Structured Abstract:
Objective:
To determine whether TOP5300, a novel oral follicle stimulating hormone receptor (FSHR) allosteric agonist, elicits a different cellular response than recombinant human FSH (rh-FSH) in human granulosa cells from in vitro fertilization patients.
Design:
Basic science research with a preclinical allosteric FSHR agonist.
Subjects:
Infertility patients at a single academic fertility clinic were recruited under an IRB-approved protocol. Primary granulosa cell cultures were established for 41 patients, of which 8 had normal ovarian reserve (NOR), 17 were of advanced reproductive age (ARA), 12 had a diagnosis of polycystic ovarian syndrome (PCOS), and 4 had a combination of diagnoses, such as ARA and PCOS.
Interventions:
Primary granulosa-lutein cell (GLC) cultures were treated with rh-FSH, TOP5300 or vehicle.
Main Outcome Measures:
Estradiol production by ELISA, steroid pathway gene expression of StAR and aromatase by quantitative polymerase chain reaction, and FSH receptor membrane localization by immunofluorescence were measured in human GLC.
Results:
TOP5300 consistently stimulated estradiol production among NOR, ARA and PCOS patients. Recombinant FSH was the more potent ligand in GLC from NOR patients but was ineffective in cells from ARA or PCOS patients. The lowest level of FSHR plasma membrane localization was seen in patients with ARA (p<0.0001) while FSHR localization was more abundant in cells from PCOS patients (p = 0.0299); the highest levels were present in cells from NOR patients. Localization of FSHR was not affected by TOP5300 relative to rh-FSH among any patient group. TOP5300 stimulated greater expression of StAR (p=0.008) and CYP19A1 (p=0.006) across cells from all patients (NOR, ARA and PCOS combined), while rh-FSH was unable to stimulate StAR and aromatase (CYP19A1) expression in cells from PCOS patients. TOP5300-induced expression of StAR and CYP19A1 mRNA among ARA and NOR patients were consistently lower than those observed in cells from PCOS patients.
Conclusion:
TOP5300 appears to stimulate estradiol production and steroidogenic gene expression from GLC more than rh-FSH in PCOS, relative to ARA and NOR, patients. It does not appear that localization of FSHR at cell membranes is a limiting step for TOP5300 or rh-FSH stimulation of steroidogenic gene expression and estradiol production.
Keywords: FSH receptor, allosteric agonist, granulosa cells, PCOS, IVF
Capsule:
TOP5300, an oral FSH receptor allosteric agonist (FSHR), stimulates estradiol production and steroidogenic gene expression from granulosa-lutein cells of polycystic ovarian patients differently than recombinant FSH.
Introduction:
Infertility affects 15% of couples worldwide (1–3). Demand by patients and physicians for more convenient COS-IVF cycles led to the development of corifollitropin (Elonva, a long-lasting fusion protein injection that replaces an average of 7 days of FSH injections). Among patients who had a previous COS-IVF cycle with daily FSH injections, there was a 75% preference for corifollitropin (4). However, neither oocyte retrieval rates per cycle start or clinical pregnancy rates were significantly improved by 120–240 IU of corifollitropin compared to 150 IU of daily recombinant gonadotropin in antagonist-controlled cycles (5, 6, 7). In an effort to improve pregnancy rates of COS-IVF cycles, the addition of luteinizing hormone (LH) [HP-hMG (8) or rLH (9)] either increased pregnancy rates in women of advanced reproductive age (>35) or had no impact on clinical pregnancy rates (10). Recent meta-analyses support the addition of r-LH to r-FSH COS for discrete populations (11–12).
Oral FSH receptor allosteric agonists offer a remarkably more convenient method of administration than subcutaneously injected hormones. Beyond the recognizable convenience benefit, FSHR allosteric agonists may offer unanticipated clinical benefits (13, 14). In the 21st century, access to a single class of therapies (injectable gonadotropins) may present a barrier for personalized therapeutics for subsets of the infertility patient population. Among polycystic ovarian syndrome (PCOS) patients (15), overcoming the impediments of androgen excess and insulin resistance with a new mechanism of receptor activation may avoid low rates of maturation of small antral follicles into preovulatory follicles. Among advanced reproductive age (ARA, age >35) patients, or those with diminished ovarian reserve (DOR), overcoming low ovarian response and lower quality oocytes are the primary concern, relative to patients with normal ovarian reserve (NOR). The cause of infertility varies for each of these groups, and it is reasonable to anticipate that new personalized therapeutics will emerge to address each of their needs. Access to an allosteric agonist with a different mechanism of action than rh-FSH on its receptor may provide an opportunity for personalized therapy for some, or all, of these patient subsets.
Although administration of an oral FSHR/LHR agonist is acknowledged to provide greater patient convenience than daily injectables, evidence that an allosteric agonist contributes a unique mechanism of receptor activation that leads to a clinical benefit remains to be shown. Current evidence favors a mechanism for allosteric agonists of GPCRs to bypass the extracellular domain (glycoprotein FSH binding site) and impose changes in the transmembrane helices, extracellular loops and hinge domain helix (16–18). Although both agonists stimulate adenylyl cyclase, some allosteric agonists exert biased agonism through Gαi, PKB/Akt, and MAPK pathways (19–20), shown previously to be stimulated by rh-FSH (21). However, it is unclear if biased signaling confers a meaningful clinical advantage, partly because allosteric agonists have yet to advance beyond phase 1 clinical trials. The following experiments were conducted to identify whether TOP5300 offers a measurable biochemical benefit in granulosa cells for subsets of infertility patients prior to evaluation of a biophysical biased agonist mechanism of TOP5300.
A summary of the physical and biochemical properties of TOP5300 and rh-FSH (follitropin-α) is provided (Supplemental Table 1). Follitropin-α is 42-fold larger molecule than TOP5300, although both ligands have similar pharmacokinetic properties in rodents. The following studies were designed to test whether the two ligands stimulate the FSHR-dependent steroidogenic pathway differently in human granulosa-lutein cells (GLC) of patient populations with known differences in response to COS (ARA, PCOS or NOR).
Materials and Methods:
Collection of human granulosa cells
Patients undergoing IVF at Texas Children’s Hospital Family Fertility Center with the diagnosis of NOR, PCOS or ARA (Supplemental Table 2) were consented pre-operatively on the day of transvaginal oocyte aspiration under the Baylor College of Medicine IRB H-18323. The oocytes were collected from the follicular fluid by the embryologists and the discarded follicular fluid was transferred to research personnel. A total of 41 patients were consented, and viable primary granulosa cell cultures were established for each patient (Supplemental Table 2). Multiple endpoints (estradiol ELISA, qPCR, immunofluorescence) were measured on the same individual patient sample. Cycle characteristics are noted in Supplemental Table 3.
Granulosa-lutein culture and compound treatment
Granulosa-lutein (GL) cell cultures were established using a previously described protocol (22, 23) to re-sensitize cells to rh-FSH. Of note, patient samples were not pooled; FSHR agonist treatments were applied individually to each primary culture established for each patient. On day 7, growth media was removed from 96-well plates and replaced with stimulation media [DMEM-F12, 0.1% BSA, fungizone, penicillin (100 IU/ml), streptomycin (100 μg/ml) and 10−7 M androstenedione (Steraloids, Newport, RI)] without FBS. After a 24hr starvation period, compounds (rh-FSH or TOP5300) at varying concentrations previously shown to be effective in hGLC (23) were added. Recombinant human FSH [National Hormone Pituitary Program (AFP8468A and AFP8821A NHPP, Torrance, CA] or TOP5300 (21) were added. Following 6hr, 24hr (day 9), or 48hr (day 10) incubation, conditioned media was collected for estradiol ELISA. RNA was collected from lysed cells at 6hr for quantitative polymerase chain reaction (qPCR) studies. Additional cell cultures were fixed with 4% PFA (Thermo Fisher, Waltham, MA) after a 6hr incubation for immunofluorescence (IF) studies.
Immunofluorescence studies targeting FSHR localization
Initial immunofluorescence experiments were conducted on Chinese Hamster Ovary (CHO)-parental cells and CHO-hFSHR cells (transfected with human FSHR) to confirm binding to human FSHR by the antibody 106–105 [24; provided, courtesy of Dr. David Schomberg (Duke University)]. CHO cells (-parental and -hFSHR) were grown to 75% confluence [DMEM-F12 with 10% FBS in T75 cell culture flasks (Corning, Corning, NY)] and then trypsin-released from flasks and plated on glass-bottom 96 well plates (Greiner Bio-One, Monroe, NC), at a density of 2500 cells/well.
To confirm that cells identified by 106–105 binding were granulosa cells, FOXL2 antibody staining [Dagmar Wilhelm at University of Melbourne (courtesy of Joanne Richards, Baylor College of Medicine)] was performed. Cells were fixed in 4% PFA at room temperature for 15 min, blocked in 3% BSA in TBS and then immunolabeled with FSHR antibody (1:500) and FOXL2 (1:1000) antibodies at 4C. After washes, secondary antibodies (1:1000, Alexa488 and Alexa 647 conjugates) were applied at room temperature for 30 minutes before adding DAPI as a nuclear counterstain for 5 minutes. Imaging was performed on the CV8000 high throughput spinning disk confocal (Yokogawa, Japan) with a 20xLWD objective, 9 FOV/condition, with a 4μm z-stack and max intensity projection. Image analysis was performed automatically with CellPathfinder (Yokogawa, Japan). For double FSHR/FOXL2 immunofluorescence experiments, cells were first immunolabeled for FSHR as described above, then post-fixed to maintain the antibody binding. A second round of immunolabeling for FOXL2 was performed using saponin as a detergent to allow for nuclear labeling, and to discriminate FSHR localized to the cell membrane in cells that expressed FOXL2 in the nucleus.
First, nuclei were identified using classic thresholding based on the DAPI channel. Size and intensity filters were applied to eliminate small and bright objects (usually dead cells, mitotic cells, debris). From the DAPI mask, a median filter was applied to the FSHR channel to identify the signal boundaries irradiating from the nuclear mask. This secondary mask is considered the cell mask. Masks touching the boundaries of the image field of view were removed. Touching cells are split using a watershed algorithm. Features are then extracted from the channels using the masks described above and further analyzed. Data was exported as CSV file containing intensity features and analyzed further in Microsoft Excel and GraphPad Prism (San Diego, CA, United States). Statistical analysis involved 2-way ANOVA and paired t-tests. p values less than 0.05 were considered statistically significant.
Estradiol measurement
Conditioned media was collected following a 48hr treatment of the human granulosa cells, and stored at −80°C. Estradiol ELISA assays were performed using kit instructions (DRG International, Springfield, NJ). Optical densities were measured using a Tecan plate reader (Lifesciences, Mannedorf, Switzerland). Data was analyzed in Microsoft Excel and GraphPad Prism (San Diego, CA, United States). Progesterone concentrations in media were measured by the Immunoassay Core (University of Virginia). Values from different patients were averaged per patient group (NOR, PCOS, ARA), and per compound treatment dose.
Gene expression studies targeting steroid enzymes
Purified RNA was extracted using TRIzol (Thermo Fisher, Waltham, MA), and precipitated at −80°C following addition of 10μg of glycogen (Thermo Fisher, Waltham, MA). The RNA pellet was air-dried for 10–15 min and resuspended in 12μl of RNAse/DNAse free water (Qiagen, Hilden, Germany). TaqMan master mixes, reagents and probes targeting GAPDH, StAR and CYP19A1 were subsequently used following kit instructions (Thermo Fisher, Waltham, MA). Data was analyzed in Microsoft Excel and GraphPad Prism (San Diego, CA, United States). Results with standard error are presented. Analysis of variance was performed, and p< 0.05 was considered statistically significant.
Results:
Clinical features
Total units of FSH administered during COS (recombinant and urinary) were similar for NOR and PCOS patients, while ARA patients received approximately 1000 IU more FSH over the COS protocol (p=0.008; [Mean +/− SEM] NOR: 2350+/−305; ARA: 3337+/−351; PCOS: 2169+/−233 IU). Menopur was administered at similar doses to all patient groups ([Mean +/−SEM] NOR: 1500+/−189; ARA: 1746+/−166; PCOS: 1598+/−189), with the exception of 1 patient in the NOR group and 2 patients in the PCOS group. Plasma AMH levels provided confirmation of the ovarian reserve anticipated for patients recruited for this study (NOR: 4.3+/−0.64, n=8; PCOS: 6.97+/−3.96, n=12; and ARA: 2.60+/−0.46, n=17) and conform to the reference values for NOR (3–6) PCOS (>6) and ARA (1–3) at Texas Children’s Hospital Family Fertility Center. Plasma levels of AMH were significantly different between NOR and PCOS patients (p=0.002, one-tail t-test), but were not different between NOR and ARA groups (p=0.5; Supplemental Table 2).
Steroid production by FSH receptor agonists
We confirmed in a small set of patients that TOP5300 stimulated estrogen production from human GLC in vitro with a response that was similar to our previous report (23) and maintained visibly different responses for TOP5300 compared to recombinant FSH, after 48hr (Figure 1; compare to Ref 23, Fig 10). Estradiol production from GLC of NOR patients was comparable between rh-FSH and TOP5300, while the EC50 of rh-FSH was 100-fold lower (more potent) than the EC50 of TOP5300. In this small set of patients, TOP5300 led to greater production of estradiol when compared to rh-FSH in cells obtained from PCOS patients (Figure 1). Progesterone production by GL was stimulated 2–3-fold by both TOP5300 and rh-FSH, was independent of patient diagnosis, but was markedly influenced by ovulation trigger (Supplemental Table 4). Because steroid production over 48hr was highly variable among patients, we redirected our analyses towards expression of 2 critical steroidogenesis genes over 6 hours culture with FSHR ligands.
Figure 1.
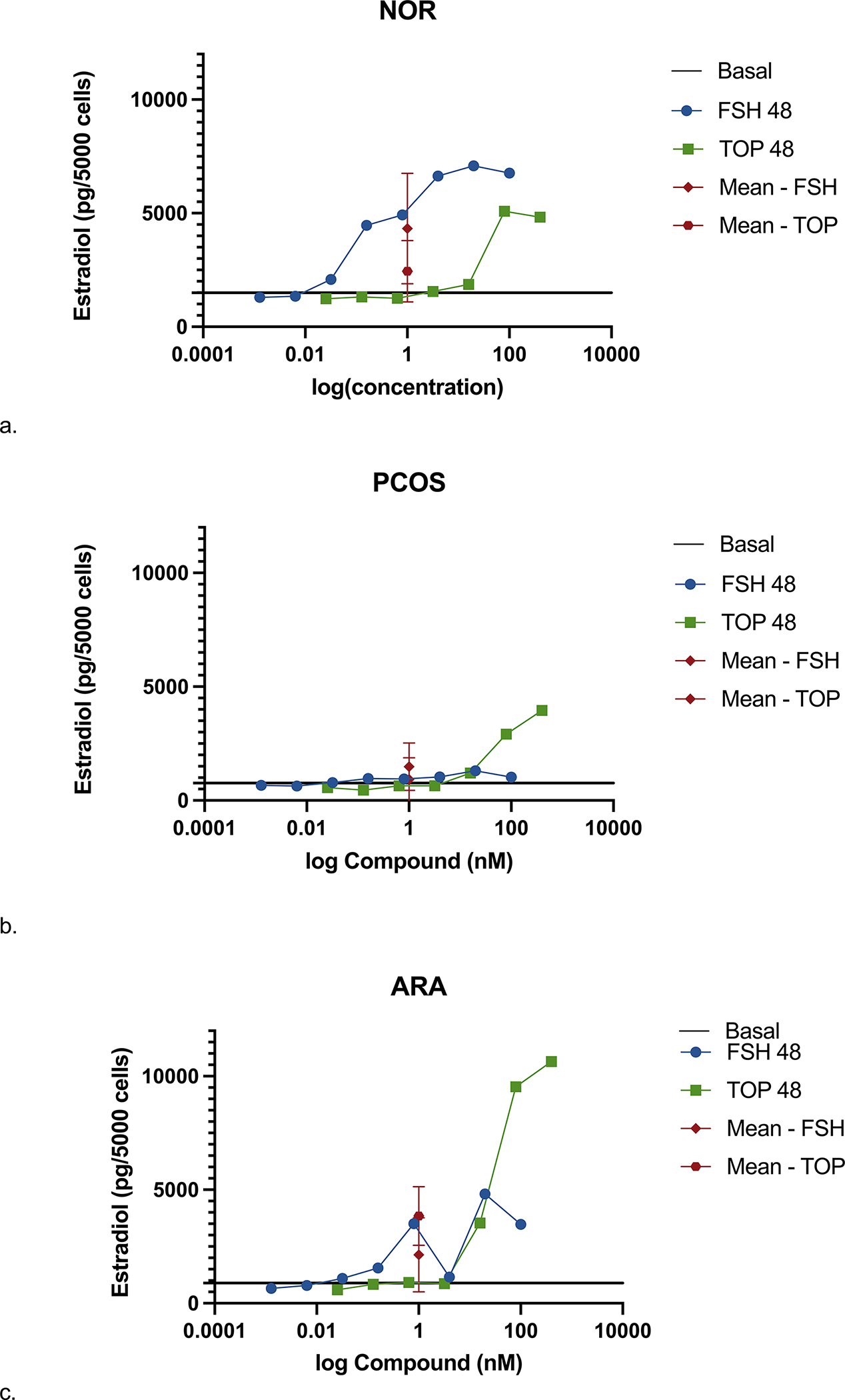
Steroidogenic response of GL to TOP5300 and rh-FSH varies among 3 patient populations evaluated. Estradiol secretion in response to ligands was measure after 48h stimulation in NOR (A), PCOS (B) and ARA (C) patients. Data is mean +/− SD. (a) NOR patients, n=2; (b) PCOS patients, n=4; (c) ARA patients, n=4.
Immunofluorescence with FSHR antibody 106–105
The FSHR antibody 106–105 confirmed localization of the FSHR at the plasma membrane of CHO-FSHR cells as compared to CHO-parental cells (Supplemental Figure 1). Patient-derived hGL cells identified by 106–105 antibody were confirmed to be granulosa cells with a second granulosa cell-specific antibody that recognizes FOXL2 (visible as nuclear red signal; Supplemental Figure 2). A consistent trend for FSHR expression in GL among rh-FSH and TOP5300 was modest expression of FSHR at cell membranes and within cells under basal conditions. NOR patients had the highest detectable FSHR levels at the cell surface (Figure 2) while PCOS patients had moderately less FSHR localization, and ARA patients had significantly less FSHR localization compared to NOR. Quantitative analysis of FSHR detection in GL suggested that localization of FSHR in GLC from patients was not influenced by ligand type (rh-FSH, TOP5300) (Figure 3). Incubation with up to 100nM rh-FSH or 400 nM TOP5300 had minimal effect on FSHR localization, eliminating competition between rh-FSH and antibody 106–105 as a potential cause for changes in antibody binding to the extracellular region of the receptor. Representative FSHR-only stained GLCs are presented in Supplemental Figure 3. Of note, the cells were not permeabilized to enable anti-FSHR antibody to reach intracellular sites; a membrane marker was not used given that detergent was not used during the FSHR immunolabeling indicating that the signal detected was most likely from cell surface or near cell surface antigens.
Figure 2.
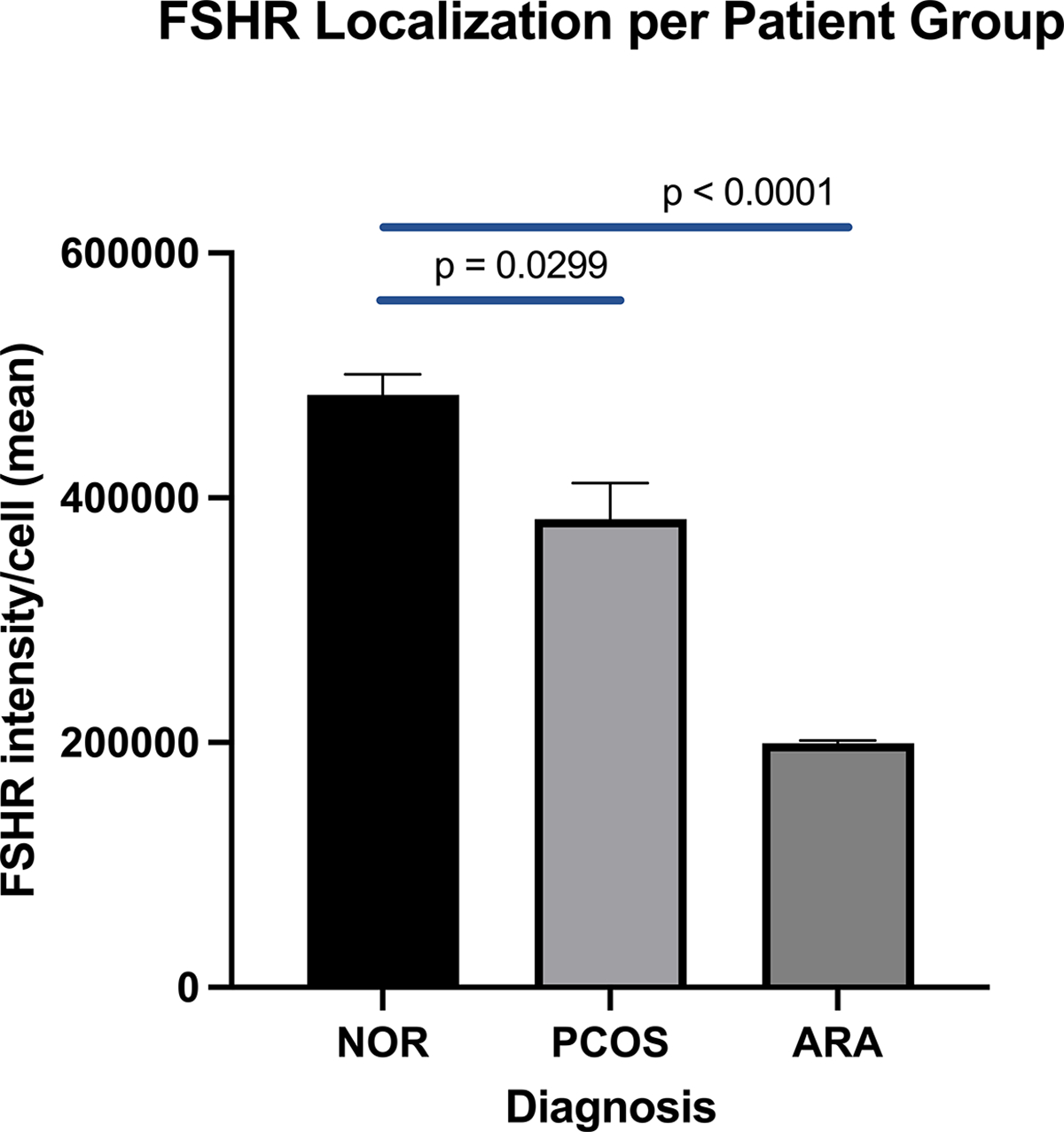
The abundance of FSHR localized to the plasma membrane within cultured GL was significantly influenced by patient diagnosis. FSHR localized to the plasma membrane of GLC were quantitated for each patient diagnosis group and comparisons were made across patient groups. [Average total intensity of fluorescence of the FSHR per cell captured at 20x/LWD on a high throughput spinning disk confocal and analyzed using CellPathfinder]. Statistical analysis involved 2-way ANOVA and paired t-tests. Patients with ARA have significantly less FSHR localization in comparison to the NOR group, p < 0.0001.
Figure 3.
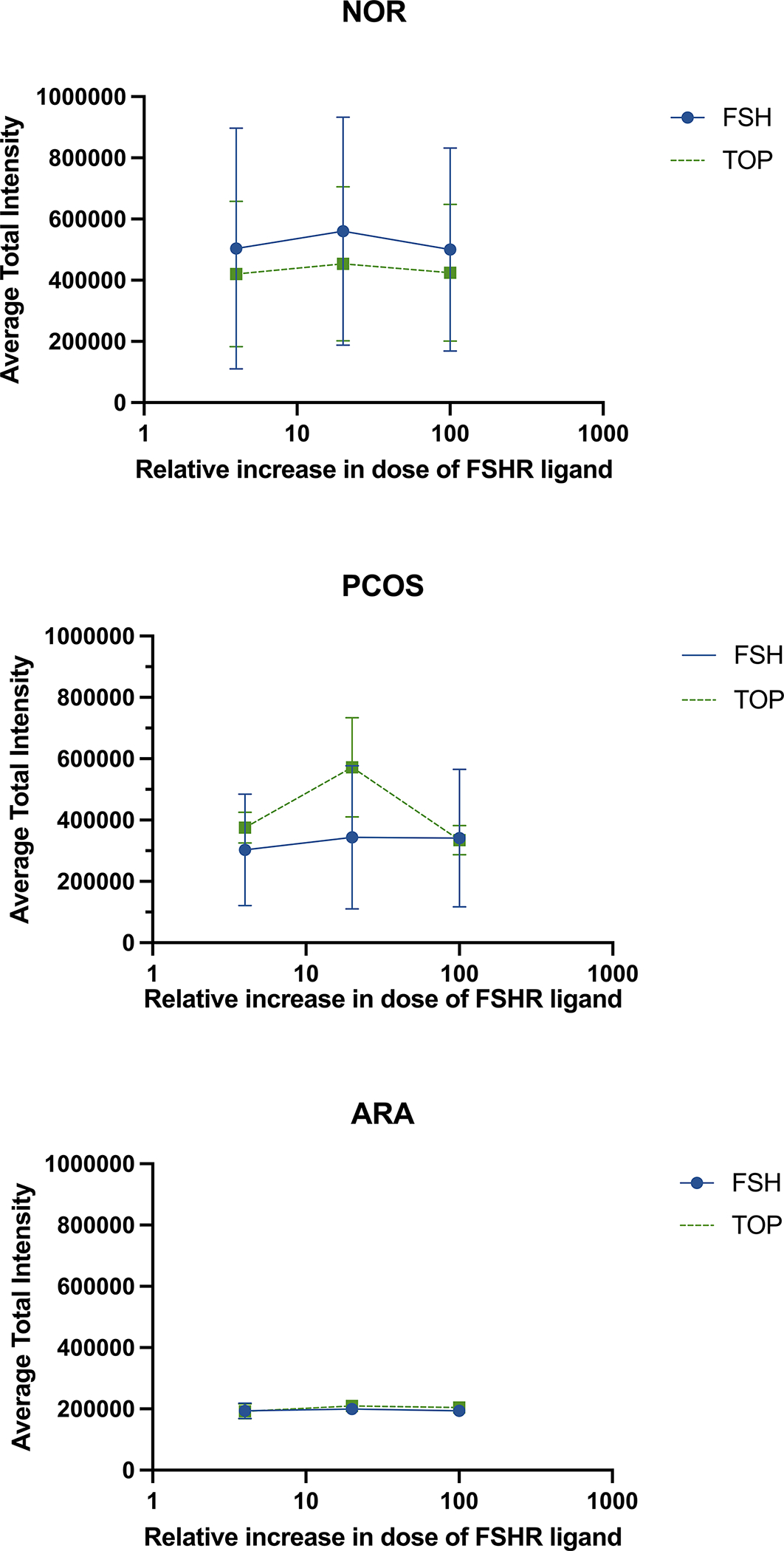
FSHR localization after FSHR ligand treatment demonstrates minimal changes in FSHR at the plasma membrane. Granulosa cells were treated with either rh-FSH or TOP5300 for 6 hours and average total intensity of FSHR at the plasma membrane was measured as in Figure 2. The effect of ligand-induced FSHR localization was not significantly different for either rh-FSH or TOP5300. NOR n=4, PCOS n=7, ARA n=4. Standard errors are plotted.
Gene expression: qPCR results
Levels of mRNA for StAR and CYP19A1 were determined by qPCR and normalized to GAPDH to determine fold-change in the steroidogenic pathway mRNAs by TOP5300 relative to rh-FSH. As a first step in this analysis, collective changes in gene expression caused by 400 nM TOP5300 or 100 nM rh-FSH relative to basal levels were measured among all patients. Among all patient groups, TOP5300 stimulated a 6-fold increase in relative StAR expression compared to vehicle control (p=0.0002) and rhFSH stimulated a 3-fold increase (p=0.008) in StAR (Figure 4a). Similarly, TOP5300 stimulated a 4-fold increase in CYP19A1 (p=0.0017), while rh-FSH stimulated a 2-fold increase (p=0.006; Figure 4b). These results suggested that TOP5300 (400nM) was more effective agonist of steroidogenic gene expression than rh-FSH (100nM). Next, ligand-specific responses among the 3 selected patient populations (2-way ANOVA, ligand x patient population) were determined to be significantly different (p=0.0105) across the 2 steroidogenic enzyme mRNAs (both StAR and CYP19A1). TOP5300 stimulated increased expression of StAR and CYP19A1 mRNA (6-fold; p=0.0412) in GLC from PCOS patients, while rh-FSH was without effect (p=0.765; Figure 4c). In contrast, both rh-FSH (3-fold; p=0.007) and TOP5300 (5-fold; p<0.0001) stimulated increased expression of StAR and CYP19A1 among NOR and ARA patient cells (Figure 4d). Heterogeneity of GL cell responses of PCOS patients to TOP5300 and low patient volume during 2019–2021 (COVID-19 restrictions) limited our ability to statistically test for independent changes in StAR from effects of CYP19A1 among the 3 patient populations.
Figure 4.
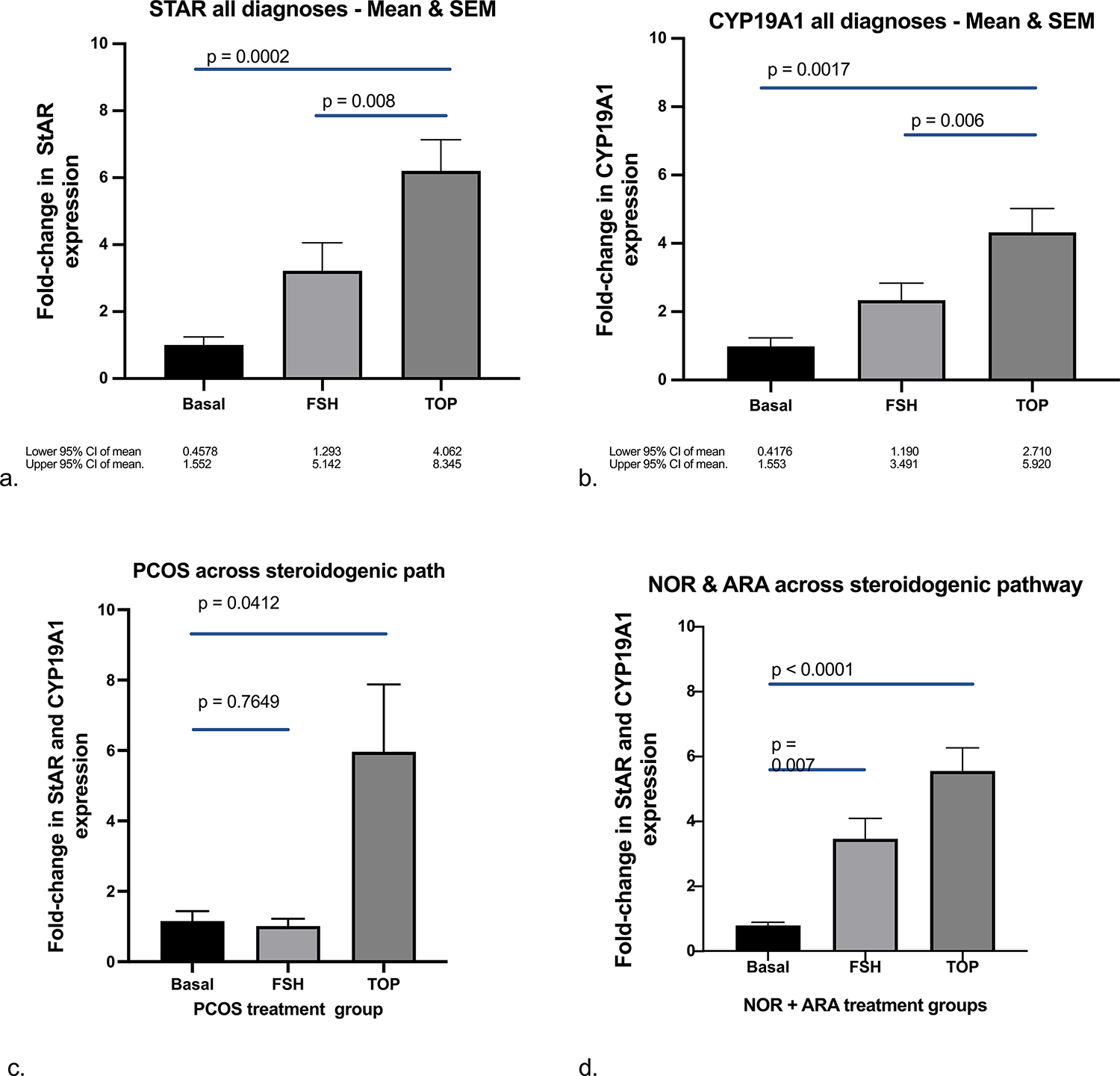
TOP5300 stimulates greater expression of STAR and CYP19A1 than rh-FSH following 6 hrs of culture and stimulates more robust response among PCOS patients compared to NOR or ARA patients. Steroid enzyme expression (determined by qPCR; see Materials & Methods) for basal, rh-FSH and TOP5300 treated cells were analyzed (ANOVA) for each compound and comparisons were made among patient diagnoses and FSHR agonist treatments. Expression of STAR (A) and CYP19A1 (B) in GLC was found to be significantly higher in TOP5300 treated cells than rh-FSH-treated cells (n = 6). (c) Integrated expression of StAR and CYP19A1 was significantly higher in TOP5300 treated cells compared to rh-FSH-treated cells for PCOS patients, while rh-FSH was similar to basal levels (n = 6). (d) Integrated expression of StAR and CYP19A1 was stimulated significantly above basal by both TOP5300 and rh-FSH, while the difference between the two ligands was not different in cells from NOR or ARA patients (n = 6). Mixed effects, 2-way ANOVA.
Discussion:
There are 4 key observations from this study that are an expansion of our efforts to discover and characterize novel FSHR allosteric agonists. First, we have extended our previous observation on steroid production; TOP5300 stimulated estradiol production in a concentration-dependent manner in GLC, in a manner similar to rh-FSH. Second, the localization of FSHR at plasma membranes in GLC is significantly reduced in ARA relative to NOR patients, and the mechanism of action of TOP5300 does not appear to rely on changes in receptor levels at the cell membrane relative to rh-FSH. Third, steroidogenic enzyme expression (StAR and CYP19A1) by rh-FSH and TOP5300 was increased similarly in NOR and ARA patients, while TOP5300 had unique potential to increase expression of StAR and CYP19A1 in cells from a limited number of PCOS patients compared to rh-FSH. Fourth, results with TOP5300 suggest there is underlying heterogeneity in the response of cells from patients to FSHR allosteric agonists, suggesting that in the future, favorable responses in a subset of patients (e.g., PCOS, Rotterdam criteria) may be identified with FSHR allosteric agonists that cannot be identified with rh-FSH.
The COS-IVF protocols used across the 3 patient populations incorporated peptide GnRH antagonist-controlled cycles. Among recruited patients, plasma AMH levels (in addition to antral follicle counts) provided confirmation of the ovarian reserve and conformed to the reference values for NOR (3–6) PCOS (>6) and ARA (1–3) at the Family Fertility Center in Houston, TX. Doses of follitropin (recombinant or urinary) used for COS followed anticipated responses of patient populations, i.e., more FSH was required for ARA than NOR and PCOS.
TOP5300 leads to greater estradiol production in PCOS patients
Differences in estradiol production and steroidogenic enzyme expression between patient groups treated with rh-FSH were anticipated, based on prior results (23). GLC from PCOS patients were responsive to TOP5300 while these same cells were not responsive to rh-FSH. In this small set of patient-derived cell cultures, TOP5300 stimulated 3–10-fold greater levels of estradiol in culture media from PCOS and ARA patients than was achieved with rh-FSH. The variability in estradiol release from GL cultures (48hr treatment) among all patient subgroups influenced our decision to shift our sample analyses towards gene expression (6hr compound treatment), rather than to continue to collect cells for estradiol production. Although TOP5300 has some CG/LHR activity (85% FSHR/15% LH/CGR activity; EC50 rat GC = 868 nM; EC50 rat LHR = 6,516 nM; EC50 human granulosa-lutein cells = 474 nM), TOP5668, is a highly specific FSHR agonist (no detectable LHR activity in vitro or in vivo), and it was previously shown to be the more potent FSHR agonist in human GL cultures (23). TOP5300 and TOP5668 represent single chemical entities suitable for either FSHR+LH/CG-R combined stimulation with a single molecular entity, or FSHR-only stimulation as convenient products for future evaluation in COS-OI and COS-IVF protocols.
TOP5300 leads to greater expression of steroid enzymes
Previous research with freshly-harvested granulosa cells from control and PCOS patients showed 2-fold higher expression of CYP19A1 and 2.5-fold higher expression of StAR in GLC retrieved from PCOS patients after COS compared to control patients (25). These observations are opposite of those reported in previous analysis of CYP19A1 expression from GLC of PCOS and non-PCOS patients (26). In line with the Owens report (25), our limited results replicates that rh-FSH increases expression of both StAR and CYP19A1 across NOR, PCOS and ARA patient groups. Our additional observation was that TOP5300 induced greater expression of both steroid enzymes compared to rh-FSH (in vitro, Figure 4) across all patient subgroups. It is possible that the greater impact of TOP5300 on steroidogenic gene expression reported in this study could be explained by its 15% additional activity on LHR, on top of its allosteric effects on FSHR, in a manner previously described (27). Our results with only a limited set of patients suggest that more extensive evaluation of the effects of TOP5300 and TOP5668 in larger sets of patient-derived GLC is needed to more clearly define clinical potential for these compounds in patient subsets (PCOS patients suggested by this work) that can be distinguished from effects of rh-FSH.
Documented molecular contributors to dysregulated steroidogenesis in GLC from PCOS patients include hyperandrogenemia, insulin resistance, and elevated AMH. Higher concentrations of androgen precursors, total androgens, and lower concentrations of estrogens have been measured in the follicular fluid of PCOS patients (days 4–7 in the follicular phase) (26, 28–29). A potential contributor to hyperandrogenemia is higher expression of LH receptor in smaller antral follicles (25). The integrity of insulin signaling is essential for optimal expression of FSHR-dependent biochemical responses. FSH-stimulated increase in IRS-2 mRNA is associated with activation of PI3K/p-Akt, however, IRS-2 expression and activation of these pathways are defective in cultured GL from PCOS patients (25–26, 29–30). The essential involvement of PI3K/p-Akt, IGF-1R and IRS-1, and associated phosphatases, has been established for the FSHR (25, 29), and disruption of IRS-1/IRS-2 impacts metabolic control of follicular development. AMH inhibits progression of primary and secondary to antral follicles. Serum AMH levels have been reported to be 2–5 times higher in women with PCOS, including levels that are higher in women with anovulatory cycles than women with ovulatory PCOS (31–38). AMH has been shown to inhibit both FSH-stimulated and LH-stimulated StAR and CYP19A1 gene expression in GLC cultures (39). Summarizing for PCOS, all three molecular pathways contributing to the PCOS phenotype are likely to influence the reduced responsiveness to rh-FSH in GLC in these experiments. It remains to be determined in larger sets of GLC results whether TOP5300 relative to rh-FSH may bypass signaling impediments within follicular cells (35–41) associated with the molecular phenotypes of PCOS.
Conclusions:
We have provided early evidence that TOP5300 induces robust steroidogenic enzyme gene expression in GLC obtained from patients with PCOS, compared to GLC from NOR or ARA patients (summarized in Supplemental Figure 4). Changes in estradiol secretion from cultured GLC followed the effects of TOP5300 relative to rh-FSH on gene expression. The efficacy of TOP5300 relative to rh-FSH in PCOS patients was independent of ligand-dependent changes in FSHR localization to plasma membranes observed for ARA patients. TOP5300 may provide a possible alternative to rh-FSH for COS for PCOS patients, based on its apparent ability to stimulate greater estradiol production and lead to greater expression of steroidogenic genes. These results inspire translation of TOP5300 into clinical measures of efficacy and safety.
Limitations of the present work
First, this work was designed to present a first glimpse into the clinical potential for novel FSHR ligands to cause responses that may address unmet needs for couples seeking COS solutions in the future. Second, although conduct of these studies with less-luteinized cells would be preferred over mural (36), the various comparisons of TOP5300 relative to rh-FSH could not have been possible with limited numbers of cells/patient.
Supplementary Material
Supplemental Figure 1. FSHR antibody 106–105 is specific for human FSHR. DAPI = blue. FSHR antibody = green. (b) FSHR expression appears in the plasma membrane of CHO-human FSHR cells as expected and (a) this is not visualized in the CHO-parental cells.
Supplemental Figure 2. Granulosa cell identity confirmed by co-immunolabeling with FSHR antibody 106–105 and FOXL2 antibody. DAPI = blue. FSHR antibody = green. FOXL2 antibody = red.
Supplemental Figure 3. Sets of FSHR localization in GLC obtained from NOR, PCOS, or ARA patients in the absence or presence of 100 nM rh-FSH or 400 nM TOP5300.
Supplemental Figure 4. Summary Model. Relative to rh-FSH, TOP5300 stimulated increased levels of steroidogenic endpoints of follicular maturation that estimate oocyte viability that was independent of changes in FSHR localization to plasma membrane.
Acknowledgements:
This work would not be possible without the contributions of the following collaborators: Reagents were provided by James Dias, Ph.D., at Albany Medical College, who generated the FSHR antibody, and David Schomberg, Ph.D., at Duke University, who provided the FSHR antibody for use in immunofluorescence experiments. Dagmar Wilhelm, Ph.D., at University of Melbourne generated the FOXL2 antibody and Joanne Richards, Ph.D., at Baylor College of Medicine provided the FOXL2 antibody for use in immunofluorescence experiments. Physicians and scientists within the Baylor College of Medicine (Stephanie Pangas, Ph.D.) and Texas Children’s Hospital Family Fertility Center (Drs. Laurie McKenzie, M.D., Blesson Selvanesan, Ph.D.) provided suggestions for experimental design.
Funding Statement:
JZG was supported by the Clinical Scientist Training Program (CSTP). SSP and JZG were supported by NIH R01HD032067. Imaging for this project was supported by the Integrated Microscopy Core at Baylor College of Medicine and the Center for Advanced Microscopy and Image Informatics (CAMII) with funding from NIH (DK56338, CA125123, ES030285), and CPRIT (RP150578, RP170719).
Footnotes
Disclosure Statement: The authors have no disclosures.
Attestation Statement: Data regarding any of the subjects in the study has not been previously published unless specified. Data will be made available to the editors of the journal for review or query upon request.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Practice Committee of the American Society for Reproductive Medicine. Diagnostic evaluation of the infertile female: a committee opinion Fertility and Sterility. 2015;103:e44–50. [DOI] [PubMed] [Google Scholar]
- 2.Practice Committee of the American Society for Reproductive Medicine. Definitions of infertility and recurrent pregnancy loss: a committee opinion. Fertility and Sterility. 2013;99:63. [DOI] [PubMed] [Google Scholar]
- 3.Sunderam S KD, Crawford SB, Folger SG, Boulet SL, Warner L, Barfield WD. Assisted reproductive technology surveillance - United States, 2015. CDC. 2018;67(3):1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Requena A CM, Collado D. Evaluation of the degree of satisfaction in oocyte donors using sustained-release FSH corifollitropin alpha. Reproductive BioMedicine Online. 2013;26:253–9. [DOI] [PubMed] [Google Scholar]
- 5.Tsakiridis I, Najdecki R, Tatsi P, Timotheou E, Kalinderi K, Michos G, Virgiliou A, Yarali H, Athanasiadis A, Papanikolaou EG. Evaluation of the safety and efficacy of corifollitropin alfa combined with GnRH agonist triggering in oocyte donation cycles. A prospective longitudinal study. JBRA Assist Reprod. 2020. Oct 6;24(4):436–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zahiri Sorouri Z, Pourmarzi D, Safar Khah N. Corifollitropin- α compared to daily r-FSH in for patients undergoing intracytoplasmic sperm injection: Clinical trial study. Int J Reprod Biomed. 2019. Mar 3;17(1):23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cozzolino M, Vitagliano A, Cecchino GN, Ambrosini G, Garcia-Velasco JA. Corifollitropin alfa for ovarian stimulation in in vitro fertilization: a systematic review and meta-analysis of randomized controlled trials. Fertil Steril. 2019. Apr;111(4):722–733. [DOI] [PubMed] [Google Scholar]
- 8.Marrs R, Meldrum D, Muasher S, Schoolcraft W, Werlin L, Kelly E. Randomized trial to compare the effect of recombinant human FSH (follitropin alfa) with or without recombinant human LH in women undergoing assisted reproduction treatment. Reprod Biomed Online. 2004. Feb;8(2):175–82. [DOI] [PubMed] [Google Scholar]
- 9.Bordewijk EM, Mol F, van der Veen F, Van Wely M. Required amount of rFSH, HP-hMG and HP-FSH to reach a live birth: a systematic review and meta-analysis. Hum Reprod Open. 2019 Jun 1;2019(3):hoz008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.König TE, van der Houwen LE, Overbeek A, Hendriks ML, Beutler-Beemsterboer SN, Kuchenbecker WK, Renckens CN, Bernardus RE, Schats R, Homburg R, Hompes PG, Lambalk CB. Recombinant LH supplementation to a standard GnRH antagonist protocol in women of 35 years or older undergoing IVF/ICSI: a randomized controlled multicentre study. Hum Reprod. 2013. Oct;28(10):2804–12. [DOI] [PubMed] [Google Scholar]
- 11.Conforti A, Esteves SC, Humaidan P, Longobardi S, D’Hooghe T, et al. , 2021. Recombinant human luteinizing hormone co-treatment in ovarian stimulation for assisted reproductive technology in women of advanced reproductive age: a systematic review and meta-analysis of randomized controlled trials. Reprod Biol Endocrinol. 19(1):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conforti A, Esteves SC, Di Rella F, Strina I, De Rosa P, Fiorenza A, et al. , 2019. The role of recombinant LH in women with hypo-response to controlled ovarian stimulation: a systematic review and meta-analysis. Reprod Biol Endocrinol. 17(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chandra A, Copen CE, Stephen EH. Infertility service use in the United States: data from the National Survey of Family Growth, 1982–2010. Natl Health Stat Report. 2014. Jan 22;(73):1–21. [PubMed] [Google Scholar]
- 14.van Eekelen R, Wang R, Danhof NA, Mol F, Mochtar M, Mol BW, van Wely M. Cost-effectiveness of ovarian stimulation agents for IUI in couples with unexplained subfertility. Hum Reprod. 2021. Apr 20;36(5):1288–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenfield RL. Current concepts of polycystic ovary syndrome pathogenesis. Curr Opin Pediatr. 2020. 32(5):698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang X DJ, He X. Structural biology of glycoprotein hormones and their receptors: insights to signaling. Mol Cell Endocrinol. 2014;382:424–51. [DOI] [PubMed] [Google Scholar]
- 17.Jiang X, Fischer D, Chen X, McKenna SD, Liu H, Sriraman V, Yu HN, Goutopoulos A, Arkinstall S, He X. Evidence for Follicle-stimulating Hormone Receptor as a Functional Trimer. J Biol Chem. 2014. May 16;289(20):14273–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma N, Nivedha AK, Vaidehi N. Allosteric communication regulates ligand-specific GPCR activity. FEBS J. 2021. Apr;288(8):2502–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sposini S DPF, Richardson R, Sayers NS, Perrais D, Yu H, Palmer S, Nataraja S, Reiter E, Hanyaloglu AC. Pharmacological programming of endosomal signaling activated by small molecule ligands of the follicle stimulating hormone receptor. Frontiers in Clinical Pharmacology. 2020. Nov; 30(11):593492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Landomiel F, De Pascali F, Raynaud P, Jean-Alphonse F, Yvinec R, Pellissier LP, Bozon V, Bruneu G, Crepieux P, Poupon A, Reiter E. Biased signaling and allosteric modulation at the FSHR. Front Endocrinol. 2019. Mar; 10:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Law NC, Donaubauer EM, Zeleznik AJ, Hunzicker-Dunn M. How Protein Kinase A Activates Canonical Tyrosine Kinase Signaling Pathways To Promote Granulosa Cell Differentiation. Endocrinology. 2017. Jul 1;158(7):2043–2051. doi: 10.1210/en.2017-00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amsterdam A, Tajima K, Frajese V, and Seger R. Analysis of signal transduction stimulated by gonadotropins in granulosa cells. Mol. Cell. Endocrinol. 2003. 202:77–80. [DOI] [PubMed] [Google Scholar]
- 23.Nataraja S, Yu H, Guner J, Palmer S. Discovery and preclinical development of orally active small molecules that exhibit highly selective follicle stimulating hormone receptor agonism. Frontiers in Clinical Pharmacology. 2021. Mar; 31(12):672778. [Google Scholar]
- 24.Lindau-Shepard B, Brumberg HA, Peterson AJ, Dias JA. Reversible immunoneutralization of human follitropin receptor. J Reprod Immunol. 2001. Jan;49(1):1–19. [DOI] [PubMed] [Google Scholar]
- 25.Owens LA, Kristensen SG, Lerner A, Christopoulos G, Lavery S, Hanyaloglu AC, Hardy K, Andersen CY, Franks S. Gene expression in granulosa cells from small antral follicles from women with or without polycystic ovaries. J Clin Endocrinol Metab. 2019. 104(12):6182–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naessen T, Kushnir MM, Chaika A, Nosenko J, Mogilevkina I, Rockwood AL, Carlstrom K, Bergquist J, Kirilovas D. Steroid profiles in ovarian follicular fluid in women with and without polycystic ovary syndrome, analyzed by liquid chromatography-tandem mass spectrometry. Fertil Steril. 2010. Nov;94(6):2228–33. [DOI] [PubMed] [Google Scholar]
- 27.Casarini L, Riccetti L, De Pascali F, Nicoli A, Tagliavini S, Trenti T, La Sala GB, Simoni M. Follicle-stimulating hormone potentiates the steroidogenic activity of chorionic gonadotropin and the anti-apoptotic activity of luteinizing hormone in human granulosa-lutein cells in vitro. Mol Cell Endocrinol. 2016. Feb;422:103–114. [DOI] [PubMed] [Google Scholar]
- 28.Panghiyangani R, Soeharso P, Andrijono, Suryandari DA, Wiweko B, Kurniati M, Pujianto DA. CYP19A1 Gene Expression in Patients with Polycystic Ovarian Syndrome. J Hum Reprod Sci. 2020. Apr-Jun;13(2):100–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anjali G, Kaur S, Lakra R, Taneja J, Kalsey GS, Nagendra A, Shrivastav TG, Devi MG, Malhotra N, Kriplani A, Singh R. FSH stimulates IRS-2 expression in human granulosa cells through cAMP/SP1, an inoperative FSH action in PCOS patients. Cell Signal. 2015. Dec;27(12):2452–66. [DOI] [PubMed] [Google Scholar]
- 30.Baumgarten SC, Convissar SM, Fierro MA, Winston NJ, Scoccia B, Stocco C. IGF1R signaling is necessary for FSH-induced activation of AKT and differentiation of human Cumulus granulosa cells. J Clin Endocrinol Metab. 2014. Aug;99(8):2995–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dewailly D, Robin G, Peigne M, Decanter C, Pigny P, Catteau-Jonard S. Interactions between androgens, FSH, anti-Müllerian hormone and estradiol during folliculogenesis in the human normal and polycystic ovary. Hum Reprod Update. 2016. Nov;22(6):709–724. [DOI] [PubMed] [Google Scholar]
- 32.Robin G, Deknuydt M, Barbotin AL, Pigny P, Catteau-Jonard S, Dewailly D. Anti-Müllerian hormone as a driving force of polycystic ovary syndrome, independently from insulin resistance. Reprod Biomed Online. 2021. May;42(5):1023–1031. [DOI] [PubMed] [Google Scholar]
- 33.Pigny P, Merlen E, Robert Y, Cortet-Rudelli C, Decanter C, Jonard S, Dewailly D. Elevated serum level of anti-mullerian hormone in patients with polycystic ovary syndrome: relationship to the ovarian follicle excess and to the follicular arrest. J Clin Endocrinol Metab. 2003. Dec;88(12):5957–62. [DOI] [PubMed] [Google Scholar]
- 34.Laven JS, Mulders AG, Visser JA, Themmen AP, De Jong FH, Fauser BC. Anti-Müllerian hormone serum concentrations in normoovulatory and anovulatory women of reproductive age. J Clin Endocrinol Metab. 2004. Jan;89(1):318–23. [DOI] [PubMed] [Google Scholar]
- 35.Pellatt L, Rice S, Dilaver N, Heshri A, Galea R, Brincat M, Brown K, Simpson ER, Mason HD. Anti-Müllerian hormone reduces follicle sensitivity to follicle-stimulating hormone in human granulosa cells. Fertil Steril. 2011. Nov;96(5):1246–51. [DOI] [PubMed] [Google Scholar]
- 36.Alebić MŠ, Stojanović N, Duhamel A, Dewailly D. The phenotypic diversity in per-follicle anti-Müllerian hormone production in polycystic ovary syndrome. Hum Reprod. 2015. Aug;30(8):1927–33. [DOI] [PubMed] [Google Scholar]
- 37.Dilaver N, Pellatt L, Jameson E, Ogunjimi M, Bano G, Homburg R, D Mason H, Rice S. The regulation and signaling of anti-Müllerian hormone in human granulosa cells: relevance to polycystic ovary syndrome. Hum Reprod. 2019. Dec 1;34(12):2467–2479. [DOI] [PubMed] [Google Scholar]
- 38.Regan SLP, Knight PG, Yovich JL, Stanger JD, Leung Y, Arfuso F, Dharmarajan A, Almahbobi G. Infertility and ovarian follicle reserve depletion are associated with dysregulation of the FSH and LH receptor density in human antral follicles. Molecular and Cellular Endocrinology. 2017. 446:40–51. [DOI] [PubMed] [Google Scholar]
- 39.Sacchi S, D’Ippolito G, Sena P, Marsella T, Tagliasacchi D, Maggi E, et al. , 2016. The anti-Müllerian hormone (AMH) acts as a gatekeeper of ovarian steroidogenesis inhibiting the granulosa cell response to both FSH and LH. J Assist Reprod Genet. 33(1):95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Regan SLP, Knight PG, Yovich JL, Stanger JD, Leung Y, Arfuso F, Almahbobi G, Dharmarajan A. The effect of ovarian reserve and receptor signaling on granulosa cell apoptosis during human follicle development. Molecular and Cellular Endocrinology. 2018. 470:219–227. [DOI] [PubMed] [Google Scholar]
- 41.Hayes E, Kushnir V, Ma X, Biswas A, Prizant H, Gleicher N, Sen A. Intra-cellular mechanism of Anti-Müllerian hormone (AMH) in regulation of follicular development. Mol Cell Endocrinol. 2016. Sep 15;433:56–65. [DOI] [PubMed] [Google Scholar]
- 42.Wu YG, Barad DH, Kushnir VA, Wang Q, Zhang L, Darmon SK, Albertini DF, Gleicher N. With low ovarian reserve, Highly Individualized Egg Retrieval (HIER) improves IVF results by avoiding premature luteinization. J Ovarian Res. 2018. Mar 16;11(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hua G, George JW, Clark KL, Jonas KC, Johnson GP, Southekal S, Guda C, Hou X, Blum HR, Eudy J, Butnev VY, Brown AR, Katta S, May JV, Bousfield GR, Davis JS. Hypo-glycosylated hFSH drives ovarian follicular development more efficiently than fully-glycosylated hFSH: enhanced transcription and PI3K and MAPK signaling. Hum Reprod. 2021. Jun 18;36(7):1891–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. FSHR antibody 106–105 is specific for human FSHR. DAPI = blue. FSHR antibody = green. (b) FSHR expression appears in the plasma membrane of CHO-human FSHR cells as expected and (a) this is not visualized in the CHO-parental cells.
Supplemental Figure 2. Granulosa cell identity confirmed by co-immunolabeling with FSHR antibody 106–105 and FOXL2 antibody. DAPI = blue. FSHR antibody = green. FOXL2 antibody = red.
Supplemental Figure 3. Sets of FSHR localization in GLC obtained from NOR, PCOS, or ARA patients in the absence or presence of 100 nM rh-FSH or 400 nM TOP5300.
Supplemental Figure 4. Summary Model. Relative to rh-FSH, TOP5300 stimulated increased levels of steroidogenic endpoints of follicular maturation that estimate oocyte viability that was independent of changes in FSHR localization to plasma membrane.


