Abstract
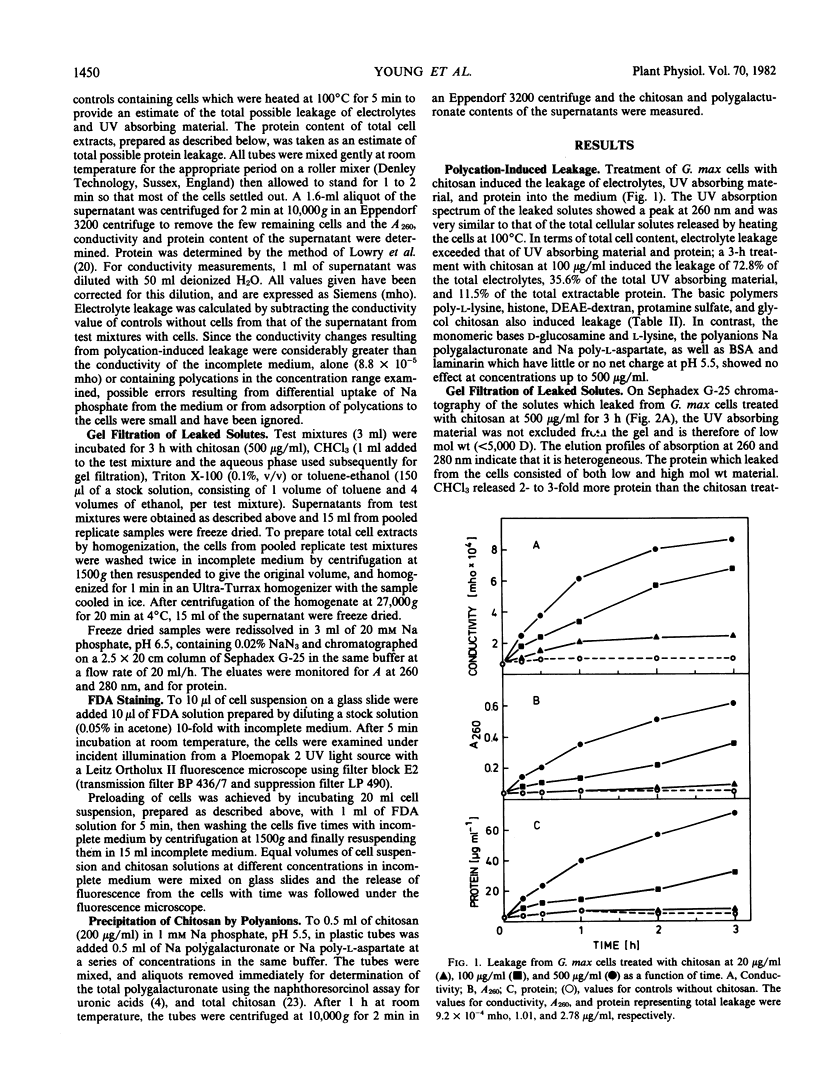
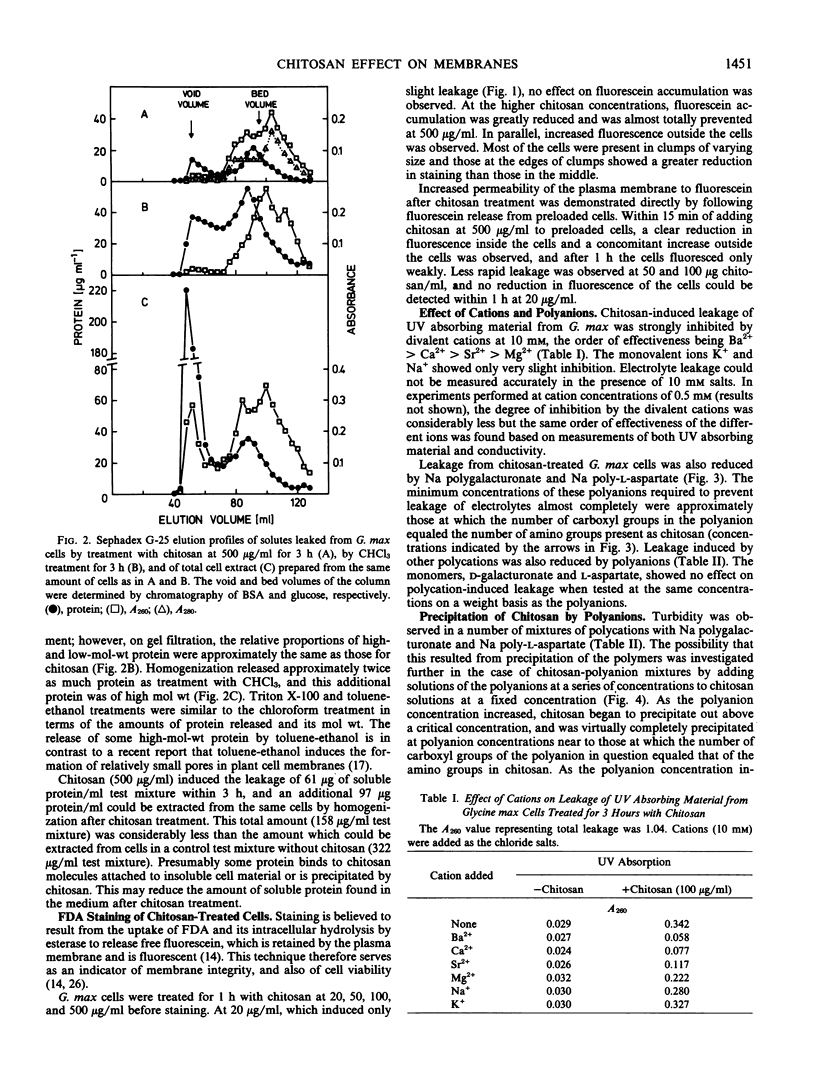
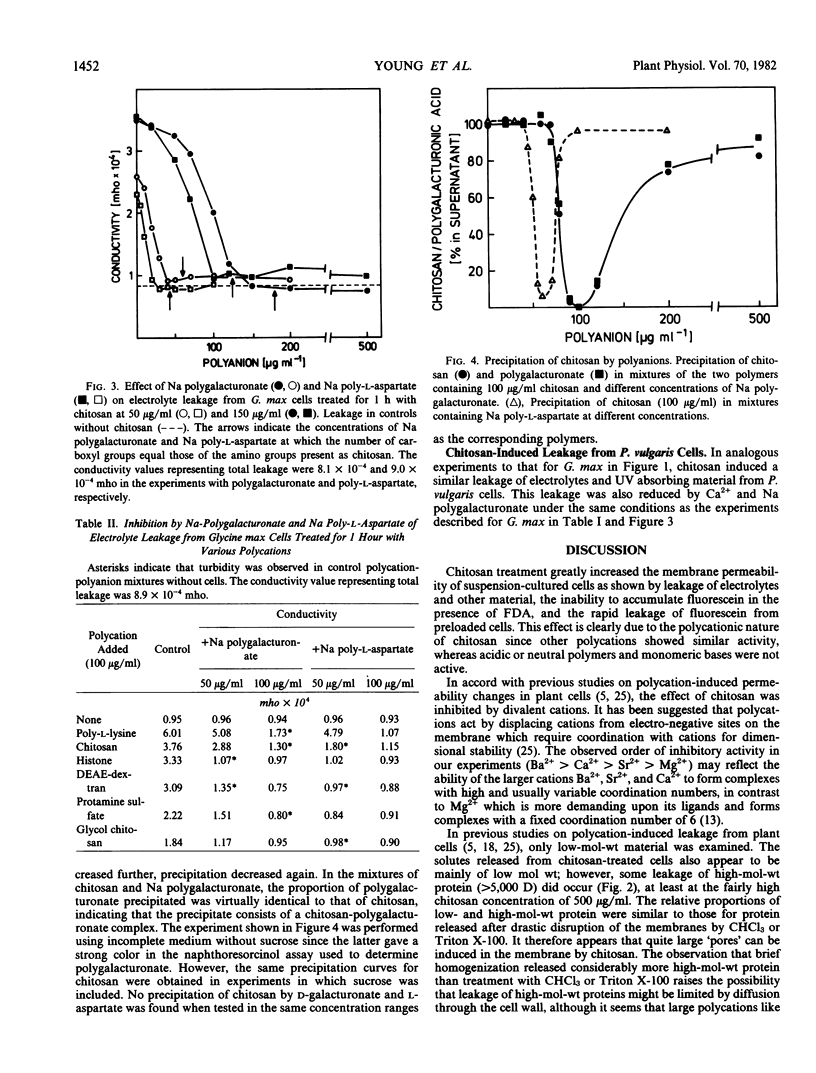
Treatment of suspension-cultured Glycine max cv Harosoy 63 cells with soluble chitosan (20-500 micrograms per milliliter) increased membrane permeability as shown by leakage of electrolytes, protein, and UV absorbing material. Severe damage to the cell membrane by chitosan (100 and 500 μg/ml) was also indicated by reduced staining with fluorescein diacetate and the leakage of fluorescein from preloaded cells. Other basic polymers (poly-l-lysine, histone, DEAE-dextran, protamine sulfate, and glycol chitosan) also increased permeability, whereas the basic monomers l-lysine and d-glucosamine, and acidic or neutral polymers were not active. Chitosan-induced leakage was inhibited by divalent cations, the order of effectiveness being Ba2+ > Ca2+ > Sr2+ > Mg2+. Na polygalacturonate and Na poly-l-aspartate also reduced polycation-induced leakage, probably by formation of polycation-polyanion complexes. A chitosan-polygalacturonate complex precipitated on mixing solutions of the two polymers containing approximately equal numbers of galacturonate and glucosamine residues, but not with either polymer in excess. A similar concentration-dependent precipitation of chitosan by Na poly-l-aspartate was found. Leakage from Phaseolus vulgaris cv Grandessa cells was also induced by chitosan, and was inhibited by Ca2+ and Na polygalacturonate.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araki Y., Ito E. A pathway of chitosan formation in Mucor rouxii. Enzymatic deacetylation of chitin. Eur J Biochem. 1975 Jun 16;55(1):71–78. doi: 10.1111/j.1432-1033.1975.tb02139.x. [DOI] [PubMed] [Google Scholar]
- Dürr M., Boller T., Wiemken A. Polybase induced lysis of yeast spheroplasts. A new gentle method for preparation of vacuoles. Arch Microbiol. 1975 Nov 7;105(3):319–327. doi: 10.1007/BF00447152. [DOI] [PubMed] [Google Scholar]
- Ebel J., Schaller-Hekeler B., Knobloch K. H., Wellman E., Grisebach H., Hahlbrock K. Coordinated changes in enzyme activities of phenylpropanoid metabolism during the growth of soybean cell suspension cultures. Biochim Biophys Acta. 1974 Oct 8;362(3):417–424. doi: 10.1016/0304-4165(74)90137-8. [DOI] [PubMed] [Google Scholar]
- Gamborg O. L., Miller R. A., Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res. 1968 Apr;50(1):151–158. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- Hadwiger L. A., Beckman J. M., Adams M. J. Localization of Fungal Components in the Pea-Fusarium Interaction Detected Immunochemically with Anti-chitosan and Anti-fungal Cell Wall Antisera. Plant Physiol. 1981 Jan;67(1):170–175. doi: 10.1104/pp.67.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadwiger L. A., Beckman J. M. Chitosan as a Component of Pea-Fusarium solani Interactions. Plant Physiol. 1980 Aug;66(2):205–211. doi: 10.1104/pp.66.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heslop-Harrison J., Heslop-Harrison Y. Evaluation of pollen viability by enzymatically induced fluorescence; intracellular hydrolysis of fluorescein diacetate. Stain Technol. 1970 May;45(3):115–120. doi: 10.3109/10520297009085351. [DOI] [PubMed] [Google Scholar]
- Kojime M., Kawakita K., Uritani I. Studies on a Factor in Sweet Potato Root Which Agglutinates Spores of Ceratocystis fimbriata, Black Rot Fungus. Plant Physiol. 1982 Feb;69(2):474–478. doi: 10.1104/pp.69.2.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lerner H. R., Ben-Bassat D., Reinhold L., Poljakoff-Mayber A. Induction of "pore" formation in plant cell membranes by toluene. Plant Physiol. 1978 Feb;61(2):213–217. doi: 10.1104/pp.61.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]


