Abstract
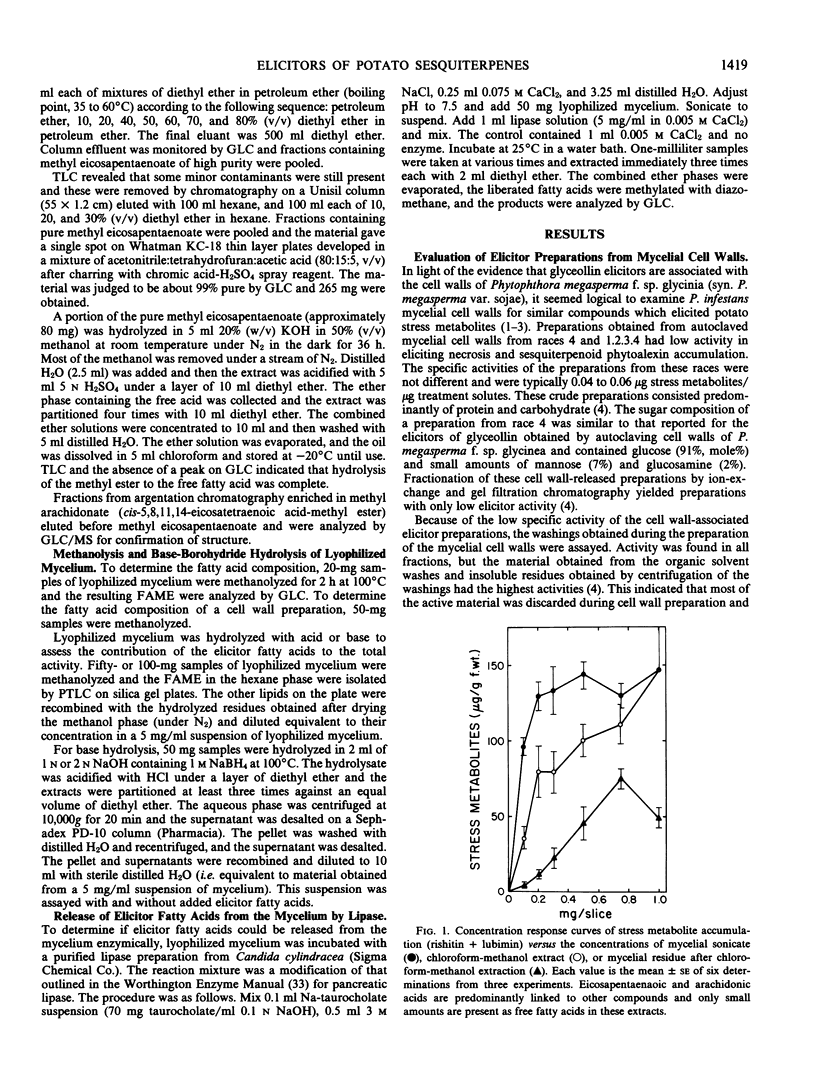
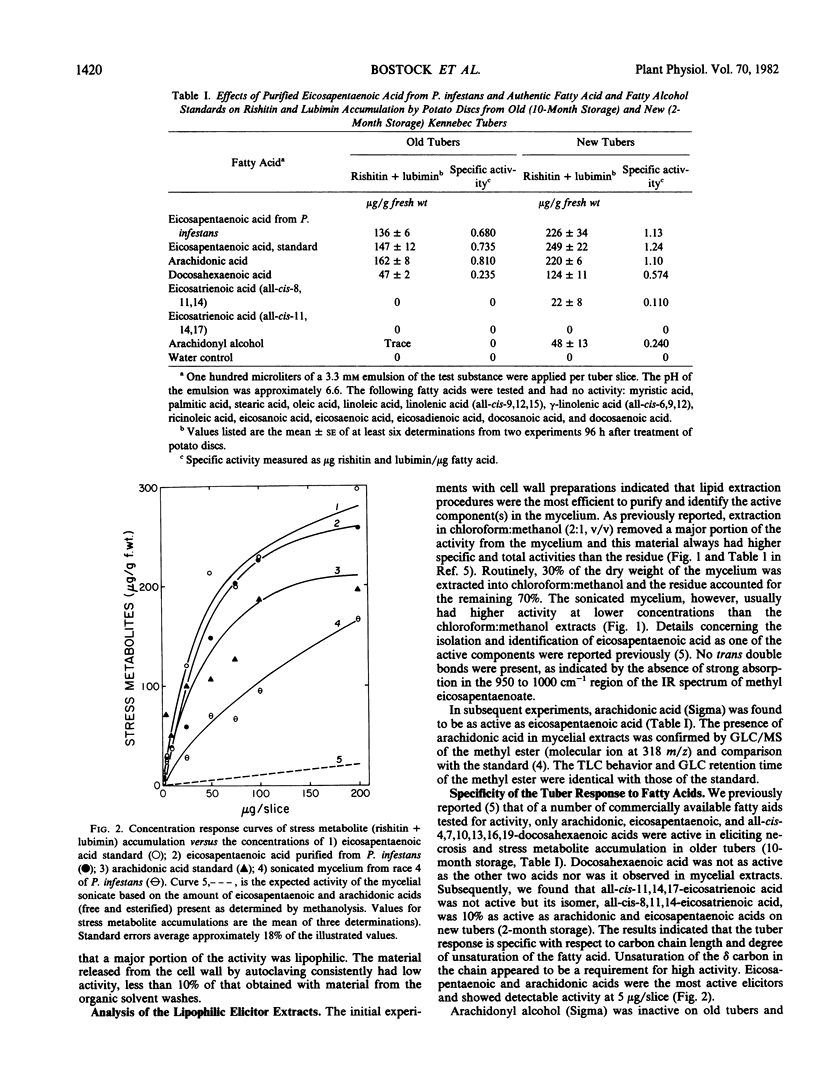
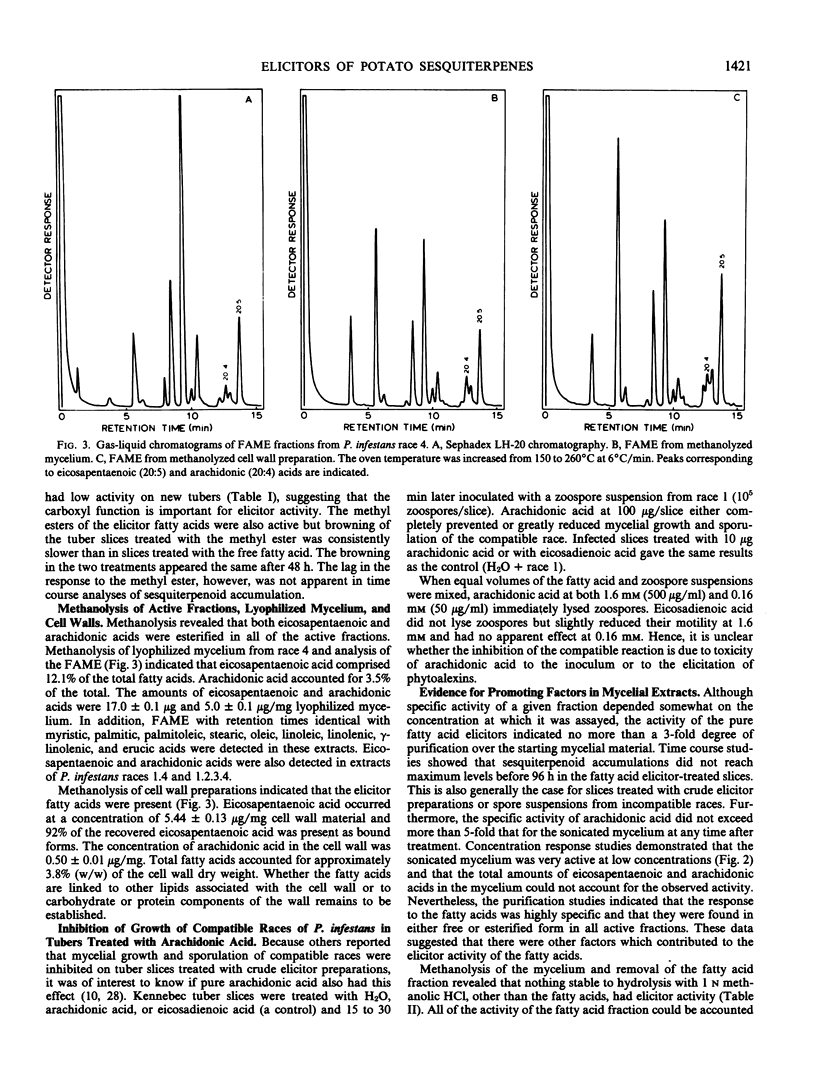
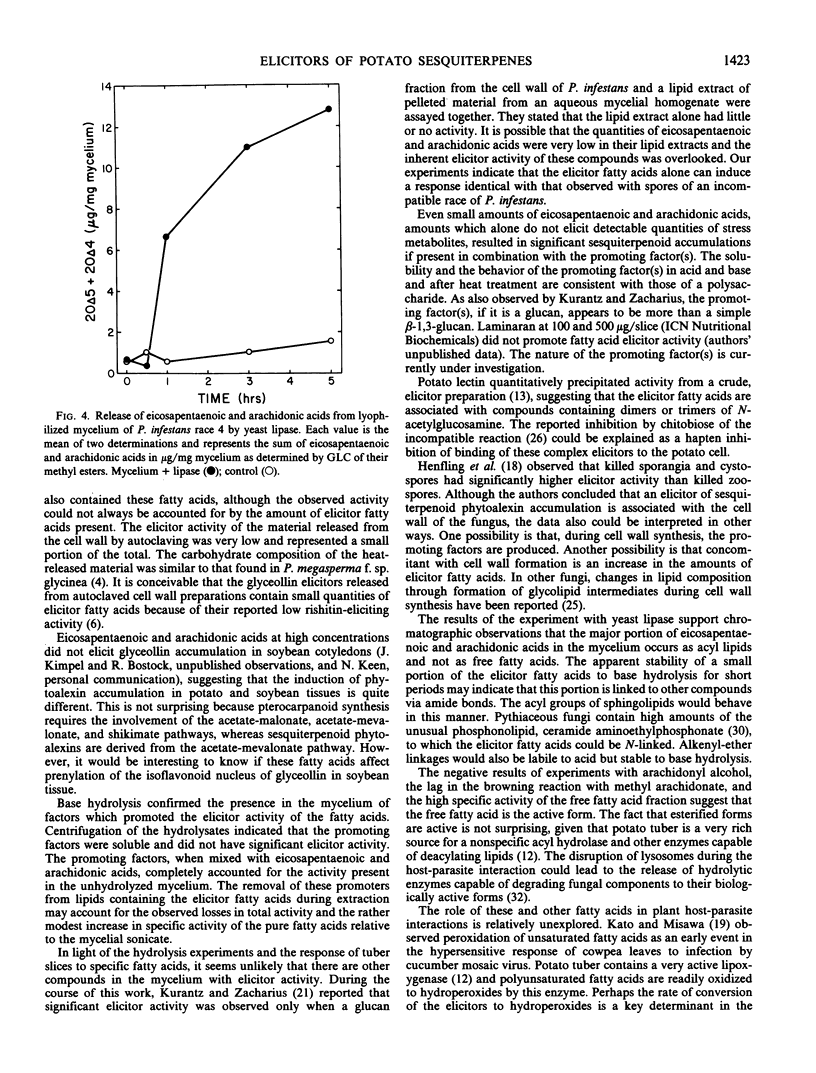
Eicosapentaenoic and arachidonic acids in extracts of Phytophthora infestans mycelium were identified as the most active elicitors of sesquiterpenoid phytoalexin accumulation in potato tuber slices. These fatty acids were found free or esterified in all fractions with elicitor activity including cell wall preparations. Yeast lipase released a major portion of eicosapentaenoic and arachidonic acids from lyophilized mycelium. Concentration response curves comparing the elicitor activity of the polyunsaturated fatty acids to a cell-free sonicate of P. infestans mycelium indicated that the elicitor activity of the sonicated mycelium exceeded that which would be obtained by the amount of eicosapentaenoic and arachidonic acids (free and esterified) present in the mycelium. Upon acid hydrolysis of lyophilized mycelium, elicitor activity was obtained only from the fatty acid fraction. However, the fatty acids accounted for only 21% of the activity of the unhydrolyzed mycelium and the residue did not enhance their activity. Centrifugation of the hydrolysate, obtained from lyophilized mycelium treated with 2n NaOH, 1 molarity NaBH4 at 100°C, yielded a supernatant fraction with little or no elicitor activity. Addition of this material to the fatty acids restored the activity to that which was present in the unhydrolyzed mycelium. The results indicate that the elicitor activity of the unsaturated fatty acids is enhanced by heat and base-stable factors in the mycelium.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albersheim P., Valent B. S. Host-pathogen interactions in plants. Plants, when exposed to oligosaccharides of fungal origin, defend themselves by accumulating antibiotics. J Cell Biol. 1978 Sep;78(3):627–643. doi: 10.1083/jcb.78.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayers A. R., Ebel J., Valent B., Albersheim P. Host-Pathogen Interactions: X. Fractionation and Biological Activity of an Elicitor Isolated from the Mycelial Walls of Phytophthora megasperma var. sojae. Plant Physiol. 1976 May;57(5):760–765. doi: 10.1104/pp.57.5.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayers A. R., Valent B., Ebel J., Albersheim P. Host-Pathogen Interactions: XI. Composition and Structure of Wall-released Elicitor Fractions. Plant Physiol. 1976 May;57(5):766–774. doi: 10.1104/pp.57.5.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostock R. M., Kuc J. A., Laine R. A. Eicosapentaenoic and Arachidonic Acids from Phytophthora infestans Elicit Fungitoxic Sesquiterpenes in the Potato. Science. 1981 Apr 3;212(4490):67–69. doi: 10.1126/science.212.4490.67. [DOI] [PubMed] [Google Scholar]
- Cline K., Wade M., Albersheim P. Host-Pathogen Interactions: XV. Fungal Glucans Which Elicit Phytoalexin Accumulation in Soybean Also Elicit the Accumulation of Phytoalexins in Other Plants. Plant Physiol. 1978 Dec;62(6):918–921. doi: 10.1104/pp.62.6.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Gellerman J. L., Schlenk H. Methyl-directed desaturation of arachidonic to eicosapentaenoic acid in the fungus, Saprolegnia parasitica. Biochim Biophys Acta. 1979 Apr 27;573(1):23–30. doi: 10.1016/0005-2760(79)90169-3. [DOI] [PubMed] [Google Scholar]
- Golbeck J. H., Cammarata K. V. Spinach Thylakoid Polyphenol Oxidase : ISOLATION, ACTIVATION, AND PROPERTIES OF THE NATIVE CHLOROPLAST ENZYME. Plant Physiol. 1981 May;67(5):977–984. doi: 10.1104/pp.67.5.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen N. T. Specific elicitors of plant phytoalexin production: detenninants of race specificity in pathogens? Science. 1975 Jan 10;187(4171):74–75. doi: 10.1126/science.187.4171.74. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Wassef M. K., Hendrix J. W. Ceramide aminoethylphosphonate in the fungus Pythium prolatum. Biochim Biophys Acta. 1976 Jan 18;486(1):172–178. doi: 10.1016/0005-2760(77)90081-9. [DOI] [PubMed] [Google Scholar]


