Abstract
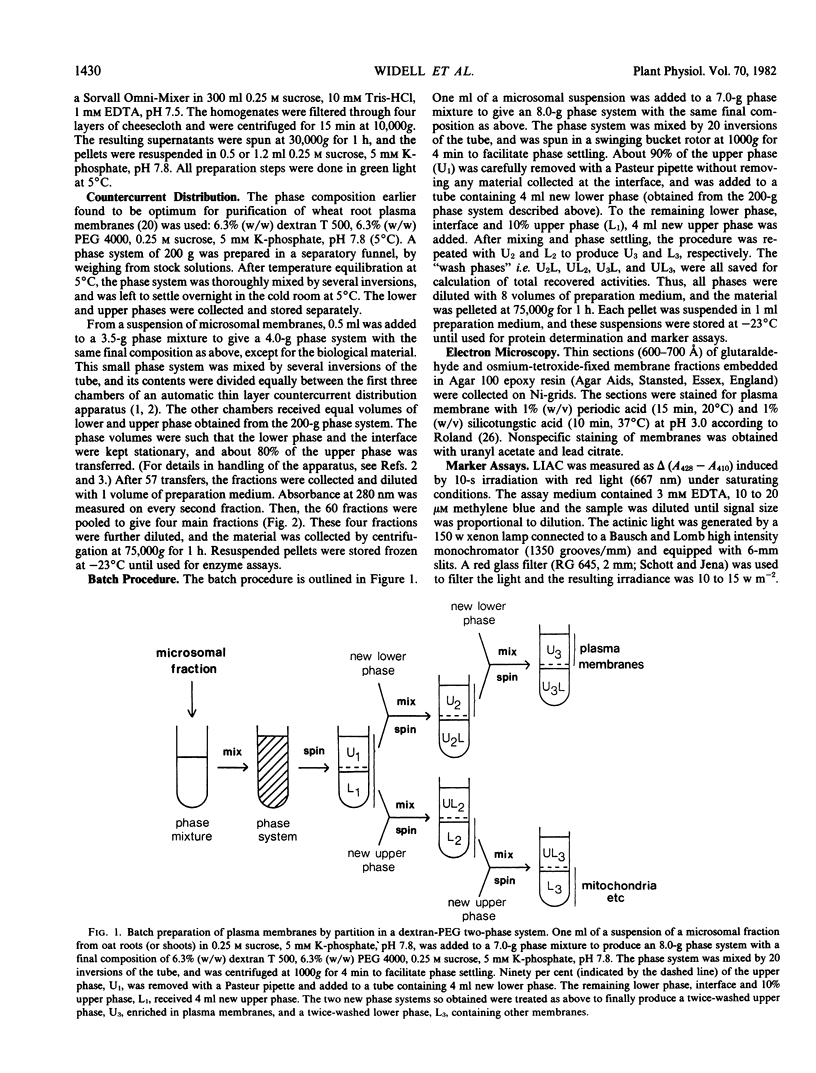
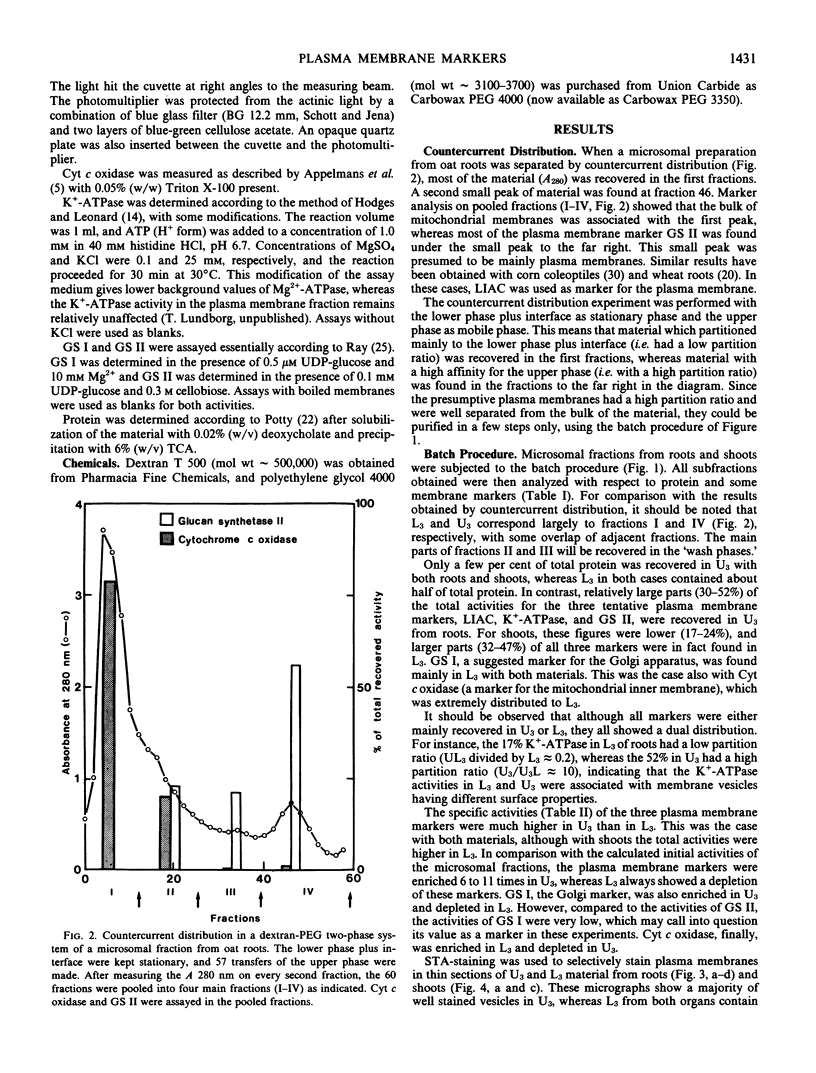
Presumptive plasma membrane fractions have been prepared from oat (Avena sativa L. cv. Brighton) roots and shoots, respectively, by partition of microsomal fractions in a dextran-polyethylene glycol two-phase system. The plasma membranes had a high affinity for the polyethylene glycol-rich upper phase, whereas membranes from mitochondria and other organelles partitioned in the dextran-rich lower phase or at the interface. Thus, relatively pure plasma membranes were obtained by only two partition steps, and within 3 hours from homogenization of the material.
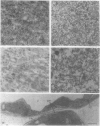
The plasma membranes from both organs were enriched in K+-stimulated Mg2+-dependent ATPase and glucan synthetase II, two tentative markers for the plant plasma membrane. Silicotungstic acid, an indicative stain for the plasma membrane, stained the vesicles recovered from the upper phase, but failed to stain the membranes partitioning in the lower phase or at the interface.
The plasma membranes were also enriched in a light-reducible b-cytochrome. This b-cytochrome can be measured by its light-induced absorbance change and may serve as a marker for the plant plasma membrane.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APPELMANS F., WATTIAUX R., DE DUVE C. Tissue fractionation studies. 5. The association of acid phosphatase with a special class of cytoplasmic granules in rat liver. Biochem J. 1955 Mar;59(3):438–445. doi: 10.1042/bj0590438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertsson P. A., Andersson B., Larsson C., Akerlund H. E. Phase partition--a method for purification and analysis of cell organelles and membrane vesicles. Methods Biochem Anal. 1982;28:115–150. doi: 10.1002/9780470110485.ch2. [DOI] [PubMed] [Google Scholar]
- Albertsson P. A. Partition of cell particles and macromolecules in polymer two-phase systems. Adv Protein Chem. 1970;24:309–341. doi: 10.1016/s0065-3233(08)60244-2. [DOI] [PubMed] [Google Scholar]
- Andersson B., Akerlund H. E. Inside-out membrane vesicles isolated from spinach thylakoids. Biochim Biophys Acta. 1978 Sep 7;503(3):462–472. doi: 10.1016/0005-2728(78)90145-7. [DOI] [PubMed] [Google Scholar]
- Brain R. D., Freeberg J. A., Weiss C. V., Briggs W. R. Blue light-induced Absorbance Changes in Membrane Fractions from Corn and Neurospora. Plant Physiol. 1977 May;59(5):948–952. doi: 10.1104/pp.59.5.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher J. D., Hansen D., Hodges T. K. Correlation between ion fluxes and ion-stimulated adenosine triphosphatase activity of plant roots. Plant Physiol. 1970 Dec;46(6):812–814. doi: 10.1104/pp.46.6.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallberg M., Larsson C. Compartmentation and export of 14CO2 fixation products in mesophyll protoplasts from the C4-plant Digitaria sanguinalis. Arch Biochem Biophys. 1981 Apr 15;208(1):121–130. doi: 10.1016/0003-9861(81)90130-2. [DOI] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T., Bracker C. E., Keenan T. W. Purification of an ion-stimulated adenosine triphosphatase from plant roots: association with plasma membranes. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3307–3311. doi: 10.1073/pnas.69.11.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T. Purification of a plasma membrane-bound adenosine triphosphatase from plant roots. Methods Enzymol. 1974;32:392–406. doi: 10.1016/0076-6879(74)32039-3. [DOI] [PubMed] [Google Scholar]
- Larsson C., Collin C., Albertsson P. A. Characterization of three classes of chloroplasts obtained by counter-current distribution. Biochim Biophys Acta. 1971 Sep 7;245(2):425–438. doi: 10.1016/0005-2728(71)90160-5. [DOI] [PubMed] [Google Scholar]
- Leonard R. T., Vanderwoude W. J. Isolation of plasma membranes from corn roots by sucrose density gradient centrifugation: an anomalous effect of ficoll. Plant Physiol. 1976 Jan;57(1):105–114. doi: 10.1104/pp.57.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M. Auxin-binding Sites of Maize Coleoptiles Are Localized on Membranes of the Endoplasmic Reticulum. Plant Physiol. 1977 Apr;59(4):594–599. doi: 10.1104/pp.59.4.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland J. C., Lembi C. A., Morré D. J. Phosphotungstic acid-chromic acid as a selective electron-dense stain for plasma membranes of plant cells. Stain Technol. 1972 Jul;47(4):195–200. doi: 10.3109/10520297209116484. [DOI] [PubMed] [Google Scholar]
- Van Der Woude W. J., Lembi C. A., Morré D. J. beta-Glucan Synthetases of Plasma Membrane and Golgi Apparatus from Onion Stem. Plant Physiol. 1974 Sep;54(3):333–340. doi: 10.1104/pp.54.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]