Abstract
The blood-cerebrospinal fluid barrier (BCSFB), formed by the choroid plexus epithelial (CPE) cells, plays an active role in removing drugs and metabolic wastes from the brain. Recent functional studies in isolated mouse choroid plexus (CP) tissues suggested the presence of organic anion transporting polypeptides (OATPs, encoded by SLCOs) at the apical membrane of BCSFB, which may clear large organic anions from the cerebrospinal fluid (CSF). However, the specific OATP isoform involved is unclear. Using quantitative fluorescence imaging, we showed that the fluorescent anions sulforhodamine 101 (SR101), fluorescein methotrexate (FL-MTX), and 8-fluorescein-cAMP (fluo-cAMP) are actively transported from the CSF to the subepithelial space in CP tissues isolated from wild-type mice. In contrast, transepithelial transport of these compounds across the CPE cells was abolished in Oatp1a/1b−/− mice due to impaired apical uptake. Using transporter-expressing cell lines, SR101, FL-MTX, and fluo-cAMP were additionally shown to be transported by mouse OATP1A5 and its human counterpart OATP1A2. Kinetic analysis showed that estrone-3-sulfate and SR101 are transported by OATP1A2 and OATP1A5 with similar Michaelis-Menten constants (Km). Immunofluorescence staining further revealed the presence of OATP1A2 protein in human CP tissues. Together, our results suggest that large organic anions in the CSF are actively transported into CPE cells by apical OATP1A2 (OATP1A5 in mice), then subsequently effluxed into the blood by basolateral multidrug resistance-associated proteins (MRPs). As OATP1A2 transports a wide array of endogenous compounds and xenobiotics, the presence of this transporter at the BCSFB may imply a novel clearance route for drugs and neurohormones from the CSF.
SIGNIFICANCE STATEMENT
Drug transporters at the blood–cerebrospinal fluid (CSF) barrier play an important but understudied role in brain drug disposition. This study revealed a functional contribution of rodent organic anion transporting polypeptide (OATP) 1A5 towards the CSF clearance of organic anions and suggested a similar role for OATP1A2 in humans. Delineating the molecular mechanisms governing CSF organic anion clearance may help to improve the prediction of central nervous system (CNS) pharmacokinetics and identify drug candidates with favorable CNS pharmacokinetic properties.
Introduction
Neurologic disorders are the primary cause of disability and the second leading cause of death worldwide (Feigin et al., 2020). Consequently, there is a need to develop novel treatments and therapies targeting the central nervous system (CNS). However, CNS drugs face many hurdles in drug development, and the attrition rate for CNS drugs is very high (Gribkoff and Kaczmarek, 2017). One challenge of CNS drug development is the difficulty in predicting CNS drug pharmacokinetics and exposure, which drive efficacy and toxicity. The pharmacokinetic and pharmacodynamic relationship within the CNS is driven by the unbound brain drug concentration, which is influenced by multiple transport and distribution mechanisms at the blood-brain barrier and the blood–cerebrospinal fluid barrier (BCSFB) (de Lange, 2013a,b).
The BCSFB is formed by the choroid plexuses (CPs), which consist of polarized choroid plexus epithelial (CPE) cells that surround a core of blood capillaries (Lun et al., 2015; Sun and Wang, 2021). As the CP blood capillaries are fenestrated and lack tight junctions, the barrier function lies with the tight junction-linked CPE cells (Hurley et al., 1981; Johanson et al., 2011). The CPE cells express multispecific transporters and enzymes that contribute to xenobiotic and endogenous compound clearance from the cerebrospinal fluid (CSF) (Sun and Wang, 2021). However, transporters at the human BCSFB are understudied with respect to function and activity. Functional studies are largely performed in preclinical species due to the lack of human in vitro systems, and characterization of transporter protein expression at the human BCSFB is limited. Thus, the overall significance of BCSFB transporters in brain drug disposition is still not fully understood.
The organic anion transporting polypeptide (OATP/SLCO) transporters are a superfamily of drug transporters involved in the uptake and disposition of a wide array of xenobiotics and endogenous substrates. Among the 11 human OATPs and 15 murine OATPs, members of the OATP1A/1B family are especially important in drug transport due to their broad substrate specificity (Hagenbuch and Stieger, 2013). The pharmacokinetic relevance of OATPs has been well studied in the liver, where OATP1B1 and 1B3 play an important role in hepatic uptake and elimination of amphipathic drugs such as statins, repaglinide, and other compounds (Kalliokoski and Niemi, 2009). Interestingly, although some human OATPs have direct rodent orthologs, this is not the case for OATP1A and OATP1B transporters. In mice, there are four members in the OATP1A family (OATP1A1, OATP1A4, OATP1A5, and OATP1A6), whereas humans only have one member (OATP1A2). In the liver-specific OATP1B family, mice only have one member (OATP1B2), whereas humans have two isoforms (OATP1B1 and OATP1B3) (Hagenbuch and Stieger, 2013). In human and mice CPs, the mRNA expression of OATP1A4, OATP1A5, and OATP1A2 have been reported (Hu et al., 2022). The OATP1A4 protein has been localized to the basolateral membrane (Gao et al., 1999), whereas OATP1A5 protein is localized apically (Ohtsuki et al., 2003). In addition, human OATP1A2 and rodent OATP1A4 are expressed at the blood-brain barrier (Abdullahi et al., 2017).
Our laboratory recently applied confocal microscopy and live-tissue imaging techniques to study the transcellular transport mechanisms for organic cations and organic anions at the BCSFB (Hu et al., 2022; Sun and Wang, 2022). Using live CP tissues isolated from the mouse brain, we observed that the CPE cells lack an intact transcellular transport system for organic cations but possess a highly functional CSF-to-blood transport system for large and amphipathic organic anions. Pharmacological inhibition studies suggested that the anion transport system likely consists of OATPs at the apical (CSF-facing) membrane and the multidrug resistance-associated proteins 1/4 (MRP1/4) at the basolateral (blood-facing) membrane (Hu et al., 2022). Using quantitative analysis and real-time imaging, we further showed that apical uptake of fluorescent organic anions [e.g., fluorescein methotrexate (FL-MTX), fluorescein–cyclic AMP (fluo-cAMP)], is the rate-limiting step in the overall CSF-to-blood transport process and highly sensitive to the OATP inhibitor rifampin (Hu et al., 2022; Sun and Wang, 2022). However, definitive data establishing the role of OATPs in CPE cell apical uptake is lacking, and the specific OATP isoform(s) involved in this rate-limiting clearance step remain unknown. Furthermore, the protein expression, localization, and functional relevance of OATP1A2 are unknown in the human CP.
In this study, we investigated the molecular mechanisms underlying transepithelial transport of large organic anions using fluorescent OATP probes sulforhodamine 101 (SR101), FL-MTX, and fluo-cAMP in freshly isolated murine CP tissues. The specific activity of OATP1A/1B transporters was defined using a cluster knockout (KO) mouse model. The transport of various fluorescent probes by human OATP1A2 and mouse OATP1A5 was further characterized and compared using cell lines overexpressing individual transporters. Lastly, the expression and localization of OATP1A2 was determined in human CP samples.
Materials and Methods
Materials
[3H]estrone-3-Sulfate (49.19 Ci/mmol) was procured from PerkinElmer. Sulforhodamine 101 was procured from Sigma-Aldrich (St. Louis, MO). Fluorescein-methotrexate was procured from Biotium (San Francisco, CA). 8-Fluorescein-cAMP was procured from Axxora (Farmingdale, NY). Cell culture media and reagents were procured from Thermo Fisher Scientific (Waltham, MA). Cell culture plastic wares were procured from Grenier Bio One (Monroe, NC) or Thermo Fisher Scientific. Unless otherwise specified, all chemicals were procured from Sigma-Aldrich (St. Louis, MO).
Animals and Choroid Plexus Tissue Collection
Animal experiments were carried out in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the US National Institutes of Health. All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Washington. Animals were maintained in the specific pathogen-free facility at the University of Washington and maintained under standard conditions, with food and water available ad libitum. Adult (10- to 14-week-old) male FVB wild-type and Slco1a/1b−/− (referred to as Oatp1a/1b−/−) mice were obtained from Taconic Biosciences (Germantown, NY). Oatp1a/1b−/− mice have a gene cluster deletion removing Slco1a1, Slco1a4, Slco1a5, Slco1a6, and Slco1b2 that respectively encodes OATP1A1, OATP1A4, OATP1A5, OATP1A6, and OATP1B2 proteins in mice. Generation and physiologic characteristics of this strain were described previously (van de Steeg et al., 2010). Lateral ventricle CPs were isolated from murine brains as previously described (Sun and Wang, 2022). The CP tissue was either snap frozen for mRNA analysis or transferred to pregassed artificial CSF (aCSF) for transport imaging studies.
Quantification of Transporter mRNA Expression by Real-Time Polymerase Chain Reaction
After CP isolation, tissues were then flash frozen in liquid nitrogen and stored in a −80°C freezer until further processing. Frozen tissue was then homogenized by bead disruptor, and total RNA was extracted by RNeasy Mini Kit (Qiagen, Hilden, Germany). Total RNA was then converted to cDNA by reverse transcription using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Waltham, MA). Expression of transporters at the CP was quantified as described previously (Duan and Wang, 2013; Hu et al., 2022). The relative mRNA levels of these transporters in CP were normalized to glyceraldehyde-3-phosphate dehydrogenase (Gapdh). The list of TaqMan Gene Expression Assays can be found in Supplemental Table 1.
Confocal Imaging and Transport Studies in Freshly Isolated CP Tissues from Wild-Type and Oatp1a/1b−/− Mice
Transport studies were conducted in freshly isolated mouse CP as described previously (Hu et al., 2022; Sun and Wang, 2022). Isolated CPs maintained vitality and transport activity for up to 2 to 3 hours, and all transport studies were performed within 2 hours after tissue isolation (Sun and Wang, 2022). Transport studies were initiated by adding a fluorescent substrate into the aCSF. Fluorescent signals were recorded in CPE cells and adjacent subepithelial region. For real-time recording, fluorescent signals were recorded every minute for 20 minutes. Image acquisition were performed using a Zeiss LSM 710 confocal microscope as previously described (Sun and Wang, 2022). Samples containing FL-MTX or fluo-cAMP were illuminated using a 488-nm fixed wavelength argon laser. Samples containing SR101 were illuminated using a 594-nm fixed wavelength helium-neon laser. Appropriate corresponding dichroic and emission filters were used to detect the emission of the fluorescent probes. Digital image analysis was conducted using Fiji ImageJ (1.53t) (Schindelin et al., 2012) as described previously (Sun and Wang, 2022).
Generation of OATP1A5 and OATP1A2 Stable Cell Lines and Cell Culture
OATP1A5/Slco1a5 expression vector (MMM1013-202762445) was obtained from Horizon Discovery (Waterbeach, UK) and subsequently stably transfected into Flp-In human embryonic kidney 293 (HEK293) cells using a modified approach as previously described (Duan and Wang, 2010). Briefly, the expression vector was digested by KpnI and XhoI (New England Biolabs, Ipswich, MA) to obtain the Slco1a5 cDNA of interest, which was subsequently subcloned into the KpnI/XhoI sites of the pcDNA5/FRT vector (Invitrogen, Waltham, MA). The insert of the OATP1A5 expression vector was sequenced and aligned with the National Center for Biotechnology Information (NCBI) GenBank sequence BC013594 to ensure fidelity. This newly generated OATP1A5 expression vector was then cotransfected with pOG44 expressing the Flp-recombinase into the Flp-In HEK293 cell line using Lipofectamine 3000 (Thermo Fisher Scientific). Transfected cells were selected by hygromycin B treatment (250 μg/ml), whereby the optimal hygromycin B concentration was determined by a kill curve in Flp-In HEK293 cells (data not shown). HEK293 cells expressing the empty pcDNA5/FRT vector were previously generated in our laboratory (Duan and Wang, 2010). Transfected HEK293 cells stably expressing the human OATP1A2 and empty vector were generated using a lentiviral construct in the Hagenbuch laboratory as follows. Lenti ORF particles encoding OATP1A2 (Myc-DDK tagged) along with Lenti ORF control particles pLenti-C-Myc-DDK-P2A-Puro were purchased from OriGene (Rockville, MD). HEK293 cells were transduced following a protocol supplied by OriGene. Briefly, HEK293 cells were seeded at 50,000 cells per well on a 24-well plate. Twenty-four hours later, the cells were transduced with a multiplicity of infection of 5 in the presence of polybrene (8 μg/ml). The next day, the medium was changed to normal medium. On day 4, the cells were split onto 6-cm plates in a medium containing 2 μg/ml puromycin. Once confluent, the cells were split onto 10-cm plates, and single colonies were isolated by limited dilution. The clone with the highest uptake of estrone-3-sulfate was used for further characterization. OATP1A2 and empty vector cells were maintained in Dulbecco’s modified Eagle’s medium (11054-020) supplemented with 10% fetal bovine serum, 2% GlutaMAX, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 μg/ml puromycin, and a final concentration of 25 mM D-glucose. Generated OATP1A5 and empty vector cells were maintained in Dulbecco’s modified Eagle’s medium (11995-065) supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and 150 μg/ml hygromycin B. The surface of the flasks was coated with 0.01% poly-D-lysine in phosphate-buffered saline to promote HEK293 cell attachment. Cells were cultured and maintained in a 37°C incubator with 5% CO2.
Uptake and Inhibition Assays in HEK293 Cells
Uptake and inhibition assays were conducted using a modified approach as previously described (López Quiñones et al., 2020). Briefly, cells were seeded on 96-well plates and grown to greater than 90% confluency. Cells were washed with 37°C Krebs-Ringer-HEPES buffer (125 mM NaCl, 4.8 mM KCl, 1.2 mM MgCl2, 1.2 mM CaCl2, 5.6mM glucose, 25 mM HEPES, 1.2 mM KH2PO4). Experiments were initiated by the addition of 100 μl of Krebs-Ringer-HEPES buffer containing substrate compound with or without an inhibitor. Uptake was quenched by removing the substrate-containing buffer and washing the cells with ice-cold buffer. After washing three times, cells were lysed with 10% acetonitrile, and the resulting lysate was used to determine the total protein amount by the bicinchoninic acid method and to quantify intracellular substrate concentrations. Fluorescence measurements of SR101 were performed from a top-read position in a Synergy HTX plate reader (BioTek, Winooski, VT) using a 585/10-nm excitation and 620/40-nm emission filter set. FL-MTX and fluo-cAMP were similarly detected using a 485/20 excitation and 528/20 emission filter set. Fluorescence values were background subtracted by fluorescence readings in wells containing cells without added fluorescent substrate. Transporter-specific uptake was determined by subtracting the uptake in empty vector-transfected cell controls from that of transporter-expressing cells. To quantify intracellular SR101 concentrations and validate the linearity of the fluorescent signal, the intracellular fluorescent signal was converted to concentrations using standard curves prepared in cell lysate and spiked with known quantities of SR101. The fluorescent signal was linear (R > 0.999) in all experiments (data not shown). [3H]estrone-3-sulfate radioactivity was measured by liquid scintillation counting.
Immunofluorescence Staining of Human Choroid Plexus
Deidentified human CP tissue sections from normal brains were obtained from Northwest BioSpecimen (NWBioSpecimen, Seattle, WA). CP tissue were preserved as formalin-fixed, paraffin-embedded tissue blocks that were then cut into 4-μm sections and mounted on positively charged microscope glass slides. To prepare samples for immunofluorescence, slides were deparaffinized and rehydrated in xylene and a series of ethanol. Antigen retrieval was performed using 10 mM citrate buffer containing 0.05% Tween 20 (pH 6.0) for 15 minutes at 98°C. Afterward, tissues were maintained in low-flow, cold deionized water for 10 minutes to reform the antigenic sites. CP tissues were then treated with 0.1% Sudan Black B for 20 minutes to quench autofluorescence and subsequently treated with Image-iT FX Signal Enhancer (Thermo Fisher Scientific) for 15 minutes.
After washing, the tissues were blocked for 1 hour using a solution of 10% normal donkey serum (NDS) with 22.52 mg/ml glycine in PBS with Tween 20 (PBS-T) and then incubated overnight at 4°C in 1% NDS in PBS-T containing 2 μg/ml anti-OATP1A2 antibody (ab221804; Abcam) and 10 μg/ml anti-Na+/K+-ATPase (ab7671; Abcam). The specificity of the anti-OATP1A2 antibody was validated in OATP1A2 and empty vector transfected HEK293 cell lines (Supplemental Fig. 1). Negative controls were maintained in blocking buffer. The following day, tissues were washed and then incubated with 2.5% NDS in PBS-T containing 10 μg/ml Alexa Fluor 488-conjugated donkey anti-mouse (A21202; Invitrogen) and 10 μg/ml Alexa Fluor 555-conjugated donkey anti-rabbit (A31572; Invitrogen) for 2 hours at room temperature. The tissues were then washed, mounted (ab104139; Abcam), and coverslipped for subsequent confocal imaging.
Imaging of immunofluorescence stains was captured similarly with the following modifications: the Zeiss LSM 710 was fitted with a Zeiss 63×, NA 1.4 oil immersion objective (total magnification: 630×). 4′,6-diamidino-2-phenylindole (DAPI) stains were illuminated using a 405-nm laser diode equipped with an appropriate emission filter. Alexa Fluor 488-conjugated antibodies were illuminated using a 488-nm fixed wavelength argon laser. Alexa 555-conjugated antibodies were illuminated using a 561-nm diode-pumped solid-state laser. Appropriate corresponding dichroic and emission filters were used to detect the fluorescence emission. Confocal images were obtained as 19s scans at 512 × 512 resolution, 16 frames line averaged, with a pixel dwell of 1.27 μs and a pinhole of 60 μm. Tissue images were z-stacked using an average intensity projection approach. Linear brightness adjustments were performed consistently across immunofluorescence images in accordance with the Office of Research Integrity.
Statistical Analysis and Data Fitting
Uptake and inhibition studies were performed in triplicate and repeated three times independently. The data were fitted by nonlinear regression using GraphPad Prism 7.0 (GraphPad Software Inc., La Jolla, CA) to obtain graphs and kinetic parameters. The uptake kinetics data were fitted to the Michaelis-Menten equation. For the dose-dependent inhibition of transporter-mediated uptake of SR101, the IC50 was calculated using a four-parameter equation. Statistical significance was determined using an unpaired Student’s t test or a one-way ANOVA followed by Dunnett’s test as specified in the figure legends. A P value less than 0.05 was considered statistically significant.
Results
mRNA Expression of OATP/Slco 1a and 1b Isoforms in FVB Mouse CP
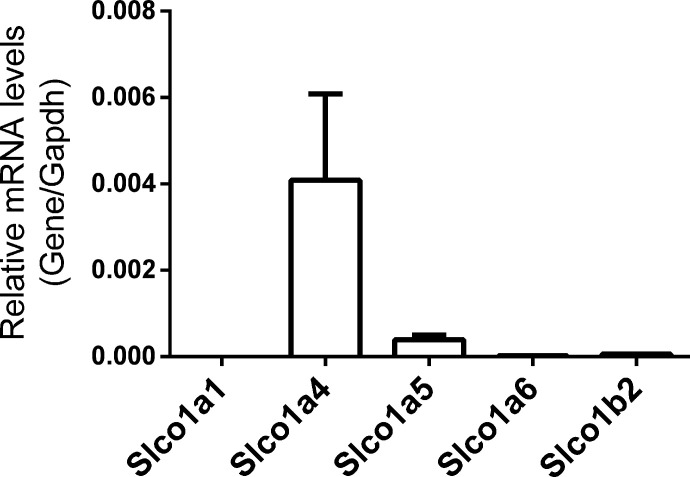
We first determined the mRNA expression of the rodent Slco 1a and 1b isoforms, encoding the OATP 1A and 1B proteins, in the CP of wild-type FVB mice using quantitative reverse-transcription polymerase chain reaction (RT-qPCR) (Fig. 1). FVB CPs express mRNA for Slco1a4 and Slco1a5, whereas expression of Slco1a1, Slco1a6, and Slco1b2 mRNA were minimal or undetectable. Although Slco1a4 is expressed at higher levels than Slco1a5, the OATP1A4 protein was previously localized to the basolateral membrane of CPE cells and hence may not contribute to apical uptake of large organic anions from the CSF (Gao et al., 1999). In addition, mRNA for Abcc1 and Abcc4 encoding basolateral efflux MRP transporters, along with the mRNAs for uptake transporters of the OATP/Slco family, Slco1c1 and Slco3a1, were highly expressed at the CPs of FVB mice (Supplemental Fig. 2). These results are consistent with our previous findings in the C57BL/6 strain (Hu et al., 2022).
Fig. 1.
Relative mRNA expression of OATP/Slco 1a/1b organic anion transporters in FVB mouse lateral ventricle CP tissues (n = 9, pooled groups of 3). Expression levels are normalized to the housekeeping gene Gapdh. Values are means ± S.D. across three pools of tissues, with each pool containing CP tissues from three mice.
Large Organic Anion Uptake from the CSF is Likely Mediated by OATP1A5 at the Mouse BCSFB
Based on RT-qPCR results and available localization data, we hypothesized that OATP1A5 may contribute to the uptake of organic anions from the CSF into CPE cells. To assess the contribution of OATPs toward organic anion uptake, CP tissues were incubated with SR101 in the absence or presence of bromosulphophthalein (BSP), a pan-OATP inhibitor (Fig. 2, A and B). SR101 was recently characterized as a fluorescent substrate for human OATP1A2 and OATP1C1 (Bakos et al., 2020). Representative graphical, differential interference contrast, and confocal images of mouse CP depicting morphologic structure and subepithelial accumulation of SR101 are shown in Supplemental Fig. 3. Real-time analysis showed that in wild-type CP, SR101 rapidly and primarily accumulates in the subepithelial space, demonstrating rapid transepithelial flux across the CPE cells (Fig. 3A). In age-matched Oatp1a/1b−/− mice (Fig. 2, C and D; Fig. 3B), subepithelial accumulation in the KO tissue is reduced by 66% (95% CI: 42% to 90%) compared with the wild-type (Fig. 3C). BSP reduced the subepithelial accumulation of SR101 by 92% (95% CI: 68% to 115%) in wild-type mice but had no effect in KO mice (Fig. 3C). No change in CPE cell accumulation was observed. These results suggest that OATP-mediated apical uptake of SR101 is abolished at the BCSFB in the Oatp1a/1b−/− animals. We then tested the transport of two additional OATP fluorescent substrates, FL-MTX and fluo-cAMP. We observed an 82% (95% CI: 38% to 126%) reduction of FL-MTX accumulation and an 90% (95% CI: 55% to 125%) reduction of fluo-cAMP accumulation in the subepithelial compartment in KO CP (Fig. 4). Taken together, the data demonstrated that members of the OATP1A and 1B family are responsible for apical uptake of large organic anions at the murine BCSFB. As OATP1B transporters are liver specific (Csanaky et al., 2011) and OATP1A5 is the only OATP1A member localized to the apical membrane in rodent CPE cells (Ohtsuki et al., 2003), OATP1A5 is likely the major mediator transporting large organic anions from CSF into CPE cells.
Fig. 2.
Representative confocal images of SR101 (2 μM) with or without BSP (100 μM) in CP obtained from wild-type (A and B) and Oatp1a/1b−/− (C and D) mice after 20 minutes. The fluorescent signal is primarily observed in the subepithelial compartment, indicating transepithelial flux from the CSF, across the CPE cells, and into the subepithelial compartment.
Fig. 3.
Representative time courses of quantified SR101 accumulation in CPE cell and subepithelial compartments from CP obtained from (A) wild-type and (B) Oatp1a/1b−/− mice. (C) Changes in subepithelial accumulation between treatment groups. Data are normalized to SR101 subepithelial accumulation in wild-type tissues. Values are means ± S.D. across three biologic replicates for all treatment groups unless otherwise noted. Statistical significance was determined using a one-way ANOVA followed by Dunnett’s test (*P < 0.05). †Values for [KO] + BSP are means ± S.D. across two biologic replicates
Fig. 4.
Representative confocal images of (A) FL-MTX (2 μM) and (B) fluo-cAMP (2 μM) in wild-type and (C) FL-MTX (2 μM) and (D) fluo-cAMP (2 μM) in Oatp1a/1b−/− CP tissue. (E) Changes in subepithelial accumulation between treatment groups. Data are normalized to subepithelial accumulation in wild-type tissues. Values are means ± S.D. across six biologic replicates for all treatment groups. Statistical significance was determined using an unpaired Student’s t test, not corrected for multiple comparisons (*P < 0.05).
SR101, FL-MTX, and Fluo-cAMP Are Substrates of Mouse OATP1A5 and Human OATP1A2
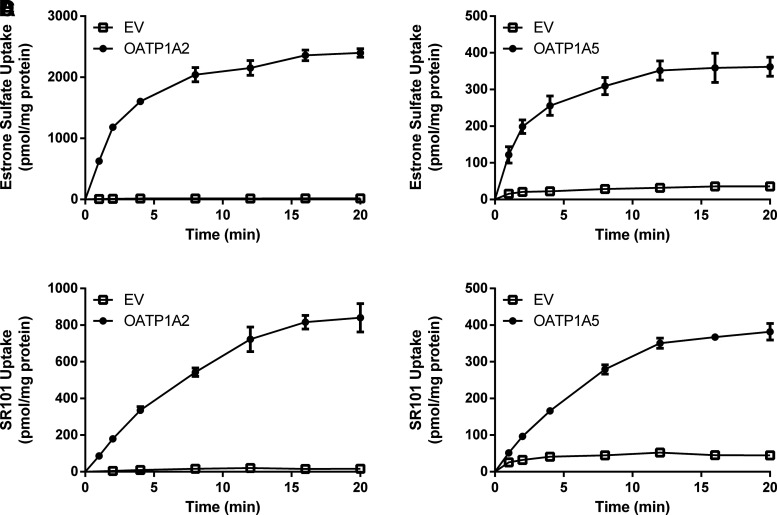
Although we have demonstrated strong evidence toward OATP1A5 function at the BCSFB, the molecular and functional properties of mouse OATP1A5 are poorly understood. We thus sought to characterize the transport properties of the fluorescent substrates used in our ex vivo CP experiments toward the mouse OATP1A5. Furthermore, we were interested in clarifying the functional similarity of mouse OATP1A5 and OATP1A2, the OATP1A homolog in humans. To address these questions, we generated HEK293 cell lines stably expressing mouse OATP1A5 and human OATP1A2. The uptake of FL-MTX, fluo-cAMP, SR101, and the classic OATP substrate estrone-3-sulfate was measured. Compared with the vector control, uptake of all four substrates increased in OATP1A5-expressing cells after a 20-minute incubation (Fig. 5), demonstrating that the substrates transported ex vivo in CP tissues are indeed substrates of OATP1A5. All of the substrates were also transported by OATP1A2, suggesting similar substrate selectivity between human OATP1A2 and mouse OATP1A5.
Fig. 5.
Uptake of (A) estrone-3-sulfate, (B) FL-MTX, (C) fluo-cAMP, and (D) SR101 in OATP1A2- and OATP1A5-overexpressing cell lines. Uptake of 1 μM substrate was measured in both transporter-expressing and empty vector–expressing HEK293 cells. Uptake was measured after 20 minutes of incubation at 37°C. Data are presented as the means ± S.D. from three independent experiments. The uptake in transporter-expressing cells was compared with empty vector (EV)-expressing cells. Statistical significance was determined by using an unpaired Student’s t test, not corrected for multiple comparisons (*P < 0.05, **P < 0.01).
Kinetic Characterization and Comparison of Estrone-3-Sulfate and SR101 Uptake by OATP1A5 and OATP1A2
The transport kinetics of estrone-3-sulfate and SR101 by OATP1A2 and OATP1A5 were further investigated. To determine the initial linear range of uptake by OATP1A2 and OATP1A5, time-dependent uptake of the two substrates was examined. Both compounds demonstrated linear uptake in the early time points before reaching a plateau by 20 minutes (Fig. 6). Transporter-mediated uptake was linear in the first 2 minutes for estrone-3-sulfate and the first 8–12 minutes for SR101 by OATP1A2 and OATP1A5. As a result, concentration-dependent kinetic studies were performed using a 2-minute incubation time for estrone-3-sulfate and a 4-minute incubation time for SR101. Transporter-mediated uptake of estrone-3-sulfate and SR101 displayed typical Michaelis-Menten kinetics for both OATP1A2 and OATP1A5 (Fig. 7). The Michaelis-Menten constant (Km) and Vmax were derived from nonlinear regression fitting and are summarized in Table 1. The Km values for estrone-3-sulfate and SR101 were very similar between OATP1A5 and OATP1A2, indicating a similar affinity for the studied substrates. We then performed a dose-dependent inhibition assay of SR101 uptake by BSP in both OATP1A2- and OATP1A5-expressing cell lines (Fig. 8). Assays were performed using a 4-minute incubation time at substrate concentrations below the Km. Consistent with the ex vivo CP inhibition data presented above, BSP inhibited the OATP1A5-mediated uptake of SR101 with an IC50 of 1.6 ± 0.4 μM. The IC50 for OATP1A2 is 15 ± 5.8 μM, 9.4-fold higher than that of OATP1A5.
Fig. 6.
Time course of 1 μM estrone-3-sulfate (A and B) and SR101 (C and D) uptake by OATP1A2 and OATP1A5. Time courses were performed independently three times, and results from one representative experiment are shown. Data points represent the means ± S.D. in triplicate.
Fig. 7.
Concentration-dependent uptake of estrone-3-sulfate (A and B) and SR101 (C and D) by OATP1A2 and OATP1A5. Transporter-specific uptake was obtained by subtracting the activity in control cells from the activity in transporter-expressing cells after a 2-minute incubation for estrone-3-sulfate and a 4-minute incubation for SR101. The data were fitted to a standard Michaelis-Menten equation for both compounds. Concentration dependent uptake was performed independently three times, and results from one representative experiment are shown. Data points represent the means ± S.D. in triplicate.
TABLE 1.
Kinetic parameters of estrone-3-sulfate and SR101 uptake by OATP1A2 and OATP1A5
Values shown in the table are means (S.D.) of the Km or Vmax from three independent experiments. Units: Km, μM; Vmax, pmol/mg protein/min.
| OATP1A2 | OATP1A5 | |||
|---|---|---|---|---|
| Km | Vmax | Km | Vmax | |
| Estrone-3-Sulfate | 15 ± 7 | 3100 ± 1500 | 17 ± 7 | 650 ± 140 |
| SR101 | 6.1 ± 0.5 | 830 ± 270 | 5.6 ± 4 | 49 ± 26 |
Fig. 8.
Dose-dependent inhibition of SR101 uptake in OATP1A2- and OATP1A5-overexpressing cells by BSP. Uptake of 1 μM SR101 in the absence and presence of BSP was measured in empty vector–, OATP1A2-, and OATP1A5-expressing HEK293 cells for 4 minutes. Transporter-specific uptake was obtained by subtracting the activity in empty vector cells from the activity in transporter-expressing cells. The results are expressed as a percentage of SR101 uptake in the absence of BSP and fitted to a four-parameter inhibition model. Each data point represents the means ± S.D. from three independent experiments.
Expression and Localization of OATP1A2 at the Human Blood-CSF Barrier
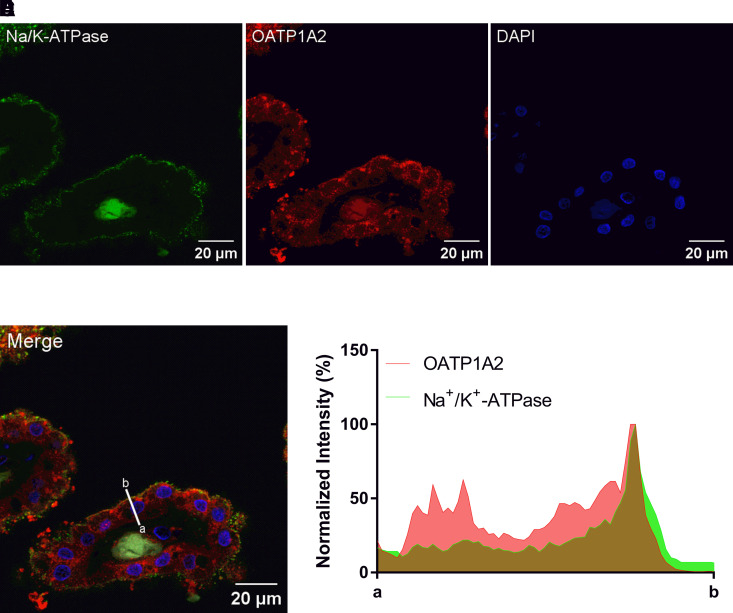
The above data suggest that large organic anion uptake from the CSF is likely mediated by Oatp1a5 at the rodent BCSFB, contributing to a clearance route for large organic anions from CSF to blood in rodents. However, it is currently unknown if a similar clearance pathway is present at the human BCSFB. Although OATP1A2 mRNA expression has been previously reported at the human BCSFB (Hu et al., 2022), its protein expression and localization are unknown. We therefore sought to determine the expression and localization of OATP1A2 protein at the human BCSFB through immunofluorescence staining of human CP sections using an OATP1A2 polyclonal antibody. As shown in Fig. 9, OATP1A2 protein is indeed expressed in human CP. Colocalization was observed with Na+/K+-ATPase, an established apical membrane marker in CPE cells (Praetorius and Nielsen, 2006; Kimura et al., 2007), suggesting OATP1A2 localizes to the apical membrane in human CP (Fig. 9, D and E). Besides apical staining, OATP1A2 signal was also observed intracellularly and possibly at the basolateral membrane. Minimal fluorescence signal was observed in CP tissue controls prepared without the primary antibodies (Supplemental Fig. 4). Expression and localization of OATP1A2 was consistent in the two human CP samples examined (Fig. 9; Supplemental Fig. 5). OATP1A2 signal was minimally observed in the ventricular ependyma (Supplemental Fig. 6), suggesting that the expression of OATP1A2 is enriched in CP relative to the surrounding tissue.
Fig. 9.
Immunofluorescence staining for (A) Na+/K+-ATPase, (B) OATP1A2, and (C) DAPI in human CP. (D) Merged image showing colocalization of OATP1A2 with apical Na+/K+-ATPase. (E) Normalized intensity of OATP1A2 and Na+/K+-ATPase fluorescence across the human CPE cell. Deidentified CP was sourced from a 60-year-old male.
Discussion
Previous work from our laboratory indicated that OATP transporters at the apical membrane of CPE cells work cooperatively with basolateral MRPs to mediate CSF-to-blood clearance of large amphipathic anions in mice. Several OATP/SLCO isoforms are expressed in rodent and human CP, but the specific contribution of individual OATP/SLCO to this process is unclear. In this study, we clarified the role of OATP1A/SLCO members toward organic anion uptake at the BCSFB using Oatp1a/1b−/− mice, investigated the functional overlap between mouse OATP1A5 and human OATP1A2, and established the presence of the OATP1A2 protein at the human BCSFB. Findings from our study provided novel insights into the molecular mechanisms underlying BCSFB clearance of large amphipathic organic anions from the CSF.
Our laboratory recently demonstrated that the apical uptake of FL-MTX and fluo-cAMP at the BCSFB is the rate-limiting step in the transepithelial transport process and plays an important kinetic role in the transport of large organic anions from the CSF into the blood (Hu et al., 2022; Sun and Wang, 2022). Apical uptake was highly sensitive to the OATP inhibitor rifampin (Hu et al., 2022; Sun and Wang, 2022). Consistent with our earlier study, we also detected OATP/Slco mRNA expression of Slco1a4/5, Slco1c1, and Slco3a1 in mouse CP tissues (Hu et al., 2022) (Fig. 1; Supplemental Fig. 2). OATP1C1 is a thyroid hormone transporter and is expressed basolaterally at the BCSFB, where it contributes to entry of thyroid hormones into the brain (Roberts et al., 2008; Mayerl et al., 2012). OATP3A1 is expressed on both membranes of the BCSFB but primarily transports prostaglandins (Huber et al., 2007). Thus, we hypothesized that members of the OATP1A subfamily are most likely to be involved in apical uptake of large fluorescent anions previously observed in live CP tissues. Indeed, transport of SR101, FL-MTX, and fluo-cAMP was abolished in CPs isolated from Oatp1a/1b−/− mice (Figs. 2 and 3). Further, SR101 transport in CP tissue of KO mice was not affected by BSP, which has been reported to inhibit rat OATP1C1 with an IC50 of 4.18 μM (Sugiyama et al., 2003), suggesting that OATP1C1 does not play a role in apical uptake of SR101. As OATP1A4 is localized basolaterally in rodent CP (Gao et al., 1999), our data using the Oatp1a/1b−/− model and RT-qPCR (Figs. 1, 3, and 4), alongside the reported apical localization of OATP1A5 (Ohtsuki et al., 2003), suggest that the CPE cell uptake of large organic anions is predominantly mediated by OATP1A5 in rodents (Fig. 10). Future studies using a single-gene knockout of Slco1a5 or an Slco1a5 knockin model on the Oapt1a/1b null background will further clarify the role of this transporter at the BCSFB.
Fig. 10.
Proposed roles of OATP and MRP transporters in BCSFB clearance at the rodent and human BCSFB. Models are based on data compiled from previous studies (Gao et al., 1999; Rao et al., 1999; Ohtsuki et al., 2003; Leggas et al., 2004) and the present study.
Efflux of large organic anions at the basolateral membrane of the CPE cell is mediated by MRP1/Abcc1 and MRP4/Abcc4, previously established in knockout models (Wijnholds et al., 2000; Leggas et al., 2004). The apical uptake is the rate-limiting step of large organic anion transport across the BCSFB in the tested compounds, indicating that the basolateral efflux clearance mediated by MRP1 and MRP4 is much greater than the apical uptake clearance likely mediated by OATP1A5. This is also consistent with the higher mRNA expression of Abcc1 and Abcc4 than Slco1a5 in mouse CP (Fig. 1; Supplemental Fig. 2). OATP1A4 is also localized basolaterally (Gao et al., 1999), but its function at the BCSFB has not been clearly understood. As OATPs are bidirectional transporters, OATP1A4 may contribute to some efflux of organic anions from the CPE cell to blood. Further studies utilizing an Slco1a4 knockout model and functional comparison of OATP1A4 with human OATP1A2 are needed to clarify the role of OATP1A4 at the BCSFB.
Outside of the mouse, studies have shown that the CP can actively uptake large organic anions in several other species. In rat CP, the fluorescent molecules FL-MTX, Texas Red, and fluo-cAMP were all transported across the tissue and accumulated in the subepithelial compartment (Breen et al., 2004; Reichel et al., 2008, 2010). Similar trends were also observed for Texas Red and FL-MTX in dogfish shark CP (Baehr et al., 2006; Reichel et al., 2008). Although mice have four members in the OATP1A subfamily, larger animals including humans only have one 1A member, OATP1A2. Regardless of this difference, the expression of OATP1A transporters at the BCSFB appears to be present across different animal species. OATP1A5 protein is very highly expressed in rat CP, and OATP1A2 protein is expressed in dog and pig CP (Uchida et al., 2015, 2020; Braun et al., 2017). Our laboratory has extended these findings into the human CP. We recently found OATP1A2 mRNA expression at the human BCSFB (Hu et al., 2022), and the data presented in this study demonstrate that OATP1A2 protein is also expressed, appearing to localize to the apical membrane. (Fig. 9). In this study, we also directly compared the transport by the poorly characterized mouse OATP1A5 and human OATP1A2 using overexpressing cells. We observed that FL-MTX, fluo-cAMP, SR101, and estrone-3-sulfate were all transported by both OATP1A5 and OATP1A2 (Fig. 5). Further kinetic studies revealed similar Km values for SR101 and estrone-3-sulfate between the human and mouse transporters (Table 1). The data suggest that mouse OATP1A5 and human OATP1A2 share functional similarity and could both mediate endobiotic and xenobiotic clearance at the BCSFB. Taken together, we thus postulate that, like rodents, apical OATP1A2 and basolateral MRP1/4 exist at the human BCSFB to mediate CSF-to-blood transport and clearance of large organic anions (Fig. 10). OATP1A2 expression is also observed intracellularly and possibly basolaterally, although further immunostaining is needed to validate this observation (Figs. 9 and 10).
A myriad of endobiotics and toxins are substrates of OATP1A2, and hence CSF concentrations of these compounds could be regulated by OATP1A2 at the BCSFB. For instance, dehydroepiandrosterone sulfate (DHEAS), a substrate of OATP1A2 (Kullak-Ublick et al., 1998), is an important neurosteroid and neutrophin, with multiple neurobiological effects in the brain. DHEAS acts as a modulator for GABAA, N-methyl-D-aspartate (NMDA), and other receptors in the brain (Maninger et al., 2009). Additionally, the steroid hormone is associated with neurogenesis and neuronal survival (Maninger et al., 2009). DHEAS is believed to be synthesized in the brain (Corpéchot et al., 1981), and OATP1A2 at the BCSFB may contribute to its clearance out of the CNS. OATP1A2 also transports the thyroid hormones thyroxine (T4) and triiodothyronine (T3), suggesting that this transporter may play a secondary role in regulating thyroid hormone levels in the CNS alongside of the prototype thyroid hormone transporters OATP1C1 and monocarboxylate transporter 8 (Mayerl et al., 2012; Roth et al., 2012).
The BCSFB remains an important but understudied blood-CNS interface for brain drug disposition. For some diseases, the CSF can be considered a directly relevant pharmacokinetic and pharmacodynamic compartment. This is the case for bacterial meningitis, where the bacteria replicate within the CSF after penetrating the CNS (Leib and Täuber, 1999). Interestingly, ceftriaxone, a cephalosporin antibiotic used to treat some forms of bacterial meningitis, has poor CSF penetration and is an OATP substrate (Lutsar and Friedland, 2000; Yamaguchi et al., 2011), which may allude to the contribution of OATP1A2 to its clearance from the CSF. OATP1A2 additionally transports a variety of other drugs that are therapeutically relevant in the CNS. Triptans, which have been identified as substrates in OATP1A2-transduced HEK293 cells, are used in the treatment of migraines and cluster headaches (Cheng et al., 2012). In addition, OATP1A2 transports a range of antivirals and antineoplastics (Roth et al., 2012). The CNS can often be a sanctuary site for human immunodeficiency virus (HIV) or malignant cancer cells, and OATP1A2 at the CP may reduce therapeutic drug concentrations at the CSF (Aragon-Ching and Zujewski, 2007; Whyte-Allman and Bendayan, 2020). Future studies may clarify the relevance of OATP1A2 toward the CNS disposition and pharmacokinetics of these drugs.
Understanding the role of OATP1A2 at the BCSFB may also be highly relevant for CNS-active drug candidates in development. The CSF is often the only clinically accessible CNS compartment to obtain information on the free drug concentrations in the human brain (de Lange, 2013b). However, numerous studies have suggested disconnects between CSF and unbound brain extracellular fluid drug concentrations, possibly due to transport processes at the BCSFB and blood-brain barrier. This has been observed for substrates of P-glycoprotein (P-gp), which is expressed luminally at the blood-brain barrier and apically or subapically at the BCSFB. For instance, Nagaya et al. (2020) demonstrated in monkeys that poor substrates of P-gp had a Kp,uu ratio between CSF and brain interstitial fluid close to unity, whereas good substrates may have CSF concentrations 3-fold or higher than brain interstitial fluid. Understanding mechanisms regulating CSF drug concentrations may help to fill the gaps in predicting unbound brain drug concentrations using mechanism-based modeling approaches such as physiologically based pharmacokinetic (PBPK) modeling.
In summary, we have clarified the molecular mechanism of OATP1A transport in rodent BCSFB and demonstrated the expression and apical localization of OATP1A2 protein at the human BCSFB. Our study suggests that at the BCSFB, organic anions in the CSF can be actively transported into CPE cells by apical OATP1A2 (OATP1A5 in rodents) followed by MRP1/4-mediated basolateral efflux into the blood. As this process can influence CSF drug concentrations and brain exposure to CNS active compounds, delineating the molecular mechanisms and the specific impact of OATP1A2 on anionic drug clearance from the CSF may help to improve the prediction of CNS pharmacokinetics and identification of drug candidates with favorable CNS pharmacokinetic properties.
Acknowledgments
The authors would like to thank Nathaniel Peters at the University of Washington Keck Microscopy Center for his help and insights in confocal microscopy practice and Vicky Sun for her assistance on the fluorescence quantification method development and the generation of OATP1A5-overexpressing cells.
Data Availability
All data supporting the study are available upon request.
Abbreviations
- BCSFB
blood-cerebrospinal fluid barrier
- BSP
bromosulphophthalein
- CNS
central nervous system
- CP
choroid plexus
- CPE
choroid plexus epithelial
- CSF
cerebrospinal fluid
- DHEAS
dehydroepiandrosterone sulfate
- FL-MTX
fluorescein methotrexate
- fluo-cAMP
8-fluorescein-cAMP
- HEK293
human embryonic kidney 293
- Km
Michaelis-Menten constant
- KO
knockout
- MRP/ABCC
multidrug resistance-associated protein
- NDS
normal donkey serum
- OATP/SLCO
organic anion transporting polypeptide
- PBS-T
PBS with Tween 20
- RT-qPCR
quantitative reverse-transcription polymerase chain reaction
- SR101
sulforhodamine 101
Authorship Contributions
Participated in research design: Sun, Wang.
Conducted experiments: Sun.
Contributed new reagents or analytic tools: Sun, Hagenbuch, Kelly.
Performed data analysis: Sun, Wang.
Wrote or contributed to the writing of the manuscript: Sun, Hagenbuch, Wang.
Footnotes
This work was supported in part by National Institutes of Health National Institute on Aging [Grant R21AG071827] (to J.W.) and National Center for Advancing Translational Sciences [Grant TL1TR002319] (to A.S.).
No author has an actual or perceived conflict of interest with the contents of this article.
 This article has supplemental material available at molpharm.aspetjournals.org.
This article has supplemental material available at molpharm.aspetjournals.org.
References
- Abdullahi W, Davis TP, Ronaldson PT (2017) Functional expression of P-glycoprotein and organic anion transporting polypeptides at the blood-brain barrier: understanding transport mechanisms for improved CNS drug delivery? AAPS J 19:931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragon-Ching JB, Zujewski JA (2007) CNS metastasis: an old problem in a new guise. Clin Cancer Res 13:1644–1647. [DOI] [PubMed] [Google Scholar]
- Baehr CH, Fricker G, Miller DS (2006) Fluorescein-methotrexate transport in dogfish shark (Squalus acanthias) choroid plexus. Am J Physiol Regul Integr Comp Physiol 291:R464–R472. [DOI] [PubMed] [Google Scholar]
- Bakos É, Német O, Patik I, Kucsma N, Várady G, Szakács G, Özvegy-Laczka C (2020) A novel fluorescence-based functional assay for human OATP1A2 and OATP1C1 identifies interaction between third-generation P-gp inhibitors and OATP1A2. FEBS J 287:2468–2485. [DOI] [PubMed] [Google Scholar]
- Braun C, Sakamoto A, Fuchs H, Ishiguro N, Suzuki S, Cui Y, Klinder K, Watanabe M, Terasaki T, Sauer A (2017) Quantification of transporter and receptor proteins in dog brain capillaries and choroid plexus: relevance for the distribution in brain and CSF of selected BCRP and P-gp substrates. Mol Pharm 14:3436–3447. [DOI] [PubMed] [Google Scholar]
- Breen CM, Sykes DB, Baehr C, Fricker G, Miller DS (2004) Fluorescein-methotrexate transport in rat choroid plexus analyzed using confocal microscopy. Am J Physiol Renal Physiol 287:F562–F569. [DOI] [PubMed] [Google Scholar]
- Cheng Z, Liu H, Yu N, Wang F, An G, Xu Y, Liu Q, Guan CB, Ayrton A (2012) Hydrophilic anti-migraine triptans are substrates for OATP1A2, a transporter expressed at human blood-brain barrier. Xenobiotica 42:880–890. [DOI] [PubMed] [Google Scholar]
- Corpéchot C, Robel P, Axelson M, Sjövall J, Baulieu EE (1981) Characterization and measurement of dehydroepiandrosterone sulfate in rat brain. Proc Natl Acad Sci U S A 78:4704–4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csanaky IL, Lu H, Zhang Y, Ogura K, Choudhuri S, Klaassen CD (2011) Organic anion-transporting polypeptide 1b2 (Oatp1b2) is important for the hepatic uptake of unconjugated bile acids: studies in Oatp1b2-null mice. Hepatology 53:272–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange ECM (2013a) The mastermind approach to CNS drug therapy: translational prediction of human brain distribution, target site kinetics, and therapeutic effects. Fluids Barriers CNS 10:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange ECM (2013b) Utility of CSF in translational neuroscience. J Pharmacokinet Pharmacodyn 40:315–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan H, Wang J (2010) Selective transport of monoamine neurotransmitters by human plasma membrane monoamine transporter and organic cation transporter 3. J Pharmacol Exp Ther 335:743–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan H, Wang J (2013) Impaired monoamine and organic cation uptake in choroid plexus in mice with targeted disruption of the plasma membrane monoamine transporter (Slc29a4) gene. J Biol Chem 288:3535–3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigin VL, Vos T, Nichols E, Owolabi MO, Carroll WM, Dichgans M, Deuschl G, Parmar P, Brainin M, Murray C (2020) The global burden of neurological disorders: translating evidence into policy. Lancet Neurol 19:255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B, Stieger B, Noé B, Fritschy J-M, Meier PJ (1999) Localization of the organic anion transporting polypeptide 2 (Oatp2) in capillary endothelium and choroid plexus epithelium of rat brain. J Histochem Cytochem 47:1255–1264. [DOI] [PubMed] [Google Scholar]
- Gribkoff VK, Kaczmarek LK (2017) The need for new approaches in CNS drug discovery: why drugs have failed, and what can be done to improve outcomes. Neuropharmacology 120:11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenbuch B, Stieger B (2013) The SLCO (former SLC21) superfamily of transporters. Mol Aspects Med 34:396–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu T, Zha W, Sun A, Wang J (2022) Live tissue imaging reveals distinct transcellular pathways for organic cations and anions at the blood-cerebrospinal fluid barrier. Mol Pharmacol 101:334–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber RD, Gao B, Sidler Pfändler M-A, Zhang-Fu W, Leuthold S, Hagenbuch B, Folkers G, Meier PJ, Stieger B (2007) Characterization of two splice variants of human organic anion transporting polypeptide 3A1 isolated from human brain. Am J Physiol Cell Physiol 292:C795–C806. [DOI] [PubMed] [Google Scholar]
- Hurley JV, Anderson RM, Sexton PT (1981) The fate of plasma protein which escapes from blood vessels of the choroid plexus of the rat--an electron microscope study. J Pathol 134:57–70. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Stopa EG, McMillan PN (2011) The blood-cerebrospinal fluid barrier: structure and functional significance. Methods Mol Biol 686:101–131. [DOI] [PubMed] [Google Scholar]
- Kalliokoski A, Niemi M (2009) Impact of OATP transporters on pharmacokinetics. Br J Pharmacol 158:693–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T, Allen PB, Nairn AC, Caplan MJ (2007) Arrestins and spinophilin competitively regulate Na+,K+-ATPase trafficking through association with a large cytoplasmic loop of the Na+,K+-ATPase. Mol Biol Cell 18:4508–4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullak-Ublick G-A, Fisch T, Oswald M, Hagenbuch B, Meier PJ, Beuers U, Paumgartner G (1998) Dehydroepiandrosterone sulfate (DHEAS): identification of a carrier protein in human liver and brain. FEBS Lett 424:173–176. [DOI] [PubMed] [Google Scholar]
- Leggas MAdachi MScheffer GLSun DWielinga PDu GMercer KEZhuang YPanetta JCJohnston B, et al. (2004) Mrp4 confers resistance to topotecan and protects the brain from chemotherapy. Mol Cell Biol 24:7612–7621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leib SL, Täuber MG (1999) Pathogenesis of bacterial meningitis. Infect Dis Clin North Am 13:527–548. [DOI] [PubMed] [Google Scholar]
- López Quiñones AJ, Wagner DJ, Wang J (2020) Characterization of Meta-iodobenzylguanidine (mIBG) transport by polyspecific organic cation transporters: implication for mIBG therapy. Mol Pharmacol 98:109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lun MP, Monuki ES, Lehtinen MK (2015) Development and functions of the choroid plexus-cerebrospinal fluid system. Nat Rev Neurosci 16:445–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutsar I, Friedland IR (2000) Pharmacokinetics and pharmacodynamics of cephalosporins in cerebrospinal fluid. Clin Pharmacokinet 39:335–343. [DOI] [PubMed] [Google Scholar]
- Maninger N, Wolkowitz OM, Reus VI, Epel ES, Mellon SH (2009) Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS). Front Neuroendocrinol 30:65–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayerl S, Visser TJ, Darras VM, Horn S, Heuer H (2012) Impact of Oatp1c1 deficiency on thyroid hormone metabolism and action in the mouse brain. Endocrinology 153:1528–1537. [DOI] [PubMed] [Google Scholar]
- Nagaya Y, Katayama K, Kusuhara H, Nozaki Y (2020) Impact of P-glycoprotein-mediated active efflux on drug distribution into lumbar cerebrospinal fluid in nonhuman primates. Drug Metab Dispos 48:1183–1190. [DOI] [PubMed] [Google Scholar]
- Ohtsuki S, Takizawa T, Takanaga H, Terasaki N, Kitazawa T, Sasaki M, Abe T, Hosoya K, Terasaki T (2003) In vitro study of the functional expression of organic anion transporting polypeptide 3 at rat choroid plexus epithelial cells and its involvement in the cerebrospinal fluid-to-blood transport of estrone-3-sulfate. Mol Pharmacol 63:532–537. [DOI] [PubMed] [Google Scholar]
- Praetorius J, Nielsen S (2006) Distribution of sodium transporters and aquaporin-1 in the human choroid plexus. Am J Physiol Cell Physiol 291:C59–C67. [DOI] [PubMed] [Google Scholar]
- Rao VV, Dahlheimer JL, Bardgett ME, Snyder AZ, Finch RA, Sartorelli AC, Piwnica-Worms D (1999) Choroid plexus epithelial expression of MDR1 P glycoprotein and multidrug resistance-associated protein contribute to the blood-cerebrospinal-fluid drug-permeability barrier. Proc Natl Acad Sci U S A 96:3900–3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel V, Kläs J, Fricker G, Masereeuw R (2010) Fluo-cAMP is transported by multidrug resistance-associated protein isoform 4 in rat choroid plexus. J Neurochem 115:200–208. [DOI] [PubMed] [Google Scholar]
- Reichel V, Miller DS, Fricker G (2008) Texas Red transport across rat and dogfish shark (Squalus acanthias) choroid plexus. Am J Physiol Regul Integr Comp Physiol 295:R1311–R1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts LM, Woodford K, Zhou M, Black DS, Haggerty JE, Tate EH, Grindstaff KK, Mengesha W, Raman C, Zerangue N (2008) Expression of the thyroid hormone transporters monocarboxylate transporter-8 (SLC16A2) and organic ion transporter-14 (SLCO1C1) at the blood-brain barrier. Endocrinology 149:6251–6261. [DOI] [PubMed] [Google Scholar]
- Roth M, Obaidat A, Hagenbuch B (2012) OATPs, OATs and OCTs: the organic anion and cation transporters of the SLCO and SLC22A gene superfamilies. Br J Pharmacol 165:1260–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin JArganda-Carreras IFrise EKaynig VLongair MPietzsch TPreibisch SRueden CSaalfeld SSchmid B, et al. (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama D, Kusuhara H, Taniguchi H, Ishikawa S, Nozaki Y, Aburatani H, Sugiyama Y (2003) Functional characterization of rat brain-specific organic anion transporter (Oatp14) at the blood-brain barrier: high affinity transporter for thyroxine. J Biol Chem 278:43489–43495. [DOI] [PubMed] [Google Scholar]
- Sun A, Wang J (2021) Choroid plexus and drug removal mechanisms. AAPS J 23:61. [DOI] [PubMed] [Google Scholar]
- Sun A, Wang J (2022) Evaluation of blood-CSF barrier transport by quantitative real time fluorescence microscopy. Pharm Res 39:1469–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida Y, Goto R, Takeuchi H, Łuczak M, Usui T, Tachikawa M, Terasaki T (2020) Abundant expression of OCT2, MATE1, OAT1, OAT3, PEPT2, BCRP, MDR1, and xCT transporters in blood-arachnoid barrier of pig and polarized localizations at CSF- and blood-facing plasma membranes. Drug Metab Dispos 48:135–145. [DOI] [PubMed] [Google Scholar]
- Uchida Y, Zhang Z, Tachikawa M, Terasaki T (2015) Quantitative targeted absolute proteomics of rat blood-cerebrospinal fluid barrier transporters: comparison with a human specimen. J Neurochem 134:1104–1115. [DOI] [PubMed] [Google Scholar]
- van de Steeg E, Wagenaar E, van der Kruijssen CMM, Burggraaff JEC, de Waart DR, Elferink RPJO, Kenworthy KE, Schinkel AH (2010) Organic anion transporting polypeptide 1a/1b-knockout mice provide insights into hepatic handling of bilirubin, bile acids, and drugs. J Clin Invest 120:2942–2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte-Allman S-K, Bendayan R (2020) HIV-1 sanctuary sites-the role of membrane-associated drug transporters and drug metabolic enzymes. AAPS J 22:118. [DOI] [PubMed] [Google Scholar]
- Wijnholds J, deLange ECM, Scheffer GL, van den Berg D-J, Mol CAAM, van der Valk M, Schinkel AH, Scheper RJ, Breimer DD, Borst P (2000) Multidrug resistance protein 1 protects the choroid plexus epithelium and contributes to the blood-cerebrospinal fluid barrier. J Clin Invest 105:279–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H, Takeuchi T, Okada M, Kobayashi M, Unno M, Abe T, Goto J, Hishinuma T, Shimada M, Mano N (2011) Screening of antibiotics that interact with organic anion-transporting polypeptides 1B1 and 1B3 using fluorescent probes. Biol Pharm Bull 34:389–395. [DOI] [PubMed] [Google Scholar]