Abstract
The effects of aminoacetonitrile (a competitive inhibitor of glycine oxidation) on net photosynthesis, glycolate pathway intermediates, and ribulose-1,5-bisphosphate (RuBP) levels have been investigated at different O2 and CO2 concentrations with soybean (Glycine max)[L] Merr. cv Pioneer 1677) leaf discs floated on 25 millimolar aminoacetonitrile (AAN) for 50 minutes prior to assay.
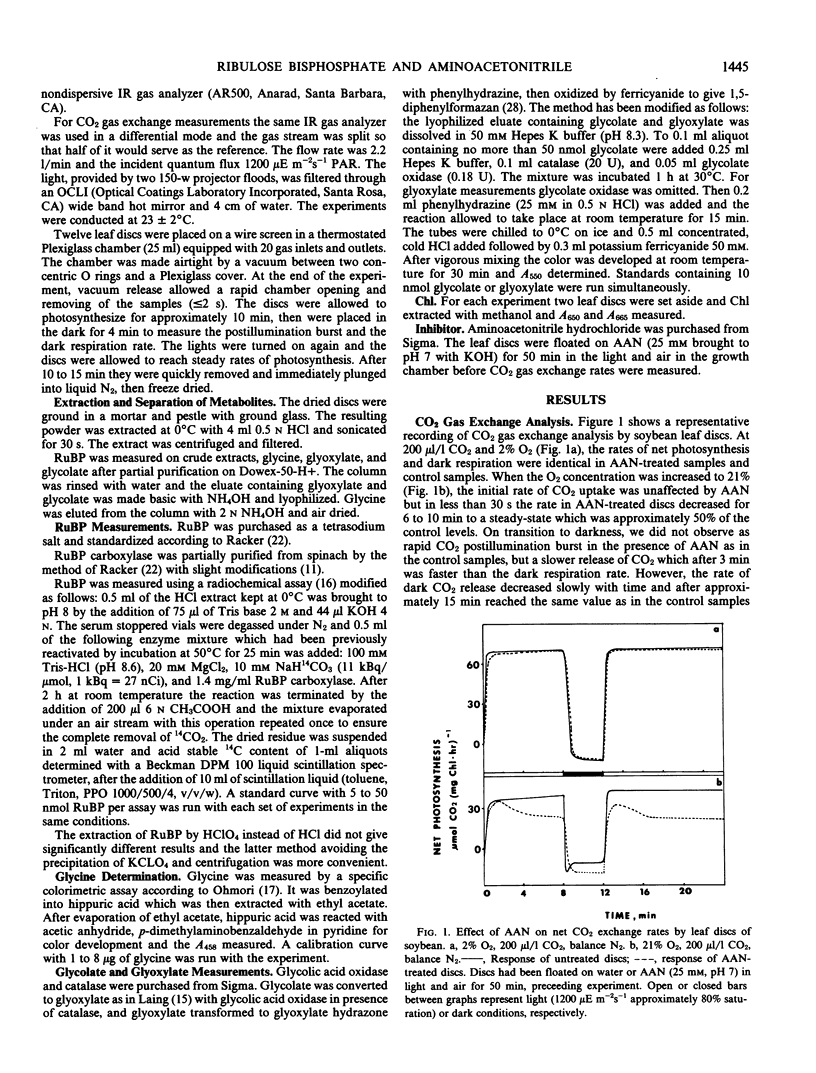
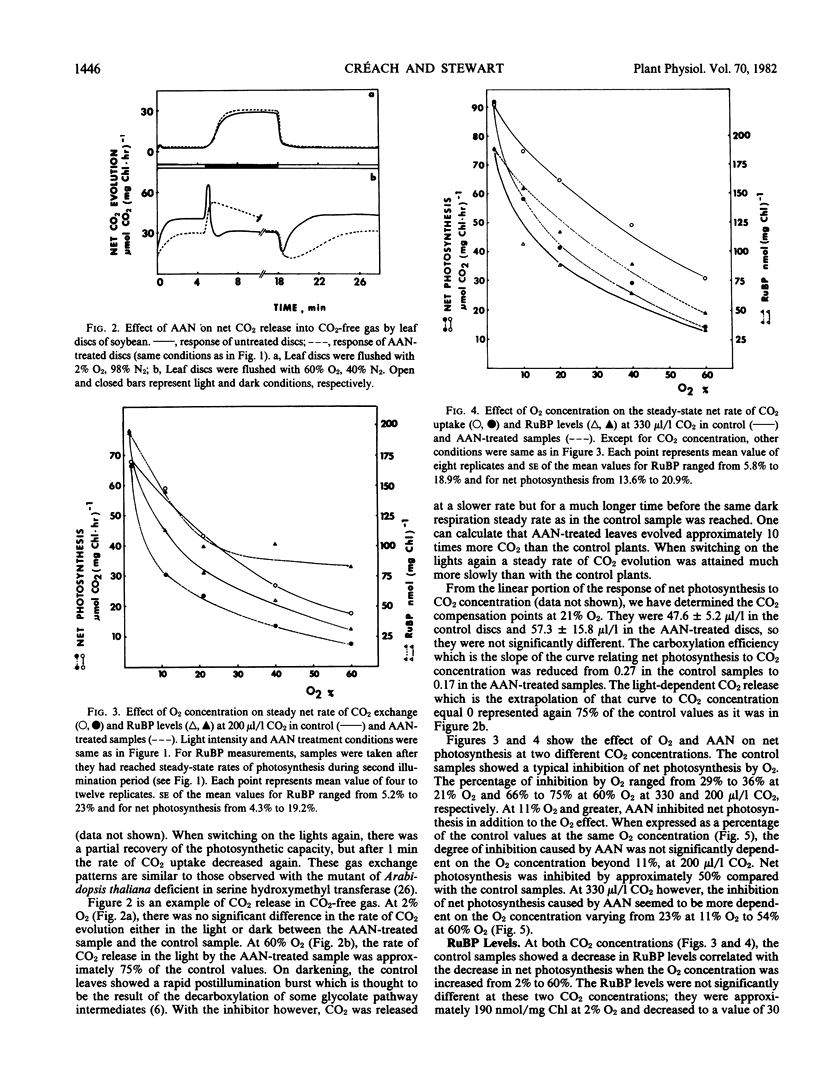
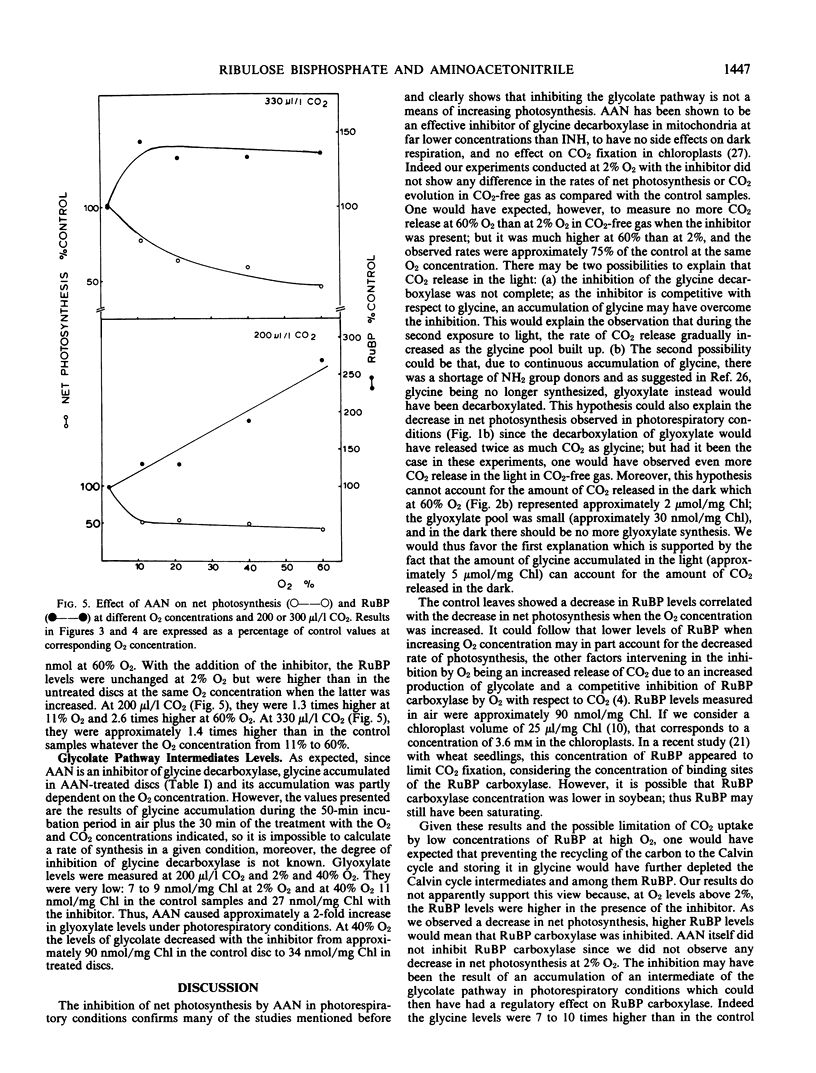
At 2% O2 and 200 or 330 microliters per liter CO2, the inhibitor had no effect on the rate of net photosynthesis and RuBP levels when compared with the control levels. At 11% to 60% O2, AAN caused a decrease in net photosynthesis in addition to the inhibition by O2. This extra inhibition ranged from 22% to 59% depending on the O2 and CO2 concentrations. The levels of RuBP, however, were 1.3 to 2.7 times higher than in the control plants at the same O2 concentrations. At 40% O2 and 200 microliters per liter CO2, the inhibitor caused a 6-fold increase in glycine and more than 2-fold increase in glyoxylate levels, whereas those of glycolate decreased by approximately one-half.
The decrease in net photosynthesis observed with AAN is not the result of the depletion of the RuBP pool due to the lack of recycling of carbon from the glycolate pathway to the Calvin cycle. The higher levels of RuBP caused by AAN in photorespiratory conditions, suggest that RuBP carboxylase was inhibited. Glyoxylate could be a possible candidate for the inhibition of the enzyme but what is known so far about its inhibitory properties in vitro may not fit the existing in vivo conditions. An alternative explanation for the inhibition is proposed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews T. J., Lorimer G. H., Tolbert N. E. Ribulose diphosphate oxygenase. I. Synthesis of phosphoglycolate by fraction-1 protein of leaves. Biochemistry. 1973 Jan 2;12(1):11–18. doi: 10.1021/bi00725a003. [DOI] [PubMed] [Google Scholar]
- Doravari S., Canvin D. T. Effect of butyl 2-hydroxy-3-butynoate on sunflower leaf photosynthesis and photorespiration. Plant Physiol. 1980 Oct;66(4):628–631. doi: 10.1104/pp.66.4.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt H. W., Sauer F. The inner membrane of the chloroplast envelope as the site of specific metabolite transport. Biochim Biophys Acta. 1971 Apr 6;234(1):83–91. doi: 10.1016/0005-2728(71)90133-2. [DOI] [PubMed] [Google Scholar]
- Hitz W. D., Stewart C. R. Oxygen and Carbon Dioxide Effects on the Pool Size of Some Photosynthetic and Photorespiratory Intermediates in Soybean (Glycine max [L.] Merr.). Plant Physiol. 1980 Mar;65(3):442–446. doi: 10.1104/pp.65.3.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmori S., Ikeda M., Watanabe Y., Hirota K. A simple and specific determination of glycine in biological samples. Anal Biochem. 1978 Oct 15;90(2):662–670. doi: 10.1016/0003-2697(78)90159-8. [DOI] [PubMed] [Google Scholar]
- Oliver D. J. Role of Glycine and Glyoxylate Decarboxylation in Photorespiratory CO(2) Release. Plant Physiol. 1981 Nov;68(5):1031–1034. doi: 10.1104/pp.68.5.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D. J. The effect of glyoxylate on photosynthesis and photorespiration by isolated soybean mesophyll cells. Plant Physiol. 1980 May;65(5):888–892. doi: 10.1104/pp.65.5.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D. J., Zelitch I. Increasing photosynthesis by inhibiting photorespiration with glyoxylate. Science. 1977 Jun 24;196(4297):1450–1451. doi: 10.1126/science.867040. [DOI] [PubMed] [Google Scholar]
- Perchorowicz J. T., Raynes D. A., Jensen R. G. Light limitation of photosynthesis and activation of ribulose bisphosphate carboxylase in wheat seedlings. Proc Natl Acad Sci U S A. 1981 May;78(5):2985–2989. doi: 10.1073/pnas.78.5.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathnam C. K., Chollet R. Photosynthetic and Photorespiratory Carbon Metabolism in Mesophyll Protoplasts and Chloroplasts Isolated from Isogenic Diploid and Tetraploid Cultivars of Ryegrass (Lolium perenne L.). Plant Physiol. 1980 Mar;65(3):489–494. doi: 10.1104/pp.65.3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville C. R., Ogren W. L. Photorespiration mutants of Arabidopsis thaliana deficient in serine-glyoxylate aminotransferase activity. Proc Natl Acad Sci U S A. 1980 May;77(5):2684–2687. doi: 10.1073/pnas.77.5.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville C. R., Ogren W. L. Photorespiration-deficient Mutants of Arabidopsis thaliana Lacking Mitochondrial Serine Transhydroxymethylase Activity. Plant Physiol. 1981 Apr;67(4):666–671. doi: 10.1104/pp.67.4.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogels G. D., Van der Drift C. Differential analyses of glyoxylate derivatives. Anal Biochem. 1970 Jan;33(1):143–157. doi: 10.1016/0003-2697(70)90448-3. [DOI] [PubMed] [Google Scholar]
- Zelitch I. Increased rate of net photosynthetic carbon dioxide uptake caused by the inhibition of glycolate oxidase. Plant Physiol. 1966 Dec;41(10):1623–1631. doi: 10.1104/pp.41.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]


