Abstract
Background and aims:
Lipoprotein(a) [Lp(a)] is an independent risk factor for atherosclerotic cardiovascular disease (ASCVD) but is not included in the Pooled Cohort Equations (PCE). We aimed to assess how well the PCE predict 10-year event rates in individuals with elevated Lp(a), and whether the addition of Lp(a) improves risk prediction.
Methods:
We compared observed versus PCE-predicted 10-year ASCVD event rates, stratified by Lp(a) level and ASCVD risk category using Poisson regression, and evaluated the association between Lp(a) > 50 mg/dL and ASCVD risk using Cox proportional hazards models in the Multi-Ethnic Study of Atherosclerosis (MESA). We evaluated the C-index and net reclassification improvement (NRI) with addition of Lp(a) to the PCE.
Results:
The study population included 6639 individuals (20%, n = 1325 with elevated Lp(a)). The PCE accurately predicted 10-year event rates for individuals with elevated Lp(a) with observed event rates falling within predicted limits. Elevated Lp(a) was associated with increased risk of CVD events overall (HR 1.27, 95% CI 1.00–1.60), particularly in low (HR 2.45, 95% CI 1.40–4.31), and high-risk (HR 1.41, 95% CI 1.02–1.96) individuals. Continuous NRI (95% CI) with the addition of Lp(a) to the PCE for CVD was 0.0963 (0.0158–0.1953) overall, and 0.2999 (0.0876, 0.5525) among low-risk individuals.
Conclusions:
The PCE performs well for event rate prediction in individuals with elevated Lp(a). However, Lp(a) is associated with increased CVD risk, and the addition of Lp(a) to the PCE improves risk prediction, particularly among low-risk individuals. These results lend support for increasing use of Lp(a) testing for risk assessment.
Keywords: Lipoprotein(a), Risk prediction, Cardiovascular disease, ASCVD, PCE
1. Introduction
Lipoprotein(a) [Lp(a)] is causally associated with cardiovascular disease (CVD) through multiple mechanisms [1]. Elevated levels of Lp(a) > 50 mg/dL, which are primarily genetically determined, are present in approximately 25% of the population [2]. In the United States, the Pooled Cohort Equations (PCE) for calculating 10-year atherosclerotic cardiovascular disease (ASCVD) risk have become the standard for clinical risk assessment for primary prevention of ASCVD [3]. Although elevated Lp(a) is an independent ASCVD risk factor and recognized as a risk enhancing factor by the ACC/AHA, it is not incorporated into the PCE [3]. Guideline recommendations for Lp(a) measurement differ significantly, ranging from testing to aid clinical decision making in intermediate risk individuals [4] to universal lifetime testing [5,6]. The relative lack of outcomes data demonstrating that lowering Lp(a) reduces cardiovascular events may explain the discrepancy in recommendations.
Prior studies have shown that the addition of Lp(a) to risk scores, including the Framingham Risk Score (FRS), Reynolds Risk Score (RRS), and the European Systematic Coronary Risk Evaluation (SCORE), improves risk prediction [7,8]. The addition of Lp(a) to the PCE has also been shown to improve risk prediction in European individuals [8]; however, this has not been studied in a diverse, multi-ethnic population using the most recent guideline recommended risk categories (5%, 7.5%, and 20%) with 7.5% representing an important threshold for recommendation of statin therapy [3].
Given the high prevalence of elevated Lp(a), its importance as an independent risk factor, and new drug development which may lead to targeted therapy for primary prevention, it is important to understand the performance of the PCE in individuals according to Lp(a) levels and the incremental value of Lp(a) measurement in addition to the PCE. In this study, we evaluated the use of the PCE in a multi-ethnic cohort of individuals with elevated Lp(a), who were free of baseline CVD, and hypothesized that the addition of Lp(a) to the PCE would improve risk prediction according to varying categories of ASCVD risk.
2. Patients and methods
2.1. Study cohort
We used data from the Multi-Ethnic Study of Atherosclerosis (MESA), a prospective cohort study. Details of the MESA design have been published previously [9]. Briefly, the MESA recruited 6814 adults, free of known baseline CVD, between 2000 and 2002 at six centers across the United States. The study was approved by the institutional review boards at each center, all participants provided written informed consent, and the study conforms to the Declaration of Helsinki. Participants were followed prospectively with follow-up examinations and for clinical events. For this study, individuals with missing data for Lp(a) testing, components of the PCE, or follow-up for clinical events were excluded.
2.2. Cohort characterization and outcomes
Participants in MESA completed standardized questionnaires at recruitment for the collection of demographics and medical history. Cigarette smoking was defined as current, former, or never. Fasting blood samples were collected for laboratory measurements including total cholesterol and high-density lipoprotein cholesterol (HDL-C). LDL-C was calculated by the Friedewald equation [10]. Lp(a) was measured using a latex-enhanced turbidimetric immunoassay (Denka Seiken) of mass concentration and reported in mg/dL. Diabetes was defined using the 2003 American Diabetes Association criteria. Systolic blood pressure (SBP) was collected as the average of multiple seated blood pressure measurements. The 10-year ASCVD risk score was calculated using the PCE based on established methods [11].
The primary outcomes for this study were coronary heart disease events (CHD; composite of fatal CHD, non-fatal myocardial infarction (MI) and resuscitated cardiac arrest), cardiovascular disease events (CVD; composite of CHD and stroke), which most closely mirrors the ASCVD outcome used for the development of the PCE [11], and MI. Adjudication of cardiovascular events was available through 2018.
2.3. Statistical analysis
Baseline characteristics were compared by Lp(a) level (using the guideline recommended threshold of 50 mg/dL4) and by race/ethnicity. Continuous variables were compared using t-tests or Mann-Whitney U tests, and categorical variables were compared using chi square tests.
The cohort was stratified by baseline ASCVD 10-year risk into 3 categories: low (<7.5%), intermediate (7.5-<20%) and high (≥20%) risk. Additional analyses were conducted with the <7.5% category further stratified into <5% and 5-<7.5% (borderline) risk which aligns with guideline recommended categories [3]. For analyses of Lp(a) level, a threshold of 50 mg/dL was used. For analyses of events, events up to 10 years were counted. For each risk category, 10-year CVD event rates by Lp(a) level were calculated by dividing the number of first events over MESA follow-up by person time at risk, then converting to a 10-year rate. Observed 10-year event rates were compared statistically using Poisson regression with adjustment for age and sex and time offset. We constructed cumulative incidence curves comparing risk for CVD events by Lp(a) level within each risk category.
We then evaluated the association between Lp(a) level and CVD events, CHD events and MI within each ASCVD risk category using Cox proportional hazards models. Models were adjusted for components of the PCE (age, sex, total cholesterol, HDL-C, SBP, diabetes mellitus, current cigarette smoking, hypertension treatment) and race/ethnicity. Of note, the PCE was developed in cohorts of predominantly White and Black individuals, while MESA additionally includes Hispanic and Chinese individuals. Total cholesterol, HDL-C, SBP, diabetes mellitus and statin use were treated as time-dependent covariates over the full MESA follow-up. The multiplicative interaction between Lp(a) level and ASCVD risk category was tested in these models. Statin users were not excluded given high rates of baseline and subsequent statin use in MESA and that risk associated with Lp(a) is similar with and without statin use [12] and is present regardless of baseline LDL-C [13]. Instead, statin use was treated as a time-dependent covariate to account for statin use over the entire study period. However, we performed two sensitivity analyses: (1) excluding baseline statin users, and (2) including baseline statin users and not adjusting for statin use in regression models. Finally, we also performed the primary analysis stratified by race/ethnicity.
We assessed the change in predictive value for CVD and CHD events when adding Lp(a) level to the PCE. We assessed concordance by Harrell’s C-index for Cox proportional hazards models including just the PCE, and the PCE + Lp(a) level, stratified by ASCVD risk category. We also calculated a continuous NRI for CVD and CHD at 10-years, stratified by ASCVD risk category with confidence intervals estimated using 200 bootstrap samples, comparing elevated Lp(a) + PCE to the PCE alone, as well as a categorical NRI using risk thresholds of 7.5% and 20%.
As a sensitivity analysis, we performed similar analyses with other guideline recommended risk enhancers [4] available in MESA for their association with CVD events by ASCVD risk category to compare with Lp(a). These included chronic kidney disease [CKD] (defined as estimated glomerular filtration rate <60 mL/min/1.73 m2), high-sensitivity C-reactive protein (hsCRP) ≥2 mg/L, ankle brachial index (ABI) < 0.9, apolipoproteinB (apoB) ≥130 mg/dL, family history (FH) of MI in a 1st degree relative, CAC score and waist circumference and fasting glucose. We also performed an analysis of the association between Lp(a) and CVD events when accounting for coronary artery calcium (CAC) score which was natural log (ln)-transformed [14]. Analyses were performed using R (R Foundation for Statistical Computing, Vienna, Austria). A two-tailed p-value <0.05 was considered statistically significant.
3. Results
After excluding individuals with missing data (n = 175), the study cohort was composed of 6639 individuals. There were 1325 (20%) individuals with Lp(a) > 50 mg/dL. Those with Lp(a) > 50 mg/dL had a greater burden of ASCVD risk factors including higher age, SBP, total cholesterol, LDL-C and prevalence of male sex, hypertension, and diabetes. Those with Lp(a) > 50 mg/dL had higher HDL-C and prevalence of statin use. Participants of Chinese, Hispanic or White race/ethnicity were less likely to have Lp(a) > 50 mg/dL, while Black participants were more likely to have elevated Lp(a). In those with Lp(a) > 50 mg/dL, median ASCVD risk score was higher (9.9% [4.3–20.0%] vs 9.0% [3.6–19.2%], p = 0.02), and there was a higher 10-year incidence of CHD (5.4% vs 3.8%, p = 0.01), CVD (8.1% vs 6.2%, p = 0.01) and MI (3.8% vs 2.7%, p = 0.02) with elevated Lp(a) (Supplemental Table 1). In general, the burden of ASCVD risk factors varied significantly by race/ethnicity with Black individuals generally having the highest prevalence. Lp(a) levels were also highest in Black individuals and similar among other groups. Median ASCVD risk score was highest in Black individuals (11.6 [5.7–20.0]%, p < 0.001), and more Black participants were in the intermediate risk category (41.5%), while more participants were in the low risk category for the other ethnicities (46.3–48.0%, p < 0.001). Black and Hispanic individuals had the highest incidence of CVD events (7.2 and 7.3%, respectively), followed by White (6.5%) and Chinese (3.8%, p = 0.006) individuals (Table 1).
Table 1.
Baseline cohort characteristics by race/ethnicity.
| Black (n = 1814) | Chinese (n = 788) | Hispanic (n = 1475) | White (n = 2562) | p | |
|---|---|---|---|---|---|
| Age, years | 62.1 (10.1) | 62.3 (10.4) | 61.2 (10.3) | 62.5 (10.3) | 0.001 |
| Female sex | 1003 (55.3) | 408 (51.8) | 762 (51.7) | 1331 (52.0) | 0.10 |
| Hypertension | 1079 (59.5) | 292 (37.1) | 610 (41.4) | 987 (38.5) | <0.001 |
| Diabetes | 316 (17.4) | 101 (12.8) | 257 (17.4) | 154 (6.0) | <0.001 |
| Current smoking | 323 (17.8) | 45 (5.7) | 202 (13.7) | 296 (11.6) | <0.001 |
| Hypertension medication use | 910 (50.2) | 225 (28.6) | 480 (32.5) | 847 (33.1) | <0.001 |
| Baseline statin use | 283 (15.7) | 102 (12.9) | 176 (11.9) | 424 (16.6) | <0.001 |
| Systolic blood pressure, mmHg | 131.7 (21.6) | 124.4 (21.6) | 126.6 (21.9) | 123.3 (20.4) | <0.001 |
| Total cholesterol, mg/dL | 189.5 (36.1) | 192.4 (31.8) | 197.7 (37.1) | 195.6 (35.2) | <0.001 |
| High-density lipoprotein cholesterol, mg/dL | 52.3 (15.2) | 49.7 (12.8) | 47.6 (13.1) | 52.3 (15.7) | <0.001 |
| Low-density lipoprotein cholesterol, mg/dL | 116.5 (32.9) | 114.8 (28.9) | 119.3 (32.5) | 116.9 (30.2) | 0.008 |
| Lp(a), mg/dL | 35.6 [20.0, 64.0] | 12.4 [6.7, 23.1] | 13.1 [6.0, 29.1] | 12.1 [5.5, 29.8] | <0.001 |
| ASCVD 10-year risk, % | 11.6 [5.7, 20.0] | 8.2 [2.9, 18.7] | 8.4 [3.3, 20.1] | 8.1 [3.1, 18.1] | <0.001 |
| ASCVD Risk Category | <0.001 | ||||
| <7.5% | 605 (33.4) | 376 (47.7) | 683 (46.3) | 1230 (48.0) | |
| 7.5 - <20% | 752 (41.5) | 234 (29.7) | 417 (28.3) | 772 (30.1) | |
| ≥20% | 457 (25.2) | 178 (22.6) | 375 (25.4) | 560 (21.9) | |
| CHD events | 80 (4.4) | 19(2.4) | 66 (4.5) | 106 (4.1) | 0.08 |
| CVD events | 131 (7.2) | 30 (3.8) | 108 (7.3) | 166 (6.5) | 0.006 |
| MI events | 40 (2.2) | 13 (1.6) | 53 (3.6) | 86 (3.4) | 0.008 |
Values are presented as n (%), mean (SD) or median (IQR). ASCVD = atherosclerotic cardiovascular disease, CHD = coronary heart disease, CVD = cardiovascular disease, Lp(a) = Lipoprotein(a), MI = myocardial infarction.
3.1. Predicted versus actual risk
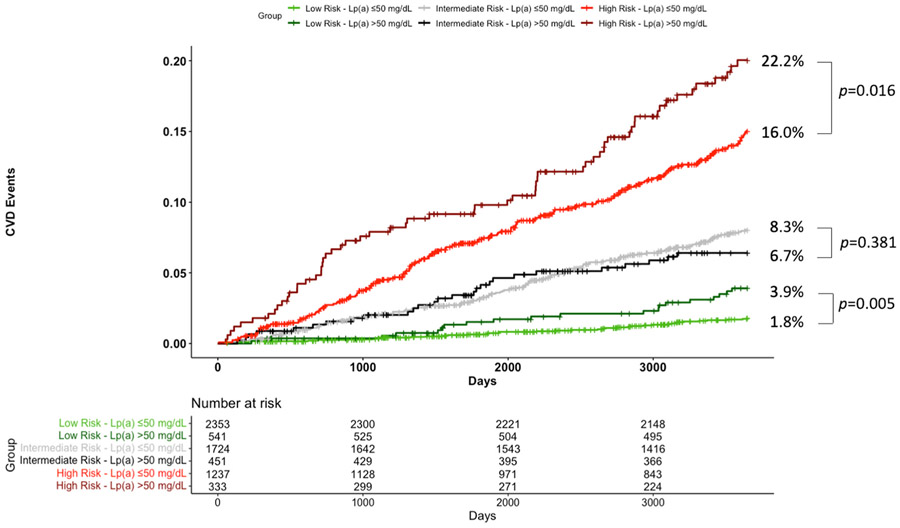
Within the study population, 43.6% (n = 2894) had <7.5%, 32.8% (n = 2175) had 7.5-<20%, and 23.6% (n = 1570) had ≥20% predicted risk. When the <7.5% category was further stratified, 32.2% (n = 2135) had <5% and 11.4% (n = 759) had 5-<7.5% predicted risk. Observed 10-year CVD event rates fell within the limits of the predicted categories of <7.5%, 7.5-<20% and ≥20% (Fig. 1). However, elevated Lp(a) was associated with an increased event rate in the low risk (<7.5%) category (RR 2.17, 95% CI 1.24–3.67) and high risk (≥20%) category (RR 1.45, 95% CI 1.06–1.94). When <7.5% risk was further stratified, there was a trend towards increased event rate with elevated Lp(a) in the <5% risk (RR 2.11, 95% CI 0.95–4.34) and 5-<7.5% risk (RR 2.05, 95% CI 0.91–4.38) categories (Supplemental Table 2).
Fig. 1. Ten year CVD event rate, stratified by ASCVD risk category and Lp(a) level.
Observed 10-year CVD event rates, stratified by predicted 10-year event rates and Lp(a) level, are shown. p-values were determined by Poisson regression. Among those with low (<7.5%), intermediate (7.5-<20%), and high (≥20%) estimated risk, calculated event rates fell within the predicted range, but were significantly higher for those with Lp(a) > 50 mg/dL among those with low and high risk.
3.2. Lp(a) and CVD risk
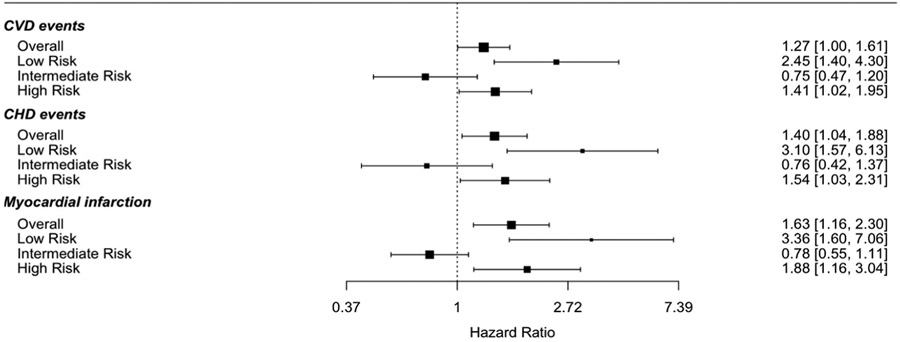
Lp(a) > 50 mg/dL was associated with significantly increased risk for CVD (HR 1.27, 95% CI 1.00–1.60), CHD (HR 1.40, 95% CI 1.04–1.87) and MI (HR 1.63, 95% CI 1.16–2.30) overall in multivariable models adjusted for components of the PCE. There was a statistically significant interaction between Lp(a) > 50 mg/dL and PCE risk category for CVD events (p = 0.006). Risk for first CVD event was significantly greater among those with Lp(a) > 50 mg/dL and predicted risk of <7.5% (HR 2.45, 95% CI 1.40–4.31) and ≥20% (HR 1.41, 95% CI 1.02–1.96) but not in intermediate-risk individuals. Risk for first CHD event was greater among those with Lp(a) > 50 mg/dL and predicted risk of <7.5% (HR 3.10, 95% CI 1.57–6.14) and predicted risk ≥20% (HR 1.54, 95% CI 1.03–2.31, Fig. 2). Similar results were seen for MI (Supplemental Table 3). When low risk was further stratified, elevated Lp(a) was associated with increased CVD and CHD (HR 2.55, 95% CI 1.18–5.52) risk in the borderline risk category (5-<7.5%), and borderline associated with increased CVD risk in the <5% risk category (Supplemental Table 4). Similar results were seen when excluding baseline statin use (HR 2.14, 95% CI 1.18, 3.88 in low risk; HR 1.43, 95% CI 0.98–2.09 in high risk) or not adjusting for statin use (HR 2.25, 95% CI 1.29–3.92 in low risk; HR 1.43, 95% CI 1.03–1.97 in high risk).
Fig. 2. Association between Lp(a) > 50 mg/dL and CVD events by ASCVD risk category.
Models adjusted for PCE components: age, sex, race, total cholesterol (time dependent), high-density lipoprotein cholesterol (time dependent), systolic blood pressure (time dependent), diabetes mellitus (time dependent), current smoking, hypertension medications, and statin use (time dependent).
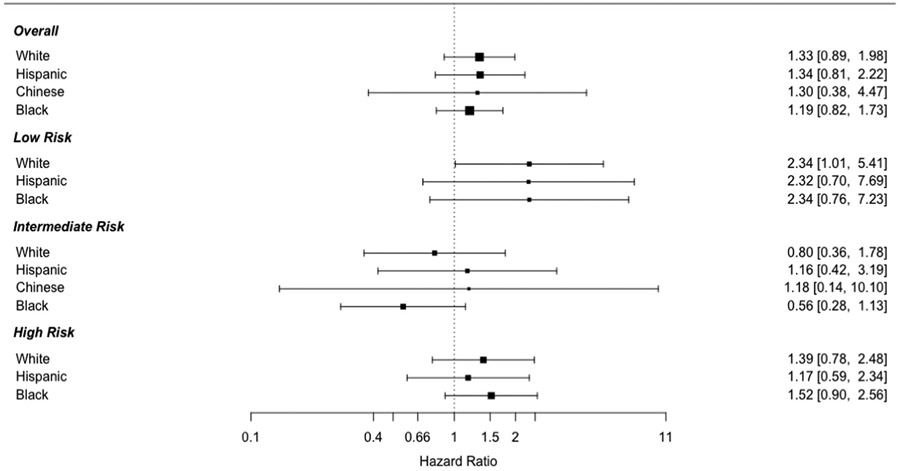
There was no significant interaction between Lp(a) level >50 mg/dL and sex (p = 0.95) or race/ethnicity (p = 0.92) for CVD events. However, given the known association between race/ethnicity and Lp(a) levels, we performed the above analysis stratified by race/ethnicity. The positive association between Lp(a) > 50 mg/dL and CVD events was consistent across racial/ethnic groups overall, and in those in the low and high-risk groups. Adjusted analyses could not be performed in Chinese individuals in the low and high-risk groups due to low number of events, but the associations were consistent in unadjusted analyses. In the intermediate risk group, however, results were more discordant, though none were significant. Lp(a) > 50 mg/dL was inversely associated with events in White and Black individuals, but positively associated in Hispanic and Chinese individuals (Fig. 3).
Fig. 3. Association between Lp(a) > 50 mg/dL and CVD events by ASCVD risk category and race/ethnicity.
Models adjusted for PCE components: age, sex, total cholesterol (time dependent), high-density lipoprotein cholesterol (time dependent), systolic blood pressure (time dependent), diabetes mellitus (time dependent), current smoking, hypertension medications, and statin use (time dependent).
3.3. Risk prediction with addition of Lp(a) to the PCE
With the addition of Lp(a) > 50 mg/dL to the PCE, the C-index for CVD or CHD events was not significantly different compared to the PCE alone for the overall cohort. There was improvement in the C-index for CVD among low risk (0.683, SE 0.033 vs 0.659, SE 0.035) and high-risk individuals (0.609, SE 0.019 vs 0.592, SE 0.020) and for CHD among low risk (0.687, SE 0.044 vs 0.649, SE 0.046) and high-risk individuals (0.598, SE 0.026 vs 0.573, SE 0.024), but not intermediate risk individuals (Table 2). With further stratification of the <7.5% risk category, the C-index for CVD was improved among those at <5% risk (0.644, SE 0.044 vs 0.613, SE 0.044) and 5-<7.5% risk (0.649, SE 0.046 vs 0.586, SE 0.037). Greater improvement was seen for CHD prediction (Supplemental Table 4).
Table 2.
Model performance for CHD and CVD events with addition of Lp(a) level to the PCE.
| CVD | ||||
|---|---|---|---|---|
| Overall | Low risk (<7.5%) | Intermediate risk (7.5-<20%) | High risk (≥20%) | |
| C-index (SE) | ||||
| PCE alone | 0.739 (0.011) |
0.659 (0.035) |
0.594 (0.022) |
0.592 (0.020) |
| PCE + Lp(a) > 50 mg/dL | 0.739 (0.011) |
0.683 (0.033) |
0.595 (0.022) |
0.609 (0.019) |
| PChange | 0.000 | +0.024 | +0.001 | +0.017 |
| Continuous NRI (95% CI) | ||||
| Overall |
0.0963
(0.0158, 0.1953) |
0.2999
(0.0876, 0.5525) |
0.0747 (−0.0130, 0.2283) |
0.1249
(0.0032, 0.2437) |
| Events |
−0.5114
(−0.5851, −0.4122) |
−0.3328 (−0.5258, 0.0462) |
0.6543 (−0.6059, 0.7898) |
−0.4711
(−0.5818, −0.3058) |
| Non-events |
0.6077
(0.5895, 0.6269) |
0.6327
(0.5949, 0.6626) |
−0.5795 (−0.6111, 0.6073) |
0.5959
(0.5460, 0.6427) |
| CHD | ||||
| Overall | Low risk (<7.5%) | Intermediate risk (7.5-<20%) | High risk (≥20%) | |
| C-index (SE) | ||||
| PCE alone | 0.735 (0.014) |
0.649 (0.046) |
0.607 (0.026) |
0.573 (0.026) |
| PCE + Lp(a) > 50 mg/dL | 0.737 (0.013) |
0.687 (0.044) |
0.609 (0.026) |
0.598 (0.024) |
| Change | +0.002 | +0.038 | +0.002 | +0.025 |
| Continuous NRI (95% CI) | ||||
| Overall |
0.1248
(0.0321, 0.2405) |
0.4432
(0.0717, 0.7827) |
0.0667
(0.0001, 0.2337) |
0.1295
(0.0052, 0.2661) |
| Events |
−0.4816
(−0.5691, −0.3748) |
−0.1889 (−0.5583, 0.1317) |
0.6488 (−0.5839, 0.8066) |
−0.4597
(−0.5891, −0.2872) |
| Non-events |
0.6064
(0.5879, 0.6297) |
0.6321
(0.6035, 0.6603) |
−0.5821 (−0.6108, 0.6158) |
0.5892
(0.5372, 0.6352) |
NRI = Net Reclassification Improvement. PCE = Pooled Cohort Equations.
In the whole cohort, continuous NRI with the addition of Lp(a) > 50 mg/dL to the PCE was significant for CVD events (0.0963, 95% CI 0.0158, 0.1953) and CHD events (0.1248, 95% CI 0.0321–0.2405) overall. Among those at low risk (<7.5%), the continuous NRI was 0.2999 (95% CI 0.0876–0.5525) for CVD events and 0.4432 (0.0717–0.7827) for CHD events. There was also significant NRI for CVD and CHD events among high-risk individuals. Improvement in risk prediction was achieved primarily by reclassifying non-cases as lower risk (Table 2). When <7.5% risk was further stratified, there was a significant NRI for CVD in both <5% risk (0.2708, 95% CI 0.0109–0.5484) and 5-<7.5% risk (0.3124, 95% CI 0.0291–0.6466). Similar results were seen for CHD with a greater improvement in the 5-<7.5% risk group (Supplemental Table 4). Similar results were seen for C-index improvement and continuous NRI when excluding statin users.
3.4. Evaluation of other risk enhancers
When other risk enhancers were evaluated, ABI, CAC score, and FH of MI, were associated with increased CVD risk in the overall cohort; however, only CAC resulted in a meaningful improvement in C-index and categorical NRI, while CAC, FH MI, hsCRP and waist circumference + fasting glucose resulted in significant continuous NRI. Among low-risk individuals, only CAC score and FH MI were associated with CVD risk, only CAC improved the C-index more than Lp(a), and only CAC and FH MI resulted in greater continuous NRI (Supplemental Table 5). The association between Lp(a) > 50 mg/dL and CVD events among those with <7.5% predicted risk was slightly attenuated, but persisted after additional adjustment for CAC score, however (HR 2.21, 95% CI 1.25–3.90, Supplemental Table 6).
4. Discussion
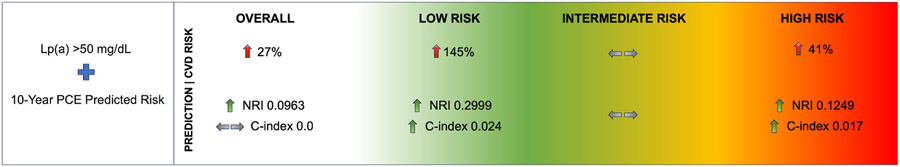
In a multi-ethnic, primary prevention population in the United States, the PCE accurately predicted 10-year event rates in individuals with elevated Lp(a). Elevated Lp(a) was associated with increased CVD and CHD risk overall and in those at low and high-risk, which was consistent across racial/ethnic groups. The addition of Lp(a) to the PCE improves risk prediction for both CVD and CHD events, particularly among those at low and high-risk (Fig. 5). This has important clinical implications as low-risk individuals are generally not offered statin therapy. Lp(a) compared favorably to several other guideline recommended risk enhancers, and study results were also consistent when accounting for CAC score. Our results suggest that Lp(a) should be accounted for in risk stratification methods for primary prevention, and more widespread Lp(a) testing should be considered.
Fig. 5.
When added to the Pooled Cohort Equations (PCE), Lp(a) > 50 mg/dL is associated with increased CVD risk and improvement in risk prediction overall. Lp(a) > 50 mg/dL is also associated with increased risk and improvement in risk prediction among low risk (<7.5%) and high risk (≥20%), but not intermediate risk (7.5%-<20%) individuals.
Previous studies have demonstrated that the addition of Lp(a) to traditional CVD risk factors improves risk prediction. Adding Lp(a) improved the c-statistic for CVD risk slightly, but not the NRI in a pooled study of participants from 37 cohorts [15]. It improved risk classification for MI and CHD risk, particularly among those with intermediate risk (10–20%) in a Danish population [16]. A composite of biomarkers, including Lp(a), resulted in improved risk classification for MI in a study in Norway [17]. In another study of European individuals, the addition of Lp(a) slightly improved the c-statistic for CHD and CVD events, but the NRI was not significant [18].
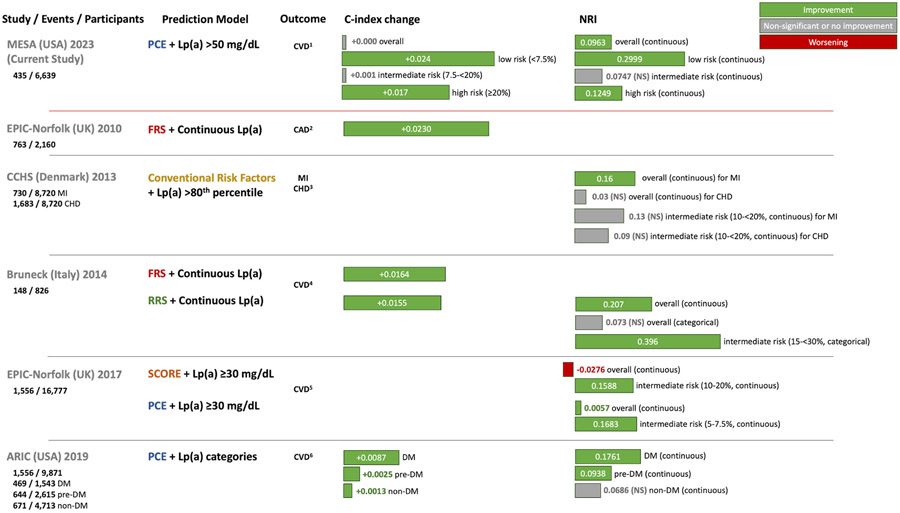
The addition of Lp(a) to various clinically used risk scores has also been shown to improve risk prediction (Fig. 4). The addition of Lp(a) to the FRS improved the c-statistic for CAD in a study of individuals in the European Prospective Investigation of Cancer (EPIC)-Norfolk [19]. In a study involving participants from the Bruneck Study, the addition of Lp(a) levels to the FRS and RRS improved CVD risk discrimination and reclassification, particularly among those at intermediate risk [7]. However, the PCE subsequently supplanted prior quantitative risk scores for ASCVD risk assessment in U.S. guidelines, initially using risk thresholds of 5% and 7.5% [20]. In another study of individuals in the EPIC-Norfolk study, the addition of Lp(a) levels to the PCE and the Systematic Coronary Risk Evaluation (SCORE) score improved risk prediction among intermediate risk individuals. However, intermediate risk was defined as 5–7.5% for the PCE [8]. Similar to our results, these studies demonstrated improvement in risk prediction primarily by reclassifying non-cases as lower risk [7,8]. This is likely because most individuals have low Lp(a), and are thus more likely to be reclassified downward. These prior studies were conducted with less diverse populations in Europe, are older with recruitment taking place in the 1990s, and utilized risk scores which are now less commonly used (FRS, RRS) or used previous risk thresholds for the PCE. Practice guidelines regarding the PCE were further refined to include risk thresholds of 5%, 7.5% (now threshold for intermediate risk) and 20% for lipid management; statin therapy was recommended at 7.5% risk (class I recommendation) in the context of other risk factors. Consideration of statin therapy guided by risk enhancing factors was also recommended at a risk of 5% to <7.5% with a weaker recommendation (class IIb) [4]. Finally, a recent study demonstrated that the addition of Lp(a) to PCE variables improved the c-statistic and risk classification for CVD events in participants in the biracial ARIC (Atherosclerosis Risk in Communities) cohort, particularly in those with diabetes and pre-diabetes [21]. Our study expands on this literature, and is the first study, to our knowledge, to evaluate the use of the most currently recommended method for risk stratification in the U.S. (the PCE) using up to date risk thresholds (refined in 2018) in a multi-ethnic U.S. population.
Fig. 4. Studies of improvement in CVD risk prediction with the addition of Lp(a) to risk scores.
A summary of the current study as well as prior studies (EPIC-Norfolk 2010 [19], CCHS [16], Bruneck [7], EPIC-Norfolk 2017 [8], ARIC [21]) is shown illustrating the base score used, how Lp(a) was added, and the results for change in C-index and continuous NRI. In the present study, Lp(a) > 50 mg/dL was associated with increased CVD risk and the addition of Lp(a) > 50 mg/dL to the Pooled Cohort Equations resulted in improved predictive value overall and in those at low and high 10-year risk, but not in those at intermediate risk. ARIC = Atherosclerosis Risk in Communities. CCHS = Copenhagen City Heart Study. DM = diabetes. EPIC = European Prospective Investigation of Cancer. FRS = Framingham Risk Score. MESA = Multi-Ethnic Study of Atherosclerosis. NRI = net reclassification improvement. PCE = Pooled Cohort Equations. RRS = Reynolds Risk Score. SCORE = Systematic Coronary Risk Evaluation. Definition of outcomes [1]: cardiovascular disease events: fatal coronary heart disease, non-fatal myocardial infarction, resuscitated cardiac arrest, stroke [2]; coronary artery disease events: unstable angina, stable angina, myocardial infarction [3]; coronary heart disease events: angina, myocardial infarction, other ischemic heart disease [4]; cardiovascular disease events: vascular death, nonfatal myocardial infarction, unstable angina, acute coronary intervention, ischemic stroke [5]; cardiovascular disease events: non-fatal myocardial infarction, coronary heart disease death, stroke [6]; cardiovascular disease events: coronary heart disease death, myocardial infarction, coronary revascularization, embolic or thrombotic stroke.
Our results demonstrated that Lp(a) compared favorably to other risk enhancers. Elevated Lp(a) was associated with CVD risk overall, resulted in improvement in the C-index in low and high-risk individuals, and significant NRI overall and in low and high-risk individuals. Several other risk enhancers assessed were not associated with overall CVD risk and resulted in lesser degrees of improvement in predictive value with the primary exceptions of CAC and FH of MI. Additionally, our results were consistent when accounting for CAC. This aligns with previous results showing that Lp(a) and CAC score are independently associated with CVD risk [22]. Thus, the increased risk of CVD events with elevated Lp(a) persists with consideration of CAC score.
Prior studies have demonstrated increased risk associated with Lp(a) across risk categories, and that not including Lp(a) in risk assessment leads to an underestimation of risk. In a study utilizing data from participants of European ancestry in the UK biobank, increasing levels of Lp (a) (from 7 mg/dL to 150 mg/dL) were associated with progressively increasing risk across categories of estimated lifetime ASCVD risk. This was even true in the lowest risk category of 5% lifetime risk – the event rate for those with Lp(a) of 30 mg/dL was 1.1% versus 8.6% for those with Lp(a) of 150 mg/dL [23]. Similarly, a risk calculator developed to estimate the risk of an MI or stroke up to the age of 80 years illustrates the increased risk when accounting for elevated Lp(a) versus not accounting for it [24].
While Lp(a) was associated with increased CVD risk overall and in low and high-risk individuals, it was not associated with increased risk in intermediate risk individuals. There are a few potential reasons for this. The risk groups varied in their racial/ethnic composition; among Black individuals, intermediate risk was most common, while low risk was most common in the other groups. This may impact our findings as the stratified analysis, though limited by power, demonstrated differential associations between Lp(a) and risk in the intermediate risk group by racial/ethnic group. Additionally, while prior studies have observed increased risk associated with Lp(a) and improved risk prediction in intermediate risk individuals, these studies, as noted above, either used different risk scores or different risk thresholds – the Bruneck study utilized FRS and RRS and defined intermediate risk as 15-<30% and EPIC-Norfolk defined intermediate as 10–20% for SCORE, and 5–7.5% for the PCE; our study used the PCE and defined intermediate risk as 7.5-<20% (Fig. 4). Another potential reason is the emphasis on 10-year risk prediction, which is the current paradigm recommended by major society guidelines. In a study utilizing the UK Biobank, increasing Lp(a) levels were associated with increased ASCVD risk across categories of lifetime risk [23]. Finally, though risk enhancers are typically considered in intermediate risk individuals, we also observed that only four of the other eight risk enhancers evaluated were associated with increased risk in this group, and only three resulted in a significant continuous NRI. Further studies are needed with larger populations to clarify these issues further.
Our study has several clinical implications. First, for CVD event rate prediction, the PCE performs well for individuals at low, intermediate, or high risk with elevated Lp(a), with calculated 10-year event rates falling within the limits of the predicted rates based on the PCE. However, elevated Lp(a) is associated with increased risk, and the addition of Lp(a) to the PCE significantly improved risk prediction overall, particularly among low and high-risk individuals. These findings were consistent across racial/ethnic groups. Thus, Lp(a) appears to add the most useful information for risk prediction in those at low risk. This is clinically relevant as the ACC/AHA guideline recommendation is to consider Lp(a) as a risk enhancing factor in borderline and intermediate risk individuals. Though limited by power, our results appeared consistent when low risk was stratified into 5-<7.5% (borderline) risk and <5% risk; thus, Lp(a) testing may be considered more broadly among those at low risk. Additionally, the strength of recommendation for statin prescription is significantly different between those at borderline and intermediate risk in clinical guidelines, and statin therapy is not recommended for <5% risk [3]. Reclassification of risk among low/borderline risk individuals with elevated Lp(a) may also change clinical decision making with regard to treatment for hypertension and aspirin therapy for primary prevention based on current guideline thresholds [3]. Importantly, the C-index for the PCE alone for CVD in the overall cohort was consistent with previously published results in MESA [25]. These findings may also have significance with the advent of targeted therapy for Lp(a) lowering [26]. With the potential for future therapy for primary prevention in people with elevated Lp(a), risk stratification tools will need to additionally account for Lp(a) to help guide this therapy. Further study is needed to address the development of risk stratification tools which account for Lp(a) and improve risk prediction among those with and without elevated Lp(a). Elevated Lp(a) was also associated with risk for events among individuals at high (≥20%) predicted 10-year risk. However, given that these individuals are already at high risk and should be offered aggressive preventive therapy, this result likely does not change clinical management.
Another potentially significant finding is that the PCE overestimates risk for CVD events among individuals at borderline and high predicted risk without elevated Lp(a). This is clinically relevant given that consideration of statin therapy starts at the 5% risk threshold and is more strongly recommended for high risk in the current guidelines [4]. Prior studies have demonstrated that the PCE may overestimate risk in general [27]. Our study demonstrates that this may partially be explained by, and potentially improved, by accounting for Lp(a) as the PCE overestimates risk in those with normal Lp(a).
Our study has limitations. As an observational study, our results are subject to residual confounding. Additionally, MESA covers a time period during which changes have occurred in primary prevention including management of lipids, hypertension and diabetes and event rates may differ if the baseline exams were conducted today. Additionally, there was a high rate of statin use at baseline and in subsequent exams in MESA. We attempted to address this with the use of time dependent covariates in multivariable regression models and in sensitivity analyses. While 20% of individuals in the cohort had Lp(a) > 50 mg/dL, only 5% had Lp(a) > 100 mg/dL, potentially limiting the generalizability of our results in individuals with very elevated Lp(a) levels and limiting our ability to evaluate other Lp(a) thresholds. Though this is the most ethnically diverse study of Lp(a) and risk stratification tools to date, all ethnic groups are not well represented. In particular, South Asians, known to be at higher CVD risk and to have higher Lp(a) levels, are not represented. There was also insufficient power to perform analyses stratified by race/ethnicity, though the interaction test between Lp(a) and race/ethnicity was not significant. Finally, Lp(a) assays were reported in mass units and measuring Lp(a) in molar concentration is now the preferred method. This is unlikely to affect the conclusions of this study as mass units underestimate Lp(a) values and are more likely to underestimate risk [28].
4.1. Conclusions
The PCE performs well for predicting event rates in individuals with elevated Lp(a). The addition of Lp(a) to the PCE, however, significantly improves risk prediction. Lp(a) should be considered as a risk factor in risk stratification tools, particularly with the potential for Lp(a)-targeted therapy in the future, and Lp(a) testing should be more widely performed.
Supplementary Material
Acknowledgements
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. HB and ST had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Financial support
HB was supported by National Institutes of Health, Grants 1KL2TR001444 and 5T32HL079891. RR is supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number T32HL076132. ST is supported by NIH R01 HL159156. The MESA was supported by contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.atherosclerosis.2023.117217.
Declaration of competing interest
ST is a co-inventor and receives royalties from patents owned by UCSD and is a co-founder and has an equity interest in Oxitope and Kleanthi Diagnostics, and has a dual appointment at UCSD and Ionis Pharmaceuticals. Although these relationships have been identified for conflict-of-interest management based on the overall scope of the project, the research findings included in this particular publication may not necessarily relate to the interests of the above companies. The terms of this arrangement have been reviewed and approved by the University of California, San Diego in accordance with its conflict-of-interest policies. MDS – Scientific advisory boards for Amgen, Novartis, Novo Nordisk; Consultant for Regeneron; HB received consulting fees from Kaneka Medical America. The other co-authors have nothing to disclose.
References
- [1].Tsimikas S, A test in context: lipoprotein(a): diagnosis, prognosis, controversies, and emerging therapies, J. Am. Coll. Cardiol 69 (2017) 692–711. [DOI] [PubMed] [Google Scholar]
- [2].Varvel S, McConnell JP, Tsimikas S, Prevalence of elevated lp(a) mass levels and patient thresholds in 532 359 patients in the United States, Arterioscler. Thromb. Vasc. Biol 36 (2016) 2239–2245. [DOI] [PubMed] [Google Scholar]
- [3].Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, et al. , ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American college of cardiology/American heart association task force on clinical practice guidelines, J. Am. Coll. Cardiol 74 (2019) e177–e232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, et al. , AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American college of cardiology/American heart association task force on clinical practice guidelines, J. Am. Coll. Cardiol 73 (2018) e285–e350. [DOI] [PubMed] [Google Scholar]
- [5].Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, et al. , ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk, Eur. Heart J 41 (2019) 111–188. [DOI] [PubMed] [Google Scholar]
- [6].Pearson GJ, Thanassoulis G, Anderson TJ, Barry AR, Couture P, Dayan N, Francis GA, Genest J, Grégoire J, Grover SA, et al. , Canadian cardiovascular society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in adults, Can. J. Cardiol 37 (2021) 1129–1150. [DOI] [PubMed] [Google Scholar]
- [7].Willeit P, Kiechl S, Kronenberg F, Witztum JL, Santer P, Mayr M, Xu Q, Mayr A, Willeit J, Tsimikas S, Discrimination and net reclassification of cardiovascular risk with lipoprotein(a): prospective 15-year outcomes in the Bruneck Study, J. Am. Coll. Cardiol 64 (2014) 851–860. [DOI] [PubMed] [Google Scholar]
- [8].Verbeek R, Sandhu MS, Hovingh GK, Sjouke B, Wareham NJ, Zwinderman AH, Kastelein JJP, Khaw KT, Tsimikas S, Boekholdt SM, Lipoprotein(a) improves cardiovascular risk prediction based on established risk algorithms, J. Am. Coll. Cardiol 69 (2017) 1513–1515. [DOI] [PubMed] [Google Scholar]
- [9].Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr., Kronmal R, Liu K, et al. , Multi-ethnic study of atherosclerosis: objectives and design, Am. J. Epidemiol 156 (2002) 871–881. [DOI] [PubMed] [Google Scholar]
- [10].Friedewald WT, Levy RI, Fredrickson DS, Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge, Clin. Chem 18 (1972) 499–502. [PubMed] [Google Scholar]
- [11].Goff DC, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, et al. , ACC/AHA guideline on the assessment of cardiovascular risk, Circulation 129 (2013) S49–S73. [DOI] [PubMed] [Google Scholar]
- [12].Willeit P, Ridker PM, Nestel PJ, Simes J, Tonkin AM, Pedersen TR, Schwartz GG, Olsson AG, Colhoun HM, Kronenberg F, et al. , Baseline and on-statin treatment lipoprotein(a) levels for prediction of cardiovascular events: individual patient-data meta-analysis of statin outcome trials, Lancet 392 (2018) 1311–1320. [DOI] [PubMed] [Google Scholar]
- [13].Rikhi R, Hammoud A, Ashburn N, Snavely AC, Michos ED, Chevli P, Tsai MY, Herrington D, Shapiro MD, Relationship of low-density lipoprotein-cholesterol and lipoprotein(a) to cardiovascular risk: the Multi-Ethnic Study of Atherosclerosis (MESA), Atherosclerosis 363 (2022) 102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, et al. , Coronary calcium as a predictor of coronary events in four racial or ethnic groups, N. Engl. J. Med 358 (2008) 1336–1345. [DOI] [PubMed] [Google Scholar]
- [15].Di Angelantonio E, Gao P, Pennells L, Kaptoge S, Caslake M, Thompson A, Butterworth AS, Sarwar N, Wormser D, Saleheen D, et al. , Lipid-related markers and cardiovascular disease prediction, JAMA 307 (2012) 2499–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kamstrup PR, Tybjærg-Hansen A, Nordestgaard BG, Extreme lipoprotein(a)levels and improved cardiovascular risk prediction, J. Am. Coll. Cardiol 61 (2013) 1146–1156. [DOI] [PubMed] [Google Scholar]
- [17].Wilsgaard T, Mathiesen EB, Patwardhan A, Rowe MW, Schirmer H, Løchen ML, Sudduth-Klinger J, Hamren S, Bønaa KH, Njølstad I, Clinically significant novel biomarkers for prediction of first ever myocardial infarction: the Tromsø Study, Circ Cardiovasc Genet 8 (2015) 363–371. [DOI] [PubMed] [Google Scholar]
- [18].Waldeyer C, Makarova N, Zeller T, Schnabel RB, Brunner FJ, Jørgensen T, Linneberg A, Niiranen T, Salomaa V, Jousilahti P, et al. , Lipoprotein(a) and the risk of cardiovascular disease in the European population: results from the BiomarCaRE consortium, Eur. Heart J 38 (2017) 2490–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Tsimikas S, Mallat Z, Talmud PJ, Kastelein JJ, Wareham NJ, Sandhu MS, Miller ER, Benessiano J, Tedgui A, Witztum JL, et al. , Oxidation-specific biomarkers, lipoprotein(a), and risk of fatal and nonfatal coronary events, J. Am. Coll. Cardiol 56 (2010) 946–955. [DOI] [PubMed] [Google Scholar]
- [20].Stone NJ, Robinson JG, Lichtenstein AH, Merz CNB, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, et al. , ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults, Circulation 129 (2013) S1–S45. [DOI] [PubMed] [Google Scholar]
- [21].Saeed A, Sun W, Agarwala A, Virani SS, Nambi V, Coresh J, Selvin E, Boerwinkle E, Jones PH, Ballantyne CM, et al. , Lipoprotein(a) levels and risk of cardiovascular disease events in individuals with diabetes mellitus or prediabetes: the Atherosclerosis Risk in Communities study, Atherosclerosis 282 (2019) 52–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mehta A, Vasquez N, Ayers CR, Patel J, Hooda A, Khera A, Blumenthal RS, Shapiro MD, Rodriguez CJ, Tsai MY, et al. , Independent association of lipoprotein (a) and coronary artery calcification with atherosclerotic cardiovascular risk, J. Am. Coll. Cardiol 79 (2022) 757–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kronenberg F, Mora S, Stroes ESG, Ference BA, Arsenault BJ, Berglund L, Dweck MR, Koschinsky M, Lambert G, Mach F, et al. , Lipoprotein(a) in atherosclerotic cardiovascular disease and aortic stenosis: a European Atherosclerosis Society consensus statement, Eur. Heart J 43 (2022) 3925–3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kronenberg F, Mora S, Stroes ESG, Ference BA, Arsenault BJ, Berglund L, Dweck MR, Koschinsky ML, Lambert G, Mach F, et al. , Frequent questions and responses on the 2022 lipoprotein(a) consensus statement of the European Atherosclerosis Society, Atherosclerosis 374 (2023) 107–120. [DOI] [PubMed] [Google Scholar]
- [25].Whelton SP, Marshall CH, Cainzos-Achirica M, Dzaye O, Blumenthal RS, Nasir K, McClelland RL, Blaha MJ, Pooled Cohort Equations and the competing risk of cardiovascular disease versus cancer: multi-Ethnic study of atherosclerosis, Am. J. Prev. Cardiol 7 (2021), 100212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tsimikas S, Karwatowska-Prokopczuk E, Gouni-Berthold I, Tardif JC, Baum SJ, Steinhagen-Thiessen E, Shapiro MD, Stroes ES, Moriarty PM, Nordestgaard BG, et al. , Lipoprotein(a) reduction in persons with cardiovascular disease, N. Engl. J. Med 382 (2020) 244–255. [DOI] [PubMed] [Google Scholar]
- [27].DeFilippis AP, Young R, McEvoy JW, Michos ED, Sandfort V, Kronmal RA, McClelland RL, Blaha MJ, Risk score overestimation: the impact of individual cardiovascular risk factors and preventive therapies on the performance of the American Heart Association-American College of Cardiology-Atherosclerotic Cardiovascular Disease risk score in a modern multi-ethnic cohort, Eur. Heart J 38 (2017) 598–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Marcovina SM, Albers JJ, Lipoprotein (a) measurements for clinical application, J. Lipid Res 57 (2016) 526–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.