Abstract
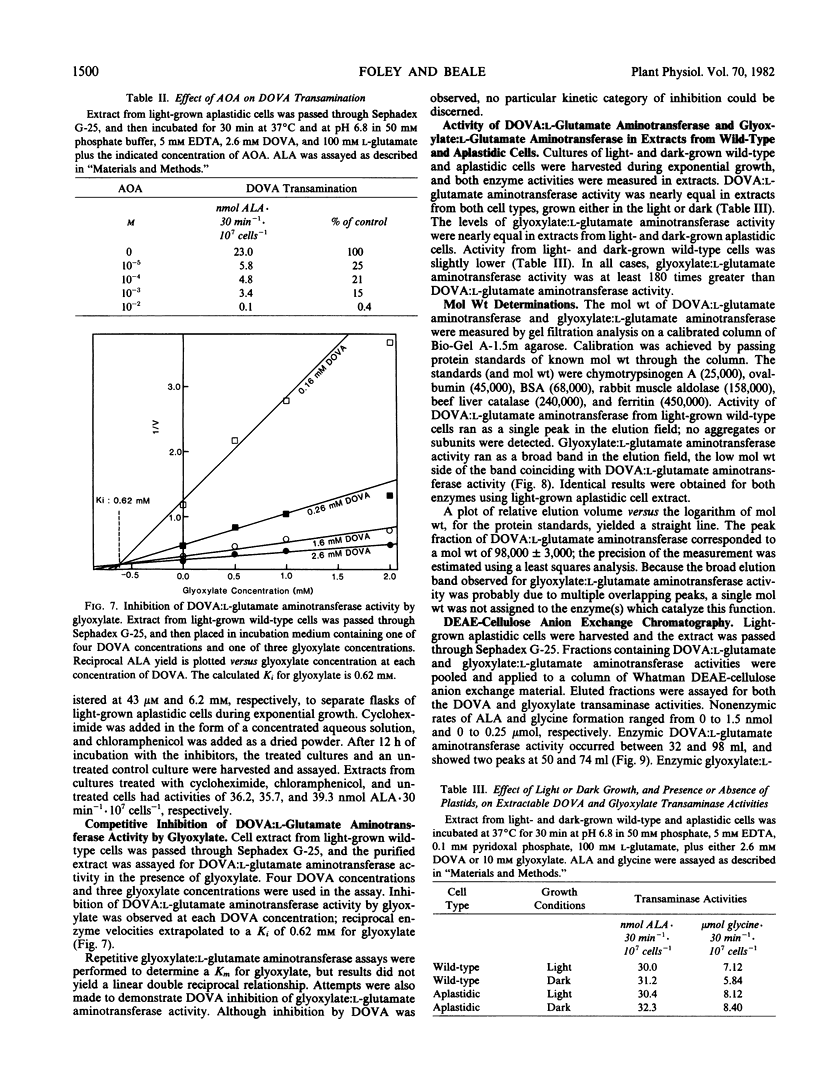
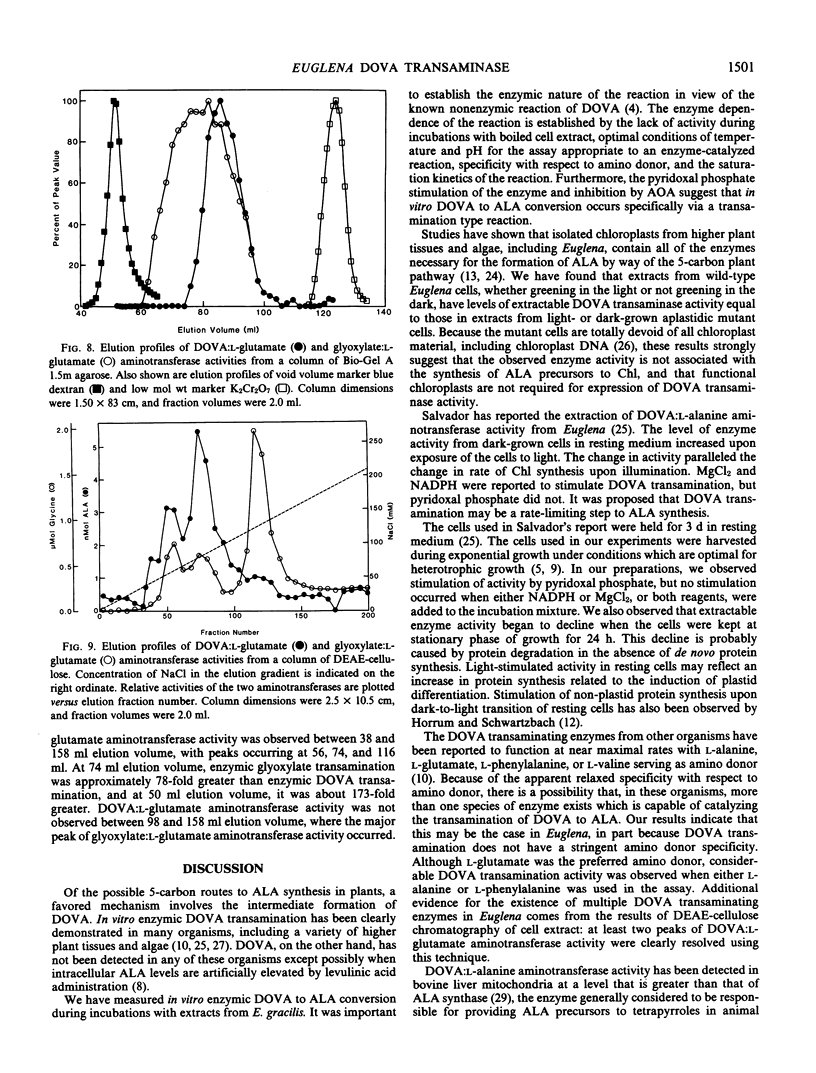
γ,δ-Dioxovaleric acid (DOVA) has been proposed as a precursor to heme and chlorophyll in plants and algae. DOVA transaminase activity was found in extracts of the unicellular green alga Euglena gracilis Klebs strain Z Pringsheim. Optimum conversion of DOVA to δ-aminolevulinic acid (ALA) occurred at pH 6.8. ALA formation was linear with time for at least 30 minutes at 37° C and was proportional to amount of cell extract in the incubation mixture. Boiled cell extract was inactive. DOVA transaminase from either wild-type or aplastidic derivative strain W14ZNaIL ran as a single band in agarose gel permeation chromatography, with a calculated molecular weight of 98,000 ± 3,000. l-Glutamic acid was the most effective amino donor. d-Glutamic acid was inactive. Km values for l-glutamic acid and DOVA were 11 and 1.1 millimolar, respectively. Pyridoxal phosphate stimulated activity maximally at 30 micromolar, and (aminooxy)acetate was strongly inhibitory. Glyoxylic acid was a competitive inhibitor with respect to DOVA, with an inhibition constant of 0.62 millimolar. Wild-type and aplastidic cells vielded equal activity, 31 ± 1 nanomoles ALA per 30 minutes per 107 cells, whether grown in light or dark. DOVA transaminase could not be separated from glyoxylate transaminase activity by agarose gel permeation or diethylaminoethyl-cellulose column chromatography. In all fractions, glyoxylate transaminase activity was at least 75 times greater than DOVA transaminase activity. DOVA transamination appears to be catalyzed by glyoxylate transaminase, and not to be of physiological significance with respect to chlorophyll synthesis in Euglena.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beale S. I., Castelfranco P. A. The Biosynthesis of delta-Aminolevulinic Acid in Higher Plants: II. Formation of C-delta-Aminolevulinic Acid from Labeled Precursors in Greening Plant Tissues. Plant Physiol. 1974 Feb;53(2):297–303. doi: 10.1104/pp.53.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beale S. I., Foley T., Dzelzkalns V. delta-Aminolevulinic acid synthase from Euglena gracilis. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1666–1669. doi: 10.1073/pnas.78.3.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beale S. I., Gough S. P., Granick S. Biosynthesis of delta-aminolevulinic acid from the intact carbon skeleton of glutamic acid in greening barley. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2719–2723. doi: 10.1073/pnas.72.7.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Collins N., Merrett M. J. The localization of glycollate-pathway enzymes in Euglena. Biochem J. 1975 May;148(2):321–328. doi: 10.1042/bj1480321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dörnemann D., Senger H. The synthesis and properties of 4,5-dioxovaleric acid, a possible intermediate in the biosynthesis of 5-aminolaevulinic acid, and its in vivo formation in Scenedesmus obliquus. Biochim Biophys Acta. 1980 Feb 21;628(1):35–45. doi: 10.1016/0304-4165(80)90349-9. [DOI] [PubMed] [Google Scholar]
- Foley T., Dzelzkalns V., Beale S. I. delta-Aminolevulinic Acid Synthase of Euglena gracilis: Regulation of Activity. Plant Physiol. 1982 Jul;70(1):219–226. doi: 10.1104/pp.70.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassman M., Pluscec J., Bogorad L. delta-Aminolevulinic Acid Transaminase in Chlorella vulgaris. Plant Physiol. 1968 Sep;43(9):1411–1414. doi: 10.1104/pp.43.9.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granick S., Beale S. I. Hemes, chlorophylls, and related compounds: biosynthesis and metabolic regulation. Adv Enzymol Relat Areas Mol Biol. 1978;46:33–203. doi: 10.1002/9780470122914.ch2. [DOI] [PubMed] [Google Scholar]
- Kisaki T., Tolbert N. E. Glycolate and glyoxylate metabolism by isolated peroxisomes or chloroplasts. Plant Physiol. 1969 Feb;44(2):242–250. doi: 10.1104/pp.44.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissel H. J., Heilmeyer L., Jr Nachweis und Bestimmung von gamma-delta-Dioxovaleriansäure: reversible Umwandlung von gamma-delta-Dioxovaleriansäure und delta-Aminolävulinsäure in Ratten. Biochim Biophys Acta. 1969 Feb 18;177(1):78–87. [PubMed] [Google Scholar]
- MAUZERALL D., GRANICK S. The occurrence and determination of delta-amino-levulinic acid and porphobilinogen in urine. J Biol Chem. 1956 Mar;219(1):435–446. [PubMed] [Google Scholar]
- Noguchi T., Mori R. Biosynthesis of porphyrin precursors in mammals. Identity of alanine: gamma, delta-dioxovalerate aminotransferase with alanine:glyoxylate aminotransferase. J Biol Chem. 1981 Oct 25;256(20):10335–10339. [PubMed] [Google Scholar]
- POGO B. G., POGO A. O. INHIBITION BY CHLORAMPHENICOL OF CHLOROPHYLL AND PROTEIN SYNTHESIS AND GROWTH IN EUGLENA GRACILIS. J Protozool. 1965 Feb;12:96–100. doi: 10.1111/j.1550-7408.1965.tb01820.x. [DOI] [PubMed] [Google Scholar]
- Porra R. J., Klein O., Dörnemann D., Senger H. A simple method for the rapid determination of 4,5-dioxovaleric acid in the presence of 2-oxoglutarate and other 2-oxocarboxylic acids. Hoppe Seylers Z Physiol Chem. 1980;361(2):187–190. [PubMed] [Google Scholar]
- Rognstad R., Clark D. G. Effects of aminooxyacetate on the metabolism of isolated liver cells. Arch Biochem Biophys. 1974 Apr 2;161(2):638–646. doi: 10.1016/0003-9861(74)90348-8. [DOI] [PubMed] [Google Scholar]
- URATA G., GRANICK S. Biosynthesis of alpha-aminoketones and the metabolism of aminoacetone. J Biol Chem. 1963 Feb;238:811–820. [PubMed] [Google Scholar]
- Varticovski L., Kushner J. P., Burnham B. F. Biosynthesis of porphyrin precursors. Purification and characterization of mammalian L-alanine:gamma,delta-dioxovaleric acid aminotransferase. J Biol Chem. 1980 Apr 25;255(8):3742–3747. [PubMed] [Google Scholar]


