Abstract
Background
The pediatric genetic white matter disorders are characterized by a broad disease spectrum. Genetic testing is valuable in the diagnosis. However, there are few studies on the clinical and genetic spectrum of Chinese pediatric genetic white matter disorders.
Methods
The participants were enrolled from the cohort of Peking Union Medical College Hospital. They all received history collection, brain MRI and gene sequencing. Their neurologic complaints which were related to white matter disorders occurred before 18. Brain MRI indicated periventricular and/or deep white matter lesions, fazekas grade 2–3.
Results
Among the 13 subjects, there were 11 males and two females. The average age of onset was 10.0 ± 5.5 years old. The potential genetic variants were found in 84.6% (11/13) subjects. The ABCD1 showed the greatest mutation frequency (30.8%, 4/13). The EIF2B3 A151fs, EIF2B4 c.885 + 2T > G, EIF2B5 R129X and MPV17 Q142X were novel pathogenic/likely pathogenic variants. 100% (4/4) ABCD1 carriers were accompanied by visual impairment, whereas 100% (3/3) EIF2B carriers developed dysuria. 100% (4/4) ABCD1 carriers exhibited diffuse white matter hyperintensities mainly in the posterior cortical regions, while the EIF2B4 and EIF2B5 carriers were accompanied by cystic degeneration.
Conclusion
There is genotypic and phenotypic heterogeneity among Chinese subjects with pediatric genetic white matter disorders. The knowledge of these clinical and genetic characteristics facilitates an accurate diagnosis of these diseases.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13052-023-01555-z.
Keywords: Pediatric genetic white matter disorder, Genetic spectrum, ABCD1
Background
The genetic white matter disorders are a group of heterogeneous diseases that predominantly affect the white matter of central nervous system. The clinical onset usually occurs in childhood. The motor impairment is the most prevalent, followed by cognitive deficit, behavioral abnormality, seizure, etc. [1]. On MRI, there is T2 hyperintensity in the white matter with T1 hypo-, iso- or hyperintensity relative to the gray matter [2].
According to Vanderver’s definition and classification, the genetic white matter disorders involve leukodystrophies and genetic leukoencephalopathies. Leukodystrophies include X-linked adrenoleukodystrophy, metachromatic leukodystrophy and Krabbe leukodystrophy, etc. And genetic leukoencephalopathies include Fabry disease, mitochondrial disorders, etc. [2].
These diseases are heterogeneous and overlapping in phenotype. Some have characteristic enzymatic alterations, such as the elevated very long chain fatty acids (VLCFA) in X-linked adrenoleukodystrophy [3]. Some do not, such as the vanishing white matter diseases [4]. Therefore, genetic testing is valuable in the diagnosis of genetic white matter disorders.
Many studies focused on the clinical and genetic spectrum of these diseases. In a UK pediatric cohort with leukodystrophies and genetic leukoencephalopathies (n = 803), the clinical spectrum involved mucopolysaccharidoses (12.5%, 100), GM1/GM2 gangliosidoses (11.3%, 91), metachromatic leukodystrophy (9.5%, 76), adrenoleukodystrophy (9.2%, 74), Krabbe leukodystrophy (6.8%, 55), etc. [5]. In a Iranian pediatric cohort with leukodystrophies and leukoencephalopathies (n = 152), the most common disease was metachromatic leukodystrophy (12.5%, 19), followed by Canavan disease (7.9%, 12), Tay-Sachs disease (7.2%, 11), adrenoleukodystrophy (5.3%, 8), Pelizaeus–Merzbacher like disease type 1 (5.3%, 8), etc. [6]. The genetic markers involved the ABCD1, EIF2B, PLP1, ARSA, MLC1, GALC, ASPA and GFAP genes, etc. [5, 6].
However, there are few studies on the clinical and genetic spectrum of Chinese pediatric genetic white matter disorders. This is a retrospective study from the cohort of Peking Union Medical College Hospital (PUMCH). Herein, we will describe the clinical and genetic characteristics of Chinese pediatric patients with genetic white matter disorders.
Method
Participants
The participants were enrolled from PUMCH cohort. The inclusion criteria were as following: ① Intact data on clinical history, brain MRI and gene sequencing. The MRI sequences included T1/T2 weighted, fluid-attenuated inversion recovery (FLAIR) and diffusion-weighted images (DWI). ② The neurologic complaints which were related to white matter disorders occurred before 18. The neurologic symptoms or signs could be developmental delay, intellectual disability, speech difficulty, psychiatric disturbance, motor regression, gait ataxia, spasticity, hearing or visual loss, autonomic dysfunction, seizure, etc. ③ Brain MRI indicated periventricular and/or deep white matter lesions, fazekas grade 2–3. The white matter lesions presented with T2/FLAIR hyperintensity with T1 hyper-, iso- or hypointensity.
The subjects were excluded if they were better explained by acquired white matter disorders due to infection, toxicity, ischemia with large or medium vessel occlusion, nutritional deficiency, trauma, neoplasm or systemic autoimmune disease.
As illustrated in Supplement Fig. 1, there were 14 pediatric patients with genetic white matter disorders. One 17-year-old female was further excluded since she had periventricular white matter lesions (fazekas grade 2), but no clinical symptom or sign. Finally, 13 subjects were enrolled.
Gene sequencing
Three cases received whole exome sequencing. Ten subjects had targeted exome sequencing of 278 genes which were related to dementia and white matter disorders. The next-generation sequencing were performed on the Illumina platform (Illumina Inc., San Diego, CA, USA). The pathogenicity of the variants was interpreted using the standards of American College of Medical Genetics and Genomics (ACMG) [7].
Result
Demographics
As shown in Tables 1 and 13 subjects of Chinese ancestry were enrolled, including 11 males and two females. The age of onset ranged between ten months and 17 years, with an average of 10.0 ± 5.5 years old. 12 cases were APOE-ε4 non-carriers, and one was ε4ε4 genotype. The elder brother of Case 1 was diagnosed with adrenoleukodystrophy at eight and died at 10. As for the other 12 subjects, no similar clinical manifestations were shown among their first-degree or second-degree family members (Table 2).
Table 1.
Demographics of 13 participants with pediatric genetic white matter disorders
| Participants (n = 13) | |
|---|---|
| Male/Female n(%) | 11 (84.6%) / 2 (15.4%) |
| Age (years old) | 17.0 ± 9.7 |
| Age of onset (years old) | 10.0 ± 5.5 |
| Family history (+/-) n(%) | 1 (7.7%) / 12 (92.3%) |
| Motor disorder (+/-) n(%) | 9 (69.2%) / 4 (30.8%) |
| Cognitive impairment (+/-) n(%) | 11 (84.6%) / 2 (15.4%) |
| Behavioral abnormality (+/-) n(%) | 2 (15.4%) / 11 (84.6%) |
| Seizure (+/-) n(%) | 2 (15.4%) / 11 (84.6%) |
| Dysuria (+/-) n(%) | 3 (23.1%) / 10 (76.9%) |
| Visual impairment (+/-) n(%) | 4 (30.8%) / 9 (69.2%) |
| Auditory impairment (+/-) n(%) | 2 (15.4%) / 11 (84.6%) |
| APOE-ε4 (+/-) n(%) | 1 (7.7%) / 12 (92.3%) |
| Causative mutation (+/-) n(%) | 11 (84.6%) / 2 (15.4%) |
Table 2.
Clinical characteristics of 13 participants with pediatric genetic white matter disorders
| Case | Gender | Age/AOO | Clinical symptom | Brain MRI | APOE |
|---|---|---|---|---|---|
| 1 | Male | 11/10 | Progressive visual impairment, speech problem, adrenocortical insufficiency | Bilateral WMH in parieto-occipital region | ε2ε3 |
| 2 | Male | 28/13 | Progressive speech problem, memory deficit, visuospatial impairment, ataxia, adrenocortical insufficiency | Bilateral WMH in parieto-occipital region | ε3ε3 |
| 3 | Male | 11/10 | Progressive intellectual impairment, visual and auditory impairment, dysarthria, ataxia, spastic paraplegia, adrenocortical insufficiency | Bilateral WMH in parieto-occipital region, degeneration of corticospinal tracts in brainstem | ε2ε2 |
| 4 | Male | 14/12 | Progressive intellectual impairment, visual impairment, ataxia, spastic paraplegia | Bilateral WMH in tempo-parieto-occipital region, splenium of corpus callosum | ε3ε3 |
| 5 | Male | 17/16 | Intellectual impairment, dysuria after syncope | Bilateral WMH in centrum semiovale, corona radiata | ε3ε3 |
| 6 | Male | 29/7 | Progressive ataxia, rapid deterioration of cognitive function, psychosis, spastic tetraplegia, dysuria after trauma | Bilateral diffuse WMH in centrum semiovale, corona radiata, middle cerebellar peduncles, cystic degeneration | ε3ε3 |
| 7 | Female | 22/12 | Progressive intellectual impairment, spastic tetraplegia, dysarthria, dysuria, seizure | Bilateral diffuse WMH in centrum semiovale, corona radiata, middle cerebellar peduncles, cystic degeneration, restricted diffusion | ε3ε3 |
| 8 | Male | 2/1.5 | Motor regression, spastic tetraplegia, dysarthria, feeding difficulties | Bilateral diffuse WMH in centrum semiovale, corona radiata, splenium of corpus callosum, restricted diffusion, stripe-like pattern | ε3ε3 |
| 9 | Male | 28/17 | Progressive cognitive impairment, pyramidal sign | Bilateral diffuse WMH in periventricular area | ε3ε3 |
| 10 | Male | 13/3 | Motor development delay, intellectual disability, seizure | Bilateral diffuse WMH in centrum semiovale, corona radiata, corpus callosum, middle cerebellar peduncles | ε3ε3 |
| 11 | Male | 15/12 | Progressive intellectual impairment, peripheral neuropathy, pyramidal sign | Diffuse WMH in subcortical regions and cerebellum, restricted diffusion | ε3ε3 |
| 12 | Male | 1.5/0.8 | Developmental retardation, optic atrophy | Bilateral diffuse WMH in centrum semiovale, corona radiata | ε3ε3 |
| 13 | Female | 29/16 | Auditory impairment, psychosis after delivery, dysplasia of left femur head, deformity of left toe | Bilateral diffuse WMH in centrum semiovale, corona radiata, cerebellum, brainstem | ε4ε4 |
AOO, age of onset; APOE, apolipoprotein e; WMH, white matter hyperintensities
Mutation interpretation
18 variants were found, including the ABCD1 (n = 4), EIF2B3 (n = 2), EIF2B4 (n = 2), EIF2B5 (n = 2), ARSA (n = 2), GFAP (n = 1), NDUFS1 (n = 2) and MPV17 variants (n = 3) (Table 3).
Table 3.
Genetic findings of 11 participants with pediatric genetic white matter disorders
| Case | Gene | Mutation | 1000 g/ESP6500 /GnomAD |
SIFT/Polyphen2 /MutationTaster |
Clinvar | ACMG | PMID |
|---|---|---|---|---|---|---|---|
| 1 |
ABCD1 |
c.1415_1416del p.Q472fs | -/-/- | Pathogenic | Pathogenic | 7,849,718 | |
| 2 | c.520T > G p.Y174D | -/-/- | D/D/A | Pathogenic | Likely pathogenic | 7,849,723 | |
| 3 | c.796G > A p.G266R | -/-/- | D/D/A | Pathogenic | Likely pathogenic | 9,195,223 | |
| 4 | c.1028G > A p.G343D | -/-/- | D/D/D | Likely pathogenic | VUS | ||
| 5 |
EIF2B3 |
c.130G > A p.E44K | 0.0002/-/0.000008 | D/D/D | VUS | VUS | 34,755,279 |
| c.450dupA p.A151fs | -/-/- | Likely pathogenic | Likely pathogenic | ||||
| 6 |
EIF2B4 |
c.1337G > A p.R446H | -/-/0.00006 | D/D/D | VUS | 35,860,328 | |
| c.885 + 2T > G | -/-/- | Pathogenic | |||||
| 7 |
EIF2B5 |
c.C385T p.R129X | -/-/0.000004 | Likely pathogenic | Pathogenic | ||
| c.G633T p.R211S | -/-/- | D/P/D | VUS | ||||
| 8 |
ARSA |
c.448 C > T p.P150S | -/-/- | D/D/D | VUS | VUS | |
| c.242G > A p.G81D | -/-/- | D/D/D | VUS | ||||
| 9 |
GFAP |
c.1246 C > T p.R416W | -/0.0005/0.00003 | D/D/D | Pathogenic | Likely pathogenic | 16,826,512 |
| 10 |
NDUFS1 |
c.266T > A p.V89E | -/-/- | D/P/D | VUS | ||
| c.1609 A > C p.I537L | -/-/0.00003 | T/B/D | VUS | ||||
| 11 |
MPV17 |
c.A263T:p.K88M | -/-/0.00006 | D/D/D | Likely pathogenic | VUS | 22,964,873 |
| c.C424T:p.Q142X | -/-/- | Likely pathogenic | |||||
| c.A265T:p.M89L | -/-/0.00005 | D/P/D | VUS |
VUS, variants of uncertain significance
According to the ACMG criteria, there were eight pathogenic or likely pathogenic variants. Of them, the ABCD1 Q472fs, Y174D, G266R and GFAP R416W were reported before [8–11]. The EIF2B3 A151fs, EIF2B4 c.885 + 2T > G, EIF2B5 R129X and MPV17 Q142X were novel. The other ten missense variants were variants of uncertain significance (VUS). Of them, the EIF2B3 E44K, EIF2B4 R446H and MPV17 K88M were reported before [12–14]. The ABCD1 G343D, EIF2B5 R211S, ARSA P150S, G81D, NDUFS1 V89E, I537L and MPV17 M89L were novel.
ABCD1 carriers
Four male subjects harbored the ABCD1 variants. They all had elevated VLCFA. Three of them were accompanied by adrenocortical insufficiency. Brain MRI mainly showed bilateral posterior white matter hyperintensities (WMH) (Fig. 1).
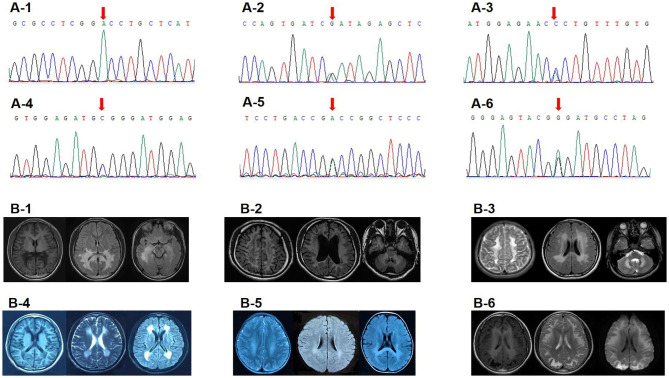
Fig. 1.
Sanger sequencing and Brain MRI examples. (A-1) Sanger sequencing showed ABCD1 c.1028G > A (p.G343D) in Case 4. (A-2/3) Sanger sequencing showed EIF2B5 c.C385T (p.R129X) and c.G633T (p.R211S) in Case 7. (A-4) Sanger sequencing showed GFAP c.1246 C > T (p.R416W) in Case 9. (A-5/6) Sanger sequencing showed ARSA c.242G > A (p.G81D) and c.448 C > T (p.P150S) in Case 8. (B-1) Brain MRI showed bilateral WMH in tempro-parieto-occipital region and splenium of corpus callosum in Case 4. (B-2) Brain MRI showed bilateral diffuse WMH in the centrum semiovale, corona radiata, middle cerebellar peduncles with cystic degeneration in Case 6. (B-3) Brain MRI showed bilateral diffuse WMH in the centrum semiovale, corona radiata, corpus callosum, middle cerebellar peduncles in Case 10. (B-4) Brain MRI showed bilateral diffuse WMH in the periventricular area in Case 9. (B-5) Brain MRI showed bilateral T2/DWI hyperintensities in the centrum semiovale, corona radiata, splenium of corpus callosum with stripe-like pattern in Case 8. (B-6) Brain MRI showed diffuse T2/DWI hyperintensities in the subcortical regions, and T1 hyperintensities in the periventricular areas in Case 11
The Q472fs carrier presented with visual and linguistic impairment at 10. He could speak fluently, but had difficulty in oral comprehension. The Y174D carrier started with difficulty in oral comprehension at 13. Gradually he presented with ataxia, memory deficit and visuospatial impairment. The G266R carrier showed intellectual impairment at 10. Gradually he exhibited ataxia, spastic paraplegia, dysarthria, visual and auditory impairment. He was bedridden at 13. The G343D carrier started with intellectual impairment at 12. Afterwards, he developed ataxia, spastic paraplegia and visual impairment. His serum cortisol and ACTH were normal.
EIF2B carriers
Three subjects harbored the EIF2B variants. They all had normal serum VLCFA, homocysteine (HCY), folic acid, vitamin B12 and organic acids. The brain MRI mainly indicated bilateral WMH in the centrum semiovale and corona radiata (Fig. 1). Two subjects had coexisting cystic degeneration.
The EIF2B3 E44K (paternal origin) and A151fs (maternal origin) were found in a 17-year-old male. He presented with intellectual impairment and dysuria after a syncope at 16. The EIF2B4 R446H (maternal origin) and c.885 + 2T > G (de novo) were in a 29-year-old male. He presented with progressive ataxia since seven. He could run and climb, but fell frequently. After an accidental trauma at 28, he showed unsteadiness while standing. Gradually, he developed memory deficit, disorientation, dyscalculia, delusion, spastic tetraplegia and dysuria. The EIF2B5 R211S (paternal origin) and R129X (de novo) were detected in a 22-year-old female. She started with seizure attack at 12. Gradually, she showed poor academic performance at school, accompanied by spastic tetraplegia, dysarthria and dysuria. At 20, she could not vocalize, eat or move.
ARSA, GFAP, NDUFS1 and MPV17 carriers
Four subjects carried the variants in the ARSA, GFAP, NDUFS1 and MPV17. They had normal serum VLCFA, HCY, folic acid, vitamin B12 and organic acids.
The ARSA P150S (paternal origin) and G81D (maternal origin) were found in a two-year-old boy. At the age of one, he was able to walk and call mom, dad. Four months later, he showed gait instability and dysarthria. Gradually he could not speak, eat or move. Physical examination indicated spastic tetraplegia. Brain MRI showed bilateral diffuse WMH in the centrum semiovale, corona radiata, splenium of corpus callosum with stripe-like pattern (Fig. 1). Serum enzyme test showed decreased Arylsulfatase A level (3nmol/17 h/mgPr).
The de novo GFAP R416W was in a 28-year-old male. He showed cognitive decline since 17. Physical examination indicated bilateral Babinski sign with normal muscle force. Brain MRI revealed bilateral diffuse WMH in the periventricular area (Fig. 1).
The NDUFS1 V89E and I537L were in a 13-year-old boy. He was able to walk at three. He showed poor intelligence and seizure attack in early childhood. Brain MRI indicated bilateral diffuse WMH in the centrum semiovale, corona radiata, corpus callosum and middle cerebellar peduncles (Fig. 1).
The MPV17 Q142X, M89L and K88M were in a 15-year-old boy. He showed poor academic performance at 12. Two years later, he developed limb weakness and gait instability. Physical examination exhibited bilateral Babinski sign, decreased muscle force and tendon reflex in the extremities, as well as reduced pin-prick sensation in the distal extremities. Electromyography revealed peripheral neuropathy. Brain MRI demonstrated T2/DWI hyperintensities in the subcortical regions, cerebellum, and T1 hyperintensities in the periventricular areas (Fig. 1).
Discussion
This is a group of subjects with pediatric genetic white matter disorders. The potential genetic variants are found in 84.6% (11/13) subjects. The ABCD1 has the greatest mutation frequency (30.8%, 4/13), followed by the EIF2B (23.1%, 3/13) and mitochondrial genes (15.4%, 2/13), which suggest the diagnosis of adrenoleukodystrophy, vanishing white matter disease and mitochondrial disease, respectively. These three diseases are also the main components of pediatric leukoencephalopathies in other countries. In a Finnish pediatric cohort with genetic white matter disorders, the most common diseases are mitochondrial (18.8%, 15/80) and adrenoleukodystrophy (7.5%, 6/80) [15]. In English and Iranian pediatric cohorts with leukoencephalopathies, the prevalence of vanishing white matter disease are 18.8% (17/903) and 2.6% (4/152), respectively [5, 6].
11 novel variants are found in this report. Of them, the EIF2B3 A151fs, EIF2B4 c.885 + 2T > G, EIF2B5 R129X, MPV17 Q142X are frameshift, splicing or stopgain variants, They are rare or absent in the 1000genome, ESP6500, GnomAD databases. The EIF2B4 c.885 + 2T > G and EIF2B5 R129X are de novo based on the pedigree analysis. The EIF2B3 A151fs and EIF2B5 R129X are determined as likely pathogenic in the Clinvar database (www.clinvar.com). Taken together, these variants are pathogenic/likely pathogenic according to the ACMG criteria.
Seven novel VUS are detected, including the ABCD1 G343D, EIF2B5 R211S, ARSA P150S and G81D, NDUFS1 V89E/I537L and MPV17 M89L. They are rare or absent in the 1000genome, ESP6500, GnomAD databases. They are deleterious from SIFT, Polyphen2 and Mutationtaster predictions. The ARSA P150S is novel, while the ARSA P150L is reported before [16]. The latter is supposed to be pathogenic/likely pathogenic in the clinvar database (www.clinvar.com). The subjects harboring the ABCD1 G343D and the ARSA P150S/G81D demonstrate increased VLCFA and decreased Arylsulfatase A levels, respectively. Taken together, these variants are VUS according to the ACMG criteria.
There are some common phenotypic features among the 13 subjects. For instance, 76.9% (10/13) cases are characterized by insidious onset and gradual progression. The most prevalent symptoms are cognitive impairment (84.6%, 11/13) and motor disorder (69.2%, 9/13). 100% (13/13) subjects have diffuse WMH in the supratentorial subcortical or periventricular regions. These are almost consistent with previous research [1, 2].
Moreover, the subjects with different gene mutations have some characteristic phenotypes. For instance, none of the three EIF2B variant carriers exhibit a typical pattern of insidious onset and gradual progression. The EIF2B5 carrier presents with an acute onset of seizure attack. The EIF2B3 and EIF2B4 carriers experience a rapid progression of neurological regression following syncope and trauma. Previous reports reveal that patients with vanishing white matter diseases can worsen rapidly under stress [4]. The mechanism is unknown.
In terms of clinical symptom, 100% (4/4) ABCD1 carriers are accompanied by visual impairment. This might be related to their occipital involvement. The MPV17 carrier has peripheral neuropathy. El-Hattab pointed out that peripheral neuropathy occurred in 18.7% (17/91) MPV17 carriers [17]. In addition, we find 100% (3/3) EIF2B carriers develop dysuria. Kar found that the mRNAs encoding the EIF2B2 were present in the axons of rat sympathetic neurons [18]. These lead to the speculation that the EIF2B genes might exert effects on autonomic nerves.
On brain MRI, 100% (4/4) ABCD1 carriers exhibit diffuse WMH mainly in the posterior cortical regions. The EIF2B4 and EIF2B5 carriers are accompanied by cystic degeneration. The ARSA carrier shows stripe-like pattern, indicating remaining tissue strands within the WMH. The T2 hyperintensity of the MPV17 carrier is primarily limited in the subcortical regions. These are similar to previous findings [3, 4, 19, 20]. However, the mechanism remains unclear.
There are two subjects carrying the variants related to mitochondrial diseases. The NDUFS1 carrier presents with motor development delay, intellectual disability, seizure with diffuse cerebral and cerebellar WMH. According to Björkman’s finding, these clinical and imaging features are common in the NDUFS1 carriers except for the cerebellar involvement [21]. The association between the NDUFS1 gene and cerebellum could be further investigated. Fortunately, the MPV17 carrier has a late onset without hepatic impairment. El-Hattab summarized the clinical characteristics of 100 MPV17 mutation carriers. He found that almost 96% individuals had an poor prognosis during infancy or early childhood due to hepatic failure. However, 4% subjects with later onset during late childhood or adulthood had no or minimal liver injury [17].
Conclusions
There is genotypic and phenotypic heterogeneity among Chinese pediatric genetic white matter disorders. The ABCD1, EIF2B and mitochondrial genes have high mutation frequencies. The subjects with different gene mutations exhibit characteristic manifestations, which have suggestive implications for the underlying genetic basis. The knowledge of these clinical and genetic characteristics facilitates an accurate diagnosis of these diseases. The functional verification of the novel variants should be performed in the following studies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Abbreviations
- VLCFA
Very long chain fatty acids
- PUMCH
Peking Union Medical College Hospital
- ACMG
American College of Medical Genetics and Genomics
- VUS
Variants of uncertain significance
- HCY
Homocysteine
- WMH
White matter hyperintensities
Authors’ contributions
LD and LS contributed to acquisition, analysis, interpretation of the data, and draft of the work. JG contributed to acquisition, analysis, interpretation of the data, and conception, revision of the work. BP and LC contributed to conception of the work. CL, CM, XH and SC contributed to acquisition of the data. All authors approved the submitted version.
Funding
Dr. Jing Gao was supported by grants from National Key Research and Development Program of China (No. 2020YFA0804500 and 2016YFC1306300), CAMS Innovation fund for Medical Sciences (No. 2021-1-I2M-1-020), National Natural Science Foundation of China (No. 81550021 and 30470618), and the strategic priority research program (pilot study) “Biological basis of aging and therapeutic strategies” of the Chinese Academy of Sciences (No. XDPB10).
Data Availability
The original contributions are included in the article, further dataset are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was in accordance with the ethical standards as laid down in the Declaration of Helsinki and its later amendments or comparable ethical standards. The study was approved by ethics committee of PUMCH (No. JS-1836). Written informed consent was obtained.
Consent for publication
Informed consent was obtained from all patients’ parents.
Competing interests
The authors have no conflict of interest to report.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Liling Dong and Li Shang contributed equally.
References
- 1.Sarret C. Leukodystrophies and genetic leukoencephalopathies in children. Rev Neurol (Paris) 2020;176:10–9. doi: 10.1016/j.neurol.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Vanderver A, Prust M, Tonduti D, et al. Case definition and classification of leukodystrophies and leukoencephalopathies. Mol Genet Metab. 2015;114:494–500. doi: 10.1016/j.ymgme.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moser HW, Mahmood A. Raymond GV X-linked adrenoleukodystrophy. Nat Clin Pract Neurol. 2007;3:140–51. doi: 10.1038/ncpneuro0421. [DOI] [PubMed] [Google Scholar]
- 4.van der Knaap MS, Fogli A, Boespflug-Tanguy O, Abbink T, Schiffmann R. Childhood Ataxia with Central Nervous System Hypomyelination / Vanishing White Matter. 1993.
- 5.Stellitano LA, Winstone AM, van der Knaap MS, Verity CM. Leukodystrophies and genetic leukoencephalopathies in childhood: a national epidemiological study. Dev Med Child Neurol. 2016;58:680–9. doi: 10.1111/dmcn.13027. [DOI] [PubMed] [Google Scholar]
- 6.Mahdieh N, Soveizi M, Tavasoli AR, et al. Genetic testing of leukodystrophies unraveling extensive heterogeneity in a large cohort and report of five common Diseases and 38 novel variants. Sci Rep. 2021;11:3231. doi: 10.1038/s41598-021-82778-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–24. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barcelo A, Giros M, Sarde CO, et al. Identification of a new frameshift mutation (1801delAG) in the ALD gene. Hum Mol Genet. 1994;3:1889–90. doi: 10.1093/hmg/3.10.1889. [DOI] [PubMed] [Google Scholar]
- 9.Fuchs S, Sarde CO, Wedemann H, Schwinger E, Mandel JL, Gal A. Missense mutations are frequent in the gene for X-chromosomal adrenoleukodystrophy (ALD) Hum Mol Genet. 1994;3:1903–5. doi: 10.1093/hmg/3.10.1903. [DOI] [PubMed] [Google Scholar]
- 10.Dodd A, Rowland SA, Hawkes SL, Kennedy MA, Love DR. Mutations in the adrenoleukodystrophy gene. Hum Mutat. 1997;9:500–11. doi: 10.1002/(SICI)1098-1004(1997)9:6<500::AID-HUMU2>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 11.Der Perng M, Su M, Wen SF, et al. The Alexander disease-causing glial fibrillary acidic protein mutant, R416W, accumulates into Rosenthal fibers by a pathway that involves filament aggregation and the association of alpha B-crystallin and HSP27. Am J Hum Genet. 2006;79:197–213. doi: 10.1086/504411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu L, Zhong M, Yang Y, et al. Adult-onset vanishing white matter in a patient with EIF2B3 variants misdiagnosed as multiple sclerosis. Neurol Sci. 2022;43:2659–67. doi: 10.1007/s10072-021-05710-4. [DOI] [PubMed] [Google Scholar]
- 13.Tian Y, Liu Q, Zhou Y, et al. Identification of a Novel heterozygous mutation in the EIF2B4 gene Associated with vanishing White Matter Disease. Front Bioeng Biotechnol. 2022;10:901452. doi: 10.3389/fbioe.2022.901452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garone C, Rubio JC, Calvo SE, et al. MPV17 mutations causing adult-onset multisystemic disorder with multiple mitochondrial DNA deletions. Arch Neurol. 2012;69:1648–51. doi: 10.1001/archneurol.2012.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knuutinen OA, Oikarainen JH, Suo-Palosaari MH, et al. Epidemiological, clinical, and genetic characteristics of paediatric genetic white matter disorders in Northern Finland. Dev Med Child Neurol. 2021;63:1066–74. doi: 10.1111/dmcn.14884. [DOI] [PubMed] [Google Scholar]
- 16.Biffi A, Cesani M, Fumagalli F, et al. Metachromatic leukodystrophy - mutation analysis provides further evidence of genotype-phenotype correlation. Clin Genet. 2008;74:349–57. doi: 10.1111/j.1399-0004.2008.01058.x. [DOI] [PubMed] [Google Scholar]
- 17.El-Hattab AW, Wang J, Dai H, et al. MPV17-related mitochondrial DNA maintenance defect: new cases and review of clinical, biochemical, and molecular aspects. Hum Mutat. 2018;39:461–70. doi: 10.1002/humu.23387. [DOI] [PubMed] [Google Scholar]
- 18.Kar AN, MacGibeny MA, Gervasi NM, Gioio AE, Kaplan BB. Intra-axonal synthesis of eukaryotic translation initiation factors regulates local protein synthesis and axon growth in rat sympathetic neurons. J Neurosci. 2013;33:7165–74. doi: 10.1523/JNEUROSCI.2040-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaimardanova AA, Chulpanova DS, Solovyeva VV, et al. Metachromatic leukodystrophy: diagnosis, modeling, and treatment approaches. Front Med (Lausanne) 2020;7:576221. doi: 10.3389/fmed.2020.576221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mundlamuri RC, Divate P, Satishchandra P. MPV17 gene variant mutation presenting as Leucoencephalopathy with Peripheral Neuropathy. Neurol India. 2021;69:1817–9. doi: 10.4103/0028-3886.333468. [DOI] [PubMed] [Google Scholar]
- 21.Bjorkman K, Sofou K, Darin N, et al. Broad phenotypic variability in patients with complex I deficiency due to mutations in NDUFS1 and NDUFV1. Mitochondrion. 2015;21:33–40. doi: 10.1016/j.mito.2015.01.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions are included in the article, further dataset are available from the corresponding author on reasonable request.