Abstract
Cardiovascular disease represents a leading cause of death, morbidity, and societal economic burden. The prevalence of cannabis use has significantly increased due to legalization and an increased societal acceptance of cannabis. Therefore, it is critically important that we gain a greater understanding of the effects and risks of cannabinoid use on cardiovascular diseases as well as the potential for cannabinoid-directed drugs to be used as therapeutics for the treatment of cardiovascular disease. This review summarizes our current understanding of the role of cannabinoid receptors in the pathophysiology of atherosclerosis and myocardial ischemia and explores their use as therapeutic targets in the treatment of ischemic heart disease. Endocannabinoids are elevated in patients with atherosclerosis, and activation of cannabinoid type 1 receptors (CB1Rs) generally leads to an enhancement of plaque formation and atherosclerosis. In contrast, selective activation of cannabinoid type 2 receptors (CB2Rs) appears to exert protective effects against atherosclerosis. Endocannabinoid signaling is also activated by myocardial ischemia. CB2R signaling appears to protect the heart from ischemic injury, whereas the role of CB1R in ischemic injury is less clear. This narrative review serves to summarize current research on the role of cannabinoid signaling in cardiovascular function with the goal of identifying critical knowledge gaps and future studies to address those gaps in a way that facilitates the development of new treatments and better cardiovascular health.
SIGNIFICANCE STATEMENT
Cardiovascular diseases, including atherosclerosis and myocardial infarction, are a leading cause of death. Cannabinoid drugs have well known acute effects on cardiovascular function, including tachycardia and orthostatic hypotension. The recent legalization of marijuana and cannabinoids for both medical and recreational use has dramatically increased their prevalence of use. This narrative review on the role of cannabinoid signaling in cardiovascular disease contributes to a better understanding of this topic by integrating current knowledge and identifying critical gaps.
Introduction
Cardiovascular disease is the leading cause of death in the United States. More than 20 million people in the United States have cardiovascular disease, and 697,000 people died (20% of all deaths) from cardiovascular diseases in 2020 (Tsao et al., 2022). Approximately 805,000 people in the United States experience a heart attack each year (Tsao et al., 2022). Recent changes in state laws and increased societal acceptance of cannabis have significantly increased the prevalence of cannabis use in the United States despite its schedule I classification by the Drug Enforcement Administration. However, the cardiovascular risks of cannabinoid use and the potential for cannabinoid receptors to be used as therapeutic targets for the treatment of atherosclerosis, myocardial ischemia, and other cardiovascular disorders remain unclear. This review summarizes our current understanding of the role of cannabinoid receptors in the pathophysiology of atherosclerosis and myocardial ischemia and explores their use as therapeutic targets in the treatment of ischemic heart disease.
Endocannabinoid Signaling System
Although the medicinal and recreational effects of cannabis have been known for thousands of years and were reported by Chinese Emperor Shen Nung in 2737 BC, a clear understanding of the mechanisms responsible for the effects of cannabis on the human body have only emerged over the last 50 years. In 1964, pioneering work by Gaoni and Mechoulam (1965) identified delta-9-tetrahydrocannabinol (Δ9-THC) as the psychoactive component in hashish. Two cannabinoid receptors were identified in subsequent years. These cloned receptors include the neuronal cannabinoid type 1 receptor (CB1R), which is responsible for mediating the psychoactive effects of Δ9-THC and is highly expressed in the brain (Matsuda et al., 1990). A second cannabinoid type 2 receptor (CB2R) was cloned and is highly expressed in cells of the immune system (Munro et al., 1993). Both CB1R and CB2R are expressed in cardiovascular tissues, including the heart, smooth muscle, and endothelial cells of the vasculature (Liu et al., 2000, 2003; Pacher et al., 2005; Weis et al., 2010). These receptors are typically coupled to Gαi/o proteins in neurons where activation of presynaptic CB1R results in the activation of mitogen-activated protein kinases (MAPKs) and G protein–coupled inwardly rectifying potassium channels (GIRKs) (Mackie et al., 1995) as well as inhibition of adenylyl cyclase (Howlett and Fleming, 1984) and voltage-gated calcium channels (VGCCs) (Mackie and Hille, 1992). Similarly, cannabinoid agonists have been shown to activate MAPK signaling pathways, including p38 and c-Jun N-terminal kinases (JNKs), in cardiomyocytes and vascular endothelial cells (Mukhopadhyay et al., 2010; Rajesh et al., 2010) and decrease cAMP accumulation in the isolated rat heart (Krylatov et al., 2005).
Two primary endocannabinoids, N-arachidonoylethanolamine (AEA; anandamide) (Devane et al., 1992) and 2-arachidonoylglycerol (2-AG) (Sugiura et al., 1995; Stella et al., 1997), have been identified and exert effects on cardiac function through CB1R and CB2R (Pacher et al., 2005). Endocannabinoids are produced from plasma membrane phospholipids by endocannabinoid synthesizing enzymes, whereas endocannabinoid signaling is terminated by endocannabinoid hydrolysis enzymes (Piomelli, 2003). Synthesis of anandamide can occur through at least three separate biosynthetic pathways, whereas the majority of 2-AG is produced through the conversion of diaglycerol to 2-AG by sn-1-diaglyerol lipase alpha and beta (DAGLα/β) (Bisogno et al., 2003). Breakdown of AEA is mediated by fatty acid amide hydrolase (FAAH) (Cravatt et al., 1996), whereas 2-AG is hydrolyzed predominately through the activity of monoacylglycerol lipase (MAGL) (Dinh et al., 2002). These biosynthetic and metabolic pathways may provide opportunities for the development of therapeutic targets.
Cannabinoid Signaling in Cells of the Cardiovascular System
The adult myocardium is composed of approximately 56% cardiomyocytes, 27% fibroblasts, 10% vascular smooth muscle cells, and 7% endothelial cells (Banerjee et al., 2007). Likewise, the vasculature is also composed of multiple cell types, including vascular smooth muscle cells, endothelial cells, fibroblasts, and perivascular adipocytes. In addition, macrophages can be found throughout the cardiovascular system. CB1R and CB2R are found in many of these cell types, where they mediate the effects of both endogenous and exogenous cannabinoids.
Cardiomyocytes
CB1R and CB2R are expressed at very low levels in the mouse and human myocardium (Rajesh et al., 2012, 2022; Valenta et al., 2018). However, expression of these receptors is significantly upregulated under some pathologic conditions, including obesity (Valenta et al., 2018), heart failure (van Esbroeck et al., 2020) cardiomyopathy (Matyas et al., 2020), and type I diabetes (Rajesh et al., 2022). Under conditions of doxorubicin-induced cardiomyopathy (Mukhopadhyay et al., 2010) or diabetic cardiomyopathy (Rajesh et al., 2012), CB1R signaling promotes increased oxidative/nitrosative stress, activation of p38 and JNK kinases, and increased apoptosis and cardiomyocyte death. CB1R receptor signaling also decreases isoproterenol and forskolin-induced cAMP levels (Liao et al., 2013) and suppresses the activation of L type calcium channels in isolated cardiomyocytes (Li et al., 2009). CB1R signaling results in suppression of cardiac contractile function (Bonz et al., 2003). CB2R has been reported to protect isolated cardiomyocytes from oxidative stress and to protect the heart from ischemia/reperfusion injury (Defer et al., 2009). However, CB2R has little or no impact on cardiomyocyte function under basal conditions.
Vascular Smooth Muscle Cells
CB1R and CB2R have opposing roles in regulating vascular smooth muscle cells. CB1R activation promotes smooth muscle cell proliferation and migration in vitro (Rajesh et al., 2008b) and also increases smooth muscle cell proliferation in vivo after carotid balloon injury (Molica et al., 2013). In contrast, CB2R activation suppresses smooth muscle cell proliferation after balloon injury and also suppresses proinflammatory signaling pathways [Ras, p38, MAPK, extracellular signal-regulated kinase (ERK), JNK, and protein kinase B (Akt)] in smooth muscle cells (Rajesh et al., 2008a). These data suggest that CB1R blockade or CB2R stimulation may provide novel therapeutic strategies to suppress vascular smooth muscle cell proliferation, stenosis, and vascular remodeling.
CB1R located in vascular smooth muscle does not play a major role in regulating blood pressure. Wang et al. (2022) recently reported that mice in which CNR1 was deleted from vascular smooth muscle exhibit no changes in basal blood pressure. However, these animals had significantly larger infarct volumes after ischemic stroke (Wang et al., 2022). These data indicate that CB1R expression in vascular smooth muscle is protective (perhaps via CB1R-mediated vasodilation) under conditions of cerebral ischemia. Previous work demonstrated that CB1R on cerebral vascular smooth muscle cells regulates the tone of cerebral arteries by decreasing calcium influx and promoting cerebral vasodilation (Gebremedhin et al., 1999). Others have suggested that cannabinoid receptors may play a role in cerebral vascular dysfunction associated with subarachnoid hemorrhage and traumatic brain injury (Benyó et al., 2016). The cerebrovascular actions of endocannabinoids and cannabinoid receptors were recently the topic of a detailed review (Benyó et al., 2016).
Endothelial Cells
Multiple studies have demonstrated that CB1R stimulation promotes inflammation in the vasculature. CB1R signaling in coronary artery endothelial cells is coupled to proinflammatory signaling pathways [p38, JNK, nuclear factor kappa B (NF-κB)], increased generation of reactive oxygen species, apoptosis, and cell death (Rajesh et al., 2007, 2010). More recent work demonstrated that Δ9-THC concentration dependently decreases cell viability in cultured endothelial cells and increases the synthesis of proinflammatory cytokines and production of reactive oxygen species (nitric oxide synthase-2 and NADPH oxidase) (Wei et al., 2022). These effects of CB1R signaling lead to dysfunction of vascular endothelial cells in vivo. These Δ9-THC–induced changes were blocked by CRISPR-based suppression of CB1R expression and by CB1R blockade with genistein (Wei et al., 2022), demonstrating that CB1R plays an important role in Δ9-THC–induced endothelial inflammation. This is consistent with endothelial dysfunction that occurs after exposure to marijuana smoke (Wang et al., 2016; Wei et al., 2022). CB1R signaling also enhances neointima formation after balloon-induced injury and promotes the formation of atherosclerotic lesions in apoprotein E knockout mice (Molica et al., 2013). These proinflammatory and proatherosclerotic effects of CB1R signaling are consistent with the identification of cannabis use as a risk factor for the cardiovascular disease (Skipina et al., 2022b).
In contrast to CB1R, CB2R signaling suppresses atherosclerosis by attenuating the endothelial expression of cell adhesion molecules that enable monocytes to migrate through the endothelium (Rajesh et al., 2007; Zhao et al., 2010b). CB2R signaling also inhibits endothelial production of monocyte chemoattractant protein-1 and monocyte binding to cultured human coronary artery endothelial cells (Rajesh et al., 2007). CB2R agonists also suppress tumor necrosis factor alpha (TNF-α)-induced activation of a variety of proinflammatory signaling proteins (Ras, p38, ERK, JNK, and Akt) in endothelial cells (Rajesh et al., 2008a). Thus, CB2R suppresses inflammatory processes in endothelial cells that contribute to cardiovascular disease.
Fibroblasts
CB1R signaling enhances interstitial fibrosis in the heart and worsens cardiac dysfunction under a variety of pathologic conditions, including diabetes (Rajesh et al., 2012), doxorubicin-induced cardiomyopathy (Mukhopadhyay et al., 2010), and after myocardial infarction (Slavic et al., 2013). Fibrosis is alleviated under these conditions by genetic deletion or pharmacological blockade of CB1R (Rajesh et al., 2007, 2012; Slavic et al., 2013). Rimonabant blocks interleukin-1–induced upregulation of matrixmetalloprotease-9 in isolated cardiac fibroblasts and decreases hydroxyproline and collagen content in the heart after ischemic injury (Slavic et al., 2013). These CB1R signaling events occurring in fibroblasts are associated with detrimental cardiac remodeling.
CB2R signaling in fibroblasts plays a protective role in the heart. Diabetes-induced cardiac fibrosis is attenuated by CB2R agonists and enhanced by genetic deletion of CB2R (Rajesh et al., 2022). CB2R also decreases collagen and fibronectin synthesis in the heart after an ischemic insult (Li et al., 2016). CB2R also suppressed collagen secretion, Akt phosphorylation, and oxidative stress in isolated cardiac fibroblasts after hypoxic injury (Li et al., 2016). Thus, CB1R and CB2R have opposing roles in the regulation of cardiac fibroblast function.
Macrophages
Macrophages express both CB1R and CB2R. CB1R activation on macrophages potentiates NLRP3-mediated inflammation (Jourdan et al., 2013), increases the production of proinflammatory cytokines (TNF-α, interleukin-6, MCP-1) (Mai et al., 2015), and promotes macrophage migration (Mai et al., 2015). In contrast, CB2R signaling suppresses inflammation (Denaës et al., 2016; Kumawat and Kaur, 2023) and may protect the vasculature against the formation of atherosclerotic lesions. Oxidized low-density lipoprotein stimulates the production of endocannabinoids and the expression of both CB1R and CB2R in macrophages (Jiang et al., 2009), and CB1R promotes the accumulation of intracellular cholesterol in macrophages (Jiang et al., 2009). Thus, inflammatory processes are enhanced by CB1R signaling and attenuated by CB2R signaling in macrophages.
Acute Cardiovascular Effects of Cannabinoids
Studies have demonstrated that Δ9-THC acts as a partial agonist at CB1R and CB2R, whereas the endocannabinoid 2-AG and many synthetic cannabinoids such as CP55,940 and WIN55,212-2 are full agonists at these receptors (Govaerts et al., 2004). Research done in the 1970s demonstrated that oral and inhaled administration of Δ9-THC or cannabis produces a number of acute cardiovascular effects, including dose-dependent tachycardia (Renault et al., 1971), reduced peripheral vascular resistance (Benowitz et al., 1979), and episodes of orthostatic hypotension (Benowitz and Jones, 1975) and syncope (Benowitz and Jones, 1975). Since these early studies, the Δ9-THC content in cannabis has increased dramatically from ∼3% to 6% in the 1970s to greater than 30% in cannabis strains that are available for recreational and medical use today (ElSohly et al., 2016; Pennypacker et al., 2022). Additional research from more recent studies also demonstrates robust acute effects of oral Δ9-THC or inhaled cannabis containing Δ9-THC on cardiovascular function, including blood pressure and heart rate (Martin-Santos et al., 2012; Kayser et al., 2020). These effects of Δ9-THC on heart rate that have been observed in adults have also been found to occur in adolescents (Murray et al., 2022). Pharmacokinetic and pharmacodynamic modeling found that the half-life for the effect of Δ9-THC on elevated heart rate is ∼8 minutes (Strougo et al., 2008). These studies demonstrate that Δ9-THC rapidly increases heart rate and can decrease blood pressure in adults and adolescents.
Early work suggested that 18–20 days of oral Δ9-THC administration results in a decrease in the magnitude of orthostatic hypotension, possibly due to the development of tolerance (Benowitz and Jones, 1975). However, more recent work in a carefully controlled inpatient setting found that tolerance developed to the subjective “high” effects of oral Δ9-THC but that tolerance was not observed for the cardiovascular effects (Gorelick et al., 2013). Multiple studies have unequivocally demonstrated that CB1R antagonists such as rimonabant, surinabant, or AVE1625 block the cardiovascular effects of Δ9-THC (Gorelick et al., 2006; Huestis et al., 2007; Zuurman et al., 2010; Klumpers et al., 2013). It is unclear why tolerance develops to the psychoactive effects produced by CB1R signaling but does not develop to the CB1R-mediated cardiovascular effects.
Cannabinoid-induced tachycardia increases myocardial oxygen demand, and exercise tolerance is decreased in patients with angina after inhalation of a single marijuana cigarette (Aronow and Cassidy, 1974). A case crossover study of 124 patients admitted for myocardial infarction found that inhaled cannabis transiently increases the relative risk of myocardial infarction by 4.8-fold for 1 hour after cannabis consumption (Mittleman et al., 2001). Cannabis consumption has also been found to cause transient changes in the electrocardiogram, including ST segment elevation, T wave flattening and inversions, increased P wave width, and decreased P wave amplitude (Beaconsfield et al., 1972; Kochar and Hosko, 1973). These electrophysiological changes are consistent with case reports of cardiac arrhythmias associated with cannabis smoking (Singh, 2000).
Since the actions of CB1R agonists (including Δ9-THC) in the brain cause psychoactive effects that are associated with substance abuse, the development of peripherally restricted agonists represents a drug discovery approach that might circumvent abuse liability associated with direct-acting CB1R agonists. AZD1940 and AZD1704 are two peripherally restricted CB1R agonists that were developed by AstraZeneca as novel pain therapeutics. However, the use of these compounds failed in clinical trials due to serious cardiovascular and metabolic side effects, including heart rate changes, hypotension, weight gain, and liver toxicity (Kalliomäki et al., 2013; Pacher et al., 2018). Some synthetic cannabinoids are 100 times more potent than Δ9-THC at CB1R, suggesting that the adverse cardiovascular effects of these synthetic cannabinoids are likely to be much more severe than those of Δ9-THC (Marusich et al., 2022). These cardiometabolic effects represent a significant challenge to the therapeutic use of CB1R agonists.
Under pathologic conditions involving shock (such as myocardial infarction, endotoxin exposure, and liver cirrhosis), endocannabinoids acting at vascular CB1R have been shown to contribute to vasodilation and hypotension in rodent models (Wagner et al., 1997, 2001; Varga et al., 1998). As described earlier in this review, both AEA and 2-AG are rapidly degraded by hydrolytic enzymes to terminate signaling. Degradation of AEA by FAAH produces ethanolamine and arachidonic acid (AA), an important precursor in the production of eicosanoids involved in inflammation, vasodilation, and vasoconstriction, including prostaglandins, epoxyeicosatrienoic acids, hydroxyeicosatetraenoic acids, and leukotrienes (Maccarrone, 2017; Marusich et al., 2022). AA can also be generated by degradation of 2-AG by MAGL, which causes the production of AA and glycerol (Maccarrone, 2017). These AA-derived signals can exert anti- or proinflammatory effects on the cardiovascular system that are not mediated by CB1R or CB2R and can be either cardioprotective or damaging depending on the context and the specific AA-derived signaling molecule produced (Pacher et al., 2018; Beccacece et al., 2023).
Atherosclerosis
Role of the Endocannabinoid System in Atherosclerosis
The process by which atherosclerosis develops was the subject of a recent review (Jebari-Benslaiman et al., 2022). Atherosclerotic plaque formation is initiated when low-density lipoprotein (LDL) that is trapped beneath the endothelium becomes oxidized. Oxidized LDL stimulates the synthesis of cell adhesion molecules on the overlying endothelial cells, which subsequently attract monocytes and macrophages. These immune cells move through the endothelium into the subendothelial space where they engulf oxidized LDL, resulting in the formation of lipid-filled “foam cells.” The attraction of additional macrophages and neutrophils results in the formation of a “necrotic core,” which becomes covered by a fibrous cap that is composed primarily of smooth muscle cells and collagen. The resulting atheroma is prone to rupture, resulting in platelet aggregation, activation of blood clotting proteins, and potential occlusion of the vessel.
Endocannabinoids have been implicated in multiple steps of atherogenesis (Fig. 1). Jehle et al. (2016) reported that deletion of DAGLα (the enzyme that produces 2-AG in macrophages) decreased the formation of atherosclerotic plaque and infiltration of macrophages into arterial walls. Consistent with this observation, increasing endogenous 2-AG concentrations in vascular tissues, either by genetic deletion or pharmacological inhibition of MAGL, resulted in increased plaque formation and an increase in monocyte and macrophage infiltration into the vessel wall (Vujic et al., 2016; Jehle et al., 2018). Enhancement of anandamide concentrations in mice, either by genetic deletion of FAAH (Lenglet et al., 2013) or by FAAH inhibition using URB597 (Hoyer et al., 2014), resulted in the formation of plaques with increased neutrophil infiltration, increased matrix metalloproteinase 9 expression, and decreased collagen content. These changes in plaque composition resulted in plaques that were more vulnerable to rupture compared with plaques that developed in mice with normal anandamide concentrations. Finally, elevated levels of circulating anandamide and 2-AG in patients with coronary artery disease (compared with patients without coronary artery disease) suggest a role for endocannabinoids in atherosclerotic plaque formation (Sugamura et al., 2009).
Fig. 1.
Role of endocannabinoids and CB1R signaling in atherosclerotic disease. Increasing levels of 2-AG or AEA by inhibiting their hydrolytic enzymes resulted in increased atherosclerotic plaque formation and infiltration of macrophages into arterial walls. Δ9-THC exposure induces the formation of atherosclerotic lesions in the vasculature, whereas inhibition or genetic deletion of CB1R has protective effects on the severity of atherosclerotic plaque formation.
Role of CB1R
CB1R expression is upregulated in monocytes during their differentiation into macrophages, and activation of CB1R promotes the release of proinflammatory cytokines, including interleukin-1β, interleukin-8, and TNF-α (Sugamura et al., 2009). Recent work assessing blood samples from recreational marijuana smokers confirmed a role for CB1R in marijuana-induced increases in proinflammatory cytokines (Wei et al., 2022). Δ9-THC exposure also induces endothelial cell dysfunction, oxidative stress, inflammation, and the formation of atherosclerotic lesions in the vasculature (Wei et al., 2022). These effects of Δ9-THC were attenuated by CB1R blockade with genistein, small interfering RNA (siRNA)-induced knockdown of CB1R, and CRISPR-mediated deletion of CB1R expression (Wei et al., 2022), implicating CB1R in these Δ9-THC–induced vascular changes.
Studies using atherosclerotic-prone mouse models also point to a role of CB1R in atherogenesis. Rimonabant inhibits the formation of atherosclerotic lesions and attenuates the production of proinflammatory cytokines in LDL receptor knockout (KO) mice fed a western diet (Dol-Gleizes et al., 2009). Rimonabant also decreased atherosclerotic lesion development and produced favorable changes in the serum lipid profile [decreased plasma triglycerides, increased high-density lipoprotein (HDL) cholesterol] in dyslipidemic ApoE3-Leiden-cholesteryl ester transfer protein (CETP) mice fed a western diet (van Eenige et al., 2021).
Human studies provide further evidence for the involvement of CB1R in atherosclerosis. CB1R expression is upregulated in human coronary atheromas isolated from coronary arteries of patients with unstable angina when compared with atheromas from patients with stable angina (Sugamura et al., 2009). This is consistent with the finding that CB1R expression was increased in unstable lipid-rich plaques that are more prone to rupture compared with stable fibrous plaques (Sugamura et al., 2009). A randomized, double-blind clinical trial found that rimonabant improved cardiometabolic risk factors such as body weight and waste circumference in obese patients while producing favorable changes in lipid profile (increased HDL and decreased triglyceride levels) and decreased C reactive protein levels (Nissen et al., 2008). These data suggest a facilitative role for CB1R in vascular inflammation and atherosclerosis and raise the possibility that peripherally restricted CB1R antagonists, without psychiatric side effects associated with action at CB1R in the brain, might be a possible therapeutic option for slowing the progression of atherosclerosis.
Role of CB2R
In contrast to the proatherogenic effects of CB1R signaling (Fig. 1), activation of CB2R suppresses atherogenesis (Fig. 2). Zhao et al. (2010b) reported that activation of CB2R suppresses the expression of cell adhesion molecules such as intercellular adhesion molecule 1, vascular cell adhesion molecule 1, and P selectin in endothelial cells. This decreased the infiltration of macrophages into the vascular wall and significantly reduced atherosclerotic plaque formation in the mouse aorta (Zhao et al., 2010b). Other work found that treatment with WIN55212-2, a mixed CB1R/CB2R agonist, decreased the number of macrophages, decreased NF-κB signaling, and decreased the expression of proinflammatory genes in atherosclerotic plaques from ApoE−/− mice. Importantly, these effects of WIN55,212-2 on plaque formation and macrophage infiltration were blocked by AM630, suggesting that they were mediated through CB2R (Zhao et al., 2010a). Furthermore, genetic deletion of CB2R in atherosclerosis-prone ApoE−/− mice resulted in increased infiltration of leukocytes into atherosclerotic lesions and increased the production of reactive oxygen species in the arterial wall of mice fed a high-cholesterol diet (Hoyer et al., 2011). In addition, CB2R in circulating immune cells suppresses immune cell infiltration into atherosclerotic lesions (Hoyer et al., 2011). Also, Steffens et al. (2005) identified CB2R in macrophages and T lymphocytes in atherosclerotic plaques from apoprotein E knockout mice. They found that orally administered Δ9-THC suppressed the progression of atherosclerotic plaques in these animals and that this effect was blocked by a CB2R-selective antagonist (SR144528). Finally, CB2R signaling alters the extracellular matrix composition of atherosclerotic plaques such that they have greater collagen content, decreased smooth muscle content, and increased structural stability (Netherland et al., 2010). Taken together, these studies suggest that CB2R signaling exerts protective effects on atherosclerosis by suppressing the recruitment of immune cells in the vascular wall and by promoting changes in plaque composition that increase stability and decrease the likelihood of atherosclerotic lesion rupture. This raises the possibility that CB2R-selective agonists might be useful therapeutic agents to slow the progression of atherosclerosis. This possibility is intriguing since CB2R agonists do not exert negative neuropsychiatric side effects due to limited expression of this receptor in the brain. To date, no human clinical trials have assessed the effect of CB2R-selective agonists on atherosclerosis. Human clinical trials should be performed to address this critical gap in our understanding surrounding a therapeutic role for CB2R signaling in the development of atherosclerosis in humans.
Fig. 2.
Role of CB2R in atherosclerotic disease. Activation of CB2R signaling exerts protective effects on atherogenesis by suppressing the infiltration of macrophages into the vascular wall. Genetic deletion or pharmacological inhibition of CB2R in atherosclerosis-prone ApoE−/− mice increased infiltration of leukocytes into atherosclerotic lesions.
Impact of Recreational Cannabinoids on Atherosclerosis
The impact of recreational cannabis use on atherosclerosis-related cardiovascular disease was recently the topic of a detailed review (Pacher et al., 2018). Some investigators have found that cannabis use is associated with an increase in atherosclerotic cardiovascular risk score (Skipina et al., 2022a) and an elevated risk for atherosclerosis-related cardiovascular disorders, including myocardial infarction (Chami and Kim, 2019; Wei et al., 2022), acute coronary syndrome (Richards et al., 2019), stroke (Hemachandra et al., 2016; Zhao et al., 2021), transient ischemic attack (Hemachandra et al., 2016), and chronic cardiovascular diseases (Richards et al., 2019). In contrast, other work has found no association between cannabis use and clinical markers of atherosclerosis such as carotid intima-media thickness (Jakob et al., 2021) and coronary or abdominal aorta calcium scores (Auer et al., 2018) and no association between marijuana use and stroke, transient ischemic attacks, coronary artery disease, or cardiovascular mortality (Reis et al., 2017; Dutta et al., 2021). Mahtta et al. (2021) reported that recreational use of alcohol, tobacco, amphetamine, and cannabis were each independently associated with early-onset atherosclerotic cardiovascular disease, suggesting that the increased risk of cardiovascular disease may result from other lifestyle factors associated with recreational drug use. Indeed, the American Heart Association recently published a position statement that current evidence on cardiovascular outcomes associated with recreational cannabis use is inconclusive. This report cited confounding limitations in the analysis and interpretation of current clinical research on the cardiovascular impact of cannabis use, including concurrent use of cannabis with tobacco and other recreational drugs, variations in cannabinoid content in cannabis, and biases associated with the use of hospitalized cannabis users (and hospitalized nonuser control patients) (Page et al., 2020). The scheduling of cannabis as a schedule I controlled substance has made it difficult for researchers to conduct appropriately controlled and sufficiently powered clinical studies. Most federally funded research using cannabis in the United States has used strains containing between 2% and 10% Δ9-THC cultivated at the University of Mississippi National Center for Natural Products Research. These strains are not necessarily representative of strains containing 20%–30% Δ9-THC that are commonly used by recreational cannabis users. The cardiovascular impact of the consumption of cannabis strains that have high Δ9-THC concentrations represent a critical gap in our understanding and is an area requiring further research.
In summary, CB1R and CB2R signaling plays important (and often opposing) roles in atherogenesis by modulating proinflammatory cytokine production, proatherosclerotic lipid profiles, infiltration of immune cells into the atherosclerotic wall, adhesion of monocytes to the endothelium, lipid accumulation in foam cells, and altering the composition and structural stability of atheromas. Although CB1R activation tends to enhance the development of atherosclerosis (Fig. 1), signaling by CB2R exerts a protective effect (Fig. 2) that could potentially be exploited therapeutically using treatment with CB2R-selective agonists.
Myocardial Ischemia
Endocannabinoid Signaling in the Ischemic Heart
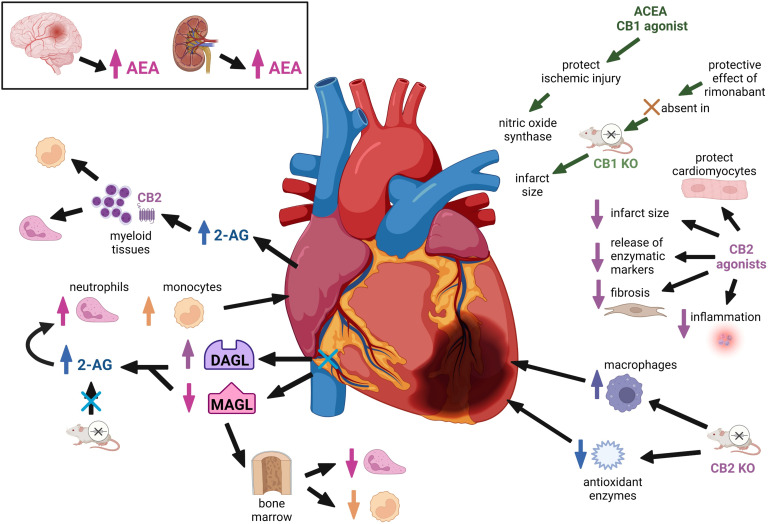
Endocannabinoid signaling has been shown to play a role in protecting the heart from ischemic injury (Fig. 3). 2-AG and anandamide concentrations are elevated in plasma from patients with coronary artery disease compared with healthy control patients (Sugamura et al., 2009). Anandamide concentrations in blood collected directly from the coronary arteries of patients with acute coronary syndrome are 10 times higher than anandamide concentrations in blood collected from systemic circulation, suggesting that anandamide is released locally from the ischemic heart (Maeda et al., 2009). Similarly, 2-AG concentrations were found to be significantly elevated in blood isolated from coronary arteries in myocardial infarction patients compared with blood collected from the coronary arteries of patients without coronary blockage (Wang et al., 2012). Consistent with these findings in the human heart, ischemia induced by ligation of the left anterior descending coronary artery elevated 2-AG concentrations in the mouse heart and plasma (Schloss et al., 2019). The increased 2-AG detected in infarcted hearts from these mice resulted from increased cardiac expression of DAGL, the biosynthetic enzyme responsible for 2-AG production, and decreased expression of MAGL, the hydrolytic enzyme that metabolizes 2-AG (Schloss et al., 2019). The increased expression of 2-AG leads to an increase in the number of neutrophils and monocytes in the blood and infarcted heart. An opposite pattern of increased DAGL mRNA expression and decreased MAGL gene expression was observed in bone marrow, suggesting that myocardial infarction might lead to a 2-AG gradient driving the recruitment of neutrophils and monocytes from bone marrow into the bloodstream and to the infarcted heart. Interestingly, the effects of 2-AG on neutrophil and monocyte recruitment were blunted in CB2R KO mice, suggesting that these protective effects of 2-AG are CB2R mediated. These human and animal studies suggest that endocannabinoids are released in response to a cardiac ischemic insult. Other work has found that anandamide is released from the ischemic brain (Muthian et al., 2004) and that 2-AG is released from the ischemic kidney, suggesting that activation of the endocannabinoid system might be a conserved and universal protective response to ischemic injury in both cardiac and noncardiac tissues.
Fig. 3.
Endocannabinoid signaling in cardiac ischemia. Endocannabinoid signaling exerts a protective effect on ischemic injury in the heart, and both 2-AG and anandamide concentrations are elevated in plasma from patients with coronary artery disease. Cardiac ischemic injury is worsened by genetic deletion or pharmacological inhibition of CB2R. CB2R-selective agonists cause a reduction in the size of myocardial infarction. Ischemic injury elevated 2-AG concentrations in the mouse heart and plasma leading to an increase in the number of neutrophils and monocytes in the blood and infarcted heart. CB1R blockade can enhance the postischemic recovery of contractile function after myocardial infarction. Several clinical studies suggest that cannabis use is associated with an increased risk of myocardial infarction.
Impact of CB1R and CB2R Signaling on the Ischemic Heart
Evidence from rodent models indicates that CB2R signaling protects the heart from ischemic injury. This is supported by work demonstrating that ischemic cardiac injury is worsened by genetic deletion of CB2R (Defer et al., 2009; Duerr et al., 2015; Hu et al., 2019) or pharmacological inhibition of CB2R using selective antagonists (Hajrasouliha et al., 2008; González et al., 2011; Yu et al., 2019). CB2R KO mice exhibit attenuated expression of antioxidant enzymes and increased infiltration of macrophages into the myocardium after an ischemic insult, indicating that endocannabinoid signaling through CB2R attenuates ischemia-induced inflammation (Duerr et al., 2015). Administration of CB2R agonists prior to ischemia or at the onset of reperfusion decreased infarct size, apoptosis, release of enzymatic markers of myocardial injury (lactate dehydrogenase and troponin), fibrosis, and inflammation after an ischemic insult (Di Filippo et al., 2004; Montecucco et al., 2009; Wang et al., 2012; Li et al., 2013b; Li et al., 2016; Yu et al., 2019; Liu et al., 2021). CB2R is coupled to an Akt–phosphatidylinositol-3-kinase–dependent signaling pathway that has been shown to inhibit opening of the mitochondrial permeability transition pore. This preserves mitochondrial integrity and reduces apoptosis and infarction size (Li et al., 2013b, 2014). CB2R signaling also protects cardiomyocytes from calcium overload–induced injury by suppressing the influx of calcium that occurs during an ischemic insult (Li et al., 2013a). In contrast to cardioprotective effects of CB2R on the myocardium, 2-AG released from the ischemic heart acts at CB2R on nearby myeloid tissues to recruit neutrophils and monocytes from bone marrow to the site of ischemic injury. Disruption of normal 2-AG signaling through CB2R in mice using systemic treatment with JZL-184, an MAGL inhibitor, exacerbated myocardial fibrosis and myocardial contractile function (Schloss et al., 2019). When considered collectively, the currently available data indicate that endocannabinoids act through CB2R-mediated mechanisms that are protective against myocardial ischemic injury.
Studies investigating the impact of CB1R have generated mixed results. In vivo studies with rimonabant demonstrated that CB1R blockade enhances the postischemic recovery of contractile function by decreasing cardiac fibrosis and facilitating cardiac remodeling after myocardial infarction (Lim et al., 2009; Slavic et al., 2013). The protective effect of rimonabant was absent in CB1R KO mice, confirming that this effect of rimonabant was mediated by CB1R. However, genetic deletion of CB1R had no impact on infarct size, suggesting that acute versus chronic disruption of CB1R signaling might have different effects on cardiac ischemic injury. Others have suggested that CB1R signaling within the heart may be cardioprotective, whereas stimulation of CB1R located in unidentified tissue(s) outside of the heart might worsen myocardial ischemic injury (Lim et al., 2009). Thus, the impact of CB1R signaling on myocardial ischemic injury remains unclear. Several avenues of future research could provide additional clarity. For example, experimental approaches that allow inducible deletion of CB1R in mice would allow investigators to parse out the temporal effects of CB1R signaling on myocardial ischemic injury, and studies with cardiac-specific CB1R KO animals would help to differentiate the roles of cardiac versus noncardiac CB1R signaling after a cardiac ischemic insult. Furthermore, a great deal of progress has been made generating positive and negative allosteric modulators of CB1R that have yet to be studied in the context of ischemic injury.
Impact of Recreational Cannabis on Myocardial Infarction
The impact of recreational cannabis on the risk of myocardial infarction was recently the topic of a detailed review (Pacher et al., 2018). Several large clinical studies suggest that cannabis use is associated with an increased risk of myocardial infarction, especially in the first hour after cannabis use when the well known tachycardic effects of cannabinoids occur (Mittleman et al., 2001; Patel et al., 2020; Ladha et al., 2021; Ma et al., 2021; Wei et al., 2022). In addition, numerous case reports have documented elevated ST segment and elevated troponin levels indicative of myocardial infarction in young healthy individuals using cannabis despite the absence of coronary atherosclerosis or other risk factors for ischemic heart disease. The use of synthetic cannabinoids, with potencies that are often more than 100 times greater than naturally occurring phytocannabinoids in cannabis, pose unique risks for cardiac events such as chest pain, dyspnea, and myocardial infarction (Armenian et al., 2018). Nevertheless, an unequivocal cause-effect relationship between cannabis use and the risk of myocardial infarction remains controversial due to concurrent tobacco use, obesity, and other cardiovascular risk factors (Mittleman et al., 2001) and also bias that is inherent to studies using hospitalized patients to study the impact of cannabis on the risk of experiencing a myocardial infarction (Page et al., 2020).
A recently published review on the mechanisms by which cannabinoids might induce myocardial infarction posited that Δ9-THC increases myocardial oxygen demand by increasing cardiac contractile force and heart rate while simultaneously decreasing the coronary flow rate and limiting blood flow to the myocardium (Weresa et al., 2022). Smoked cannabis, but not vaporized or oral cannabinoids, leads to the formation of carboxyhemoglobin, which limits the capacity of red blood cells to transport oxygen to tissues, including the heart. Thus, cannabinoids could act through multiple mechanisms to increase the risk of myocardial ischemia.
Cannabinoid System As a Therapeutic Target for Cardiovascular Diseases
Correlation between Plasma Endocannabinoid Levels and Cardiovascular Risk
Plasma 2-AG concentrations in obese men are positively correlated with cardiometabolic risk factors, including abdominal obesity, body mass index, waist girth, and plasma triglyceride and insulin levels, and negatively correlated with plasma high-density lipoprotein levels (Côté et al., 2007), In addition, AEA concentrations are significantly elevated in obese patients and correlate with coronary endothelial and circulatory dysfunction (Quercioli et al., 2011, 2012). These data suggest that increased production of endocannabinoids may provide a mechanistic link between obesity and coronary circulatory dysfunction (Al Suwaidi et al., 2001; Schindler et al., 2006).
Role of Endocannabinoids in Cardiomyopathy
Doxorubicin is a topoisomerase inhibitor that is commonly used for cancer chemotherapy. Therapeutic use of this agent is often limited by doxorubicin-induced toxicity, which is characterized by oxidative/nitrative stress, apoptosis, and declining cardiac function. Genetic deletion of FAAH, the enzyme that metabolizes AEA, enhances doxorubicin-induced oxidative/nitrative stress, increases apoptosis and cell death, and increases doxorubicin-induced cardiac dysfunction and mortality in mice (Mukhopadhyay et al., 2011). Doxorubicin-induced toxicity is attenuated by CB1R blockade both in human cardiomyocytes (Mukhopadhyay et al., 2010) and in mice (Mukhopadhyay et al., 2007). Similarly, myocardial AEA levels, CB1R expression, oxidative/nitrative stress, and apoptosis are increased in the heart under conditions of diabetic cardiomyopathy (Rajesh et al., 2012). Pharmacological blockade or genetic deletion of the CB1R suppresses diabetes-induced oxidative stress, apoptosis, and inflammation in the heart and preserves myocardial contractile function. These data provide evidence that endogenous cannabinoids acting on CB1R worsen doxorubicin- and diabetes-induced cardiomyopathy. In contrast, deletion of the CB2R worsens oxidative/nitrative stress, inflammation, apoptosis, and contractile dysfunction associated with diabetic cardiomyopathy, indicating that endocannabinoid signaling via CB2R has a cardioprotective effect under these pathologic conditions (Rajesh et al., 2022).
Clinical Impact of CB1R Blockade on Cardiovascular Risk
Dysfunction of the endocannabinoid system plays an important role in obesity. Plasma 2-AG concentrations are significantly greater in obese individuals than lean subjects and are positively correlated with visceral fat mass (Blüher et al., 2006). In addition, the number of mRNA transcripts encoding CB1R and FAAH in visceral adipose tissue is significantly lower in obese individuals compared with lean subjects (Blüher et al., 2006). Variations in genes involved in endocannabinoid signaling may predispose individuals to weight gain. A single nucleotide polymorphism in the gene encoding fatty acid amide hydrolase (P129T) results in decreased FAAH expression and activity (Chiang et al., 2004). Sipe et al. (2005) reported that people who are homozygous for this polymorphism exhibit a significantly greater body mass index than those who are heterozygotes or inherit the wild-type version of this gene, suggesting that this heritable polymorphism may play a role in obesity.
Rimonabant was approved in Europe in 2006 for the treatment of obesity. Clinical trials on rimonabant found significant improvements in cardiovascular risk factors, including decreased body weight, improved dyslipidemias, and decreased fasting glucose and insulin levels in overweight patients (Hollander, 2007; Nissen et al., 2008; Van Gaal et al., 2008b). The Rimonabant in Obesity (RIO) study found that obese patients who were prescribed rimonabant in combination with a modestly hypocaloric diet (600 kcal per day deficit) lost significantly more weight after 1 year than patients taking a placebo in concert with the hypocaloric diet. Rimonabant produced significant improvements (compared with placebo) in several cardiometabolic risk factors, including waist circumference, insulin resistance, prevalence of metabolic syndrome, A1C, HDL cholesterol, and triglycerides (Van Gaal et al., 2005, 2008a). These benefits were sustained after a second year of rimonabant treatment, indicating that chronic rimonabant therapy could produce long-term improvements in these cardiometabolic risk factors (Van Gaal et al., 2008b). The Comprehensive Rimonabant Evaluation Study of Cardiovascular Endpoints and Outcomes (CRESCENDO) trial was established to determine whether long-term rimonabant treatment decreased the risk of myocardial infarction, stroke, and other cardiovascular causes of death. This study was a double-blind, placebo-controlled clinical trial involving 974 hospitals in 42 countries and was intended to follow patients taking rimonabant or placebo for a minimum of 33 months. However, due to an increase in psychiatric side effects, including suicidal ideation, this study was terminated prematurely after 14 months with no significant cardiovascular findings (Topol et al., 2010). The prevalence and severity of these adverse psychiatric effects led to rimonabant’s removal from the European market in 2008. Two other clinical studies found no impact on clinical indicators of the progression of atherosclerosis, including atheroma volume in the coronary vasculature (Nissen et al., 2008) or carotid intima-media thickness (O’Leary et al., 2011), in patients taking rimonabant for 12–30 months. The investigators suggested that the benefits of rimonabant may have been masked by the fact that most patients had lipid profiles that were well controlled by statins prior to initiation of the study (Nissen et al., 2008). Similar to the CRESCENDO trial, both of these studies found adverse psychiatric effects in patients using rimonabant.
In light of the finding that adverse psychiatric effects are significantly increased by rimonabant, peripherally acting CB1R antagonists or monoclonal antibodies that do not enter the central nervous system (CNS) may be more therapeutically useful. Wei et al. (2022) recently identified genistein as a CB1R antagonist with minimal penetration into the CNS. Genistein reduced Δ9-THC–induced atherogenesis in vivo, providing evidence that this ligand may provide an alternative approach to targeting peripheral CB1Rs without inducing adverse effects in the CNS. Another enticing therapeutic possibility involving CB1R is the use of negative allosteric modulators that might dampen endocannabinoid signaling tone in the context of cardiovascular disease. The use of CB1R negative allosteric modulators to reduce endogenous CB1R signaling (as opposed to antagonists directed to the orthosteric binding site) is an approach that might reduce the likelihood of adverse psychiatric side effects.
Current data indicate that CB2R signaling exerts a protective effect in the context of atherosclerosis and myocardial ischemia. Therefore, the prophylactic use of selective CB2R agonists in patients with cardiovascular risk factors is a therapeutic option that warrants more study. Interestingly, CB2R is virtually nonexistent in the uninjured brain under normal conditions, and the use of CB2R agonists has not been linked to psychoactivity or adverse psychiatric effects in either rodent models or human studies. Another therapeutic possibility warranting further study is the use of inhibitors of endocannabinoid hydrolysis enzymes to enhance levels of anandamide and 2-AG. Since chronic genetic and pharmacological blockade of MAGL has been shown to desensitize and downregulate CB1R in the brain, MAGL inhibitors may be useful for treating human diseases, including cardiovascular conditions (Schlosburg et al., 2010). Similar to the use of allosteric modulators, therapeutic approaches to increase basal endocannabinoid signaling tone are less likely to produce CB1R-associated negative side effects compared with direct acting orthosteric agonists. Finally, to fully realize the potential of different cannabinoid-directed therapeutics, it is important that we gain a clear understanding of the temporal requirements for cannabinoid signaling in cardiovascular disease. For example, it is important to understand the capacity of each cannabinoid-directed therapeutic compound to prevent the establishment of cardiovascular disease as opposed to their abilities to reverse disease once it has occurred. This knowledge will shed insight on whether treatments need to be given prophylactically to patients at risk for cardiovascular disease or whether they might also be useful in treating established disease.
Acknowledgments
The authors would like to acknowledge and thank the reviewers for extremely detailed and helpful suggestions that helped to substantively improve this manuscript.
Data Availability
No data was presented in this manuscript.
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Rorabaugh, Guindon, Morgan.
Abbreviations
- AA
arachidonic acid
- AEA
anandamide
- 2-AG
2-arachidonoylglycerol
- Akt
protein kinase B
- CB1R
cannabinoid type 1 receptor
- CB2R
cannabinoid type 2 receptor
- CNS
central nervous system
- DAGL
sn-1-diaglyerol lipase
- FAAH
fatty acid amide hydrolase
- HDL
high-density lipoprotein
- JNK
c-Jun N-terminal kinase
- KO
knockout
- LDL
low-density lipoprotein
- MAGL
monoacylglycerol lipase
- MAPK
mitogen-activated protein kinase
- Δ9-THC
delta-9-tetrahydrocannabinol
- TNF-α
tumor necrosis factor alpha
Footnotes
This work was supported by funding from National Institutes of Health National Institute on Drug Abuse [Grant R01 DA044999] (to J.G. and D.J.M.) and National Heart, Lung, and Blood Institute [Grant R15 HL145546] (to B.R.R.).
The authors declare that they have no conflicts of interest with the contents of this article.
References
- Al Suwaidi J, Higano ST, Holmes DR Jr, Lennon R, Lerman A (2001) Obesity is independently associated with coronary endothelial dysfunction in patients with normal or mildly diseased coronary arteries. J Am Coll Cardiol 37:1523–1528. [DOI] [PubMed] [Google Scholar]
- Armenian P, Darracq M, Gevorkyan J, Clark S, Kaye B, Brandehoff NP (2018) Intoxication from the novel synthetic cannabinoids AB-PINACA and ADB-PINACA: a case series and review of the literature. Neuropharmacology 134:82–91. [DOI] [PubMed] [Google Scholar]
- Aronow WS, Cassidy J (1974) Effect of marihuana and placebo-marihuana smoking on angina pectoris. N Engl J Med 291:65–67. [DOI] [PubMed] [Google Scholar]
- Auer R, Sidney S, Goff D, Vittinghoff E, Pletcher MJ, Allen NB, Reis JP, Lewis CE, Carr J, Rana JS (2018) Lifetime marijuana use and subclinical atherosclerosis: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Addiction 113:845–856. [DOI] [PubMed] [Google Scholar]
- Banerjee I, Fuseler JW, Price RL, Borg TK, Baudino TA (2007) Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. Am J Physiol Heart Circ Physiol 293:H1883–H1891. [DOI] [PubMed] [Google Scholar]
- Beaconsfield P, Ginsburg J, Rainsbury R (1972) Marihuana smoking. Cardiovascular effects in man and possible mechanisms. N Engl J Med 287:209–212. [DOI] [PubMed] [Google Scholar]
- Beccacece L, Abondio P, Bini C, Pelotti S, Luiselli D (2023) The link between prostanoids and cardiovascular diseases. Int J Mol Sci 24:4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Jones RT (1975) Cardiovascular effects of prolonged delta-9-tetrahydrocannabinol ingestion. Clin Pharmacol Ther 18:287–297. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Rosenberg J, Rogers W, Bachman J, Jones RT (1979) Cardiovascular effects of intravenous delta-9-tetrahydrocannabinol: autonomic nervous mechanisms. Clin Pharmacol Ther 25:440–446. [DOI] [PubMed] [Google Scholar]
- Benyó Z, Ruisanchez É, Leszl-Ishiguro M, Sándor P, Pacher P (2016) Endocannabinoids in cerebrovascular regulation. Am J Physiol Heart Circ Physiol 310:H785–H801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno THowell FWilliams GMinassi ACascio MGLigresti AMatias ISchiano-Moriello APaul PWilliams EJ, et al. (2003) Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol 163:463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blüher M, Engeli S, Klöting N, Berndt J, Fasshauer M, Bátkai S, Pacher P, Schön MR, Jordan J, Stumvoll M (2006) Dysregulation of the peripheral and adipose tissue endocannabinoid system in human abdominal obesity. Diabetes 55:3053–3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonz A, Laser M, Küllmer S, Kniesch S, Babin-Ebell J, Popp V, Ertl G, Wagner JA (2003) Cannabinoids acting on CB1 receptors decrease contractile performance in human atrial muscle. J Cardiovasc Pharmacol 41:657–664. [DOI] [PubMed] [Google Scholar]
- Chami T, Kim CH (2019) Cannabis abuse and elevated risk of myocardial infarction in the young: a population-based study. Mayo Clin Proc 94:1647–1649. [DOI] [PubMed] [Google Scholar]
- Chiang KP, Gerber AL, Sipe JC, Cravatt BF (2004) Reduced cellular expression and activity of the P129T mutant of human fatty acid amide hydrolase: evidence for a link between defects in the endocannabinoid system and problem drug use. Hum Mol Genet 13:2113–2119. [DOI] [PubMed] [Google Scholar]
- Côté M, Matias I, Lemieux I, Petrosino S, Alméras N, Després JP, Di Marzo V (2007) Circulating endocannabinoid levels, abdominal adiposity and related cardiometabolic risk factors in obese men. Int J Obes 31:692–699. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB (1996) Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 384:83–87. [DOI] [PubMed] [Google Scholar]
- Defer NWan JSouktani REscoubet BPerier MCaramelle PManin SDeveaux VBourin MCZimmer A, et al. (2009) The cannabinoid receptor type 2 promotes cardiac myocyte and fibroblast survival and protects against ischemia/reperfusion-induced cardiomyopathy. FASEB J 23:2120–2130. [DOI] [PubMed] [Google Scholar]
- Denaës T, Lodder J, Chobert MN, Ruiz I, Pawlotsky JM, Lotersztajn S, Teixeira-Clerc F (2016) The cannabinoid receptor 2 protects against alcoholic liver disease via a macrophage autophagy-dependent pathway. Sci Rep 6:28806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R (1992) Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258:1946–1949. [DOI] [PubMed] [Google Scholar]
- Di Filippo C, Rossi F, Rossi S, D’Amico M (2004) Cannabinoid CB2 receptor activation reduces mouse myocardial ischemia-reperfusion injury: involvement of cytokine/chemokines and PMN. J Leukoc Biol 75:453–459. [DOI] [PubMed] [Google Scholar]
- Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, Kathuria S, Piomelli D (2002) Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci USA 99:10819–10824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dol-Gleizes F, Paumelle R, Visentin V, Marés AM, Desitter P, Hennuyer N, Gilde A, Staels B, Schaeffer P, Bono F (2009) Rimonabant, a selective cannabinoid CB1 receptor antagonist, inhibits atherosclerosis in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol 29:12–18. [DOI] [PubMed] [Google Scholar]
- Duerr GDHeinemann JCGestrich CHeuft TKlaas TKeppel KRoell WKlein AZimmer AVelten M, et al. (2015) Impaired border zone formation and adverse remodeling after reperfused myocardial infarction in cannabinoid CB2 receptor deficient mice. Life Sci 138:8–17. [DOI] [PubMed] [Google Scholar]
- Dutta TRyan KAThompson OLopez HFecteau NSparks MJChaturvedi SCronin CMehndiratta PNunez Gonzalez JR, et al. (2021) Marijuana use and the risk of early ischemic stroke: the stroke prevention in young adults study. Stroke 52:3184–3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ElSohly MA, Mehmedic Z, Foster S, Gon C, Chandra S, Church JC (2016) Changes in cannabis potency over the last 2 decades (1995–2014): analysis of current data in the United States. Biol Psychiatry 79:613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebremedhin D, Lange AR, Campbell WB, Hillard CJ, Harder DR (1999) Cannabinoid CB1 receptor of cat cerebral arterial muscle functions to inhibit L-type Ca2+ channel current. Am J Physiol 276:H2085–H2093. [DOI] [PubMed] [Google Scholar]
- González C, Herradón E, Abalo R, Vera G, Pérez-Nievas BG, Leza JC, Martín MI, López-Miranda V (2011) Cannabinoid/agonist WIN 55,212-2 reduces cardiac ischaemia–reperfusion injury in Zucker diabetic fatty rats: role of CB2 receptors and iNOS/eNOS. Diabetes Metab Res Rev 27:331–340. [DOI] [PubMed] [Google Scholar]
- Gorelick DAGoodwin RSSchwilke ESchwope DMDarwin WDKelly DLMcMahon RPLiu FOrtemann-Renon CBonnet D, et al. (2013) Tolerance to effects of high-dose oral δ9-tetrahydrocannabinol and plasma cannabinoid concentrations in male daily cannabis smokers. J Anal Toxicol 37:11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick DA, Heishman SJ, Preston KL, Nelson RA, Moolchan ET, Huestis MA (2006) The cannabinoid CB1 receptor antagonist rimonabant attenuates the hypotensive effect of smoked marijuana in male smokers. Am Heart J 151:754.e1–754.e5. [DOI] [PubMed] [Google Scholar]
- Govaerts SJ, Hermans E, Lambert DM (2004) Comparison of cannabinoid ligands affinities and efficacies in murine tissues and in transfected cells expressing human recombinant cannabinoid receptors. Eur J Pharm Sci 23:233–243. [DOI] [PubMed] [Google Scholar]
- Hajrasouliha AR, Tavakoli S, Ghasemi M, Jabehdar-Maralani P, Sadeghipour H, Ebrahimi F, Dehpour AR (2008) Endogenous cannabinoids contribute to remote ischemic preconditioning via cannabinoid CB2 receptors in the rat heart. Eur J Pharmacol 579:246–252. [DOI] [PubMed] [Google Scholar]
- Hemachandra D, McKetin R, Cherbuin N, Anstey KJ (2016) Heavy cannabis users at elevated risk of stroke: evidence from a general population survey. Aust N Z J Public Health 40:226–230. [DOI] [PubMed] [Google Scholar]
- Hollander P (2007) Endocannabinoid blockade for improving glycemic control and lipids in patients with type 2 diabetes mellitus. Am J Med 120 (2, Suppl 1):S18–S28, discussion S29–S32. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Fleming RM (1984) Cannabinoid inhibition of adenylate cyclase. Pharmacology of the response in neuroblastoma cell membranes. Mol Pharmacol 26:532–538. [PubMed] [Google Scholar]
- Hoyer FFKhoury MSlomka HKebschull MLerner RLutz BSchott HLütjohann DWojtalla ABecker A, et al. (2014) Inhibition of endocannabinoid-degrading enzyme fatty acid amide hydrolase increases atherosclerotic plaque vulnerability in mice. J Mol Cell Cardiol 66:126–132. [DOI] [PubMed] [Google Scholar]
- Hoyer FF, Steinmetz M, Zimmer S, Becker A, Lütjohann D, Buchalla R, Zimmer A, Nickenig G (2011) Atheroprotection via cannabinoid receptor-2 is mediated by circulating and vascular cells in vivo. J Mol Cell Cardiol 51:1007–1014. [DOI] [PubMed] [Google Scholar]
- Hu Y, Tao Y, Hu J (2019) Cannabinoid receptor 2 deletion deteriorates myocardial infarction through the down-regulation of AMPK-mTOR-p70S6K signaling-mediated autophagy. Biosci Rep 39:BSR20180650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huestis MA, Boyd SJ, Heishman SJ, Preston KL, Bonnet D, Le Fur G, Gorelick DA (2007) Single and multiple doses of rimonabant antagonize acute effects of smoked cannabis in male cannabis users. Psychopharmacology (Berl) 194:505–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakob J, von Wyl R, Stalder O, Pletcher MJ, Vittinghoff E, Tal K, Rana JS, Sidney S, Reis JP, Auer R (2021) Cumulative marijuana use and carotid intima-media thickness at middle age: the CARDIA study. Am J Med 134:777–787.e9. [DOI] [PubMed] [Google Scholar]
- Jebari-Benslaiman S, Galicia-García U, Larrea-Sebal A, Olaetxea JR, Alloza I, Vandenbroeck K, Benito-Vicente A, Martín C (2022) Pathophysiology of atherosclerosis. Int J Mol Sci 23:3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jehle JHoyer FFSchöne BPfeifer PSchild KJenniches IBindila LLutz BLütjohann DZimmer A, et al. (2016) Myeloid-specific deletion of diacylglycerol lipase α inhibits atherogenesis in ApoE-deficient mice. PLoS One 11:e0146267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jehle JSchöne BBagheri SAvraamidou EDanisch MFrank IPfeifer PBindila LLutz BLütjohann D, et al. (2018) Elevated levels of 2-arachidonoylglycerol promote atherogenesis in ApoE-/- mice. PLoS One 13:e0197751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang LS, Pu J, Han ZH, Hu LH, He B (2009) Role of activated endocannabinoid system in regulation of cellular cholesterol metabolism in macrophages. Cardiovasc Res 81:805–813. [DOI] [PubMed] [Google Scholar]
- Jourdan TGodlewski GCinar RBertola ASzanda GLiu JTam JHan TMukhopadhyay BSkarulis MC, et al. (2013) Activation of the Nlrp3 inflammasome in infiltrating macrophages by endocannabinoids mediates beta cell loss in type 2 diabetes. Nat Med 19:1132–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalliomäki J, Segerdahl M, Webster L, Reimfelt A, Huizar K, Annas P, Karlsten R, Quiding H (2013) Evaluation of the analgesic efficacy of AZD1940, a novel cannabinoid agonist, on post-operative pain after lower third molar surgical removal. Scand J Pain 4:17–22. [DOI] [PubMed] [Google Scholar]
- Kayser RR, Haney M, Raskin M, Arout C, Simpson HB (2020) Acute effects of cannabinoids on symptoms of obsessive-compulsive disorder: a human laboratory study. Depress Anxiety 37:801–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpers LE, Roy C, Ferron G, Turpault S, Poitiers F, Pinquier JL, van Hasselt JG, Zuurman L, Erwich FA, van Gerven JM (2013) Surinabant, a selective cannabinoid receptor type 1 antagonist, inhibits Δ9-tetrahydrocannabinol-induced central nervous system and heart rate effects in humans. Br J Clin Pharmacol 76:65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochar MS, Hosko MJ (1973) Electrocardiographic effects of marihuana. JAMA 225:25–27. [PubMed] [Google Scholar]
- Krylatov AV, Maslov LN, Lasukova OV, Pertwee RG (2005) Cannabinoid receptor antagonists SR141716 and SR144528 exhibit properties of partial agonists in experiments on isolated perfused rat heart. Bull Exp Biol Med 139:558–561. [DOI] [PubMed] [Google Scholar]
- Kumawat VS, Kaur G (2023) Cannabinoid receptor 2 (CB(2)) agonists and L-arginine ameliorate diabetic nephropathy in rats by suppressing inflammation and fibrosis through NF-κβ pathway. Naunyn Schmiedebergs Arch Pharmacol DOI: 10.1007/s00210-023-02597-0. [DOI] [PubMed] [Google Scholar]
- Ladha KS, Mistry N, Wijeysundera DN, Clarke H, Verma S, Hare GMT, Mazer CD (2021) Recent cannabis use and myocardial infarction in young adults: a cross-sectional study. CMAJ 193:E1377–E1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenglet S, Thomas A, Soehnlein O, Montecucco F, Burger F, Pelli G, Galan K, Cravatt B, Staub C, Steffens S (2013) Fatty acid amide hydrolase deficiency enhances intraplaque neutrophil recruitment in atherosclerotic mice. Arterioscler Thromb Vasc Biol 33:215–223. [DOI] [PubMed] [Google Scholar]
- Li Q, Cui N, Du Y, Ma H, Zhang Y (2013a) Anandamide reduces intracellular Ca2+ concentration through suppression of Na+/Ca2+ exchanger current in rat cardiac myocytes. PLoS One 8:e63386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Guo HC, Maslov LN, Qiao XW, Zhou JJ, Zhang Y (2014) Mitochondrial permeability transition pore plays a role in the cardioprotection of CB2 receptor against ischemia-reperfusion injury. Can J Physiol Pharmacol 92:205–214. [DOI] [PubMed] [Google Scholar]
- Li Q, Ma HJ, Zhang H, Qi Z, Guan Y, Zhang Y (2009) Electrophysiological effects of anandamide on rat myocardium. Br J Pharmacol 158:2022–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Wang F, Zhang Y-M, Zhou J-J, Zhang Y (2013b) Activation of cannabinoid type 2 receptor by JWH133 protects heart against ischemia/reperfusion-induced apoptosis. Cell Physiol Biochem 31:693–702. [DOI] [PubMed] [Google Scholar]
- Li X, Han D, Tian Z, Gao B, Fan M, Li C, Li X, Wang Y, Ma S, Cao F (2016) Activation of cannabinoid receptor type II by AM1241 ameliorates myocardial fibrosis via Nrf2-mediated inhibition of TGF-β1/Smad3 pathway in myocardial infarction mice. Cell Physiol Biochem 39:1521–1536. [DOI] [PubMed] [Google Scholar]
- Liao Y, Bin J, Luo T, Zhao H, Ledent C, Asakura M, Xu D, Takashima S, Kitakaze M (2013) CB1 cannabinoid receptor deficiency promotes cardiac remodeling induced by pressure overload in mice. Int J Cardiol 167:1936–1944. [DOI] [PubMed] [Google Scholar]
- Lim SY, Davidson SM, Yellon DM, Smith CC (2009) The cannabinoid CB1 receptor antagonist, rimonabant, protects against acute myocardial infarction. Basic Res Cardiol 104:781–792. [DOI] [PubMed] [Google Scholar]
- Liu J, Batkai S, Pacher P, Harvey-White J, Wagner JA, Cravatt BF, Gao B, Kunos G (2003) Lipopolysaccharide induces anandamide synthesis in macrophages via CD14/MAPK/phosphoinositide 3-kinase/NF-kappaB independently of platelet-activating factor. J Biol Chem 278:45034–45039. [DOI] [PubMed] [Google Scholar]
- Liu J, Gao B, Mirshahi F, Sanyal AJ, Khanolkar AD, Makriyannis A, Kunos G (2000) Functional CB1 cannabinoid receptors in human vascular endothelial cells. Biochem J 346:835–840. [PMC free article] [PubMed] [Google Scholar]
- Liu W, Chen C, Gu X, Zhang L, Mao X, Chen Z, Tao L (2021) AM1241 alleviates myocardial ischemia-reperfusion injury in rats by enhancing Pink1/Parkin-mediated autophagy. Life Sci 272:119228. [DOI] [PubMed] [Google Scholar]
- Ma I, Genet T, Clementy N, Bisson A, Herbert J, Semaan C, Bouteau J, Angoulvant D, Ivanes F, Fauchier L (2021) Outcomes in patients with acute myocardial infarction and history of illicit drug use: a French nationwide analysis. Eur Heart J Acute Cardiovasc Care 10:1027–1037. [DOI] [PubMed] [Google Scholar]
- Maccarrone M (2017) Metabolism of the endocannabinoid anandamide: open questions after 25 years. Front Mol Neurosci 10:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie K, Hille B (1992) Cannabinoids inhibit N-type calcium channels in neuroblastoma-glioma cells. Proc Natl Acad Sci USA 89:3825–3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie K, Lai Y, Westenbroek R, Mitchell R (1995) Cannabinoids activate an inwardly rectifying potassium conductance and inhibit Q-type calcium currents in AtT20 cells transfected with rat brain cannabinoid receptor. J Neurosci 15:6552–6561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda NOsanai TKushibiki MFujiwara TTamura YOowada SHiguma TSasaki SYokoyama JYoshimachi F, et al. (2009) Increased serum anandamide level at ruptured plaque site in patients with acute myocardial infarction. Fundam Clin Pharmacol 23:351–357. [DOI] [PubMed] [Google Scholar]
- Mahtta D, Ramsey D, Krittanawong C, Al Rifai M, Khurram N, Samad Z, Jneid H, Ballantyne C, Petersen LA, Virani SS (2021) Recreational substance use among patients with premature atherosclerotic cardiovascular disease. Heart 107:650–656. [DOI] [PubMed] [Google Scholar]
- Mai P, Yang L, Tian L, Wang L, Jia S, Zhang Y, Liu X, Yang L, Li L (2015) Endocannabinoid system contributes to liver injury and inflammation by activation of bone marrow-derived monocytes/macrophages in a CB1-dependent manner. J Immunol 195:3390–3401. [DOI] [PubMed] [Google Scholar]
- Martin-Santos RCrippa JABatalla ABhattacharyya SAtakan ZBorgwardt SAllen PSeal MLangohr KFarré M, et al. (2012) Acute effects of a single, oral dose of d9-tetrahydrocannabinol (THC) and cannabidiol (CBD) administration in healthy volunteers. Curr Pharm Des 18:4966–4979. [DOI] [PubMed] [Google Scholar]
- Marusich JA, Gamage TF, Zhang Y, Akinfiresoye LR, Wiley JL (2022) In vitro and in vivo pharmacology of nine novel synthetic cannabinoid receptor agonists. Pharmacol Biochem Behav 220:173467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI (1990) Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346:561–564. [DOI] [PubMed] [Google Scholar]
- Matyas CErdelyi KTrojnar EZhao SVarga ZVPaloczi JMukhopadhyay PNemeth BTHaskó GCinar R, et al. (2020) Interplay of liver-heart inflammatory axis and cannabinoid 2 receptor signaling in an experimental model of hepatic cardiomyopathy. Hepatology 71:1391–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechoulam R, Gaoni Y (1965) Hashish. IV. The isolation and structure of cannabinolic cannabidiolic and cannabigerolic acids. Tetrahedron 21:1223–1229. [DOI] [PubMed] [Google Scholar]
- Mittleman MA, Lewis RA, Maclure M, Sherwood JB, Muller JE (2001) Triggering myocardial infarction by marijuana. Circulation 103:2805–2809. [DOI] [PubMed] [Google Scholar]
- Molica FBurger FThomas AStaub CTailleux AStaels BPelli GZimmer ACravatt BMatter CM, et al. (2013) Endogenous cannabinoid receptor CB1 activation promotes vascular smooth-muscle cell proliferation and neointima formation. J Lipid Res 54:1360–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montecucco F, Lenglet S, Braunersreuther V, Burger F, Pelli G, Bertolotto M, Mach F, Steffens S (2009) CB(2) cannabinoid receptor activation is cardioprotective in a mouse model of ischemia/reperfusion. J Mol Cell Cardiol 46:612–620. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay PBátkai SRajesh MCzifra NHarvey-White JHaskó GZsengeller ZGerard NPLiaudet LKunos G, et al. (2007) Pharmacological inhibition of CB1 cannabinoid receptor protects against doxorubicin-induced cardiotoxicity. J Am Coll Cardiol 50:528–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay PHorváth BRajesh MMatsumoto SSaito KBátkai SPatel VTanchian GGao RYCravatt BF, et al. (2011) Fatty acid amide hydrolase is a key regulator of endocannabinoid-induced myocardial tissue injury. Free Radic Biol Med 50:179–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay P, Rajesh M, Bátkai S, Patel V, Kashiwaya Y, Liaudet L, Evgenov OV, Mackie K, Haskó G, Pacher P (2010) CB1 cannabinoid receptors promote oxidative stress and cell death in murine models of doxorubicin-induced cardiomyopathy and in human cardiomyocytes. Cardiovasc Res 85:773–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M (1993) Molecular characterization of a peripheral receptor for cannabinoids. Nature 365:61–65. [DOI] [PubMed] [Google Scholar]
- Murray CH, Huang Z, Lee R, de Wit H (2022) Adolescents are more sensitive than adults to acute behavioral and cognitive effects of THC. Neuropsychopharmacology 47:1331–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthian S, Rademacher DJ, Roelke CT, Gross GJ, Hillard CJ (2004) Anandamide content is increased and CB1 cannabinoid receptor blockade is protective during transient, focal cerebral ischemia. Neuroscience 129:743–750. [DOI] [PubMed] [Google Scholar]
- Netherland CD, Pickle TG, Bales A, Thewke DP (2010) Cannabinoid receptor type 2 (CB2) deficiency alters atherosclerotic lesion formation in hyperlipidemic Ldlr-null mice. Atherosclerosis 213:102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen SENicholls SJWolski KRodés-Cabau JCannon CPDeanfield JEDesprés JPKastelein JJSteinhubl SRKapadia S, et al. ; STRADIVARIUS Investigators (2008) Effect of rimonabant on progression of atherosclerosis in patients with abdominal obesity and coronary artery disease: the STRADIVARIUS randomized controlled trial. JAMA 299:1547–1560. [DOI] [PubMed] [Google Scholar]
- O’Leary DHReuwer AQNissen SEDesprés JPDeanfield JEBrown MWZhou RZabbatino SMJob BKastelein JJ, et al. ; AUDITOR investigators (2011) Effect of rimonabant on carotid intima-media thickness (CIMT) progression in patients with abdominal obesity and metabolic syndrome: the AUDITOR Trial. Heart 97:1143–1150. [DOI] [PubMed] [Google Scholar]
- Pacher P, Bátkai S, Kunos G (2005) Cardiovascular pharmacology of cannabinoids. Handb Exp Pharmacol 2005:599–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Steffens S, Haskó G, Schindler TH, Kunos G (2018) Cardiovascular effects of marijuana and synthetic cannabinoids: the good, the bad, and the ugly. Nat Rev Cardiol 15:151–166. [DOI] [PubMed] [Google Scholar]
- Page RL 2nd, Allen LA, Kloner RA, Carriker CR, Martel C, Morris AA, Piano MR, Rana JS, Saucedo JF; American Heart Association Clinical Pharmacology Committee and Heart Failure and Transplantation Committee of the Council on Clinical Cardiology; Council on Basic Cardiovascular Sciences; Council on Cardiovascular and Stroke Nursing; Council on Epidemiology and Prevention; Council on Lifestyle and Cardiometabolic Health; and Council on Quality of Care and Outcomes Research (2020) Medical marijuana, recreational cannabis, and cardiovascular health: a scientific statement from the American Heart Association. Circulation 142:e131–e152. [DOI] [PubMed] [Google Scholar]
- Patel RS, Manocha P, Patel J, Patel R, Tankersley WE (2020) Cannabis use is an independent predictor for acute myocardial infarction related hospitalization in younger population. J Adolesc Health 66:79–85. [DOI] [PubMed] [Google Scholar]
- Pennypacker SD, Cunnane K, Cash MC, Romero-Sandoval EA (2022) Potency and therapeutic THC and CBD ratios: U.S. cannabis markets overshoot. Front Pharmacol 13:921493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piomelli D (2003) The molecular logic of endocannabinoid signalling. Nat Rev Neurosci 4:873–884. [DOI] [PubMed] [Google Scholar]
- Quercioli APataky ZMontecucco FCarballo SThomas AStaub CDi Marzo VVincenti GAmbrosio GRatib O, et al. (2012) Coronary vasomotor control in obesity and morbid obesity: contrasting flow responses with endocannabinoids, leptin, and inflammation. JACC Cardiovasc Imaging 5:805–815. [DOI] [PubMed] [Google Scholar]
- Quercioli APataky ZVincenti GMakoundou VDi Marzo VMontecucco FCarballo SThomas AStaub CSteffens S, et al. (2011) Elevated endocannabinoid plasma levels are associated with coronary circulatory dysfunction in obesity. Eur Heart J 32:1369–1378. [DOI] [PubMed] [Google Scholar]
- Rajesh MBátkai SKechrid MMukhopadhyay PLee WSHorváth BHolovac ECinar RLiaudet LMackie K, et al. (2012) Cannabinoid 1 receptor promotes cardiac dysfunction, oxidative stress, inflammation, and fibrosis in diabetic cardiomyopathy. Diabetes 61:716–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajesh M, Mukhopadhyay P, Bátkai S, Arif M, Varga ZV, Mátyás C, Paloczi J, Lehocki A, Haskó G, Pacher P (2022) Cannabinoid receptor 2 activation alleviates diabetes-induced cardiac dysfunction, inflammation, oxidative stress, and fibrosis. Geroscience 44:1727–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajesh MMukhopadhyay PBátkai SHaskó GLiaudet LHuffman JWCsiszar AUngvari ZMackie KChatterjee S, et al. (2007) CB2-receptor stimulation attenuates TNF-alpha-induced human endothelial cell activation, transendothelial migration of monocytes, and monocyte-endothelial adhesion. Am J Physiol Heart Circ Physiol 293:H2210–H2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajesh M, Mukhopadhyay P, Haskó G, Huffman JW, Mackie K, Pacher P (2008a) CB2 cannabinoid receptor agonists attenuate TNF-alpha-induced human vascular smooth muscle cell proliferation and migration. Br J Pharmacol 153:347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajesh M, Mukhopadhyay P, Haskó G, Liaudet L, Mackie K, Pacher P (2010) Cannabinoid-1 receptor activation induces reactive oxygen species-dependent and -independent mitogen-activated protein kinase activation and cell death in human coronary artery endothelial cells. Br J Pharmacol 160:688–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajesh M, Mukhopadhyay P, Haskó G, Pacher P (2008b) Cannabinoid CB1 receptor inhibition decreases vascular smooth muscle migration and proliferation. Biochem Biophys Res Commun 377:1248–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis JP, Auer R, Bancks MP, Goff DC Jr, Lewis CE, Pletcher MJ, Rana JS, Shikany JM, Sidney S (2017) Cumulative lifetime marijuana use and incident cardiovascular disease in middle age: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Am J Public Health 107:601–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renault PF, Schuster CR, Heinrich R, Freeman DX (1971) Marihuana: standardized smoke administration and dose effect curves on heart rate in humans. Science 174:589–591. [DOI] [PubMed] [Google Scholar]
- Richards JR, Bing ML, Moulin AK, Elder JW, Rominski RT, Summers PJ, Laurin EG (2019) Cannabis use and acute coronary syndrome. Clin Toxicol (Phila) 57:831–841. [DOI] [PubMed] [Google Scholar]
- Schindler TH, Cardenas J, Prior JO, Facta AD, Kreissl MC, Zhang XL, Sayre J, Dahlbom M, Licinio J, Schelbert HR (2006) Relationship between increasing body weight, insulin resistance, inflammation, adipocytokine leptin, and coronary circulatory function. J Am Coll Cardiol 47:1188–1195. [DOI] [PubMed] [Google Scholar]
- Schlosburg JEBlankman JLLong JZNomura DKPan BKinsey SGNguyen PTRamesh DBooker LBurston JJ, et al. (2010) Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nat Neurosci 13:1113–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloss MJ, Horckmans M, Guillamat-Prats R, Hering D, Lauer E, Lenglet S, Weber C, Thomas A, Steffens S (2019) 2-Arachidonoylglycerol mobilizes myeloid cells and worsens heart function after acute myocardial infarction. Cardiovasc Res 115:602–613. [DOI] [PubMed] [Google Scholar]
- Singh GK (2000) Atrial fibrillation associated with marijuana use. Pediatr Cardiol 21:284. [DOI] [PubMed] [Google Scholar]
- Sipe JC, Waalen J, Gerber A, Beutler E (2005) Overweight and obesity associated with a missense polymorphism in fatty acid amide hydrolase (FAAH). Int J Obes 29:755–759. [DOI] [PubMed] [Google Scholar]
- Skipina TM, Patel N, Upadhya B, Soliman EZ (2022a) Cannabis use is associated with prevalent coronary artery disease. Am J Med Sci 364:304–308. [DOI] [PubMed] [Google Scholar]
- Skipina TM, Patel N, Upadhya B, Soliman EZ (2022b) Relation of cannabis use to elevated atherosclerotic cardiovascular disease risk score. Am J Cardiol 165:46–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavic SLauer DSommerfeld MKemnitz URGrzesiak ATrappiel MThöne-Reineke CBaulmann JPaulis LKappert K, et al. (2013) Cannabinoid receptor 1 inhibition improves cardiac function and remodelling after myocardial infarction and in experimental metabolic syndrome. J Mol Med (Berl) 91:811–823. [DOI] [PubMed] [Google Scholar]
- Steffens S, Veillard NR, Arnaud C, Pelli G, Burger F, Staub C, Karsak M, Zimmer A, Frossard JL, Mach F (2005) Low dose oral cannabinoid therapy reduces progression of atherosclerosis in mice [published correction appears in Nature (2005) 435:528]. Nature 434:782–786. [DOI] [PubMed] [Google Scholar]
- Stella N, Schweitzer P, Piomelli D (1997) A second endogenous cannabinoid that modulates long-term potentiation. Nature 388:773–778. [DOI] [PubMed] [Google Scholar]
- Strougo A, Zuurman L, Roy C, Pinquier JL, van Gerven JM, Cohen AF, Schoemaker RC (2008) Modelling of the concentration--effect relationship of THC on central nervous system parameters and heart rate -- insight into its mechanisms of action and a tool for clinical research and development of cannabinoids. J Psychopharmacol 22:717–726. [DOI] [PubMed] [Google Scholar]
- Sugamura KSugiyama SNozaki TMatsuzawa YIzumiya YMiyata KNakayama MKaikita KObata TTakeya M, et al. (2009) Activated endocannabinoid system in coronary artery disease and antiinflammatory effects of cannabinoid 1 receptor blockade on macrophages. Circulation 119:28–36. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K (1995) 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun 215:89–97. [DOI] [PubMed] [Google Scholar]
- Topol EJBousser MGFox KACreager MADespres JPEaston JDHamm CWMontalescot GSteg PGPearson TA, et al. ; CRESCENDO Investigators (2010) Rimonabant for prevention of cardiovascular events (CRESCENDO): a randomised, multicentre, placebo-controlled trial. Lancet 376:517–523. [DOI] [PubMed] [Google Scholar]
- Tsao CWAday AWAlmarzooq ZIAlonso ABeaton AZBittencourt MSBoehme AKBuxton AECarson APCommodore-Mensah Y, et al. (2022) Heart disease and stroke statistics-2022 update: a report from the American Heart Association. Circulation 145:e153–e639. [DOI] [PubMed] [Google Scholar]
- Valenta IVarga ZVValentine HCinar RHorti AMathews WBDannals RFSteele KKunos GWahl RL, et al. (2018) Feasibility evaluation of myocardial cannabinoid type 1 receptor imaging in obesity: a translational approach. JACC Cardiovasc Imaging 11:320–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eenige RYing ZTambyrajah LPronk ACMBlomberg NGiera MWang YCoskun Tvan der Stelt MRensen PCN, et al. (2021) Cannabinoid type 1 receptor inverse agonism attenuates dyslipidemia and atherosclerosis in APOE∗3-Leiden.CETP mice. J Lipid Res 62:100070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Esbroeck ACMVarga ZVDi Xvan Rooden EJTóth VEOnódi ZKuśmierczyk MLeszek PFerdinandy PHankemeier T, et al. (2020) Activity-based protein profiling of the human failing ischemic heart reveals alterations in hydrolase activities involving the endocannabinoid system. Pharmacol Res 151:104578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gaal L, Pi-Sunyer X, Després JP, McCarthy C, Scheen A (2008a) Efficacy and safety of rimonabant for improvement of multiple cardiometabolic risk factors in overweight/obese patients: pooled 1-year data from the Rimonabant in Obesity (RIO) program. Diabetes Care 31 (Suppl 2):S229–S240. [DOI] [PubMed] [Google Scholar]
- Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rössner S; RIO-Europe Study Group (2005) Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet 365:1389–1397. [DOI] [PubMed] [Google Scholar]
- Van Gaal LF, Scheen AJ, Rissanen AM, Rössner S, Hanotin C, Ziegler O; RIO-Europe Study Group (2008b) Long-term effect of CB1 blockade with rimonabant on cardiometabolic risk factors: two year results from the RIO-Europe Study. Eur Heart J 29:1761–1771. [DOI] [PubMed] [Google Scholar]
- Varga K, Wagner JA, Bridgen DT, Kunos G (1998) Platelet- and macrophage-derived endogenous cannabinoids are involved in endotoxin-induced hypotension. FASEB J 12:1035–1044. [DOI] [PubMed] [Google Scholar]
- Vujic NSchlager SEichmann TOMadreiter-Sokolowski CTGoeritzer MRainer SSchauer SRosenberger AWoelfler ADoddapattar P, et al. (2016) Monoglyceride lipase deficiency modulates endocannabinoid signaling and improves plaque stability in ApoE-knockout mice. Atherosclerosis 244:9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner JA, Hu K, Bauersachs J, Karcher J, Wiesler M, Goparaju SK, Kunos G, Ertl G (2001) Endogenous cannabinoids mediate hypotension after experimental myocardial infarction. J Am Coll Cardiol 38:2048–2054. [DOI] [PubMed] [Google Scholar]
- Wagner JA, Varga K, Ellis EF, Rzigalinski BA, Martin BR, Kunos G (1997) Activation of peripheral CB1 cannabinoid receptors in haemorrhagic shock. Nature 390:518–521. [DOI] [PubMed] [Google Scholar]
- Wang LN, Xing MD, Qu WT, Wang CB, Liu ZQ, Han J, Ren W, Qiao YN (2022) Impaired vessel relaxation response and increased infarct size in smooth muscle cannabinoid receptor 1 knockout mice. Microvasc Res 139:104263. [DOI] [PubMed] [Google Scholar]
- Wang PF, Jiang LS, Bu J, Huang XJ, Song W, Du YP, He B (2012) Cannabinoid-2 receptor activation protects against infarct and ischemia-reperfusion heart injury. J Cardiovasc Pharmacol 59:301–307. [DOI] [PubMed] [Google Scholar]
- Wang XDerakhshandeh RLiu JNarayan SNabavizadeh PLe SDanforth OMPinnamaneni KRodriguez HJLuu E, et al. (2016) One minute of marijuana secondhand smoke exposure substantially impairs vascular endothelial function. J Am Heart Assoc 5:e003858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei T-TChandy MNishiga MZhang AKumar KKThomas DManhas ARhee SJustesen JMChen IY, et al. (2022) Cannabinoid receptor 1 antagonist genistein attenuates marijuana-induced vascular inflammation. Cell 185:1676–1693.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis F, Beiras-Fernandez A, Sodian R, Kaczmarek I, Reichart B, Beiras A, Schelling G, Kreth S (2010) Substantially altered expression pattern of cannabinoid receptor 2 and activated endocannabinoid system in patients with severe heart failure. J Mol Cell Cardiol 48:1187–1193. [DOI] [PubMed] [Google Scholar]
- Weresa J, Pędzińska-Betiuk A, Mińczuk K, Malinowska B, Schlicker E (2022) Why do marijuana and synthetic cannabimimetics induce acute myocardial infarction in healthy young people? Cells 11:1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Jin G, Zhang J, Wei W (2019) Selective activation of cannabinoid receptor 2 attenuates myocardial infarction via suppressing NLRP3 inflammasome. Inflammation 42:904–914. [DOI] [PubMed] [Google Scholar]
- Zhao J, Chen H, Zhuo C, Xia S (2021) Cannabis use and the risk of cardiovascular diseases: a Mendelian randomization study. Front Cardiovasc Med 8:676850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Liu Y, Zhang W, Xue J, Wu YZ, Xu W, Liang X, Chen T, Kishimoto C, Yuan Z (2010a) WIN55212-2 ameliorates atherosclerosis associated with suppression of pro-inflammatory responses in ApoE-knockout mice. Eur J Pharmacol 649:285–292. [DOI] [PubMed] [Google Scholar]
- Zhao YYuan ZLiu YXue JTian YLiu WZhang WShen YXu WLiang X, et al. (2010b) Activation of cannabinoid CB2 receptor ameliorates atherosclerosis associated with suppression of adhesion molecules. J Cardiovasc Pharmacol 55:292–298. [DOI] [PubMed] [Google Scholar]
- Zuurman L, Roy C, Schoemaker RC, Amatsaleh A, Guimaeres L, Pinquier JL, Cohen AF, van Gerven JM (2010) Inhibition of THC-induced effects on the central nervous system and heart rate by a novel CB1 receptor antagonist AVE1625. J Psychopharmacol 24:363–371. [DOI] [PubMed] [Google Scholar]