Abstract
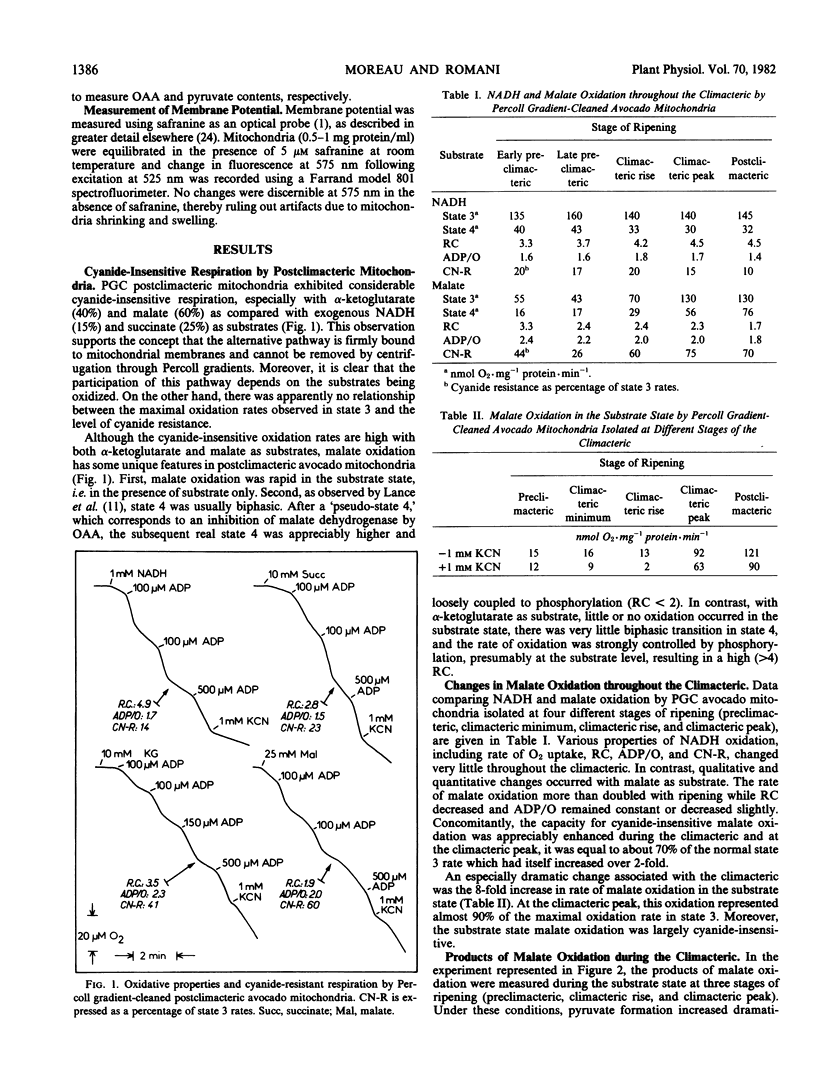
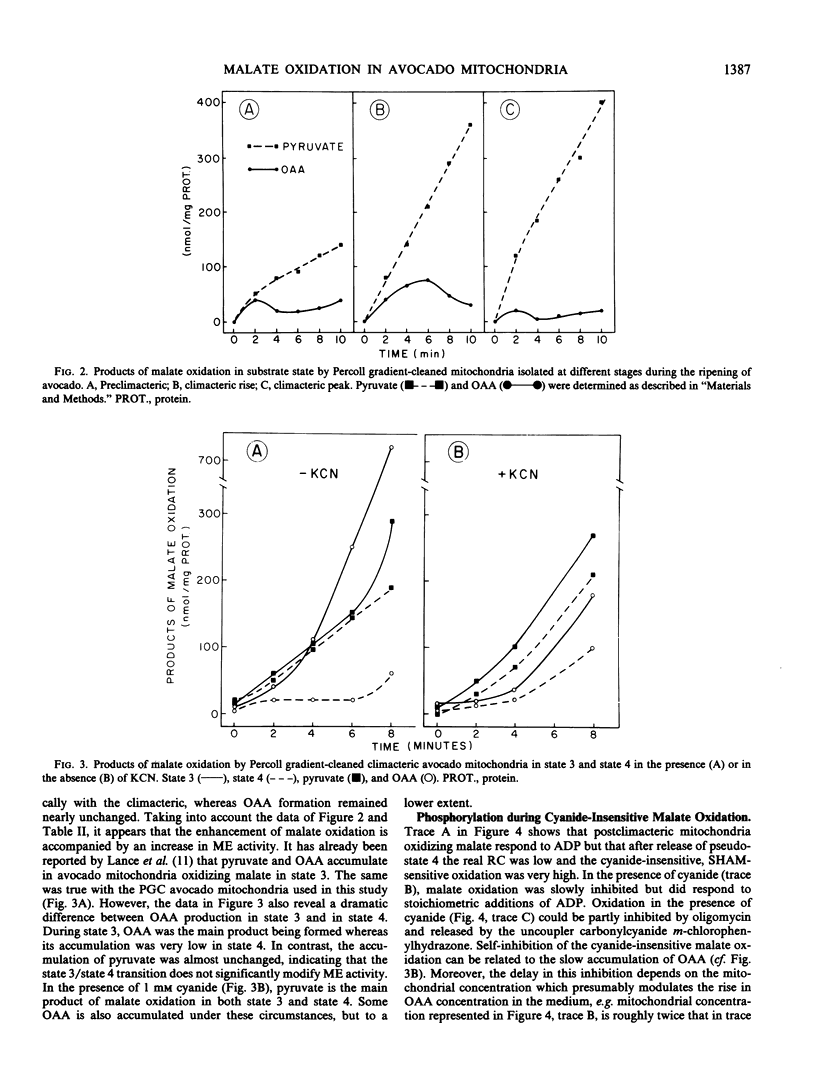
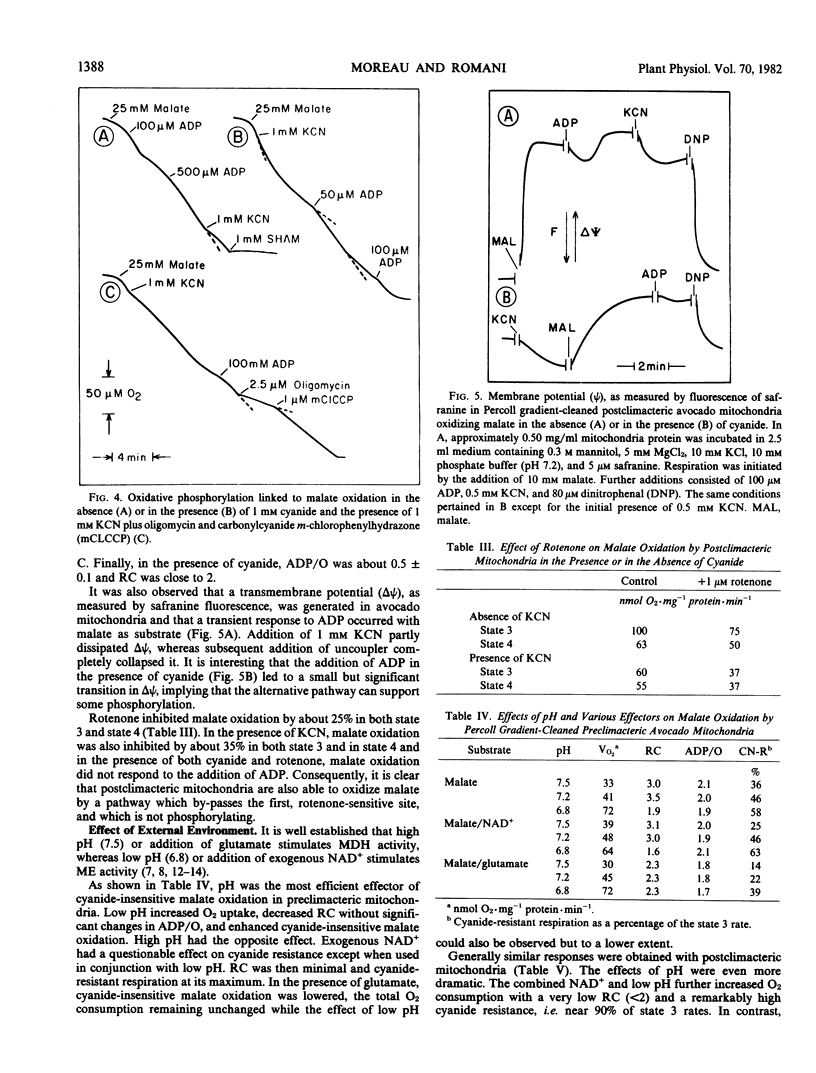
After preparation on self-generated Percoll gradients, avocado (Persea americana Mill, var. Fuerte and Hass) mitochondria retain a high proportion of cyanide-insensitive respiration, especially with α-ketoglutarate and malate as substrates. Whereas α-ketoglutarate oxidation remains unchanged, the rate of malate oxidation increases as ripening advances through the climacteric. An enhancement of mitochondrial malic enzyme activity, measured by the accumulation of pyruvate, closely parallels the increase of malate oxidation. The capacity for cyanide-insensitive respiration is also considerably enhanced while respiratory control decreases (from 3.3 to 1.7), leading to high state 4 rates.
Both malate dehydrogenase and malic enzyme are functional in state 3, but malic enzyme appears to predominate before the addition of ADP and after its depletion. In the presence of cyanide, a membrane potential is generated when the alterntive pathway is operating. Cyanide-insensitive malate oxidation can be either coupled to the first phosphorylation site, sensitive to rotenone, or by-pass this site. In the absence of phosphate acceptor, malate oxidation is mainly carried out via malic enzyme and the alternative pathway. Experimental modification of the external mitochondrial environment in vitro (pH, NAD+, glutamade) results in changes in malate dehydrogenase and malic enzyme activities, which also modify cyanide resistance. It appears that a functional connection exists between malic enzyme and the alternative pathway via a rotenone-insensitive NADH dehydrogenase and that this pathway is responsible, in part, for nonphosphorylating respiratory activity during the climacteric.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerman K. E., Wikström M. K. Safranine as a probe of the mitochondrial membrane potential. FEBS Lett. 1976 Oct 1;68(2):191–197. doi: 10.1016/0014-5793(76)80434-6. [DOI] [PubMed] [Google Scholar]
- Bahr J. T., Bonner W. D., Jr Cyanide-insensitive respiration. I. The steady states of skunk cabbage spadix and bean hypocotyl mitochondria. J Biol Chem. 1973 May 25;248(10):3441–3445. [PubMed] [Google Scholar]
- Bahr J. T., Bonner W. D., Jr Cyanide-insensitive respiration. II. Control of the alternate pathway. J Biol Chem. 1973 May 25;248(10):3446–3450. [PubMed] [Google Scholar]
- Coleman J. O., Palmer J. M. The oxidation of malate by isolated plant mitochondria. Eur J Biochem. 1972 Apr 24;26(4):499–509. doi: 10.1111/j.1432-1033.1972.tb01792.x. [DOI] [PubMed] [Google Scholar]
- Day D. A., Wiskich J. T. The oxidation of malate and exogenous reduced nicotinamide adenine dinucleotide by isolated plant mitochondria. Plant Physiol. 1974 Jan;53(1):104–109. doi: 10.1104/pp.53.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lance C., Hobson G. E., Young R. E., Biale J. B. Metabolic processes in cytoplasmic particles of the avocado fruit. IX. The oxidation of pyruvate and malate during the climacteric cycle. Plant Physiol. 1967 Apr;42(4):471–478. doi: 10.1104/pp.42.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lance C., Hobson G. E., Young R. E., Biale J. B. Metabolic processes in cytoplasmic particles of the avocado fruit. VII. Oxidative and phosphorylative activities throughout the climacteric cycle. Plant Physiol. 1965 Nov;40(6):1116–1123. doi: 10.1104/pp.40.6.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley R. M. Isolation of Functionally Intact Rhodoplasts from Griffithsia monilis (Ceramiaceae, Rhodophyta). Plant Physiol. 1981 Jan;67(1):5–8. doi: 10.1104/pp.67.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrae A. R., Moorhouse R. The oxidation of malate by mitochondria isolated from cauliflower buds. Eur J Biochem. 1970 Sep;16(1):96–102. doi: 10.1111/j.1432-1033.1970.tb01058.x. [DOI] [PubMed] [Google Scholar]
- McGahen J. W., Hoffmann C. E. Absence of mutagenic effects of 3- and 6-alkyl-5-bromouracil herbicides on a bacteriophage. Nature. 1966 Mar 19;209(5029):1241–1242. doi: 10.1038/2091241a0. [DOI] [PubMed] [Google Scholar]
- Moreau F., Romani R. Preparation of Avocado Mitochondria Using Self-Generated Percoll Density Gradients and Changes in Buoyant Density during Ripening. Plant Physiol. 1982 Nov;70(5):1380–1384. doi: 10.1104/pp.70.5.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuburger M., Douce R. Effect of bicarbonate and oxaloacetate on malate oxidation by spinach leaf mitochondria. Biochim Biophys Acta. 1980 Feb 8;589(2):176–189. doi: 10.1016/0005-2728(80)90036-5. [DOI] [PubMed] [Google Scholar]
- Rustin P., Moreau F., Lance C. Malate Oxidation in Plant Mitochondria via Malic Enzyme and the Cyanide-insensitive Electron Transport Pathway. Plant Physiol. 1980 Sep;66(3):457–462. doi: 10.1104/pp.66.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustin P., Moreau F. Malic enzyme activity and cyanide-insensitive electron transport in plant mitochondria. Biochem Biophys Res Commun. 1979 Jun 13;88(3):1125–1131. doi: 10.1016/0006-291x(79)91525-0. [DOI] [PubMed] [Google Scholar]
- Schonbaum G. R., Bonner W. D., Jr, Storey B. T., Bahr J. T. Specific inhibition of the cyanide-insensitive respiratory pathway in plant mitochondria by hydroxamic acids. Plant Physiol. 1971 Jan;47(1):124–128. doi: 10.1104/pp.47.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomos T., Laties G. G. Induction of ethylene of cyanide-resistant respiration. Biochem Biophys Res Commun. 1976 May 17;70(2):663–671. doi: 10.1016/0006-291x(76)91098-6. [DOI] [PubMed] [Google Scholar]
- Theologis A., Laties G. G. Respiratory Contribution of the Alternate Path during Various Stages of Ripening in Avocado and Banana Fruits. Plant Physiol. 1978 Aug;62(2):249–255. doi: 10.1104/pp.62.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin A., Djerdjour B., Journet E., Neuburger M., Douce R. Effect of NAD on Malate Oxidation in Intact Plant Mitochondria. Plant Physiol. 1980 Aug;66(2):225–229. doi: 10.1104/pp.66.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]



