Abstract
Background and Objective
Knowledge about exposure to cannabidiol (CBD) in breastfed infants can provide an improved understanding of potential risk. The aim was to predict CBD exposure in breastfed infants from mothers taking CBD and CBD-containing products.
Methods
Cannabidiol concentrations in milk previously attained from data collected through an existing human milk research biorepository were used to simulate infant doses and identify subgroups. A developed pediatric physiologically based pharmacokinetic model produced virtual breastfed infants administered the simulated CBD doses. Predicted breastfed infant exposures and upper area under the curve ratios were compared to the lowest therapeutic dose for approved indications in children.
Results
The existing human milk research biorepository contained 200 samples from 181 unique breastfeeding mothers for whom self-reported administration data and CBD concentrations had previously been measured. Samples that were above the lower limit of quantification with only one maternal administration type revealed that administration type, i.e., joint/blunt or edible versus oil or pipe, resulted in significantly different subgroups in terms of milk concentrations. Resulting simulated infant doses (ng/kg) were described by lognormal distributions with geometric means and geometric standard deviations: 0.61 ± 2.41 all concentrations, 0.10 ± 0.37 joint/blunt or edible, and 2.23 ± 8.15 oil or pipe. Doses administered to breastfed infants had exposures magnitudes lower than exposures in children aged 4–11 years administered the lowest therapeutic dose for approved indications, and low upper area under the curve ratios.
Conclusions
Based on real-world use, breastfeeding infants are predicted to receive very small exposures of CBD through milk. Studies examining adverse reactions will provide further insight into potential risk.
1. Introduction
The American College of Obstetrics and Gynecologists and the American Academy of Pediatrics recommend avoiding cannabidiol (CBD) and CBD-containing products during breastfeeding because of potential neurodevelopmental risks to the infant [1, 2]. Cannabidiol use is widespread and increasing among adults, especially for medical purposes [3, 4]. However, information on CBD risk to the breastfed infant is largely unknown owing to limited and variable existing evidence [5]. To support the strength of recommendations, more knowledge is required on the dose–exposure–response relationship of CBD in breastfed infants. Such information would lead to a better understanding of whether observed CBD concentrations in milk consumed by infants (dose) lead to relevant systemic concentrations in breastfed infants (exposure) associated with neurodevelopmental delays (response).
Beginning in 2014, Mommy’s Milk Human Milk Biorepository (HMB) investigators (CDC and KAB) sought to improve the understanding of maternal exposure to various agents, including marijuana and its metabolites, during breastfeeding and the potential for infant exposure to specific agents and subsequent adverse infant outcomes. The HMB is a USA- and Canada-wide study that collects human milk samples from mothers who were or were not taking medications and recreational drugs, including marijuana, CBD, and CBD-containing products [6]. The HMB investigators continue to study breastfeeding exposures and potential infant outcomes through administration of neurodevelopmental questionnaires and face-to-face testing. In the present secondary data analysis, we seek to fill a gap by further defining CBD exposures to breastfed infants. In this work, we leverage real-world CBD concentrations in breastmilk from the HMB, knowledge of breastmilk intake as a function of infant age [7], dose and type of administration, and physiologically based pharmacokinetic (PBPK) models to translate CBD dose through breastfeeding into neonatal exposures. Physiologically based pharmacokinetic modeling is a mathematical tool used to predict drug exposures based on the physicochemical properties of a compound, and the anatomy and physiology of organisms. We sought to answer, among breastfeeding mothers taking CBD based on real-world use, what is the predicted exposure and its associated variability in breastfed infants?
2. Methods
2.1. Software Used and Data Source
The open-source PBPK modeling platform, PK-Sim version 11 (Open Systems Pharmacology Suite, https://www.open-systems-pharmacology.org/) was used to perform PBPK modeling. Plot Digitizer version 2.6.8 (by Joseph Huwaldt) was used to digitize published pharmacokinetic profiles to obtain concentration–time data. R (R Core Team, 2019, Vienna, Austria) was used to curate the HMB dataset, analyze subgroups, and simulate infant daily doses.
The HMB was established in 2014 at the University of California San Diego for research purposes. The HMB collects voluntary human milk samples from lactating women who are or are not exposed to any medication, recreational drug, or environmental chemical primarily in the 2 weeks prior to sample collection. Detailed information on recruitment, data collection, and sample preparation and analysis methods have been presented previously [6]. Participants complete an interview to provide their demographics, maternal and child health history, breastfeeding habits, and all exposures focused in the previous 2 weeks prior to sample collection. Exposure information from women who reported marijuana use at any time since giving birth included type of administration, frequency of use, dose, and time since last use before milk sample collection. Milk samples were previously measured for metabolites, including CBD concentrations and the date and time of the milk collection were ascertained. Cannabidiol concentrations in human milk were determined by liquid chromatography with tandem mass spectrometry. The analytical range of the assay was 0.1–200 ng/mL. The method was validated in human milk by establishing the accuracy and precision of three sets of calibration curves and quality-control samples over 3 days. Acceptability criteria for accuracy (within a run and between runs) was ±15% of nominal concentrations except ±20% at the lower limit of quantification (LLOQ). Acceptability criteria for precision (within a run and between runs) was ±15% coefficient of variation, except ±20% coefficient of variation at LLOQ. This present study received ethics clearance from the parent study through the UC San Diego Human Research Protections Program, and for a secondary data analysis through the University of Waterloo Research Ethics Board (REB# 42860).
2.2. Dose Determination
2.2.1. CBD Concentrations in Milk
Information on maternal demographics, exposures, and measured CBD concentrations in milk collected and assayed by the parent study between 2015 and 2021 were extracted from the existing HMB dataset. The dataset was organized to describe: all concentrations in milk (Dataset 1); and concentrations by self-reported maternal frequency, dose, and type of administration (Dataset 2). From the existing data for the sample on quantification of CBD, three methods were assessed to account for below the limit of quantification (BLQ) values: (1) BLQ = LLOQ/2, (2) BLQ are drawn from uniform distributions of 0 to LLOQ, and (3) BLQ = LLOQ.
For concentrations from samples with a maternal reported type of administration (Dataset 2), only concentrations with one type of maternal administration were retained. Missing end time of exposure was replaced with the time of concentration sample collection and vice versa. The effects of administration type, time after last dose (TAD), and dose frequency on concentration were assessed. Administration type was a categorical variable defined as: edible, joint, oil, pipe, or other (vaporizer, topical), and N/A (not reported) categories. As a continuous variable, TAD was described as time in hours elapsed from the end of maternal administration to milk sample collection for concentration measurement. Time after last dose was calculated by subtracting the date and time of sample collection by date and time of the last reported date of maternal administration. To account for the varying ways in which dose and frequency of CBD and CBD-containing products were consumed (e.g., number of puffs per day versus milligrams per week), dose frequency was categorized as low, medium, and high based on the data of each week-normalized dose type. To compare these subgroups, the exposure-concentration subset (Dataset 2) was considered with and without BLQ values. A linear regression model to predict log-concentrations was obtained after testing the significance of subgroups on CBD in milk concentrations including TAD, administration type, and interactions between TAD and administration type, and administration type and dose frequency. Model goodness of fit was evaluated through a standard residual analysis. Post-hoc pairwise comparisons of estimated marginal means of the significant subgroups were performed using various p value adjustment methods (from most to least conservative: Bonferroni, Holm, and Tukey) owing to the lack of a gold standard method.
2.2.2. Volume of Milk Intake
The volume of milk intake that an infant typically receives on a weight-normalized basis and as a function of postnatal age was drawn from a literature review-derived milk intake model described in our previous work [7, 8].
2.2.3. Dose Simulation
To simulate weight-normalized doses received by each virtual breastfed infant, daily milk intake volume (mL/kg) was multiplied with an observed or simulated CBD milk concentration (ng/mL). For all concentrations, random sampling with replacement was performed on the full dataset (Dataset 1). For the significant subgroups (Dataset 2), above LLOQ concentrations were simulated from a log-normal distribution using the mean and variance from the subgroup log-concentrations. Concentrations that were BLQ were simulated based on the estimated probabilities obtained from a logistic regression model. The Hosmer–Lemeshow goodness-of-fit test was used for model assessment. Milk intake volumes were selected from a normal distribution with a mean [7] obtained from a non-linear age-dependent equation and a standard deviation [8] specific to the age group of the infant.
2.3. Model Development and Evaluation
The pediatric PBPK model was developed according to the workflow of Maharaj et al. [9]. An adult oral CBD PBPK model established from our previous work [10] was scaled to simulate CBD exposure in virtual breastfeeding infants. Two key modifications were made to the published model based on information presented by Bansal et al. [11]. First, the percent contributions of cytochrome P450 and UGT enzymes to CBD clearance were redefined as 20% and 80%, respectively, based on updated in vitro human liver microsome studies [11]. Second, UGT1A7 was removed as an important contributing enzyme and the relative percent contributions of UGT1A9 and UGT2B7 to overall CBD clearance were redefined (16% and 64%, respectively). To capture these changes, the percent contributions to CBD clearance as determined from each study [11–13] were updated, and the fraction metabolized in the liver via the enzymes were adjusted to the revised percent contributions. While the update in CBD clearance pathways and fraction metabolized did not affect the overall pharmacokinetic simulations with CBD administered alone, it did increase accuracy regarding the drug–drug interaction predictions (geometric mean treatment ratios for itraconazole, fluconazole, and rifampicin: 1.24, 1.45, and 0.49 [10] and 1.06, 1.10, and 0.77 [this study], respectively). As a result, we chose to use the updated literature for extrapolating to pediatrics. Following these modifications, anatomy and physiology were scaled for different infant ages, and growth and maturation of relevant processes including metabolic capacity, glomerular filtration rate, protein binding, and body composition, were adjusted for. Variability was applied to the anatomy and physiology to produce a virtual infant population. For the user defined protein, UGT1A9, activity was found not to be age dependent, and thus ontogeny was described with a linear function and geometric standard deviation of 1.5 [10].
For model evaluation, two studies reported on the pharmacokinetics of CBD administered in children; however, the experimental data were not consistent [14, 15]. Particularly, the area under the curve on day 1 presented by Wheless et al. [14] vastly differed from the reported in adults [16–21] and children aged 4–11 years reported by Devinsky et al. [15] with similar weight-normalized doses. Thus, evaluation of the pediatric PBPK model was performed with Devinsky et al. [15], where children aged 4–11 years were randomized to receive one of three doses of CBD oral solution daily (5, 10, or 20 mg/kg).
2.4. Exposure Predictions
Using the developed pediatric PBPK model, infant populations of 200 individuals using the National Health and Nutrition Examination Survey population [22, 23] (50% female) were simulated per age group in days: > 0 to 7, > 7 to 14, > 14 to 30, > 30 to 60, and > 60 to 365. Administration of CBD to these virtual breastfed infants differed from that given to adults [10]. In the adult oral model, CBD solution was described as a dissolution-precipitation process that was dose specific and fit to describe the data. For extrapolation to pediatric populations, CBD was assumed to remain as a solution because of the small doses that breastfed infants receive. As CBD exhibits non-linear kinetics, each infant was assigned a daily dose of CBD solution until steady state was reached and , where hours, was taken. This process to simulate doses was performed with all CBD concentrations in milk and for each of the subgroups.
Simulated was determined for 200 virtual breastfed infants per age group and 100 virtual children administered the lowest therapeutic dose of 5 mg/kg/day [15, 24] for approved indications as a comparison. The upper area under the curve ratio (UAR) was calculated for each breastfed infant age group using the following equation [8]:
The median therapeutic was calculated from the 100 virtual children administered 5 mg/kg/day based on observed data in Devinsky et al. [15].
3. Results
The HMB dataset contained 200 breast milk samples of CBD concentrations (42% BLQ) obtained from 181 unique breastfeeding mothers. Participant demographics of the 181 mothers presented per breast milk sample are provided in Table 1. Of these samples, 124 (45% BLQ) from 118 participants had only one maternal type of administration. The three methods to account for BLQ values produced similar results. Thus, LLOQ/2 with LLOQ as 0.1 ng/mL was applied. Only concentrations above the LLOQ were used in the subgroup analyses as BLQ values tended to produce unsatisfactory residual distributions when incorporated into the log-linear regression models. The proportion of BLQ values were similar across subgroups (33–54%). Descriptive plots of each assessed subgroup using the exposure-concentration subset (Dataset 2) while accounting for BLQ values are presented in Fig. 1.
Table 1.
Demographics of participants (N = 181) breastfeeding during CBD or CBD-containing product use reported by a breast milk sample (N = 200)
| Characteristic | Number of samples (%)a |
|---|---|
|
| |
| Age, years | |
| 19–25 | 28 (14) |
| 26–30 | 54 (27) |
| 31–35 | 69 (34.5) |
| 36–40 | 37 (18.5) |
| 41–42 | 12 (6) |
| BMI, kg/m2 | |
| < 18.5 | 3 (1.5) |
| 18.5–24.9 | 78 (39) |
| 25–29.9 | 72 (36) |
| 30–34.9 | 27 (13.5) |
| 35–39.9 | 14 (7) |
| ≥ 40 | 6 (3) |
| Ethnicity | |
| Hispanic | 37 (18.5) |
| Non-Hispanic | 163 (81.5) |
| Race | |
| Asian | 4 (2) |
| Black | 9 (4.5) |
| Caucasian | 174 (87) |
| Native American | 11 (5.5) |
| Pacific Islander/Alaska Native | 2 (1) |
| Education | |
| Partial high school | 2 (1) |
| High school graduate/GED | 17 (8.5) |
| Some college/specialization | 88 (44) |
| College graduate | 54 (27) |
| Post-graduate | 37 (18.5) |
| Not reported | 2 (1) |
CBD cannabidiol, BMI body mass index, GED general educational development
N = 17 participants contributed two or more breast milk samples
Fig. 1.
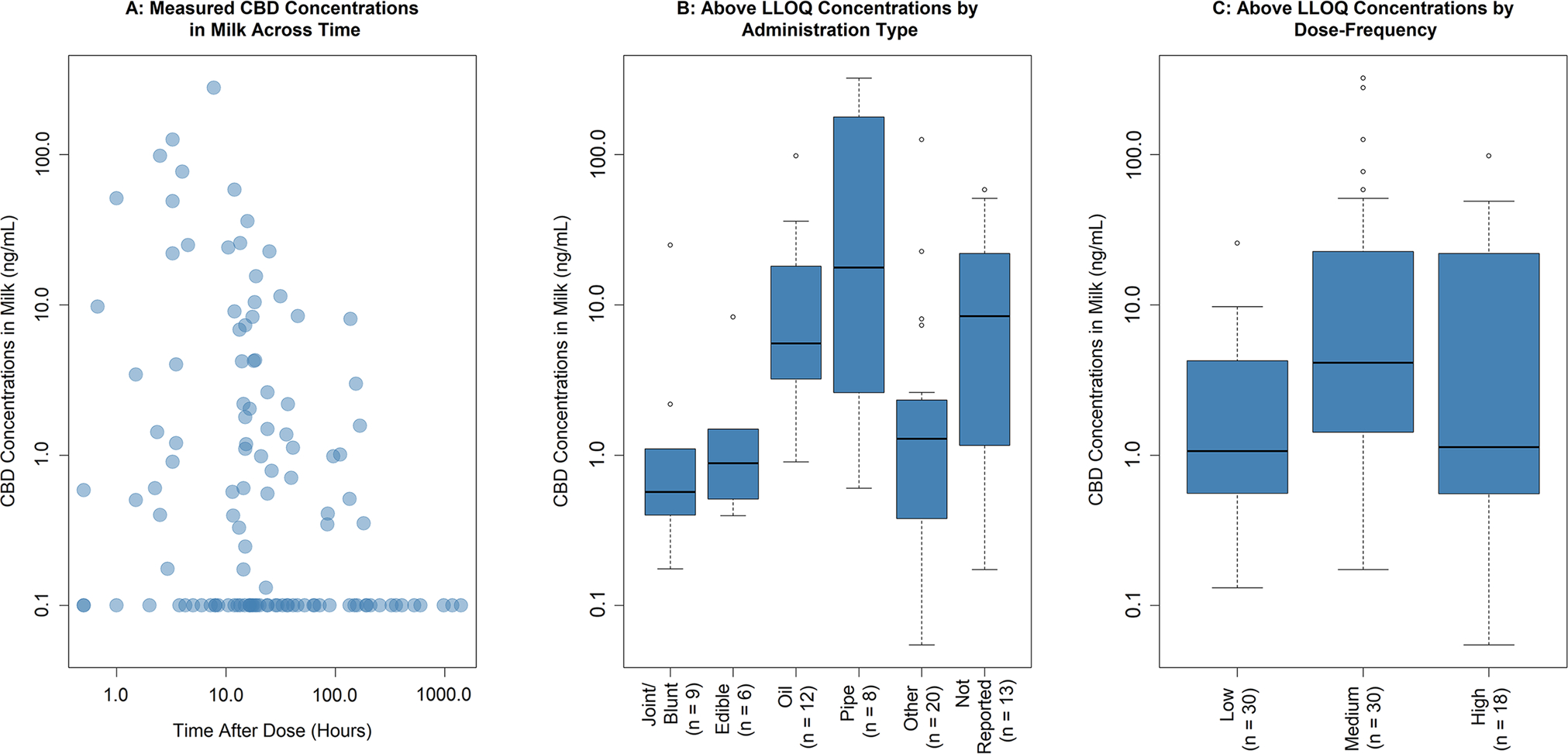
Descriptive plots to assess potential subgroups from the exposure-concentration subset (Dataset 2). A Below the limit of quantification (BLQ) values reported as 0.1 ng/mL. Five concentrations (0.055 ng/mL, 1.16 ng/mL, 325 ng/mL, BLQ, and BLQ) at the time after dose = 0 h not shown. B, C Below the limit of quantification values were not included. The number of samples in each subgroup is presented in brackets. CBD cannabidiol, LLOQ lower limit of quantification
A backward step-wise elimination procedure was performed involving TAD, administration type, and their interactions. An interaction with administration type and dose frequency was not feasible for model testing because of the low sample size. The final model included administration type, which exhibited satisfactory residual behavior. Post-hoc pairwise comparisons between administration types across the three p value adjustment methods suggested that oil versus joint/blunt, joint/blunt versus pipe, and edible versus pipe had significantly different estimated marginal means. Therefore, administration type was grouped into two contrasting subgroups, oil or pipe and joint/blunt or edible, for subsequent dose simulations. Goodness-of-fit plots, estimated marginal means, their 95% confidence intervals, and model estimates are presented in Figs. S1 and S2, and Table S1 of the Electronic Supplementary Material.
The logistic regression model used to simulate the probabilities of BLQ concentrations found that TAD was significant. Administration type was not found to be significant after controlling for TAD, and thus BLQ values had the same chance of occurring for all administration types. The model performed well with the Hosmer–Lemeshow test resulting in a p value of 0.768.
The distributions of CBD in milk concentrations and administered doses to virtual breastfed infants are presented in Table 2. The developed pediatric PBPK model evaluation with Devinsky et al. [15] results are shown in Table 3. Predicted were comparable to observed , which provided confidence in the ability of the model to accurately predict exposures in pediatrics.
Table 2.
Characteristics of CBD in milk concentrations and dose distributions
| Dataset | Distribution | Geometric mean | Geometric SD |
|---|---|---|---|
|
| |||
| Concentrations for sampling | |||
| Full dataset | Resampling from 200 concentrations | N/A | N/A |
| Joint/blunt or edible only | Log-normal | 0.94 ng/mL | 3.67 |
| Oil or pipe only | Log-normal | 9.74 ng/mL | 6.31 |
| Doses administered to virtual breastfed infants | |||
| Full dataset | Log-normal | 0.61 ng/kg | 2.41 |
| Joint/blunt or edible only | Log-normal | 0.10 ng/kg | 0.37 |
| Oil or pipe only | Log-normal | 2.23 ng/kg | 8.15 |
CBD cannabidiol, N/A not reported, SD standard deviation
Table 3.
Pediatric physiologically based pharmacokinetic model-predicted versus observed AUC 0-τa
| Study | 5-mg/kg/day target dose | 10-mg/kg/day target dose | 20-mg/kg/day target dose |
|---|---|---|---|
|
| |||
| Day 1 at 1.25 mg/kg | |||
| Devinsky et al. [13] | 70.6 (20.4) (N = 10) | 66.4 (121) (N = 8) | 73.7 (96.6) (N = 9) |
| This study | 76.5 (59.4) | 76.5 (59.4) | 76.5 (59.4) |
| Day 22 at target dose via BID | |||
| Devinsky et al. [13] | 241 (101) (N = 10) | 722 (79.9) (N = 8) | 963 (93.4) (N = 9) |
| This study | 207 (64.6) | 401 (60.0) | 748 (57.3) |
AUC area under the curve, BID twice daily, N number of patients
τ: 5 hours, presented as a geometric mean in ng⋅h/mL (% coefficient of variation)
Pediatric PBPK model-predicted, daily, steady-state of breastfed infants across the age groups for all CBD concentrations, joint/blunt or edible exposure only, and oil or pipe exposure only compared to children administered the CBD therapeutic dose are presented in Fig. 2. Calculated UARs for each age group are shown in Table 4.
Fig. 2.
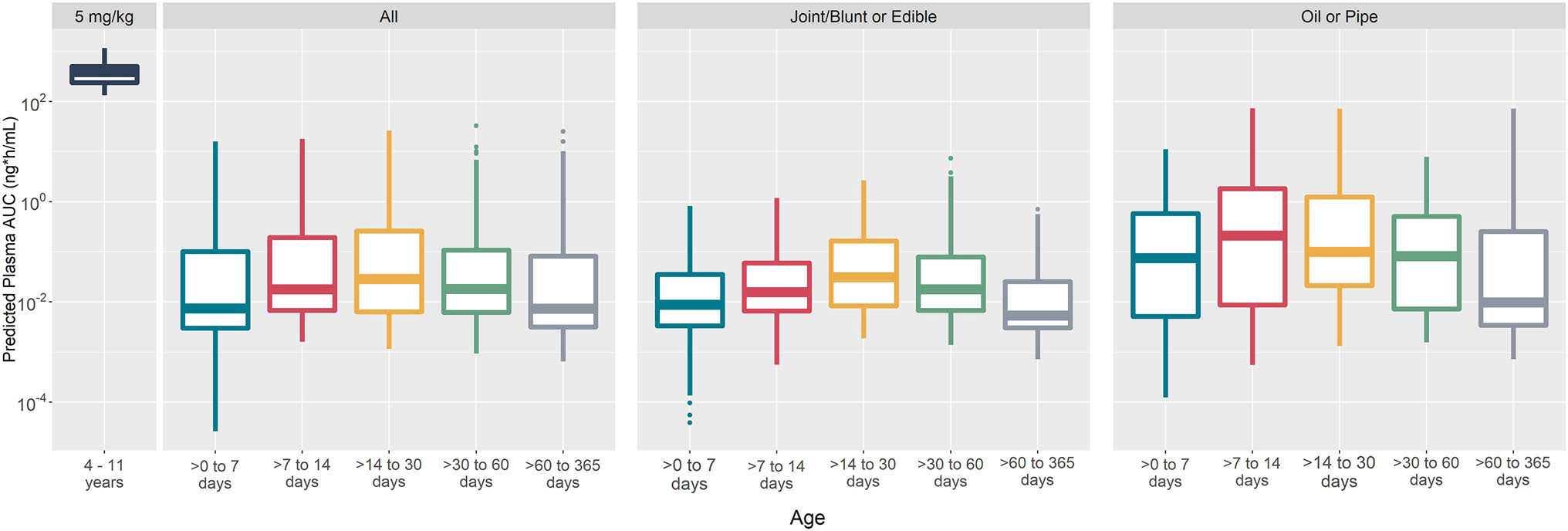
Cannabidiol (CBD) physiologically based pharmacokinetic model-predicted, daily, steady-state area under the curve (AUC) of children (N = 100, receiving 5 mg/kg/day) compared to breastfed infants across age groups (N = 200 per group, receiving CBD in milk doses). All: CBD in breast milk concentrations from the full human milk biorepository dataset; joint/blunt or edible: CBD in breast milk concentrations from joint/blunt or edible exposures; oil or pipe: CBD in breast milk concentrations from oil or pipe exposures
Table 4.
UAR of infants (N = 200 per age group) breastfed by mothers during real-world use of CBD and CBD-containing products
| Parameter | > 0 to 7 days old | > 7 to 14 days old | > 14 to 30 days old | > 30 to 60 days old | > 60 to 365 days old |
|---|---|---|---|---|---|
|
| |||||
| All concentrations | |||||
| 95th percentile of simulated breastfeeding infants (ng∙h/mL) | 1.87 | 3.60 | 4.50 | 4.60 | 1.23 |
| UARa | 0.0024 | 0.0047 | 0.0058 | 0.0060 | 0.0016 |
| Joint/blunt or edible exposure | |||||
| 95th percentile of simulated breastfeeding infants (ng∙h/mL) | 0.22 | 0.40 | 0.69 | 0.50 | 0.25 |
| UARa | 0.00029 | 0.00047 | 0.00090 | 0.00065 | 0.00033 |
| Oil or pipe exposure | |||||
| 95th percentile of simulated breastfeeding infants (ng∙h/mL) | 3.23 | 35.25 | 17.09 | 4.56 | 4.00 |
| UARa | 0.0042 | 0.046 | 0.022 | 0.0059 | 0.0052 |
AUC area under the curve, CBD cannabidiol, UAR upper area under the curve ratio
UAR denominator consists of the simulated median based on the children aged 4–11 years from Devinsky et al. [13] receiving therapeutic doses for approved indications
4. Discussion
Through use of real-world CBD concentrations in breastmilk, this study provided additional information on potential concentrations of CBD exposure in breastfed infants. By examining the relationship between the maternal type of administration and concentrations in breast milk, it was determined that oil or pipe tended to result in higher predicted concentrations as compared with joint/blunt or edible forms. Additionally, this work found that a significant proportion (a little less than half) of breast milk samples contained BLQ values that likely contributed to the low predicted exposures to breastfed infants. The longer the TAD, the greater the presence of BLQ concentrations were in breast milk. Moreover, BLQ values had the same chance of occurring for all administration types. Knowledge about the impact of TAD on BLQ concentrations across administration types could have clinical advice implications, such as the existence of optimal breastfeeding times when taking CBD and CBD-containing products.
A strength of this study was based on the ability of the PBPK model to predict reasonably in adults [10]. This increased our confidence especially in the predictions in children aged 4–11 years for model evaluation. Although geometric mean was predicted to be 1.7-fold less than observed in Devinsky et al. [15] for 10-mg/kg/day dosing, our findings were relatively in line with Wheless et al. [14] (659.6 ng•h/mL). It is worth noting that the pediatric CBD PBPK model developed by Bansal et al. [11] predictions were in line with Devinsky et al. [15] at this dose, which suggests a potential significant role of accumulation and time-dependent auto-inhibition. Yet, since their model tended to overpredict in all other doses in children, and our model predictions were acceptable in adults, further research is needed to confirm the potential CBD impact on enzymes responsible for its own metabolism.
Beyond the ability to predict exposures, the UAR accounts for the anatomy and physiology of breastfeeding infants; age-dependent factors, such as milk intake volumes as a function of age; and variability in the infant and maternal population, such as maternal pharmacogenotypes that could lead to an increased presence of medication in breast milk. The UAR was calculated using the pediatric PBPK model-predicted exposures in virtual breastfed infants. This novel metric offers an improvement over current metrics that focus solely on the potential dose received by the breastfed infant, without accounting for exposure (i.e., infant plasma concentrations). The UAR calculated for CBD revealed that even the exposures of the most vulnerable breastfed infant (95th percentile on the higher exposure end) are well below the exposures of children aged 4–11 years receiving the lowest approved dose for approved indications. This finding serves as additional exposure information to healthcare providers to consider when discussing CBD use by mothers in relation to their breastfeeding infants.
For context, our group has simulated breastfeeding exposures for lamotrigine [8] and escitalopram [25] in previous work. Predicted breastfeeding infant exposures tended to reach levels of exposure from adults taking therapeutic doses for lamotrigine, but not for escitalopram. The UAR was also calculated for lamotrigine and was determined to be relatively high for some age groups. These observations were in line with adverse reactions reported for lamotrigine and escitalopram, with more observed in the former than the latter. Thus, the UAR serves as a useful tool to anticipate potential responses in breastfeeding infants. In regard to CBD, it would be of interest to follow-up in future studies assessing breastfeeding infant adverse reactions and effects on neurodevelopment to understand the relationship between the UAR results of this study with response information.
This work recognizes the great uncertainty of CBD bioavailability in breastfed infants. In adults, bioavailability is low and greatly impacted by food. To address this issue, we used the idea that breastfed infants receive small doses of CBD and thus the precipitation-dissolution-precipitation cycle experienced in adults was not expected. Therefore, CBD was given as an oral solution without the dissolution complexities. Moreover, because a solution is already dissolved, the food effect was not relevant in our virtual breastfed infants. As a result, our work was conservative with the pediatric PBPK model-predicted 0- to 1-year-old infant bioavailability being 0.54–0.68, as compared with 0.24 in adults administered the 200-mg oral solution. Even with this larger infant bioavailability, the UAR was still very low.
The low sample size per subgroup serves as a limitation to this study. Although administration type was found to be a significant subgroup, further data to support this finding are warranted. Likewise, larger sample sizes are needed to assess other potential subgroups (a power issue), such as those given by dose frequency. It is possible that oil or a pipe maternal type of administration tended to have higher dose frequencies. Similarly, the relationship between TAD and BLQ concentrations in milk could be influenced by dose frequency. However, analyses with the limited dose frequency information we had suggest this not to be the case.
A limitation of the parent study is that maternal exposure information on dose, timing, and type of administration relied on a maternal report and may therefore be inaccurate. Furthermore, maternal administration information was typically measured in the previous 2 weeks prior to milk sample collection. As a result, less data were acquired on the long-term frequency of use, which may contribute to the infant dose.
A further limitation to this study relates to the inability to validate our workflow with CBD concentrations measured in breastfed infant plasma. As these data have not been reported in the literature, we were not able to check whether the pediatric PBPK model-predicted infant plasma concentrations were in line with observed concentrations. Future studies should focus on collecting and analyzing plasma concentrations from infants breastfed by mothers taking CBD or CBD-containing products to confirm our results.
Because the study of CBD in milk concentrations was based on highly dispersed observational data, it can only shed light on the potential association between concentrations and administration types and any statement on causality should be avoided. Nevertheless, this study was able to draw conclusions on infant exposures from the real-world maternal use of CBD and CBD-containing products, which can be insightful to healthcare providers in advising breastfeeding mothers taking CBD and CBD-containing products. Future studies investigating the relationship of maternal administered CBD doses to potential breastfed infant responses, particularly neurodevelopmental delay, will add to our understanding of the CBD dose–exposure–response relationship in infants. For example, low predicted exposures of CBD in breastfed infants as compared with children receiving therapeutic doses for approved indications may still have the potential for adverse effects, if infants are more susceptible in early brain development. Another future direction includes studying CBD metabolites, especially 7-hydroxycannabidiol, which is known to have the activity and potential to accumulate in breast milk. Further cannabinoids are another area of focus. Notably, tetrahydrocannabinol is observed to have substantially greater concentrations in milk as compared with CBD [6]. Similar studies to our presented work can be applied to 7-hydroxycannabidiol and tetrahydrocannabinol to provide a fuller perspective on cannabis use during breastfeeding.
5. Conclusions
Predicted CBD exposures in breastfed infants were magnitudes lower than exposures based on observed children (aged 4–11 years) administered the lowest approved CBD dose. Although this main study finding does not change recommendations from organizations such as the American College of Obstetrics and Gynecologists and the American Academy of Pediatrics, it allows healthcare providers to be better informed to discuss the use of CBD and CBD-containing products in breastfeeding mothers from an exposure perspective. This study combined with future work studying infant responses to CBD exposure via breast milk can lead to a better understanding of the entire dose–exposure–response pathway for improved breastfeeding advice.
Supplementary Material
Key Points.
Guidelines recommend that cannabidiol (CBD) and CBD-containing products not be taken during breastfeeding. Cannabidiol is given in therapeutic doses to children for the treatment of seizures associated with two forms of epilepsy, who have a range of exposures with notable effects.
Physiologically based pharmacokinetic model-predicted exposures in infants from real-world CBD concentrations in breast milk were magnitudes lower than exposures in children administered the lowest therapeutic dose for approved indications. This suggests that infants only receive small exposures of CBD via breast milk.
Healthcare providers will be better informed to discuss the use of CBD and CBD-containing products in breastfeeding mothers from an exposure perspective.
Funding
All phases of this study were supported by the Canadian Institutes of Health Research (CIHR) Project Grant, PJT-159782; and CIHR Frederick Banting and Charles Best Canada Graduate Scholarships Doctoral Award (CGS-D), a Canada Scholarship to Honour Nelson Mandela, DF2-171445. Collection of the original samples and data used for this secondary analysis was supported by funding to the University of California San Diego through the National Institutes of Health, Grant UL1TR001442 (PI Firestein), and the Gerber Foundation, projects 4998 and 6488 (PI Chambers).
Footnotes
Declarations
Conflicts of interest/competing interests Cindy H.T. Yeung, Kerri A. Bertrand, Brookie M. Best, Edmund Capparelli, Christina D. Chambers, Dagmar M. Hajducek, Abdullah Hamadeh, Shinya Ito, Jeremiah D. Momper, and Andrea N. Edginton have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval This present study received ethics clearance from the parent study through the UC San Diego Human Research Protections Program, and for the secondary data analysis through the University of Waterloo Research Ethics Board (REB# 42860).
Consent to participate Not applicable.
Consent for publication Not applicable.
Code availability Not applicable.
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s40262-023-01307-6.
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Availability of data and material
A de-identified dataset can be shared with the appropriate approvals. The requestor is required to contact the PI (Dr. Christina Chambers, chchambers@health.ucsd.edu) with the request.
References
- 1.Committee on Obstetric Practice. Committee Opinion No. 722: marijuana use during pregnancy and lactation. Obstetrics Gynecol. 2017;130(4):e205–9. [DOI] [PubMed] [Google Scholar]
- 2.Meek JY, Noble L. Policy statement: breastfeeding and the use of human milk. Pediatrics. 2022;150(1): e2022057988. [DOI] [PubMed] [Google Scholar]
- 3.Leung J, Chan G, Stjepanović D, Chung JYC, Hall W, Hammond D. Prevalence and self-reported reasons of cannabis use for medical purposes in USA and Canada. Psychopharmacology. 2022;239(5):1509–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goodman S, Wadsworth E, Schauer G, Hammond D. Use and perceptions of cannabidiol products in Canada and in the United States. Cannabis Cannabinoid Res. 2022;7(3):355–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Navarrete F, García-Gutiérrez MS, Gasparyan A, Austrich-Olivares A, Femenía T, Manzanares J. Cannabis use in pregnant and breastfeeding women: behavioral and neurobiological consequences. Front Psychiatry. 2020;11: 586447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertrand KA, Hanan NJ, Honerkamp-Smith G, Best BM, Chambers CD. Marijuana use by breastfeeding mothers and cannabinoid concentrations in breast milk. Pediatrics. 2018;142(3): e20181076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yeung CHT, Fong S, Malik PRV, Edginton AN. Quantifying breast milk intake by term and preterm infants for input into paediatric physiologically based pharmacokinetic models. Matern Child Nutr. 2020;16(2): e12938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeung CHT, Ito S, Autmizguine J, Edginton AN. Incorporating breastfeeding-related variability with physiologically based pharmacokinetic modeling to predict infant exposure to maternal medication through breast milk: a workflow applied to lamotrigine. AAPS J. 2021;23(4):70. [DOI] [PubMed] [Google Scholar]
- 9.Maharaj AR, Barrett JS, Edginton AN. A workflow example of PBPK modeling to support pediatric research and development: case study with lorazepam. AAPS J. 2013;15(2):455–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yeung CHT, Beers JL, Jackson KD, Edginton AN. Verifying in vitro-determined enzyme contributions to cannabidiol clearance for exposure predictions in human through physiologically-based pharmacokinetic modeling. CPT Pharmacometr Syst Pharmacol. 2023;12(3):320–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bansal S, Ladumor MK, Paine MF, Unadkat JD. A physiologically-based pharmacokinetic model for cannabidiol in healthy adults, hepatically-impaired adults, and children. Drug Metab Dispos. 2023;51(6):743–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beers JL, Fu D, Jackson KD. Cytochrome P450-catalyzed metabolism of cannabidiol to the active metabolite 7-hydroxy-cannabidiol. Drug Metab Dispos. 2021;49:882–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mazur A, Lichti CF, Prather PL, Zielinska AK, Bratton SM, Gallus-Zawada A, et al. Characterization of human hepatic and extrahepatic UDP-glucuronosyltransferase enzymes involved in the metabolism of classic cannabinoids. Drug Metab Dispos. 2009;37(7):1496–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wheless JW, Dlugos D, Miller I, Oh DA, Parikh N, Phillips S, et al. Pharmacokinetics and tolerability of multiple doses of pharmaceutical-grade synthetic cannabidiol in pediatric patients with treatment-resistant epilepsy. CNS Drugs. 2019;33(6):593–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devinsky O, Patel AD, Thiele EA, Wong MH, Appleton R, Harden CL, et al. Randomized, dose-ranging safety trial of cannabidiol in Dravet syndrome. Neurology. 2018;90(14):e1204–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tayo B, Taylor L, Sahebkar F, Morrison G. A phase I, open-label, parallel-group, single-dose trial of the pharmacokinetics, safety, and tolerability of cannabidiol in subjects with mild to severe renal impairment. Clin Pharmacokinet. 2020;59(6):747–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor L, Crockett J, Tayo B, Morrison G. A phase 1, open-label, parallel-group, single-dose trial of the pharmacokinetics and safety of cannabidiol (CBD) in subjects with mild to severe hepatic impairment. J Clin Pharmacol. 2019;59(8):1110–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crockett J, Critchley D, Tayo B, Berwaerts J, Morrison G. A phase 1, randomized, pharmacokinetic trial of the effect of different meal compositions, whole milk, and alcohol on cannabidiol exposure and safety in healthy subjects. Epilepsia. 2020;61(2):267–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Center for Drug Evaluation and Research. Other review(s): sponsor’s submission, GWEP1541. 2018.
- 20.Schoedel KA, Szeto I, Setnik B, Sellers EM, Levy-Cooperman N, Mills C, et al. Abuse potential assessment of cannabidiol (CBD) in recreational polydrug users: a randomized, double-blind, controlled trial. Epilepsy Behav. 2018;88:162–71. [DOI] [PubMed] [Google Scholar]
- 21.Taylor L, Gidal B, Blakey G, Tayo B, Morrison G. A phase I, randomized, double-blind, placebo-controlled, single ascending dose, multiple dose, and food effect trial of the safety, tolerability and pharmacokinetics of highly purified cannabidiol in healthy subjects. CNS Drugs. 2018;32(11):1053–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burt VL, Harris T. The third National Health and Nutrition Examination Survey: contributing data on aging and health. Gerontologist. 1994;34(4):486–90. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey: NHANES III. https://wwwn.cdc.gov/nchs/nhanes/nhanes3/default.aspx. Accessed 17 Jan 2023.
- 24.Greenwich Biosciences Inc. Epidiiolex (cannabidiol) oral solution label; 6/2018. Report No. 4282447.
- 25.Delaney SR, Malik PRV, Stefan C, Edginton AN, Colantonio DA, Ito S. Predicting escitalopram exposure to breastfeeding infants: integrating analytical and in silico techniques. Clin Pharmacokinet. 2018;57(12):1603–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
A de-identified dataset can be shared with the appropriate approvals. The requestor is required to contact the PI (Dr. Christina Chambers, chchambers@health.ucsd.edu) with the request.


