defines a new DNA damage checkpoint protein. Curr. Biol. 11, 1934-1944. 10.1016/S0960-9822(01)00604-2 [DOI] [PubMed] [Google Scholar]
- Alpi, A., Pasierbek, P., Gartner, A. and Loidl, J. (2003). Genetic and cytological characterization of the recombination protein RAD-51 in Caenorhabditis elegans. Chromosoma 112, 6-16. 10.1007/s00412-003-0237-5 [DOI] [PubMed] [Google Scholar]
- Bhatia, V., Barroso, S. I., García-Rubio, M. L., Tumini, E., Herrera-Moyano, E. and Aguilera, A. (2014). BRCA2 prevents R-loop accumulation and associates with TREX-2 mRNA export factor PCID2. Nature 511, 362-365. 10.1038/nature13374 [DOI] [PubMed] [Google Scholar]
- Blobel, G. (1985). Gene gating: a hypothesis. Proc. Natl. Acad. Sci. USA 82, 8527-8529. 10.1073/pnas.82.24.8527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner, S. (1974). The genetics of Caenorhabditis elegans. Genetics 77, 71-94. 10.1093/genetics/77.1.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano-Pozo, M., García-Muse, T. and Aguilera, A. (2012a). R-loops cause replication impairment and genome instability during meiosis. EMBO Rep. 13, 923-929. 10.1038/embor.2012.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano-Pozo, M., García-Muse, T. and Aguilera, A. (2012b). The Caenorhabditis elegans THO complex is required for the mitotic cell cycle and development. PLoS ONE 7, e52447. 10.1371/journal.pone.0052447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano-Pozo, M., Santos-Pereira, J. M., Rondón, A. G., Barroso, S., Andújar, E., Pérez-Alegre, M., García-Muse, T. and Aguilera, A. (2013). R loops are linked to histone H3 S10 phosphorylation and chromatin condensation. Mol. Cell 52, 583-590. 10.1016/j.molcel.2013.10.006 [DOI] [PubMed] [Google Scholar]
- Castellano-Pozo, M., Pacheco, S., Sioutas, G., Jaso-Tamame, A. L., Dore, M. H., Karimi, M. M. and Martinez-Perez, E. (2020). Surveillance of cohesin-supported chromosome structure controls meiotic progression. Nat. Commun. 11, 4345. 10.1038/s41467-020-18219-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colak, D., Zaninovic, N., Cohen, M. S., Rosenwaks, Z., Yang, W.-Y., Gerhardt, J., Disney, M. D. and Jaffrey, S. R. (2014). Promoter-bound trinucleotide repeat mRNA drives epigenetic silencing in fragile X syndrome. Science 343, 1002-1005. 10.1126/science.1245831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley, M. P., Bocek, M. and Cimprich, K. A. (2019). R-loops as cellular regulators and genomic threats. Mol. Cell 73, 398-411. 10.1016/j.molcel.2019.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Alessandro, G., Whelan, D. R., Howard, S. M., Vitelli, V., Renaudin, X., Adamowicz, M., Iannelli, F., Jones-Weinert, C. W., Lee, M. Y., Matti, V.et al. (2018). BRCA2 controls DNA:RNA hybrid level at DSBs by mediating RNase H2 recruitment. Nat. Commun. 9, 5376. 10.1038/s41467-018-07799-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dernburg, A. F., McDonald, K., Moulder, G., Barstead, R., Dresser, M. and Villeneuve, A. M. (1998). Meiotic recombination in C. elegans initiates by a conserved mechanism and is dispensable for homologous chromosome synapsis. Cell 94, 387-398. 10.1016/S0092-8674(00)81481-6 [DOI] [PubMed] [Google Scholar]
- Domínguez-Sánchez, M. S., Barroso, S., Gómez-González, B., Luna, R. and Aguilera, A. (2011a). Genome instability and transcription elongation impairment in human cells depleted of THO/TREX. PLoS Genet. 7, e1002386. 10.1371/journal.pgen.1002386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domínguez-Sánchez, M. S., Sáez, C., Japón, M. A., Aguilera, A. and Luna, R. (2011b). Differential expression of THOC1 and ALY mRNP biogenesis/export factors in human cancers. BMC Cancer 11, 77. 10.1186/1471-2407-11-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley, M. L., Baillie, D. L., Riddle, D. L. and Rose, A. M. (2006). Genetic balancers. In WormBook (ed. The C. elegans Research Community), pp. 1-32. WormBook. 10.1895/wormbook.1.89.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faza, M. B., Kemmler, S., Jimeno, S., González-Aguilera, C., Aguilera, A., Hurt, E. and Panse, V. G. (2009). Sem1 is a functional component of the nuclear pore complex-associated messenger RNA export machinery. J. Cell Biol. 184, 833-846. 10.1083/jcb.200810059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer, T., Sträßer, K., Rácz, A., Rodriguez-Navarro, S., Oppizzi, M., Ihrig, P., Lechner, J. and Hurt, E. (2002). The mRNA export machinery requires the novel Sac3p-Thp1p complex to dock at the nucleoplasmic entrance of the nuclear pores. EMBO J. 21, 5843-5852. 10.1093/emboj/cdf590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer, T., Rodríguez-Navarro, S., Pereira, G., Rácz, A., Schiebel, E. and Hurt, E. (2004). Yeast centrin Cdc31 is linked to the nuclear mRNA export machinery. Nat. Cell Biol. 6, 840-848. 10.1038/ncb1163 [DOI] [PubMed] [Google Scholar]
- Frøkjær-Jensen, C., Davis, M. W., Hopkins, C. E., Newman, B. J., Thummel, J. M., Olesen, S.-P., Grunnet, M. and Jorgensen, E. M. (2008). Single-copy insertion of transgenes in Caenorhabditis elegans. Nat. Genet. 40, 1375-1383. 10.1038/ng.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard, H., Herrera-Moyano, E. and Aguilera, A. (2013). Transcription-associated genome instability. Chem. Rev. 113, 8638-8661. 10.1021/cr400017y [DOI] [PubMed] [Google Scholar]
- Gallardo, M., Luna, R., Erdjument-Bromage, H., Tempst, P. and Aguilera, A. (2003). Nab2p and the Thp1p-Sac3p complex functionally interact at the interface between transcription and mRNA metabolism. J. Biol. Chem. 278, 24225-24232. 10.1074/jbc.M302900200 [DOI] [PubMed] [Google Scholar]
- García-Benítez, F., Gaillard, H. and Aguilera, A. (2017). Physical proximity of chromatin to nuclear pores prevents harmful R loop accumulation contributing to maintain genome stability. Proc. Natl. Acad. Sci. USA 114, 10942-10947. 10.1073/pnas.1707845114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Muse, T. and Aguilera, A. (2019). R loops: from physiological to pathological roles. Cell 179, 604-618. 10.1016/j.cell.2019.08.055 [DOI] [PubMed] [Google Scholar]
- Garcia-Muse, T. and Boulton, S. J. (2005). Distinct modes of ATR activation after replication stress and DNA double-strand breaks in Caenorhabditis elegans. EMBO J. 24, 4345-4355. 10.1038/sj.emboj.7600896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Muse, T. and Boulton, S. J. (2007). Meiotic recombination in Caenorhabditis elegans. Chromosome Res. 15, 607-621. 10.1007/s10577-007-1146-x [DOI] [PubMed] [Google Scholar]
- García-Oliver, E., García-Molinero, V. and Rodríguez-Navarro, S. (2012). mRNA export and gene expression: the SAGA-TREX-2 connection. Biochim. Biophys. Acta (BBA) Gene Regul. Mech. 1819, 555-565. 10.1016/j.bbagrm.2011.11.011 [DOI] [PubMed] [Google Scholar]
- García-Pichardo, D., Cañas, J. C., García-Rubio, M. L., Gómez-González, B., Rondón, A. G. and Aguilera, A. (2017). Histone mutants separate R loop formation from genome instability induction. Mol. Cell 66, 597-609.e5. 10.1016/j.molcel.2017.05.014 [DOI] [PubMed] [Google Scholar]
- García-Rubio, M., Aguilera, P., Lafuente-Barquero, J., Ruiz, J. F., Simon, M.-N., Geli, V., Rondón, A. G. and Aguilera, A. (2018). Yra1-bound RNA-DNA hybrids cause orientation-independent transcription-replication collisions and telomere instability. Genes Dev. 32, 965-977. 10.1101/gad.311274.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavaldá, S., Santos-Pereira, J. M., García-Rubio, M. L., Luna, R. and Aguilera, A. (2016). Excess of Yra1 RNA-binding factor causes transcription-dependent genome instability, replication impairment and telomere shortening. PLoS Genet. 12, e1005966. 10.1371/journal.pgen.1005966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-González, B. and Aguilera, A. (2019). Transcription-mediated replication hindrance: a major driver of genome instability. Genes Dev. 33, 1008-1026. 10.1101/gad.324517.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Saldivar, G., Fernandez, A., Hirano, Y., Mauro, M., Lai, A., Ayuso, C., Haraguchi, T., Hiraoka, Y., Piano, F. and Askjaer, P. (2016). Identification of conserved MEL-28/ELYS domains with essential roles in nuclear assembly and chromosome segregation. PLoS Genet. 12, e1006131. 10.1371/journal.pgen.1006131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Aguilera, C., Tous, C., Gómez-González, B., Huertas, P., Luna, R. and Aguilera, A. (2008). The THP1-SAC3-SUS1-CDC31 complex works in transcription elongation-mRNA export preventing RNA-mediated genome instability. Mol. Biol. Cell 19, 4310-4318. 10.1091/mbc.e08-04-0355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowrishankar, J. and Harinarayanan, R. (2004). Why is transcription coupled to translation in bacteria? Mol. Microbiol. 54, 598-603. 10.1111/j.1365-2958.2004.04289.x [DOI] [PubMed] [Google Scholar]
- Groh, M., Lufino, M. M. P., Wade-Martins, R. and Gromak, N. (2014). R-loops associated with triplet repeat expansions promote gene silencing in Friedreich ataxia and fragile X syndrome. PLoS Genet. 10, e1004318. 10.1371/journal.pgen.1004318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamperl, S., Bocek, M. J., Saldivar, J. C., Swigut, T. and Cimprich, K. A. (2017). Transcription-replication conflict orientation modulates R-loop levels and activates distinct DNA damage responses. Cell 170, 774-786.e19. 10.1016/j.cell.2017.07.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huertas, P. and Aguilera, A. (2003). Cotranscriptionally formed DNA:RNA hybrids mediate transcription elongation impairment and transcription-associated recombination. Mol. Cell 12, 711-721. 10.1016/j.molcel.2003.08.010 [DOI] [PubMed] [Google Scholar]
- Jani, D., Lutz, S., Hurt, E., Laskey, R. A., Stewart, M. and Wickramasinghe, V. O. (2012). Functional and structural characterization of the mammalian TREX-2 complex that links transcription with nuclear messenger RNA export. Nucleic Acids Res. 40, 4562-4573. 10.1093/nar/gks059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo-Lambert, A., Ellefson, M., Villeneuve, A. M. and Engebrecht, J. (2007). Differential timing of S phases, X chromosome replication, and meiotic prophase in the C. elegans germ line. Dev. Biol. 308, 206-221. 10.1016/j.ydbio.2007.05.019 [DOI] [PubMed] [Google Scholar]
- Krogan, N. J., Lam, M. H. Y., Fillingham, J., Keogh, M.-C., Gebbia, M., Li, J., Datta, N., Cagney, G., Buratowski, S., Emili, A.et al. (2004). Proteasome involvement in the repair of DNA double-strand breaks. Mol. Cell 16, 1027-1034. 10.1016/j.molcel.2004.11.033 [DOI] [PubMed] [Google Scholar]
- Lang, K. S., Hall, A. N., Merrikh, C. N., Ragheb, M., Tabakh, H., Pollock, A. J., Woodward, J. J., Dreifus, J. E. and Merrikh, H. (2017). Replication-transcription conflicts generate R-loops that orchestrate bacterial stress survival and pathogenesis. Cell 170, 787-799.e18. 10.1016/j.cell.2017.07.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei, E. P., Stern, C. A., Fahrenkrog, B., Krebber, H., Moy, T. I., Aebi, U. and Silver, P. A. (2003). Sac3 is an mRNA export factor that localizes to cytoplasmic fibrils of nuclear pore complex. Mol. Biol. Cell 14, 836-847. 10.1091/mbc.e02-08-0520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X. and Manley, J. L. (2005). Inactivation of the SR protein splicing factor ASF/SF2 results in genomic instability. Cell 122, 365-378. 10.1016/j.cell.2005.06.008 [DOI] [PubMed] [Google Scholar]
- Loomis, E. W., Sanz, L. A., Chédin, F. and Hagerman, P. J. (2014). Transcription-associated R-loop formation across the human FMR1 CGG-repeat region. PLoS Genet. 10, e1004294. 10.1371/journal.pgen.1004294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna, R., Rondón, A. G. and Aguilera, A. (2012). New clues to understand the role of THO and other functionally related factors in mRNP biogenesis. Biochim. Biophys. Acta (BBA) Gene Regul. Mech. 1819, 514-520. 10.1016/j.bbagrm.2011.11.012 [DOI] [PubMed] [Google Scholar]
- Marnef, A. and Legube, G. (2021). R-loops as Janus-faced modulators of DNA repair. Nat. Cell Biol. 23, 305-313. 10.1038/s41556-021-00663-4 [DOI] [PubMed] [Google Scholar]
- Marston, N. J., Richards, W. J., Hughes, D., Bertwistle, D., Marshall, C. J. and Ashworth, A. (1999). Interaction between the product of the breast cancer susceptibility gene BRCA2 and DSS1, a protein functionally conserved from yeast to mammals. Mol. Cell. Biol. 19, 4633-4642. 10.1128/MCB.19.7.4633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, J. S., Winkelmann, N., Petalcorin, M. I. R., McIlwraith, M. J. and Boulton, S. J. (2005). RAD-51-dependent and -independent roles of a Caenorhabditis elegans BRCA2-related protein during DNA double-strand break repair. Mol. Cell. Biol. 25, 3127-3139. 10.1128/MCB.25.8.3127-3139.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pispa, J., Palmén, S., Holmberg, C. I. and Jäntti, J. (2008). C. elegans dss-1is functionally conserved and required for oogenesis and larval growth. BMC Dev. Biol. 8, 51. 10.1186/1471-213X-8-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ródenas, E., González-Aguilera, C., Ayuso, C. and Askjaer, P. (2012). Dissection of the NUP107 nuclear pore subcomplex reveals a novel interaction with spindle assembly checkpoint protein MAD1 in Caenorhabditis elegans. Mol. Biol. Cell 23, 930-944. 10.1091/mbc.e11-11-0927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohner, S., Kalck, V., Wang, X., Ikegami, K., Lieb, J. D., Gasser, S. M. and Meister, P. (2013). Promoter- and RNA polymerase II-dependent hsp-16 gene association with nuclear pores in Caenorhabditis elegans. J. Cell Biol. 200, 589-604. 10.1083/jcb.201207024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondón, A. G., Jimeno, S. and Aguilera, A. (2010). The interface between transcription and mRNP export: from THO to THSC/TREX-2. Biochim. Biophys. Acta (BBA) Gene Regul. Mech. 1799, 533-538. 10.1016/j.bbagrm.2010.06.002 [DOI] [PubMed] [Google Scholar]
- Santos-Pereira, J. M., García-Rubio, M. L., González-Aguilera, C., Luna, R. and Aguilera, A. (2014). A genome-wide function of THSC/TREX-2 at active genes prevents transcription-replication collisions. Nucleic Acids Res. 42, 12000-12014. 10.1093/nar/gku906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skourti-Stathaki, K., Kamieniarz-Gdula, K. and Proudfoot, N. J. (2014). R-loops induce repressive chromatin marks over mammalian gene terminators. Nature 516, 436-439. 10.1038/nature13787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stergiou, L., Eberhard, R., Doukoumetzidis, K. and Hengartner, M. O. (2011). NER and HR pathways act sequentially to promote UV-C-induced germ cell apoptosis in Caenorhabditis elegans. Cell Death Differ. 18, 897-906. 10.1038/cdd.2010.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umlauf, D., Bonnet, J., Waharte, F., Fournier, M., Stierle, M., Fischer, B., Brino, L., Devys, D. and Tora, L. (2013). The human TREX-2 complex is stably associated with the nuclear pore basket. J. Cell Sci. 126, 2656-2667. 10.1242/jcs.118000 [DOI] [PubMed] [Google Scholar]
- Wellinger, R. E., Prado, F. and Aguilera, A. (2006). Replication fork progression is impaired by transcription in hyperrecombinant yeast cells lacking a functional THO complex. Mol Cell. Biol. 26, 3327-3334. 10.1128/MCB.26.8.3327-3334.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West, S. C. (2003). Molecular views of recombination proteins and their control. Nat. Rev. Mol. Cell Biol. 4, 435-445. 10.1038/nrm1127 [DOI] [PubMed] [Google Scholar]
- Wilmes, G. M., Bergkessel, M., Bandyopadhyay, S., Shales, M., Braberg, H., Cagney, G., Collins, S. R., Whitworth, G. B., Kress, T. L., Weissman, J. S.et al. (2008). A genetic interaction map of RNA-processing factors reveals links between Sem1/Dss1-containing complexes and mRNA export and splicing. Mol. Cell 32, 735-746. 10.1016/j.molcel.2008.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, H., Jeffrey, P. D., Miller, J., Kinnucan, E., Sun, Y., Thoma, N. H., Zheng, N., Chen, P.-L., Lee, W.-H. and Pavletich, N. P. (2002). BRCA2 function in DNA binding and recombination from a BRCA2-DSS1-ssDNA structure. Science 297, 1837-1848. 10.1126/science.297.5588.1837 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.