Abstract
Purpose
To study the efficacy and toxic effects of bepotastine besilate 1.5% preservative-free (BB-PF) and olopatadine 0.2% BAK-preserved (OL-BAK) drops on the ocular surface of patients with allergic conjunctivitis.
Patients and Methods
Ninety-seven patients with allergic conjunctivitis diagnosis participated in a prospective, multicenter, randomized, double-blind, controlled, parallel-group clinical trial. Patients received either BB-PF (n=48) or OL-BAK (n=49), both administered once daily in the morning. The patients were followed for 60 days. Ocular itching was the primary outcome measure. Secondary outcomes included ocular symptoms, signs, and non-ocular symptoms associated with rhinoconjunctivitis. Conjunctival impression cytology (CIC) was performed to evaluate histopathological changes related to the toxic effects of preservatives.
Results
BB-PF treatment was associated with a 1.30 more probability of diminished ocular itching than OL-BAK (odds ratio (OR)=1.30; 95% CI=(0.96–1.7); p=0.086). No statistically significant differences were found between treatments in the resolution of other ocular symptoms or signs, except for tearing, which was superior in the BB-PF (OR=1.37; 95% (1.26–1.47); p<0.0001). BB-PF was superior in terms of the resolution of rhinorrhea (p=0.040) and nasal itching (p=0.037). After 60 days of treatment, the BB-PF group exhibited 2.0 times higher probability of having a lower Nelson scale score compared to the OL-BAK group (OR=2.00; 95% CI=(1.19–3.34); p=0.010).
Conclusion
Both medications presented a similar efficacy in terms of the resolution of ocular signs and symptoms associated with ocular conjunctivitis. BB-PF is superior in the resolution of non-ocular symptoms and safer for the ocular surface than OL-BAK.
Keywords: allergic conjunctivitis, bepotastine besilate, olopatadine, preservative-free, ocular surface, rhinoconjunctivitis
Introduction
Allergic conjunctivitis (AC) is the most common allergic ocular disorder, affecting approximately 20% of the global population.1,2 The prevalence of AC is region-dependent, and appears to be increasing worldwide.3 This pathology is associated with seasonal pollen sensitivity, although perennial forms are associated with exposure to animal dander, mites, and molds.4 AC is a type-1, IgE-mediated hypersensitivity immune reaction that occurs in individuals previously exposed to a specific allergen. The immune response involves the release of inflammatory mediators, including histamine, leukotrienes, bradykinin, prostaglandins, proteases, and cytokines, which contribute to the development of signs and symptoms.5 Histamines from degranulated mast cells are the principal immune mediators related to early allergic responses. This molecule binds to receptors (H1, H2, H3, and H4) on vascular endothelial cells, neuronal fibers, goblet cells, immune cells, and conjunctival epithelium culminating in clinical manifestations of allergic conjunctivitis including redness, periocular swelling, chemosis, itching, and tearing.6 This is followed by a late phase that is manifested by pro-inflammatory mediators and the recruitment of immune cells including eosinophils and neutrophils.4
The keystone of the treatments is histamine antagonists (also called antihistamines), which inhibit the action of histamine by blocking histamine receptors and, consequently, inhibiting clinical manifestations related to this mediator. Mast cell stabilizers inhibit mast cell degranulation and, consequently, the release of histamine by interrupting the normal chain of intracellular signals.4,7 The first line of treatment for this condition includes antihistamines that combine both mechanisms of action: dual-action antihistamines, which present an increased safety and efficacy profile compared with older topical agents used in the treatment of AC.4
Bepotastine besilate is the latest-generation ophthalmic antihistamine with multiple mechanisms of action in both preclinical and clinical studies. This drug is a highly selective H1 receptor antagonist with potent mast cell-stabilizing action. Bepotastine besilate exerts its anti-inflammatory action through the inhibition of leukotriene B48 and the reduction and activation of eosinophil chemotaxis.9 It also inhibits the biosynthesis of proinflammatory IL-5 in vitro.9 The clinical efficacy and safety of bepotastine besilate twice a day (BID) have been demonstrated in several clinical studies.10,11 Recent studies suggest that the effect of bepotastine besilate 1.5% was maintained for up to 16 hr,12,13 so a posology of once a day in the morning can be proposed.
A large percentage of patients with AC also present nasal symptoms as a comorbidity, known as allergic rhinoconjunctivitis.4,5,7 In rhinitis epidemiology studies, it was found that approximately 90% of patients suffering from nasal symptoms also had ocular symptomatology.14–16 The conjunctiva and nasal mucosa share a similar epithelium, leading to a similar reactivity to the same allergens in both tissues.14 Some studies have supported that the use of bepotastine besilate 1.5% eye drops may have some effect on nasal symptoms associated with rhinoconjunctivitis because a significant proportion of the drug is expected to reach the nasal mucosa via the nasolacrimal duct.17,18
The formulation under study is a new bepotastine besilate 1.5% preservative-free (PF) solution developed in a new multidose device called “Ophthalmic Squeeze Dispenser” (OSD, Aptar™ Pharma). The use of preservative-free ophthalmic formulations is necessary to prevent preservative-induced toxicity. Benzalkonium chloride (BAK), an ammonium quaternary compound widely used as a preservative in ophthalmic eye drops, is well known for its toxic effects. Although the adverse effects are more problematic with chronic exposure, they also manifest after exposure for as brief as 7 days.19 Numerous documented cytotoxic effects of BAK exposure have been observed in ocular tissues. These effects include a reduction in corneal survival,20–23 conjunctival survival,20,21,23,24 trabecular meshwork (TM) impairment,25–27 decreased survival of ciliary epithelial cells,27 loss of conjunctival goblet cells,28,29 delayed corneal healing,30 lymphocyte infiltration of conjunctival epithelium and stroma,31 and an increase in inflammatory markers within ocular tissues.32,33 Furthermore, BAK exposure leads to various clinical manifestations that negatively impact the quality of life for patients. These include sensations of pain/discomfort,34–36 excessive tearing,34,36 a persistent feeling of a foreign body in the eye,35 subconjunctival inflammation/fibrosis,37,38 and signs of dry eye disease. These dry eye symptoms manifest as a reduced tear breakup time,36,39,40 diminished Schirmer test results,36,40 positive corneal and conjunctival staining,36,39,40 and a higher Ocular Surface Disease Index (OSDI) score.39,40
To our knowledge, no prospective studies have established the effectiveness and safety of bepotastine besilate 1.5% PF once a day in comparison with olopatadine 0.2% BAK preserved under environmental exposure conditions.
Our study aims to evaluate the efficacy of this ophthalmic formulation on ocular symptoms and signs and non-ocular symptoms and to determine if there is any consequence on the histology of conjunctival epithelium after 60 days of treatment with bepotastine 1.5% PF compared to Olopatadine hydrochloride 0.2% BAK-preserved once a day in patients with allergic conjunctivitis.
Materials and Methods
Design
A prospective, multicenter, double-blind, randomized, parallel-group comparative trial was conducted between March 2021 and August 2022 in 4 public hospitals in Buenos Aires, Argentina.
This study was approved by the Institutional Review Board Ethics Committees (Comité de Ética en investigación Hospital Oftalmológico Santa Lucía, Comité de Ética en investigación Hospital Alta Complejidad El Cruce, Comité de Ética Biomédica del Complejo Médico Churruca Visca) and registered at clinicaltrials.gov under identifier NCT04776096. This study adhered to the tenets of the Declaration of Helsinki and was in accordance with The Code of Ethics of the World Medical Association. Informed consent was obtained from all participants after explaining the nature and possible consequences of the study.
Study Sample
Inclusion Criteria
Subjects had to meet the following inclusion criteria to be eligible for participation in the clinical trial: (1) at least 18 years of age; (2) provided written informed consent; (3) with allergic conjunctivitis diagnosis with subject-assessed ocular itching score ≥2 described as a mild continuous itch, can be localized, without a desire to rub, and physician-assessed ocular hyperemia score ≥2 described as moderate, more apparent dilation of blood vessels; vessel color is more intense; involves the majority of the vessel bed;12 (4) no use of contact lenses and other topical medications within the duration of participation; and (5) Intraocular pressure ≤18 mmHg in both eyes.
Exclusion Criteria
Potential participants were excluded if they (1) had undergone refractive surgery within 6 months before the start of the study; (2) had significant active systemic or ocular diseases (eg, glaucoma, blepharitis, or severe cardiovascular disease); (3) had used any eye medication in the last 15 days and/or had received anti-inflammatory drugs (corticosteroids and/or NSAIDs) and/or antihistamines orally or intravenously; or (4) had a known contraindication or sensitivity to the use of any study drugs or their components. (5) Women who are breastfeeding or pregnant.
Medication Under Study
Patients who met the eligibility criteria were randomly assigned according to a computer-generated randomization list to receive one of the two treatments under study: Bepotastine besilate 1.5% preservative-free (Traler® LC, Poen Laboratories, Argentina) or olopatadine hydrochloride 0.2% with 0.001% BAK (Patanol® S, Alcon Laboratories, Argentina) in 1:1 ratio. Each patient received two bottles of the same treatment for 60 days. Patients were instructed to instill either bepotastine besilate 1.5% or olopatadine 0.2% once daily (at approximately 8 am) for 60 days. Each treatment was provided in the packing, originally approved by the local sanitary authority but relabeled to maintain double masking.
Outcomes Measures
The primary efficacy endpoint was the patient-assessed ocular itching score at baseline and at 15, 30, 45, and 60 days after randomization.
As secondary endpoints, other ocular patient-assessed symptoms were evaluated, including tearing, eye burning, and foreign-body sensation. In addition, we evaluated physician-assessed ocular signs such as conjunctival hyperemia, chemosis, ocular secretion, and palpebral swelling.
Nasal symptoms, including congestion, pruritus, and rhinorrhea, were assessed. These endpoints were evaluated at baseline and 15, 30, 45, and 60 days after randomization.
Severity scales for all variables were based on a 4-point Likert Scale stratified as absent, mild, moderate, or severe. Ocular and non-ocular symptoms were assessed using a standardized questionnaire in which patients were required to check one of the four ordinal categories. Ocular signs were physician-assessed by ocular surface specialists and evaluated using slit-lamp biomicroscopy performed at each visit.
To evaluate ocular cytotoxicity, conjunctival impression cytology was performed at baseline and 30 and 60 days after randomization. Cytology was graded using Nelson’s classification.41 This classification considered four grades from 0 (normal epithelium) to stage III (absence of goblet cells and epithelial metaplasia). Stages 0 and I were considered normal epithelium and stages II and III abnormal conjunctival epithelium, as they did not show the presence of goblet cells.
Statistical Methods
The trial was analyzed by comparing patients randomized to bepotastine vs olopatadine, with this group serving as the referent. The primary outcome was analyzed using GEE to model the relationship between groups and itching grade over time, while accounting for within-subject correlation. Specifically, we used an ordinal logistic regression model to estimate the odds ratio (OR) of having a higher itching grade with bepotastine than olopatadine. An OR greater than 1.0 indicate a more favorable outcome on the symptom or sign score among patients randomized to bepotastine than to olopatadine. OR results with a 95% CI that did not include 1.0 were considered statistically significant.
The sample size calculated was 95 patients to detect an accumulative odds ratio of 0.41 in the absence of ocular itching on day 60, with a power of 0.80, and a two-tailed alpha of 0.05. Also, 15% of drop-out was considered.
All ordinal variables were analyzed using the GEE logistic ordinal regression. Demographic data were analyzed using the t-test for continuous variables and Pearson’s chi-square test for categorical data.
Data are presented as ORs and 95% confidence intervals (CIs) for the association between treatment group and symptom/sign grade for ordinal variables. Analyses were performed using IBM SPSS software (version 22.0, IBM Corp.).
Results
Study Population
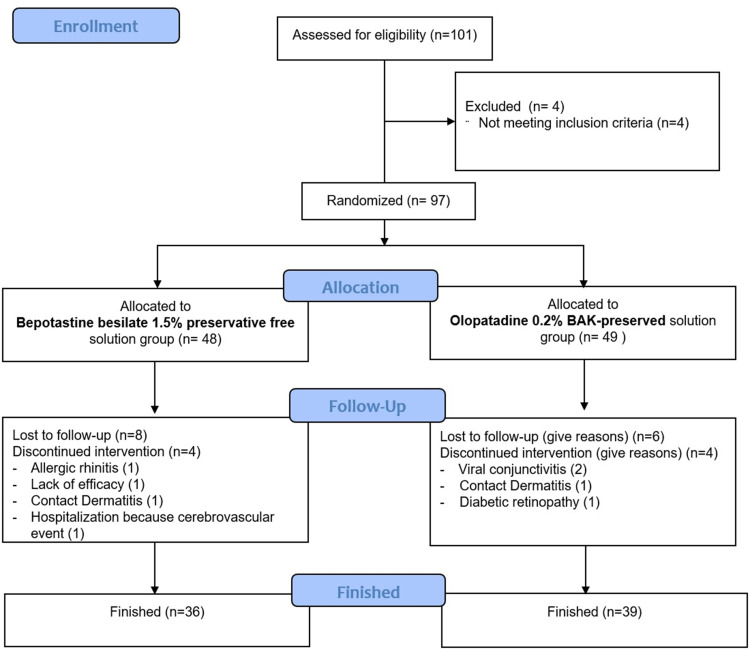
A total of 101 patients were included in this study. Four of them were not included as they did not meet the inclusion criteria. A total of 97 patients from 4 ophthalmological centers were enrolled and randomized into treatment groups at the baseline visit, of which 75 completed all study visits (Figure 1).
Figure 1.
CONSORT Flow Diagram.
Note: Flow-chart showing inclusion, randomization, and participation throughout the study.
The demographic characteristics (sex and age) of the patients were well-balanced between the treatment groups in the intention to treat (ITT) population. Pairwise comparisons between groups showed no statistically significant differences. The median age of the enrolled population (n=97) was 43.2 years for bepotastine group and 45.0 for Olopatadine group, while most patients were women in both treatment groups: 68.1% for bepotastine group and 66.7% for olopatadine group.
Ocular Symptoms
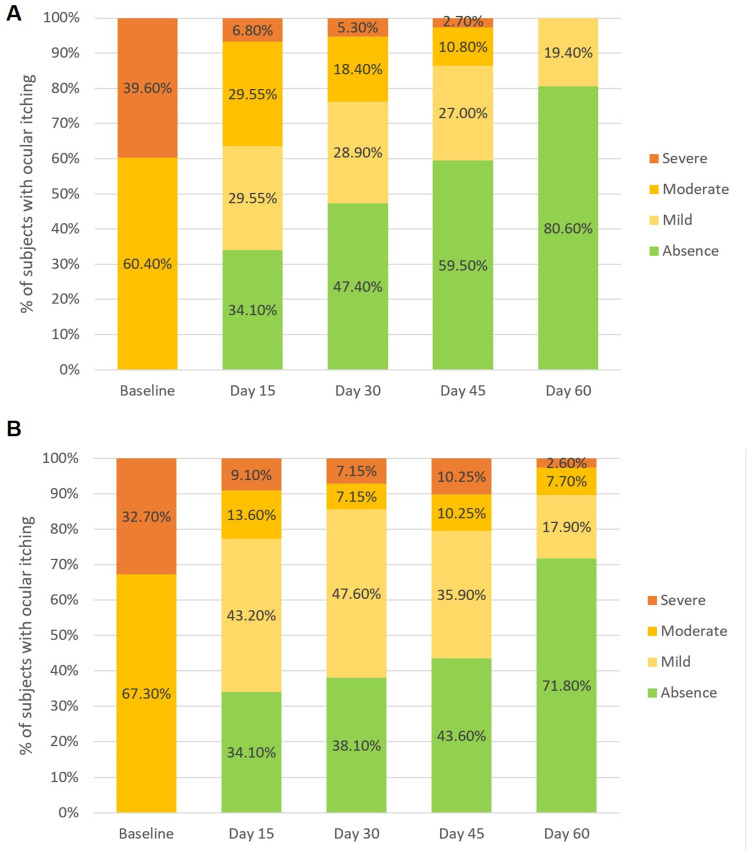
Ocular itching: The degree of ocular itching decreased significantly over time in both groups of treatment (p<0.0001). Bepotastine besilate 1.5% PF administered once daily to patients with allergic conjunctivitis significantly reduced ocular itching, with a probability 1.30 times greater than that of olopatadine 0.2% BAK-preserved (odds ratio (OR) = 1.30; 95% confidence interval (CI) = (0.96–1.70); p = 0.086). After 60 days of treatment, 80.60% of patients in the bepotastine group completely resolved the main symptoms associated with allergic conjunctivitis, whereas 71.80% of patients in the olopatadine group completely resolved this symptom. None of the patients in the bepotastine group reported a moderate or severe degree of ocular itching after 60 days of treatment (Figure 2).
Figure 2.
(A) Ocular itching severity over the time in the Bepotastine group. (B) Ocular itching over the time in the Olopatadine Group.
Notes: Ocular itching was graded on a four points-Likert scale (absence, mild, moderate, severe), p = 0.086 for the GEE ordinal logistic regression. Each bar represents the percentage of patients in each degree of ocular itching by time.
Abbreviation: GEE, Generalized estimating equation.
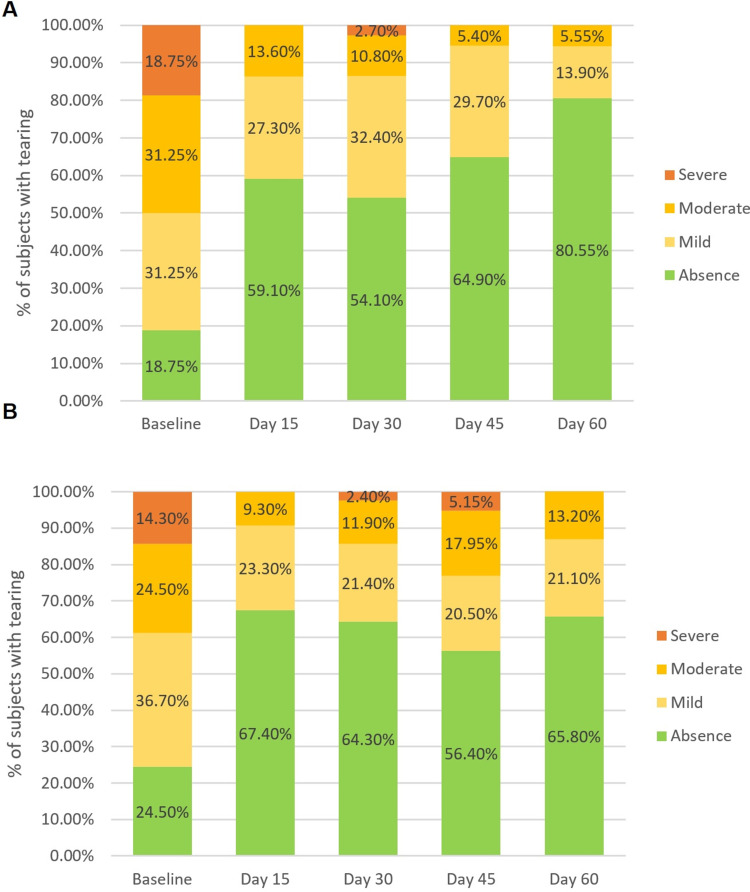
Tearing: The degree of tearing decreased significantly over time in both the treatment groups (p = 0.043). Bepotastine besilate 1.5% PF once a day reduced tearing with a probability of 1.37 times greater than olopatadine 0.2% BAK-preserved over time (OR = 1.37; 95% CI = (1.26–1.47); p < 0.0001). After 60 days of treatment, 80.55% of the patients in the bepotastine group had completely resolved tearing, while 65.80% had completely resolved this symptom in the olopatadine group (Figure 3).
Figure 3.
(A) Tearing severity over the time in the Bepotastine group. (B) Tearing severity over the time in the Olopatadine group.
Notes: Tearing was graded on a four points-Likert scale (absence, mild, moderate, severe), p > 0.001 for the GEE ordinal logistic regression. Each bar represents the percentage of patients in each degree of tearing by time.
Abbreviation: GEE, Generalized estimating equation.
Ocular burning: The degree of ocular burning decreased significantly over time in both the treatment groups (p < 0.0001). There were no statistically significant differences observed between the groups in terms of the resolution of ocular burning over time (OR = 0.78; 95% CI = (0.58–1.04); p = 0.096).
Foreign body sensation: The degree of foreign body sensation decreased significantly over time in both the treatment groups (p < 0.0001). No statistically significant differences were detected between the groups over time in the resolution of this symptom (OR = 0.86; 95% CI = (0.62–1.2); p = 0.390).
Ocular Signs
Conjunctival Hyperemia: Conjunctival hyperemia decreased significantly over time in both the treatment groups (p < 0.0001). No statistically significant differences were detected between groups over time in the resolution of this symptom (OR = 0.96; 95% CI = (0.76–1.23); p = 0.770).
Palpebral swelling: The severity of Palpebral swelling decreased significantly over time in both the treatment groups (p=0.003). No statistically significant differences were detected between the groups over time (OR = 1.16; 95% CI = (0.71–1.9); p = 0.548).
Ocular secretion: The degree of ocular secretion decreased significantly over time in both the treatment groups. There were no statistically significant differences between groups (OR = 0.54; 95% CI (0.28–1.52); p = 0.067).
Chemosis: After 15 days of treatment, all the patients who presented with this symptom at baseline showed complete resolution. No statistically significant differences were observed between the treatment groups (OR = 0.64; 95% CI = (0.11–3.6); p = 0.614).
Non-Ocular Symptoms
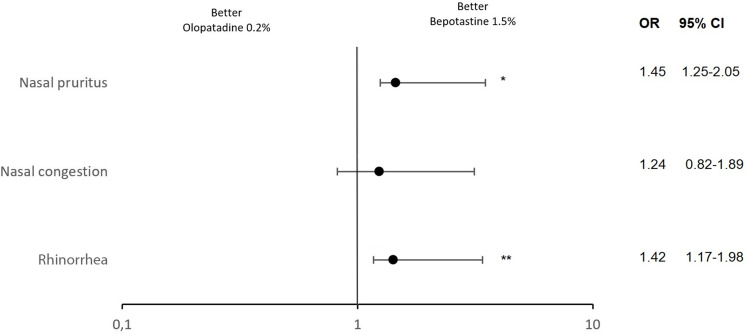
Nasal congestion: Nasal congestion severity decreased in patients who present with at least one non-ocular allergy symptom over time, with once-daily dosing in both treatment groups (p < 0.001). There were no statistically significant differences between the treatments in the reduction of the degree of nasal congestion over time (OR = 1.24; CI 95% = (0.82–1.89); p = 0.300) (Figure 4).
Figure 4.
Foster plot for non-ocular symptoms (nasal pruritus, nasal congestion, and rhinorrhea).
Notes: Nasal pruritus, nasal congestion, and rhinorrhea were graded on a four points-Likert scale (absence, mild, moderate, severe). OR is shown by closed circles and whiskers represents the 95% CI. *p=0.037 **p=0.040.
Abbreviations: OR, Odds ratio; CI, confidence interval.
Nasal pruritus: The severity of nasal pruritus significantly decreased over time in both the treatment groups (p < 0.001).
Bepotastine 1.5% LC administered once daily to patients with allergic rhinoconjunctivitis significantly reduced nasal pruritus with a probability 1.45 times greater than olopatadine 0.2% with BAK (OR = 1.45; CI 95% = (1.25–2.05); p = 0.037) (Figure 4).
Rhinorrhea: The degree of rhinorrhea in patients who at baseline presented at least one non-ocular symptom of allergy decreased significantly over time with once-daily administration in both treatment groups (p < 0.001).
Bepotastine 1.5% PF administered once daily to patients with allergic rhinoconjunctivitis significantly reduced the degree of rhinorrhea, with a probability 1.42 times greater than that of olopatadine 0.2% with BAK (OR = 1.42; 95% CI = (1.17–1.98); p = 0.040) (Figure 4).
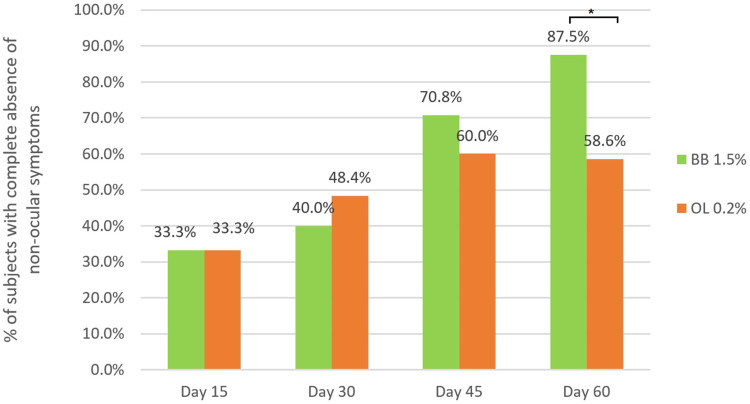
Complete resolution of non-ocular symptoms: The percentage of patients with total resolution of non-ocular symptoms increased significantly over time with once-daily dosing in both the treatment groups (p < 0.001). Bepotastine 1.5% PF, administered once daily to patients with allergic conjunctivitis, significantly alleviated non-ocular symptoms with 1.60 times higher probability compared to olopatadine 0.2% with BAK (OR = 1.60; 95% CI = 1.10–2.28; p = 0.014). At day 60 of treatment, approximately 90% of the patients treated with bepotastine besilate showed resolution of all nasal symptoms under analysis, whereas approximately 60% of the patients in the olopatadine group achieved complete resolution (p = 0.020) (Figure 5).
Figure 5.
Complete resolution of non-ocular symptoms over the time by group of treatment.
Notes: Each bar represents the percentage of patients with complete non-ocular symptoms resolution by time. *p=0.020 for the GEE binary logistic regression.
Abbreviations: BB, Bepotastine besilate 1.5% ophthalmic solution; OL, Olopatadine 0.2% ophthalmic solution.
Conjunctival Impression Cytology
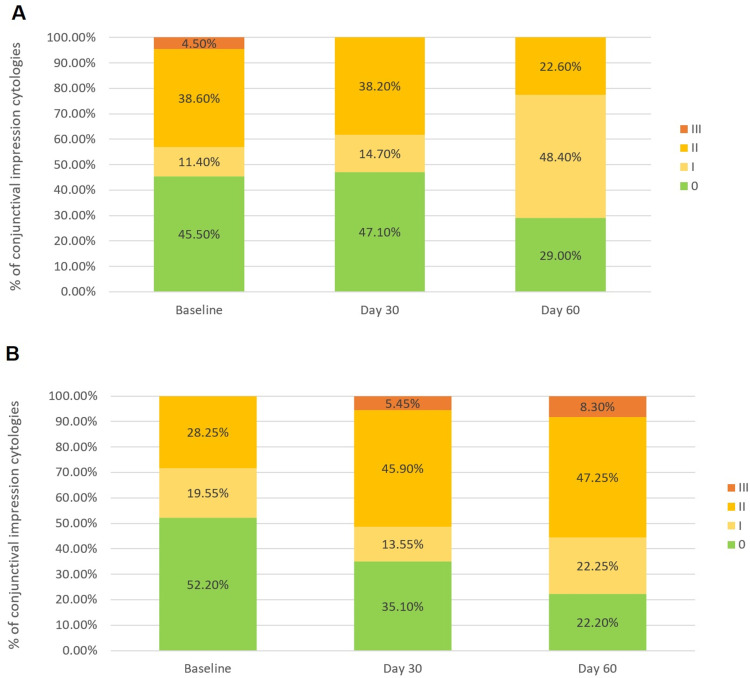
The histology of the conjunctival epithelium, classified using the Nelson scale, changed significantly over time in both treatment groups (p = 0.001).
Bepotastine 1.5% LC administered once daily improved the integrity of the conjunctival epithelium with a probability 2.00 times greater than that of olopatadine 0.2% with BAK (OR = 2.03; 95% CI = (1.19–3.34); p = 0.010). This toxic effect on the conjunctiva is likely due to the presence of BAK in the formulation. At day 60 after treatment, no citologies presented metaplasia in the bepotastine group, whereas three conjunctivas presented metaplasia in the olopatadine group (Figure 6).
Figure 6.
(A) Percentage of conjunctival impression cytology by Nelson classification over the time in the Bepotastine besilate group. (B) Percentage of conjunctival impression cytology by Nelson classification over the time in the Olopatadine group.
Note: p=0.010 for the GEE ordinal logistic regression.
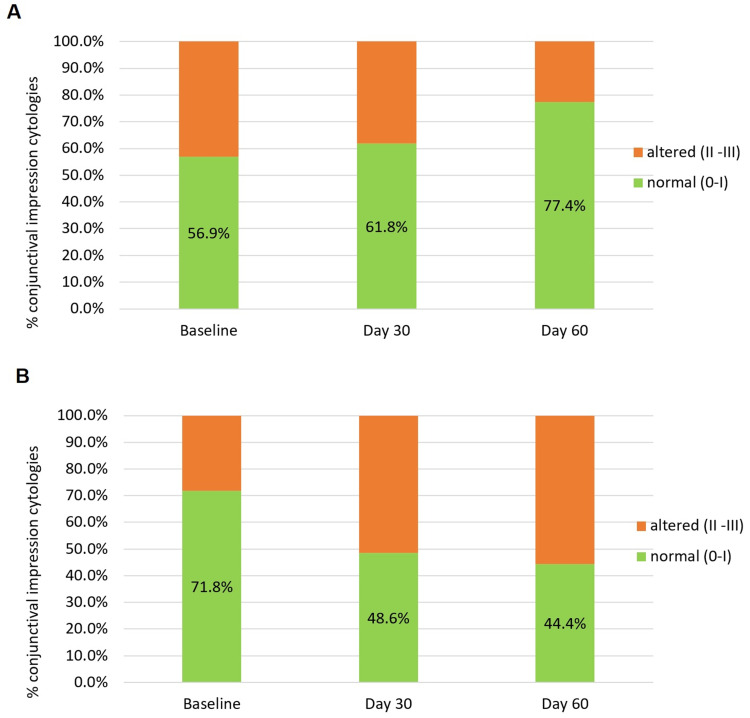
Analyzing the data set as binary outcome (normal and abnormal conjunctiva), probability of presenting with a normal epithelium over time was 2.7 greater in the bepotastine group than in the olopatadine (OR = 2.72, 95% CI = (1.50–4.94); p < 0.001). After 60 days of treatment, the normal conjunctiva in the olopatadine group decreased by 27.4%, whereas in the bepotastine group, there was an improvement of 20.5% (p = 0.006) (Figure 7).
Figure 7.
(A) Percentage of normal conjunctivas over the time in the Bepotastine besilate group. (B) Percentage of normal conjunctivas over the time in the Olopatadine group.
Notes: Normal conjunctivas were cytologies classified as 0 or I by Nelson classification. p<0.001 for the GEE binary logistic regression.
Abbreviation: GEE, Generalized estimating equation.
Safety
The safety population (n=97) was defined as subjects who received at least one dose of the test agent. Five ocular adverse events were reported: two in the bepotastine group (burning at instillation and palpebral allergic reaction) and three in the olopatadine group (periocular allergic reaction and viral conjunctivitis (2)). Four non-ocular adverse reactions reported by 3 of the 3 patients were possibly related to the treatment (dysgeusia in the bepotastine group and nausea and headache in the olopatadine group).
Discussion
Signs and symptoms of allergic conjunctivitis significantly decrease the quality of life and ability to function, sleep problems, decrease the ability to visual tasks, and social interactions. This leads to missed time at work, owing to visits to the doctor’s office and decreased productivity.42,43 Consequently, it is important to establish non-pharmacological, pharmacologically effective, and safe treatments.
Bepotastine besilate is a last-generation antihistamine drug with a high affinity for the H1 receptor without any appreciable action on other receptors associated with undesirable effects such as histamine H3, adrenergic (α1, α2, β), serotonin (5-HT2), muscarinic, and benzodiazepine receptors.44 This multi-action antiallergic drug was first approved in Japan as an oral tablet formulation indicated to allergic rhinitis (AR) treatment. Clinical trials have investigated its safety and non-sedating effects when administered orally.45 In 2009, an ophthalmic formulation was developed and approved by the FDA and Drug Administration for the treatment of ocular itching associated with allergic conjunctivitis when administered BID.46
The efficacy and safety of bepotastine besilate 1.5% ophthalmic solution were studied previously using the CAC model of allergic conjunctivitis. These studies reported positive effects, including a reduction in ocular itching, which lasted for up to 8 hr10,11 and even extended to 16 hr.12 Although the CAC model presents several advantages such as reproducibility, controlled allergen exposure, and the possibility of internal control when administered in the contralateral eye as a placebo solution, environmental models allow accurate assessment of chronic exposure to allergens with the normal fluctuating effects of geographic location, diversity in allergen sources, weather, and evidence of clinical improvements over several days or weeks, reflecting the real-life benefits of this therapeutic option.42 Carr and collaborators published a natural exposure, a placebo-control clinical trial where the efficacy of bepotastine besilate 1.5% BID over 2 weeks was evaluated. In this trial, bepotastine resulted in efficacy and safe treatment of allergic conjunctivitis administered twice a day compared with placebo solutions.47
We identified the need to compare bepotastine with other antiallergic treatments owing to the lack of published evidence. To the best of our knowledge, only two studies have compared bepotastine with other commercially available antiallergic ophthalmic solutions. Ayyappanavar et al compared bepotastine besilate 1.5% BID with olopatadine hydrochloride 0.2% and alcaftadine 0.25% administered once daily. Both antiallergic solutions were superior to Olopatadine analyzed by Total Ocular Symptom Scoring System (TOSS) after 14 days of treatments.48 McCabe et al assessed patient preferences for bepotastine besilate 1.5% administered twice daily and olopatadine 0.2% ophthalmic solution taken once daily in terms of patient-assessment relief and ocular comfort. Bepotastine besilate 1.5% BID provides better relief from ocular itching, nose itching, and morning and evening relief from ocular allergy symptoms than Olopatadine.49 However, there was no study that compared Bepotastine besilate 1.5% once a day vs any other antiallergic ophthalmic eye drop. In our study, we conducted a comparative analysis between reduced dosage of Bepotastine besilate 1.5% and olopatadine 0.2%, both administered once daily. Olopatadine is a well-established dual-action antihistamine that has been widely used worldwide for several decades. It has demonstrated a similar efficacy and safety profile in resolving symptoms and signs associated with allergic conjunctivitis. Upon evaluating the signs and symptoms related to AC, we found no statistically significant differences between this and treatments, except for tearing, where bepotastine exhibited superior effectiveness compared to Olopatadine group over time.
Symptoms of both conditions, AC and AR, coexist in more than 80% of allergic patients.17 Since there are similarities between the allergic response in the conjunctival and nasal mucosa and the pharmacological agents used to treat both conditions are the same, suggesting that bepotastine may relieve both nasal and ocular allergic symptoms. Cavet et al analyzed results from two Phase III CAC trials and a two-week natural-exposure allergen Phase IV study17 where nasal outcomes were analyzed as secondary outcomes. In both studies, bepotastine besilate administered twice a day improved the resolution of nasal symptoms associated with allergic conjunctivitis, such as nasal congestion, rhinorrhea, and nasal pruritus. In our study, we evaluated the resolution of these symptoms with a reduced posology once daily. Compared with olopatadine 0.2% administered once daily, bepotastine was superior in the resolution of non-ocular symptoms, reaching approximately 30% more patients with complete resolution of these symptoms after 60 days of treatment.
It is important to emphasize the impact of preservatives on ocular surface integrity. Long term use of BAK-preserved ophthalmic solutions is widely known to cause several impairments in superficial and deep ocular tissues.50 In this study, the comparator group (olopatadine 0.2% ophthalmic solution) was preserved with BAK 0.01%. This is one of the highest concentrations of BAK used to prepare eye drops. As documented in several experimental and clinical trials, BAK was induced in the first stage of its toxic action histopathological changes that initially remain asymptomatic but could then evolve to more severe stages of ocular surface disease.51 In our investigation, we studied by conjunctival impression cytology, the histology of patients exposed to both formulations. We found that when patients were exposed to olopatadine hydrochloride 0.2% BAK-preserved, the integrity of the conjunctival epithelium decreased by 27.4% after 60 days of treatment, while 20.5% of patients treated with preservative-free bepotastine besilate recovered normal conjunctival histology.
Since adherence to treatment regimens is related to clinical benefits and patient wellness, it is possible to administer this modern antiallergic ophthalmic solution that presents additional benefits, such as resolution of nasal symptoms associated with rhinoconjunctivitis, in a reduced posology once a day.
In addition, formulations without preservatives may turn bepotastine besilate 1.5% PF into the next gold standard treatment for AC in the near future.
This research, however, is subject to some limitations. It would be more appropriate to add an objective measurement of allergic disease resolution as IgE tear levels, although in clinical practice the evaluation of resolution of symptoms and signs associated with this pathology is usually followed by ophthalmologist evaluation of signs and patient self-reporting of symptoms.
Conclusion
In conclusion, bepotastine besilate 1.5% administered once a day is at least as effective as olopatadine hydrochloride 0.2% BAK-preserved once a day in the reduction of ocular symptoms and signs associated with allergic conjunctivitis. Additionally, it offers a more comprehensive approach to treating rhinoconjunctivitis due to its superior effectiveness in resolving non-ocular symptoms. Moreover, it stands out in terms of safety, as it exhibits lower cytotoxicity and ensures the preservation of ocular surface integrity throughout allergic treatment, thanks to its preservative-free formulation.
Acknowledgments
We would like to acknowledge Dr Cintia Nashiro, Julieta Geraldi, Mercedes O’ Farrell, Pablo Bouzon, and Gonzalo Gordillo for their contribution to the recruitment and follow-up of patients.
Abbreviations
AC, Allergic Conjunctivitis; AR, Allergic Rhinitis; BAK, Benzalkonium Chloride; BID, Twice a day; CAC, Conjunctival allergen challenge model; CIC, Conjunctival impression cytology; OSD, Ophthalmic Squeeze Dispenser; PF, Preservative-Free; TOSS, total ocular symptom scoring system.
Data Sharing Statement
Any data besides what is included in the manuscript will be shared. Data will be accessible from the corresponding author at any time.
Disclosure
Melina Sol del Papa and María Silvia Passerini are Poen Laboratories employees. Martin Berra, Fernanda Girado, and Alejandro Aguilar report on financial support provided by Poen Laboratories. Maria Cecilia Marini and Paula Albera report no conflicts of interest in this work.
References
- 1.Leonardi A, Castegnaro A, Valerio ALG, Lazzarini D. Epidemiology of allergic conjunctivitis: clinical appearance and treatment patterns in a population-based study. Curr Opin Allergy Clin Immunol. 2015;15(5):482–488. doi: 10.1097/ACI.0000000000000204 [DOI] [PubMed] [Google Scholar]
- 2.Rosario N, Bielory L. Epidemiology of allergic conjunctivitis. Curr Opin Allergy Clin Immunol. 2011;11:471–476. doi: 10.1097/ACI.0b013e32834a9676 [DOI] [PubMed] [Google Scholar]
- 3.Miyazaki D, Fukagawa K, Okamoto S, et al. Epidemiological aspects of allergic conjunctivitis. Allergol Int. 2020;69(4):487–495. doi: 10.1016/j.alit.2020.06.004 [DOI] [PubMed] [Google Scholar]
- 4.Bergmann MT, Williams JI, Gomes PJ. Treatment of allergic conjunctivitis with bepotastine besilate ophthalmic solution 1.5%. Clin Ophthalmol. 2014;8:1495–1505. doi: 10.2147/OPTH.S66637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phipatanakul W. Allergic rhinoconjunctivitis: epidemiology. Immunol Allergy Clin North Am. 2005;25(2):263–281. doi: 10.1016/j.iac.2005.03.001 [DOI] [PubMed] [Google Scholar]
- 6.Bielory L, Delgado L, Katelaris CH, Leonardi A, Rosario N, Vichyanoud P. ICON: diagnosis and management of allergic conjunctivitis. Ann Allergy Asthma Immunol. 2019;124:118–134. doi: 10.1016/j.anai.2019.11.014 [DOI] [PubMed] [Google Scholar]
- 7.Castillo M, Scott NW, Mustafa MZ, Mustafa MS, Azuara-Blanco A. Topical antihistamines and mast cell stabilisers for treating seasonal and perennial allergic conjunctivitis. Cochrane Database Syst Rev. 2015;2015(6). doi: 10.1002/14651858.CD009566.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andoh T, Kuraishi Y. Suppression by bepotastine besilate of substance P-induced itch-associated responses through the inhibition of the leukotriene B4 action in mice. Eur J Pharmacol. 2006;547(1–3):59–64. doi: 10.1016/j.ejphar.2006.07.009 [DOI] [PubMed] [Google Scholar]
- 9.Kida T, Fujii A, Sakai O, et al. Bepotastine besilate, a highly selective histamine H1 receptor antagonist, suppresses vascular hyperpermeability and eosinophil recruitment in in vitro and in vivo experimental allergic conjunctivitis models. Exp Eye Res. 2010;91(1):85–91. doi: 10.1016/j.exer.2010.04.006 [DOI] [PubMed] [Google Scholar]
- 10.Abelson MB, Torkildsen GL, Williams JI, Gow JA, Gomes PJ, McNamara TR. Time to onset and duration of action of the antihistamine bepotastine besilate ophthalmic solutions 1.0% and 1.5% in allergic conjunctivitis: a Phase III, single-center, prospective, randomized, double-masked, placebo-controlled, conjunctival allergen cha. Clin Ther. 2009;31(9):1908–1921. doi: 10.1016/j.clinthera.2009.09.001 [DOI] [PubMed] [Google Scholar]
- 11.Macejko TT, Bergmann MT, Williams JI, et al. Multicenter clinical evaluation of bepotastine besilate ophthalmic solutions 1.0% and 1.5% to treat allergic conjunctivitis. Am J Ophthalmol. 2010;150(1):122–127. doi: 10.1016/j.ajo.2010.02.007 [DOI] [PubMed] [Google Scholar]
- 12.Williams JI, Kennedy KS, Gow JA, et al. Prolonged effectiveness of bepotastine besilate ophthalmic solution for the treatment of ocular symptoms of allergic conjunctivitis. J Ocul Pharmacol Ther. 2011;27(4):385–393. doi: 10.1089/jop.2011.0005 [DOI] [PubMed] [Google Scholar]
- 13.Torkildsen GL, Gomes PJ, Williams JI, Gow JA, McNamara TR. Bepotastine besilate ophthalmic solution 1.5% reduces ocular itching in a clinical model of allergic conjunctivitis. J Allergy Clin Immunol. 2009;123(2):S57. doi: 10.1016/j.jaci.2008.12.186 [DOI] [Google Scholar]
- 14.Iordache A, Boruga M, Mușat O, Jipa DA, Tătaru CP, Mușat GC. Relationship between allergic rhinitis and allergic conjunctivitis (allergic rhinoconjunctivitis) - review. Rom J Ophthalmol. 2022;66(1):8–12. doi: 10.22336/rjo.2022.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berger W, Abelson MB, Gomes PJ, et al. Effects of adjuvant therapy with 0. 1 % olopatadine hydrochloride ophthalmic solution on quality of life in patients with allergic rhinitis using systemic or nasal therapy. Ann Allergy Asthma Immunol. 2005;95(4):361–371. doi: 10.1016/S1081-1206(10)61155-6 [DOI] [PubMed] [Google Scholar]
- 16.Bousquet J, Knani J, Hejjaoui A, Ferrando R, Cour P, Dhivert H. Heterogeneity of atopy. I. Clinical and immunologic characteristics of patients allergic to cypress pollen. Allergy. 1993;48:183–188. doi: 10.1111/j.1398-9995.1993.tb00711.x [DOI] [PubMed] [Google Scholar]
- 17.Cavet ME, Gomes PJ, Carr WW, Williams JI. Bepotastine besilate ophthalmic solution 1.5% for alleviating nasal symptoms in patients with allergic conjunctivitis. J Asthma Allergy. 2018;11:29–39. doi: 10.2147/JAA.S160687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torkildsen GL, Williams JI, Gow JA, Gomes PJ, Abelson MB, McNamara TR. Bepotastine besilate ophthalmic solution for the relief of nonocular symptoms provoked by conjunctival allergen challenge. Ann Allergy Asthma Immunol. 2010;105(1):57–64. doi: 10.1016/j.anai.2010.04.005 [DOI] [PubMed] [Google Scholar]
- 19.Goldstein MH, Silva FQ, Blender N, Tran T, Vantipalli S. Ocular benzalkonium chloride exposure: problems and solutions. Eye. 2022;36(2):361–368. doi: 10.1038/s41433-021-01668-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ammar DA, Noecker RJ, Kahook MY. Effects of benzalkonium chloride-preserved, polyquad-preserved, and sofZia-preserved topical glaucoma medications on human ocular epithelial cells. Adv Ther. 2010;27(11):837–845. doi: 10.1007/s12325-010-0070-1 [DOI] [PubMed] [Google Scholar]
- 21.Ayaki M, Iwasawa A. Cytotoxicity of prostaglandin analog eye drops preserved with benzalkonium chloride in multiple corneoconjunctival cell lines. Clin Ophthalmol. 2010;4(1):919–924. doi: 10.2147/OPTH.S13406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pauly A, Brasnu E, Riancho L, Brignole-Baudouin F, Baudouin C. Multiple endpoint analysis of BAC-preserved and unpreserved antiallergic eye drops on a 3D-reconstituted corneal epithelial model. Mol Vis. 2011;17:745–755. [PMC free article] [PubMed] [Google Scholar]
- 23.Epstein SP, Ahdoot M, Marcus E, Asbell PA. Comparative toxicity of preservatives on immortalized corneal and conjunctival epithelial cells. J Ocul Pharmacol Ther. 2009;25(2):113–119. doi: 10.1089/jop.2008.0098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tau J, Aguilar A, Berra A, Del Papa M, Passerini MS. Cytotoxicity evaluation of a novel latanoprost 0.005% nanoemulsion. Poster presentado en: 2021 European Association for Vision and Eye Research Virtual Congress; 2021.
- 25.Baudouin C, Denoyer A, Desbenoit N, Hamm G, Grise A. In vitro and in vivo experimental studies on trabecular meshwork degeneration induced by benzalkonium chloride (An American Ophthalmological Society Thesis). Trans Am Ophtalmol Soc. 2012;110:40–63. [PMC free article] [PubMed] [Google Scholar]
- 26.Izzotti A, La Maestra S, Micale RT, Longobardi MG, Saccà SC. Genomic and post-genomic effects of anti-glaucoma drugs preservatives in trabecular meshwork. Mutat Res. 2015;772:1–9. doi: 10.1016/j.mrfmmm.2014.11.006 [DOI] [PubMed] [Google Scholar]
- 27.Ammar DA, Kahook MY. Effects of glaucoma medications and preservatives on cultured human trabecular meshwork and non-pigmented ciliary epithelial cell lines. Br J Ophthalmol. 2011;95(10):1466–1469. doi: 10.1136/bjophthalmol-2011-300012 [DOI] [PubMed] [Google Scholar]
- 28.Kahook MY, Noecker R. Quantitative analysis of conjunctival goblet cells after chronic application of topical drops. Adv Ther. 2008;25(8):743–751. doi: 10.1007/s12325-008-0078-y [DOI] [PubMed] [Google Scholar]
- 29.Hedengran A, Freiberg JC, Hansen PM, et al. Generic benzalkonium chloride-preserved travoprost eye drops are not identical to the branded polyquaternium-1-preserved travoprost eye drop: effect on cultured human conjunctival goblet cells and their physicochemical properties. Acta Ophthalmol. 2022;100(7):819–827. doi: 10.1111/aos.15163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagai N, Murao T, Okamoto N, Ito Y. Comparison of corneal wound healing rates after instillation of commercially available latanoprost and travoprost in rat debrided corneal epithelium. J Oleo Sci. 2010;59(3):135–141. doi: 10.5650/jos.59.135 [DOI] [PubMed] [Google Scholar]
- 31.Liang H, Brignole-Baudouin F, Riancho L, Baudouin C. Reduced in vivo ocular surface toxicity with polyquad-preserved travoprost versus benzalkonium-preserved travoprost or latanoprost ophthalmic solutions. Ophthalmic Res. 2012;48(2):89–101. doi: 10.1159/000335984 [DOI] [PubMed] [Google Scholar]
- 32.Pauly A, Meloni M, Bringole-Baudouin F, Warnet JM, Baudouin C. Multiple endpoint analysis of the 3D-reconstituted corneal epithelium after treatment with benzalkonium chloride: early detection of toxic damage. Invest Ophthalmol Vis Sci. 2009;50:2644. doi: 10.1167/iovs.08-2992 [DOI] [PubMed] [Google Scholar]
- 33.Kim JH, Kim EJ, Kim YH, et al. In vivo effects of preservative-free and preserved prostaglandin analogs: mouse ocular surface study. Korean J Ophthalmol. 2015;29(4):270–279. doi: 10.3341/kjo.2015.29.4.270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pisella PJ, Pouliquen P, Baudouin C, Grasso CM. Prevalence of ocular symptoms and signs with preserved and preservative free glaucoma medication. Br J Ophthalmol. 2002;86:418–423. doi: 10.1136/bjo.86.4.418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jaenen N, Baudouin C, Pouliquen P, Manni G, Figueiredo A, Zeyen T. Ocular symptoms and signs with preserved and preservative-free glaucoma medications. Eur J Ophthalmol. 2007;17(3):341–349. doi: 10.1177/112067210701700311 [DOI] [PubMed] [Google Scholar]
- 36.Uusitalo H, Egorov E, Kaarniranta K, Astakhov Y, Ropo A. Benefits of switching from latanoprost to preservative-free tafluprost eye drops: a meta-analysis of two phase IIIb clinical trials. Clin Ophthalmol. 2016;10:445–454. doi: 10.2147/OPTH.S91402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang C, Wang H, Pan J, et al. Benzalkonium chloride induces subconjunctival fibrosis through the COX-2-modulated activation of a TGF-β1/Smad3 signaling pathway. Investig Ophthalmol Vis Sci. 2014;55(12):8111–8122. doi: 10.1167/iovs.14-14504 [DOI] [PubMed] [Google Scholar]
- 38.Baudouin C, Pisella PJ, Fillacier K, et al. Ocular surface inflammatory changes induced by topical antiglaucoma drugs: human and animal studies. Ophthalmology. 1999;106(3):556–563. doi: 10.1016/S0161-6420(99)90116-1 [DOI] [PubMed] [Google Scholar]
- 39.Leung EW, Medeiros FA, Weinreb RN. Prevalence of ocular surface disease in glaucoma patients. J Glaucoma. 2008;17(5):350–355. doi: 10.1097/IJG.0b013e31815c5f4f [DOI] [PubMed] [Google Scholar]
- 40.Casiraghi JF, Grigera D, Alejo Peyret J, Del Papa M, Passerini MS. Efficacy and tolerability of a new latanoprost 0.005% BAK-free nanoemulsion: a nonrandomized open-label trial. ReGEN Open. 2021;1(1):110–116. [Google Scholar]
- 41.Nelson JD. Impression Cytology. Cornea. 1988;7(1):71–81. doi: 10.1097/00003226-198801000-00012 [DOI] [PubMed] [Google Scholar]
- 42.Abelson MB, Loeffler O. Conjunctival allergen challenge: models in the investigation of ocular allergy. Curr Allergy Asthma Rep. 2003;3(4):363–368. doi: 10.1007/s11882-003-0100-z [DOI] [PubMed] [Google Scholar]
- 43.Dupuis P, Prokopich CL, Hynes A, Kim H. A contemporary look at allergic conjunctivitis. Allergy Asthma Clin Immunol. 2020;16(1). doi: 10.1186/s13223-020-0403-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kato M, Nishida A, Aga Y, et al. Pharmacokinetic and pharmacodynamic evaluation of central effect of the novel antiallergic agent bepotastine besilate. Arzneimittel. 1997;47(10):1116–1124. [PubMed] [Google Scholar]
- 45.Carrillo-martin I, Gonzalez-estrada A, Dimov V. Bepotastine besilate for the treatment of perennial allergic rhinitis. Expert Opin Pharmacother. 2018;19(15):1727–1730. doi: 10.1080/14656566.2018.1519020 [DOI] [PubMed] [Google Scholar]
- 46.Williams JI, Gow JA, Klier SM, McCue SL, Salapatek AMF, McNamara TR. Non-clinical pharmacology, pharmacokinetics, and safety findings for the antihistamine bepotastine besilate. Curr Med Res Opin. 2010;26(10):2329–2338. doi: 10.1185/03007995.2010.486753 [DOI] [PubMed] [Google Scholar]
- 47.Carr WW, Nayak AS, Ratner PH, Gow JA, McNamara TR, Williams JI. Efficacy of bepotastine besilate ophthalmic solution 1.5% for seasonal allergic conjunctivitis: a randomized, placebo-controlled, natural exposure, clinical trial. Allergy Asthma Proc. 2013;34(3):247–254. doi: 10.2500/aap.2013.34.3671 [DOI] [PubMed] [Google Scholar]
- 48.Ayyappanavar S, Sridhar S, Kumar K, et al. Comparative analysis of safety and efficacy of alcaftadine 0.25%, olopatadine hydrochloride 0.2% and bepotastine besilate 1.5% in allergic conjunctivitis. Indian J Ophthalmol. 2021;69(2):257–261. doi: 10.4103/ijo.IJO_2083_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McCabe CF, McCabe SE. Comparative efficacy of bepotastine besilate 1.5% ophthalmic solution versus olopatadine hydrochloride 0.2% ophthalmic solution evaluated by patient preference. Clin Ophthalmol. 2012;6(1):1731–1738. doi: 10.2147/OPTH.S35431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baudouin C, Labbé A, Liang H, Pauly A, Brignole-Baudouin F. Preservatives in eyedrops: the good, the bad and the ugly. Prog Retin Eye Res. 2010;29(4):312–334. doi: 10.1016/j.preteyeres.2010.03.001 [DOI] [PubMed] [Google Scholar]
- 51.Baudouin C, Kolko M, Melik-Parsadaniantz S, Messmer EM. Inflammation in glaucoma: from the back to the front of the eye, and beyond. Prog Retin Eye Res. 2021;83:100916. doi: 10.1016/j.preteyeres.2020.100916 [DOI] [PubMed] [Google Scholar]